Abstract
Background
PM01183 is a new compound that blocks active transcription, produces DNA breaks and apoptosis, and affects the inflammatory microenvironment. PM01183 showed strong antitumor activity in preclinical models of cisplatin-resistant epithelial ovarian cancer.
Patients and methods
Patients with platinum-resistant/refractory ovarian cancer were included in a two-stage, controlled, randomized (in a second stage), multicenter, phase II study. Primary endpoint was overall response rate (ORR) by RECIST and/or GCIG criteria. The exploratory first stage (n = 22) confirmed the activity of PM01183 as a single agent at 7.0 mg flat dose every 3 weeks (q3wk). The second stage (n = 59) was randomized and controlled with topotecan on days 1–5 q3wk or weekly (every 4 weeks, q4wk).
Results
ORR was 23% (95% CI, 13%–37%) for 52 PM01183-treated patients. Median duration of response was 4.6 months (95% CI, 2.5–6.9 months), and 23% (95% CI, 0%–51%) of responses lasted 6 months or more. Ten of the 12 confirmed responses were reported for 33 patients with platinum-resistant disease [ORR = 30% (95% CI, 16%–49%)]; for the 29 patients treated with topotecan in the second stage, no responses were found. Median PFS for all PM01183-treated patients was 4.0 months (95% CI, 2.7–5.6 months), and 5.0 months (95% CI, 2.7–6.9 months) for patients with platinum-resistant disease.
Grade 3/4 neutropenia in 85% of patients; febrile neutropenia in 21% and fatigue (grade 3 in 35%) were the principal safety findings for PM01183.
Conclusion
PM01183 is an active drug in platinum-resistant/refractory ovarian cancer and warrants further development. The highest activity was observed in platinum-resistant disease. Its safety profile indicates the dose should be adjusted to body surface area (mg/m2).
Trial code
EudraCT 2011-002172-16.
Keywords: lurbinectedin, topotecan, ovarian cancer, platinum resistant
Introduction
PM01183 (lurbinectedin) is a synthetic tetrahydroisoquinoline that is a selective inhibitor of active transcription. The mechanism involve the irreversible stalling of elongating RNA polymerase II (Pol II) on the DNA template and its specific degradation by the ubiquitin/proteasome machinery, with subsequent DNA breaks and apoptosis [1]. Furthermore, PM01183 affects the inflammatory microenvironment, with selective apoptotic-inducing effect on tumor-associated macrophages, and specific inhibition of inflammatory cytokines production [2]. Strong preclinical antitumor activity was observed in cisplatin-resistant epithelial ovarian cancer models [3]. This two-stage phase II trial evaluated PM01183 activity in terms of overall response rate (ORR) in patients with platinum-resistant or platinum-refractory ovarian cancer.
Materials and methods
The study was conducted at nine sites following the ICH GCP guidelines, and approved by the respective Research Ethics Committees. Written informed consent was obtained from all patients.
Eligibility criteria
Selection criteria included: age ≥18 years; histologically/cytologically confirmed epithelial ovarian, fallopian tube or primary peritoneal cancer; advanced measurable (per RECIST v.1.1) or non-measurable disease [per Gynecologic Cancer InterGroup (GCIG) criteria]; platinum-resistant (disease relapse or progression <6 months after last platinum-containing chemotherapy) or platinum-refractory (disease that did not respond during last platinum-containing chemotherapy) disease; <3 prior lines of cytotoxic-containing chemotherapy; Eastern Cooperative Oncology Group performance status (ECOG PS) score ≤2; recovery from previous toxicities; and adequate bone marrow, hepatic, renal, and metabolic function.
Criteria for exclusion were: prior treatment with topotecan; brain metastases or leptomeningeal involvement; pregnancy or lactation; prior pelvic irradiation; concomitant conditions such as unstable angina, myocardial infarction, congestive heart failure, generalized edemas or grade ≥3 ascites, immune deficiency; active uncontrolled infection, myopathy.
