The landscape of cancer treatment has undergone a vast change over the past four decades [1, 2]. Discovery of the heterogeneous molecular features of tumors and the associated microenvironment has led to the development of novel classes of targeted therapeutics [3–5], the two main types of which are small-molecule inhibitors and monoclonal antibodies (mAbs) [1–3]. These targeted drugs have furthered the development of personalized therapeutic regimens in oncology.
Cancers exhibit numerous genetic and epigenetic alterations manifesting as a diverse population of antigens that the immune system should be able to use to differentiate between tumor tissues and their healthy counterparts [6]. However, many cancers can ‘hide’ from immune surveillance (i.e. immune evasion) [7, 8]. Maintenance of self-tolerance under normal physiologic conditions is regulated by immune checkpoints, and expression of immune-checkpoint proteins can be impaired in tumor tissue [6]. Early research into the manipulation of antitumor immunity focused on T cells, and specifically blockade of cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) [9]. Clinical trials of ipilimumab, a fully humanized CTLA-4 mAb, demonstrated antitumor activity and a survival benefit in patients with unresectable or metastatic melanoma [10–12], resulting in its approval in 2011 by the United States Food and Drug Administration (FDA) for this patient population [6].
Programmed death 1 (PD-1) is another immune-checkpoint receptor; specifically, a negative costimulatory receptor that is expressed on the surface of activated T cells, B cells, natural killer T cells, and dendritic cells [6, 13–16]. Binding of PD-1 with its ligands, PD-L1 and PD-L2, inhibits the cytotoxic T-cell response [17]. The PD-1 pathway plays an important role in the induction and maintenance of immune tolerance, enabling the body to defend itself against a wide variety of pathogens while simultaneously protecting against self-reactivity (autoimmunity) [18]. In this way, expression of PD-L1 on endothelial cells may be, in part, responsible for maintaining tissue tolerance [18]. PD-L1 and PD-L2 are expressed on the surface of tumor cells in many cancer types; PD-L1 expression has been found both intracellularly and extracellularly in epithelial cancers, including melanoma and non-small cell lung cancer (NSCLC), and PD-L2 expression has been found in lymphoid malignancies such as mantle cell lymphoma [15, 19, 20], as well as in several solid tumors including head and neck squamous carcinoma (HNSCC), both with and without concomitant PD-L1 staining [21]. Furthermore, PD-1 expression is upregulated on tumor-infiltrating lymphocytes [15, 20]. While the presence of these immune-checkpoint receptors enables some tumors to escape destruction via the T-cell immune response, it also provides a promising target for antitumor therapy [6, 20].
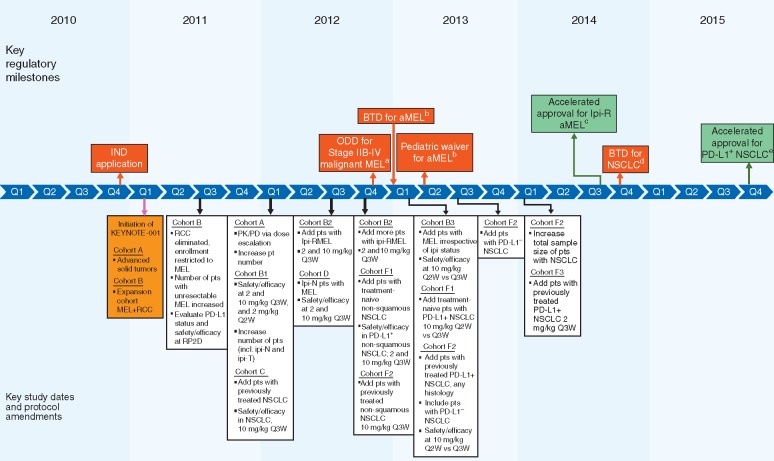