Study design
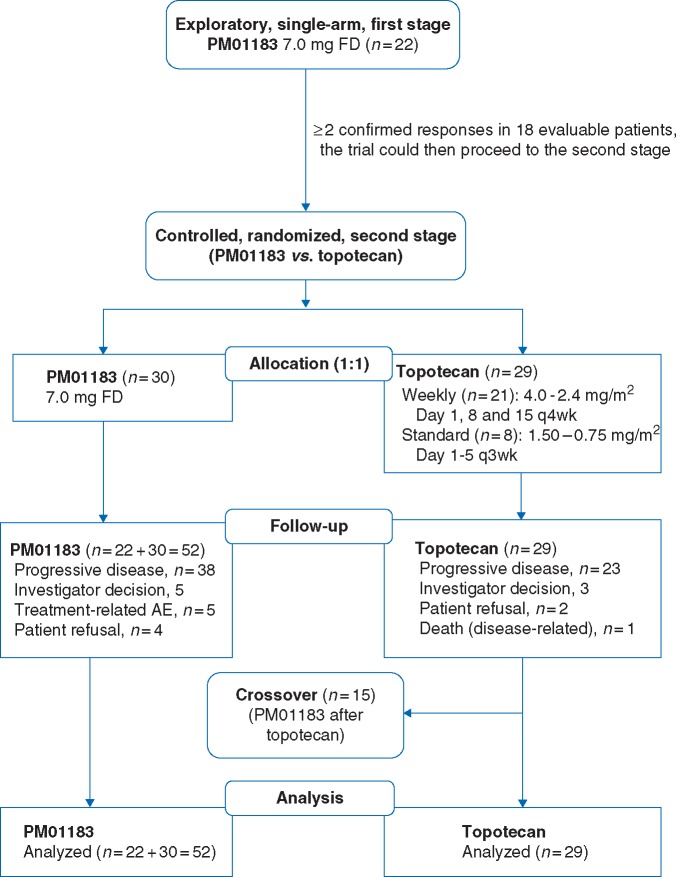
This was a two-stage phase II clinical trial (Figure 1). First stage explored the activity of PM01183 7.0 mg flat dose (FD) every 3 weeks (q3wk) in platinum-resistant/refractory advanced ovarian cancer. Second stage was randomized and controlled. Control arm initially consisted of standard pegylated liposomal doxorubicin (PLD) every 4 weeks (q4wk) or standard i.v. topotecan daily times five q3wk [4]. However, due to a worldwide shortage of PLD, PLD was replaced with topotecan, including a weekly schedule, based on research suggesting better tolerability for weekly topotecan compared with standard regimen [5]. Patients in the second stage were assigned to each treatment arm, PM01183 or topotecan, by strata (platinum refractory versus resistant disease) [6] random lists (random permuted blocks method).
Figure 1.
CONSORT flow diagram.
Study treatment
PM01183 was administered as a 7.0 mg FD 1-h intravenous (i.v.) infusion every 3 weeks (q3wk) in both stages [7]. Topotecan was administered as a 30-min i.v. infusion: the regimen, standard (days 1–5 q3wk) or weekly (days 1, 8, and 15 q4wk), was decided by the investigators. Topotecan starting dose was adjusted according to creatinine clearance and ECOG PS: 1.50–0.75 mg/m2 days 1–5 (standard regimen; referred to as ‘daily’) or 4.0–2.4 mg/m2 (weekly regimen). All patients received antiemetic prophylaxis. Granulocyte colony-stimulating factors could be added for secondary prevention of neutropenia. During the second stage, patients in the control arm with disease progression could crossover to the experimental arm.
Study assessments
Patients were assessed at baseline, and on day 1 of each new treatment cycle. Laboratory values were tested again on days 8 and 15 (cycle 1) and on day 10 (subsequent cycles). Febrile neutropenia and grade 4 neutropenia/thrombocytopenia were reassessed daily until recovery to grade ≤3 or fever resolution. Antitumor activity was evaluated every 6 weeks (±1 week) using RECIST v.1.1 [8] (measurable disease) and GCIG criteria (non-measurable disease). Laboratory abnormalities and adverse events (AEs) were graded per the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) v.4.03.
Statistical analysis
The primary objective was to test PM01183 activity; therefore, sample size was calculated based on PM1183 response rate. If enough activity was observed in the exploratory stage I (interim analysis with 18 evaluable patients), then stage II had to include additional patients treated with PM10183 and the same number of patients for the control arm.
A two-stage design to test the null hypothesis (H0) that ≤8% patients responded to PM01183 according to RECIST v.1.1 and/or GCIG criteria (P ≤ 0.08) versus the alternative hypothesis (H1) that ≥25% (P ≥ 0.25) was used (whole data, including stages I and II of this study). The type I error (alpha) was 0.025 (one-sided) and the type II error (beta) was 0.1; hence, statistical power was 90%. At the end of the study, if at least 8 of the 48 evaluable patients treated with PM01183 (18 in the first stage and 30 in the second stage) were responders, then PM01183 treatment should to be considered for further study.
All tests were two-sided (significance level at 0.05). For the evaluation of the main primary endpoint (ORR), the exact binomial estimator including its 95% confidence interval was used. Time-to-event variables were calculated following the Kaplan–Meier method.
Results
Patient characteristics
Eighty-one patients were treated between December 2011 and March 2013: 52 received PM01183 (22 in the first stage; 30 in the second stage) (Figure 1) and 29 topotecan (15 crossed over to PM01183). Most patients had ovarian cancer, serous histology, poorly differentiated carcinoma, and platinum-resistant disease (Table 1).
Table 1.
Baseline characteristics
| All PM01183 (n=52) | Randomized, controlled second stage |
||
|---|---|---|---|
| PM01183 (n=30) | Topotecan (n=29) | ||
| n (%) | n (%) | n (%) | |
| Median age (range) (years) | 59 (35–81) | 60 (35–81) | 61 (35–80) |
| ≥70 | 8 (15%) | 7 (23%) | 11 (38%) |
| ECOG PS | |||
| 0 | 26 (50%) | 17 (57%) | 11 (38%) |
| 1 | 24 (46%) | 12 (40%) | 14 (48%) |
| 2 | 2 (4%) | 1 (3%) | 4 (14%) |
| Disease evaluation | |||
| RECIST (measurable disease) | 43 (83%) | 26 (87%) | 22 (76%) |
| GCIG (non-measurable disease) | 9 (17%) | 4 (13%) | 7 (24%) |
| Primary tumor site | |||
| Ovarian | 44 (85%) | 27 (90%) | 23 (79%) |
| Peritoneal | 7 (14%) | 2 (7%) | 4 (14%) |
| Fallopian tube | 1 (2%) | 1 (3%) | 2 (7%) |
| Most common histology type | |||
| Papillary serous | 38 (73%) | 22 (73%) | 19 (66%) |
| Endometrioid | 4 (8%) | 1 (3%) | 2 (7%) |
| Undifferentiated carcinoma | 4 (8%) | 3 (10%) | 2 (7%) |
| Clear cell | 1 (2%) | - | 3 (10%) |
| Histology grade | |||
| Well differentiated | 8 (15%) | 4 (13%) | - |
| Moderately differentiated | 9 (17%) | 6 (20%) | 8 (28%) |
| Poorly differentiateda | 24 (46%) | 14 (47%) | 9 (31%) |
| Median time from first diagnosis (range) (months) | 19 (3–109) | 19 (3–109) | 14 (4–81) |
| Disease stage (inclusion) | |||
| III | 20 (39%) | 15 (50%) | 16 (55%) |
| IV | 32 (62%) | 15 (50%) | 13 (45%) |
| Ascites | 11 (21%) | 7 (23%) | 10 (35%) |
| Most common sites of current disease | |||
| Peritoneum | 35 (67%) | 21 (70%) | 23 (79%) |
| Lymph node | 24 (46%) | 14 (47%) | 17 (59%) |
| Liver | 17 (33%) | 8 (27%) | 5 (17%) |
| Prior radical surgery | 38 (73%) | 24 (80%) | 24 (83%) |
| Prior chemotherapy lines | |||
| Median | 2 (1–3) | 2 (1–3) | 2 (1–3) |
| 1 | 18 (35%) | 9 (30%) | 13 (45%) |
| 2 | 26 (50%) | 14 (47%) | 12 (41%) |
| 3 | 8 (15%) | 7 (23%) | 4 (14%) |
| Platinum status | |||
| Refractory | 19 (37%) | 13 (43%) | 13 (45%) |
| Resistant | 33 (63%) | 17 (57%) | 16 (55%) |
| Platinum resistance | |||
| Primary resistance | 30 (58%) | 17 (57%) | 18 (62%) |
| Secondary resistance | 22 (42%) | 13 (43%) | 11 (38%) |
| Platinum-free interval median (range) (months) | 3.6 (0.1–6.0) | 3.3 (0.1–5.9) | 2.3 (0–5.9) |
| Prior angiogenesis inhibitor | 13 (25%) | 5 (17%) | 11 (38%) |
| Prior bevacizumab | 6 (12%) | 3 (10%) | 9 (31%) |
No patients were previously treated with poly ADP ribose polymerase inhibitors.
One patient in the second stage PM01183 arm was previously treated with immunotherapy.
Includes undifferentiated. Unknown grades are not shown.
ECOG PS, Eastern Cooperative Oncology Group performance status; GCIG, Gynecological Cancer Intergroup criteria; RECIST, Response Evaluation Criteria in Solid Tumors.
Treatment
In the experimental arm (first/second stage, n = 52), 310 PM01183 cycles were administered (median of five cycles per patient). In the control arm (n = 29), 21 patients (72%) were treated with 53 cycles of weekly topotecan (median of two cycles per patient). Eight patients (28%) were treated with 28 cycles of daily topotecan (median of three cycles per patient).
Eighteen PM01183-treated patients (36% of patients with more than one cycle) had dose reduced in a total of 23 cycles (9% of cycles); 15 dose reductions (6% of cycles) in 14 patients (28%) were due to hematological toxicities (febrile neutropenia, n = 5) and eight due non-hematological toxicity (mostly fatigue).
Treatment-related fatigue resulted in three treatment discontinuations. Hematological toxicity was the reason for treatment discontinuation in two patients treated with PM01183 (one due to grade 3 febrile neutropenia and one due to grade 4 neutropenia/thrombocytopenia). Increased transaminases led to one dose reduction and one cycle delay.
Efficacy
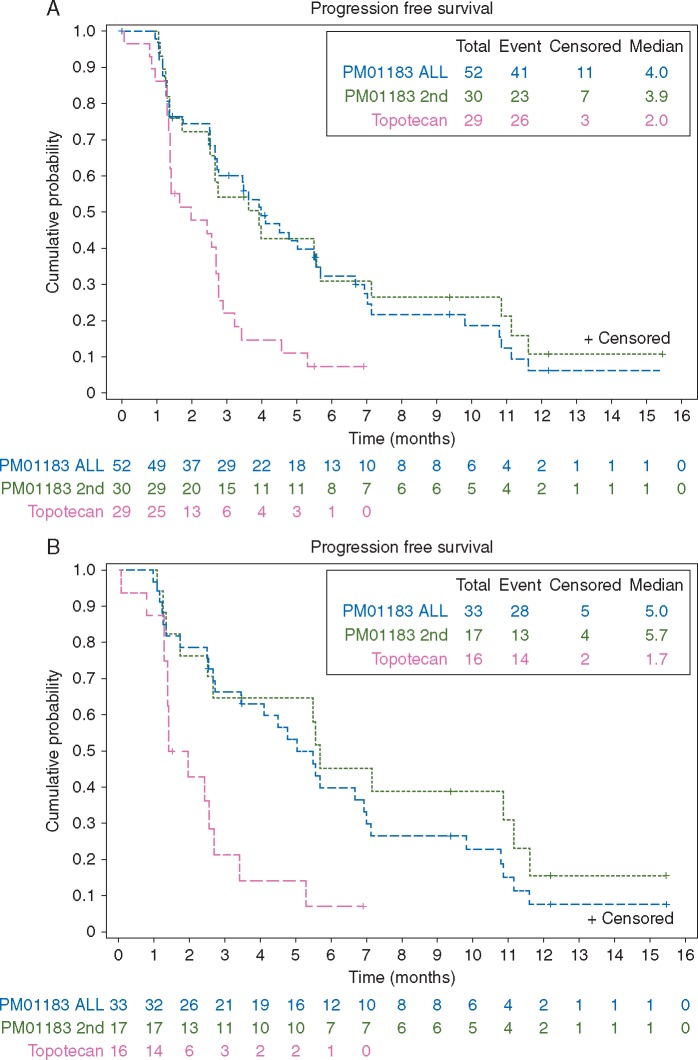
Twelve (seven in the first stage, five in the second stage) confirmed responses were observed in the 52 patients treated with PM01183: one CR (per RECIST) and 11 PRs (nine per RECIST; two per GCIG criteria). ORR for PM01183 was 23% (95% CI, 13%–37%) (Table 2). Median duration of response was 4.6 months (95% CI, 2.5–6.9 months) and 23% (95% CI, 0%–51%) of responses lasted ≥6 months. Median PFS was 4.0 months (95% CI, 2.7–5.6 months) (Figure 2A). Median OS was 10.6 months (95% CI, 9.5–18.1 months).
Table 2.
Overall response and clinical benefit (RECIST/GCIG) in all patients
| All PM01183 (n =52) | Randomized, controlled second stage |
||
|---|---|---|---|
| PM01183 (n =30) | Topotecan (n =29)a | ||
| n (%) | n (%) | n (%) | |
| Overall response rate (RECIST/GCIG) | 23% | 17% | - |
| (95% CI) | (13%–37%) | (6%–35%) | (0%–12%) |
| PM01183 versus topotecan, P =0.0033 | |||
| Complete response | 1 (2%) | 1 (3%) | - |
| Partial response | 11 (21%) | 4 (13%) | - |
| Stable disease | 26 (50%) | 15 (50%) | 15 (52%) |
| Progressive disease | 13 (25%) | 9 (30%) | 14 (48%) |
| Clinical benefit (CR+PR+SD≥4 months) | 22 (42%) | 12 (40%) | 4 (14%) |
| (95% CI) | (29%–57%) | (23%–59%) | (4%–32%) |
Weekly (n = 21): 4.0–2.4 mg/m2 days 1, 8, and 15 q4wk; standard (n = 8): 1.50–0.75 mg/m2 days 1–5 q3wk.
CI, confidence interval; CR, complete response; GCIG, Gynecologic Cancer Intergroup criteria; PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumors; SD, stable disease.
Figure 2.
Progression-free survival in (A) all patients; (B) patients with platinum-resistant disease.
Nine responders (75%) had an ECOG PS score of 0 (none scored 2) and all were free of ascites at baseline; most were <65 years old; ten of 12 responders (83%) had platinum-resistant disease (seven had primary resistance). Two patients had BRCA mutation. Median number of prior advanced chemotherapy lines was one (range, 1–2). Median progression-free interval (PFI) was 4.5 months (range, 0.9–5.95 months). Seven responders (58%) had non-platinum-based therapy as last prior line. All but one received six or more PM01183 treatment cycles. Antitumor activity was observed in three of six patients who had previously received bevacizumab.
No responses (either by RECIST or GCIG criteria) were found in the 29 patients treated with topotecan. In the second randomized stage, median PFS was significantly longer with PM01183: 3.9 months (95% CI, 2.5–5.7 months) versus 2.0 months (95% CI, 1.4–2.8 months) with topotecan (P = 0.0067). Median OS was 9.7 months (95% CI, 7.7–19.3 months) with PM01183, and 8.5 months (95% CI, 3.3–15.6 months) with topotecan (P = 0.2871).
Noteworthy antitumor activity was observed with PM01183 in platinum-resistant disease (supplementary Table S1, available at Annals of Oncology online): ORR was 30% (95% CI, 16%–49%) (10 of 33 patients treated in both study stages), with median PFS of 5.0 months (95% CI, 2.7–6.9 months) (5.7 months in the second stage versus 1.7 months in the topotecan-treated patients with platinum-resistant disease) and median OS of 13.5 months (95% CI, 9.7–22.0 months) (15.6 months in the second stage versus 8.7 months in the topotecan-treated patients). Median PFS was significantly longer with PM01183 versus topotecan for patients with platinum-resistant disease, both in the study as a whole (P = 0.0017) and in the second stage alone (P = 0.0043) (Figure 2B).
According to GCIG criteria,13 of 37 patients with tumor marker values at least two times the ULN at baseline had a >50% CA-125 decrease during PM01183 treatment; seven were subsequently confirmed (response rate of 19%; 95% CI, 8%–35%); and five responses were also confirmed response by RECIST.
Fifteen patients from the control arm (52%) crossed over to PM01183 after disease progression; PM01183 antitumor activity was observed in two patients (ORR = 13%), consisting of one PR per RECIST (SD with weekly topotecan) and one PR per GCIG criteria (PD as best response with weekly topotecan); both had platinum-resistant disease and one had BRCA mutation; one had been previously treated with bevacizumab.
Toxicity
Myelosuppression was the most frequent AE associated with PM01183 (Table 3). The most common severe hematological abnormalities were grade 3/4 neutropenia (85%; grade 4: 64%) and grade 3/4 thrombocytopenia (33%; grade 4: 23%). There were 11 patients (21%) with febrile neutropenia meaning that primary granulocyte-colony stimulating factor (G-CSF) prophylaxis would be required according to current guidelines [9]. G-CSFs (prophylactic or therapeutic) were administered in 20 (39%) of PM01183-treated patients. Transfusions were given to 19 (37%): eight received platelets (15%, all after grade 4 thrombocytopenia) and 11 (21%) RBCs. Fourteen patients (27%) received erythropoietin.
Table 3.
Grade 3/4 laboratory abnormalities and treatment-related (or relationship unknown) adverse events (or in ≥10% of patients)
| PM01183 (n =52) |
Topotecan (n =29)a |
|||||||
|---|---|---|---|---|---|---|---|---|
| NCI-CTCAE grade |
NCI-CTCAE grade |
|||||||
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Hematological laboratory abnormalities | ||||||||
| Anemia | 14 (27%) | 18 (35%) | 19 (37%) | - | 5 (17%) | 12 (41%) | 11 (38%) | - |
| Leukopenia | - | 12 (23%) | 19 (37%) | 15 (29%) | 6 (21%) | 8 (28%) | 9 (31%) | 2 (7%) |
| Lymphopenia | 13 (25%) | 24 (46% | 8 (15%) | 5 (10%) | 6 (21%) | 11 (38%) | 5 (17%) | 1 (3%) |
| Neutropenia | 1 (2%) | 1 (2%) | 11 (21%) | 33 (64%) | 5 (17%) | 7 (24%) | 3 (10%) | 8 (28%) |
| Thrombocytopenia | 16 (31%) | 4 (8%) | 5 (10%) | 12 (23%) | 11 (38%) | 4 (14%) | 4 (14%) | 3 (10%) |
| Biochemical laboratory abnormalities | ||||||||
| ALP increase | 25 (48%) | 4 (8%) | 2 (4%) | - | 13 (48%) | 4 (15%) | - | - |
| ALT increase | 23 (44%) | 13 (25%) | 8 (15%) | 1 (2%) | 14 (48%) | 1 (3%) | - | - |
| AST increase | 29 (56%) | 2 (4%) | 6 (12%) | - | 8 (28%) | 1 (3%) | - | - |
| Creatinine increase | 40 (77%) | 6 (12%) | 2 (4%) | - | 23 (79%) | 2 (7%) | - | - |
| GGT increase | 16 (31%) | 14 (27%) | 12 (23%) | 1 (2%) | 10 (37%) | 7 (26%) | 3 (11%) | - |
| Treatment-related adverse events | ||||||||
| Abdominal pain | 5 (10%) | 1 (2%) | - | - | - | 1 (3%) | - | - |
| Alopecia | 4 (8%) | - | - | - | 2 (7%) | 3 (10%) | - | - |
| Constipation | 11 (21%) | 4 (8%) | - | - | 1 (3%) | 1 (3%) | - | - |
| Decreased appetite | 7 (14%) | 1 (2%) | 2 (4%) | - | 2 (7%) | 1 (3%) | 1 (3%) | - |
| Diarrhea | 4 (8%) | 3 (6%) | - | - | 5 (17%) | 2 (7%) | - | - |
| Fatigue | 10 (19%) | 12 (23%) | 18 (35%) | - | 5 (17%) | 8 (28%) | - | - |
| Febrile neutropenia | - | - | 8 (15%) | 3 (6%) | - | - | 3 (10%) | - |
| Gastrointestinal toxicity | - | - | 1 (2%) | - | - | - | - | - |
| Nausea | 14 (27%) | 15 (29%) | 6 (12%) | - | 6 (21%) | 5 (17%) | - | - |
| Rhabdomyolysis | - | - | 1 (2%) | - | - | - | - | - |
| Vomiting | 15 (29%) | 8 (15%) | 5 (10%) | - | 4 (14%) | - | - | |
Weekly (n = 21): 4.0–2.4 mg/m2 days 1, 8, and 15 q4wk; standard (n = 8): 1.50–0.75 mg/m2 days 1–5 q3wk.
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CPK, creatine transferase; NCI-CTCAE, National Cancer Institute Common Terminology Criteria for Adverse Events.
Fatigue was the most frequent non-hematological treatment-related AE reported among PM01183-treated patients (all grades, 77%) and was also the most common severe non-hematological toxicity (grade 3, 35%; no grade 4 AEs other than febrile neutropenia were observed). Nausea was common (67%; grade 3 in 12%) as was vomiting (54%; grade 3 in 10%).
There was one event of grade 3 rhabdomyolysis in cycle 2 reported as related to PM01183; the patient’s dose was not reduced as required by protocol despite grade 4 neutropenia, grade 3 transaminases increase, and fatigue in cycle 1.
Topotecan safety profile agreed with that previously reported for both the daily and weekly regimens. Grade 3/4 neutropenia occurred in 38% of patients (grade 4 in 28%; 88% in the daily regimen compared with 5% in the weekly); and grade 3/4 thrombocytopenia in 24% of patients (grade 4 in 10%, all in the daily regimen). There were three cases of febrile neutropenia (10% overall); all of them in the daily regimen (38% of patients). G-CSF use (prophylactic or therapeutic) was required in four (14%) patients (all four following the daily regimen; 50% of patients treated with daily topotecan required G-CSFs). Non-hematological topotecan-related AEs included mild/moderate fatigue (45%), nausea (38%), vomiting (28%), and diarrhea (24%). Apart from febrile neutropenia, only decreased appetite (3%) reached grade 3 in the control arm; all grade 3 AEs occurred with the daily regimen.
No remarkable changes were seen in PM01183 safety profile in the fifteen patients who crossed over. Most AEs remained the same or slightly improved after crossover. Laboratory values did not vary greatly before and after crossover: many remained the same and noticeable shifts were in line with PM01183’s known toxicities.
There were no treatment-related deaths in the study.
Discussion
The primary endpoint of this phase II study was met: PM01183 was active in patients with platinum-resistant/refractory ovarian cancer. Twelve (of a total of 52 treated patients) responded with a median duration of response of 4.6 months. The poor prognosis of the patient population in this phase II trial needs to be borne in mind: 37% of patients had platinum-refractory disease, and 35% had previously received one prior chemotherapy line after confirmation of platinum resistant/refractory disease. The activity was not influenced by prior angiogenesis-inhibitor treatment; three of six patients with previous bevacizumab had confirmed response. Six of eighteen (33%) patients who received PM01183 as second-line after their disease became platinum-resistant/refractory had confirmed response. This response rate is superior to the 8.1% reported by Griffiths et al. [10] who evaluated the efficacy of subsequent lines of treatment in 274 patients with platinum-resistant/refractory disease.
PM01183 antitumor activity was especially noteworthy in patients with platinum-resistant disease (n = 33; both stages) with ORR = 30%, median PFS = 5.0 months, and median OS = 13.5 months. More modest activity was observed in patients with platinum-refractory disease: ORR = 10.5%; median OS = 9.3 months.
In platinum-resistant disease, ORR and PFS with PM01183 were statistically significant with respect to topotecan in the second stage; two patients with platinum-resistant disease treated initially with topotecan achieved PR after crossing over to PM01183 treatment (one had a BRCA germline mutation).
In the randomized second stage, antitumor activity with topotecan was lower with respect to PM01183: ORR = 0%, median PFS = 2.0 months, and median OS = 8.5 months. Recent studies in ovarian cancer have also shown a poor activity with topotecan. ORR (RECIST and/or GCIG) was 3.3% and median PFS was 2.1 months (95% CI, 1.9–3.3 months) in the phase III AURELIA trial (most patients treated with the weekly regimen) [11, 12]. No responses (RECIST) and median PFS of 2.6 months (95% CI, 2.1–4.3 months) were found in the phase III PENELOPE trial (n = 24 patients treated with topotecan 1.25 mg/m2 days 1–5 q3wk) [13].
The incidence of severe hematological abnormalities found here was higher than that observed among the 15 patients (47% women) with advanced solid tumors treated at 7.0 mg FD in the first-in-human study, where 53% and 7% of patients had grade 3/4 neutropenia and grade 3/4 thrombocytopenia, respectively, and without febrile neutropenia [7]. Ongoing PK/PD modeling of pooled phase I/II data has shown some covariates [ascites, albumin, aprepitant, body surface area (BSA)] that might affect neutropenia and thrombocytopenia (unpublished data). Four of six patients who received aprepitant concomitantly with PM01183 developed severe (and in some cases prolonged) pancytopenia. Aprepitant is an inhibitor of cytochrome P-450 isoenzyme 3A4 (CYP3A4) which metabolizes PM01183, and those patients had PM01183 clearance (CL) reduced and AUC increased. Currently, aprepitant is forbidden in all clinical trials with PM01183.
Preliminary PK analysis showed that PM01183 CL was unaffected by BSA, but a recent analysis of pooled phase II data showed grade 3/4 neutropenia and thrombocytopenia more frequent in patients with lower BSA [14]. BSA-based dosing (mg/m2) should now be adopted and the recommended dose reduced for future studies to improve safety.
In conclusion, PM01183 is an active drug in platinum-resistant/refractory ovarian cancer and warrants further clinical development, particularly in platinum-resistant disease, where its activity was remarkable. Currently, PM01183 is being tested in a pivotal randomized trial (CORAIL study, NCT02421588) in patients with platinum-resistant ovarian cancer [15].
Supplementary Material
Acknowledgements
The authors wish to thank Vicente Alfaro for their help as handling editor of this manuscript.
Funding
This trial was funded by Pharma Mar, S.A. No grant numbers apply.
Disclosure
AP, JMC, IRC, MP, EMGA, and AC report grants from Pharma Mar, S.A. for the conduct of this study. JA reports grants and personal fees from Pharma Mar, S.A. for the conduct of this study; and personal fees from Roche S.A. and Novartis S.A. outside the submitted work. AGM reports personal fees from Clovis, Roche, AstraZeneca and PharmaMar as local advisory board or speaker outside the submitted work. CF, IR, APH, and PB are employees of Pharma Mar, S.A. AS, CK, CFT, and CMG are employees and shareholders in Pharma Mar, S.A.
References
- 1. Santamaria Nunez G, Robles CM, Giraudon C. et al. Lurbinectedin Specifically Triggers the Degradation of Phosphorylated RNA Polymerase II and the Formation of DNA Breaks in Cancer Cells. Mol Cancer Ther 2016; 15: 2399–2412. [DOI] [PubMed] [Google Scholar]
- 2. Allavena P, Belgiovine C, Liguori M. et al. Lurbinectedin reduces tumor-associated macrophages and the production of inflammatory cytokines, chemokines, and angiogenic factors in preclinical models. In Proceedings of the 107th Annual Meeting of the American Association for Cancer Research; 2016. Apr 16-20; New Orleans, LA. Cancer Res 2016; 76: Abstract nr 1284.
- 3. Vidal A, Munoz C, Guillen MJ. et al. Lurbinectedin (PM01183), a new DNA minor groove binder, inhibits growth of orthotopic primary graft of cisplatin-resistant epithelial ovarian cancer. Clin Cancer Res 2012; 18: 5399–5411. [DOI] [PubMed] [Google Scholar]
- 4. Wilson MK, Pujade-Lauraine E, Aoki D. et al. Fifth Ovarian Cancer Consensus Conference of the Gynecologic Cancer InterGroup: Recurrent Disease. Ann Oncol 2017; 28(4): 727–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sehouli J, Stengel D, Harter P. et al. Topotecan weekly versus conventional 5-day schedule in patients with platinum-resistant ovarian cancer: a randomized multicenter phase II trial of the North-Eastern German Society of Gynecological Oncology Ovarian Cancer Study Group. J Clin Onocol 2011; 29: 242–248. [DOI] [PubMed] [Google Scholar]
- 6. McGee J, Bookman M, Harter P. et al. Fifth Ovarian Cancer Consensus Conference: individualized therapy and patient factors. Ann Oncol 2017; 28(4): 702–710. [DOI] [PubMed] [Google Scholar]
- 7. Elez ME, Tabernero J, Geary D. et al. First-in-human phase I study of lurbinectedin (PM01183) in patients with advanced solid tumors. Clin Cancer Res 2014; 20: 2205–2214. [DOI] [PubMed] [Google Scholar]
- 8. Eisenhauer EA, Therasse P, Bogaerts J. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–247. [DOI] [PubMed] [Google Scholar]
- 9. Smith TJ, Bohlke K, Lyman GH. et al. Recommendations for the use of WBC growth factors: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 2015; 33: 3199–3212. [DOI] [PubMed] [Google Scholar]
- 10. Griffiths RW, Zee YK, Evans S. et al. Outcomes after multiple lines of chemotherapy for platinum-resistant epithelial cancers of the ovary, peritoneum, and fallopian tube. Int J Gynecol Cancer 2011; 21: 58–65. [DOI] [PubMed] [Google Scholar]
- 11. Poveda AM, Selle F, Hilpert F. et al. Bevacizumab combined with weekly paclitaxel, pegylated liposomal doxorubicin, or topotecan in platinum-resistant recurrent ovarian cancer: analysis by chemotherapy cohort of the randomized phase III AURELIA trial. J Clin Oncol 2015; 33: 3836–3838. [DOI] [PubMed] [Google Scholar]
- 12. Poveda AM, Selle F, Hilpert F. et al. Weekly paclitaxel (PAC), pegylated liposomal doxorubicin (PLD) or topotecan (TOP) ± bevacizumab (BEV) in platinum (PT)-resistant recurrent ovarian cancer (OC): analysis by chemotherapy (CT) cohort in the GCIG AURELIA randomised phase III trial. Ann Oncol 2012; 23: ixe17 (abstract LBA26). [DOI] [PubMed] [Google Scholar]
- 13. Kurzeder C, Bover I, Marme F. et al. Double-blind, placebo-controlled, randomized phase III trial evaluating pertuzumab combined with chemotherapy for low tumor human epidermal growth factor receptor 3 mRNA-expressing platinum-resistant ovarian cancer (PENELOPE). J Clin Oncol 2016; 34: 2516–2525. [DOI] [PubMed] [Google Scholar]
- 14. Fernandez-Teruel C, Del Campo JM, Berton-Rigaud D. et al. Lurbinectedin (PM1183) efficacy in platinum resistant/refractory ovarian cancer (PRROC) patients correlates with drug exposure using pharmacokinetic/pharmacodynamic (PK/PD) modelling. Int J Gynecol Cancer 2015; 25: 433 (Abs Nº ESGO-0843). [Google Scholar]
- 15. Gaillard S, Ghamande SA, Pardo B. et al. CORAIL trial: Randomized phase III study of lurbinectedin (PM01183) versus pegylated liposomal doxorubicin (PLD) or topotecan (T) in patients with platinum-resistant ovarian cancer. J Clin Oncol 2016; 34(Suppl): abstr TPS5597. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.