Pembrolizumab (Keytruda; Merck & Co., Inc., Kenilworth, NJ, USA) is a humanized mAb that blocks the interaction between PD-1 and its ligands [22], thereby enabling an antitumor immune response (Figure 1). The unique clinical development of pembrolizumab began in 2010 with an investigational new drug application submitted to the FDA, followed by the initiation of a seminal phase 1 clinical trial in patients with advanced solid tumors—KEYNOTE-001 (ClinicalTrials.gov identifier: NCT01295827; Figure 2). Pembrolizumab was granted orphan drug designation for the treatment of advanced melanoma in late 2012 [23, 24] and was subsequently awarded breakthrough therapy designation for advanced melanoma in 2013 (Figure 2) [25]; this was the first FDA-granted breakthrough therapy designation for a cancer drug. Orphan drug designation is granted by the FDA for drugs intended to treat rare diseases [23]. Breakthrough therapy designation is granted by the FDA for drugs intended to treat a serious condition and for which preliminary clinical evidence has demonstrated a marked improvement in a clinically significant endpoint over existing therapies [26]. Breakthrough therapy designation enables expedited clinical development, which in the case of pembrolizumab ultimately led to its accelerated approval in the USA in 2014 for the treatment of patients with unresectable or metastatic melanoma and disease progression after ipilimumab and, if BRAFV600 mutation positive, a BRAF inhibitor [22, 23, 27]. Accelerated approval is granted by the FDA for drugs that fill an unmet medical need for a serious or life-threatening disease or condition based on a surrogate endpoint that is reasonably likely to predict clinical benefit. It allows for earlier approval, enabling the drug to be provided to patients sooner than would otherwise be possible. Confirmation of benefit is required through confirmatory trials [26]. This milestone for pembrolizumab represented the first regulatory approval for an anti-PD-1 agent in the USA.
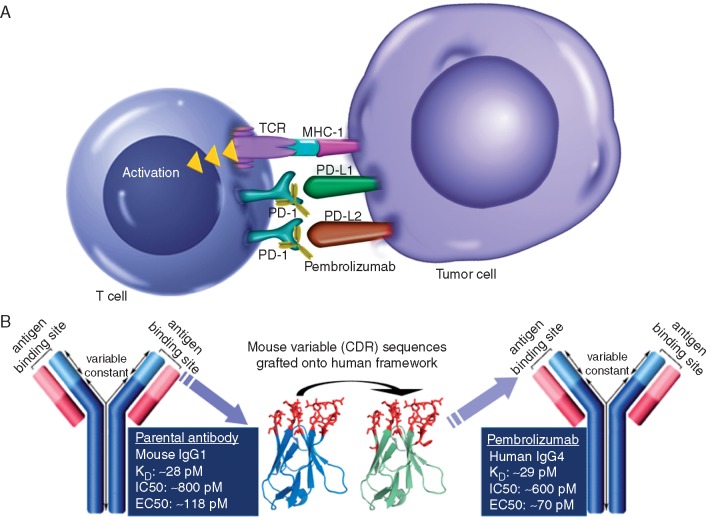
Figure 1.
(A) Engagement between programmed death 1 (PD-1) and its ligands, PD-L1 and PD-L2, can enable some tumors to evade T-cell immune surveillance. PD-1 inhibitors such as pembrolizumab can ‘unmask’ PD-L1-expressing cells from the antitumor immune response. (B) Design of the pembrolizumab monoclonal antibody. CDR, complementarity determining region; EC50, half-maximal effective concentration; IC50, half-maximal inhibitory concentration; KD, dissociation constant; MHC-1, major histocompatibility complex 1; PD-1, programmed death 1; PD-L1, programmed death ligand 1; PD-L2, programmed death ligand 2; TCR, T-cell receptor.
Figure 2.
KEYNOTE-001: timeline of key study design elements and US FDA regulatory milestones. ALK, anaplastic lymphoma kinase; aMEL, metastatic melanoma; BTD, breakthrough therapy designation; chemo, chemotherapy; EGFR, epidermal growth factor receptor; incl., including; IND, investigational new drug; Ipi(-R, -T, -N), ipilimumab (-refractory, -treated, -naive); MEL, melanoma; NSCLC, non-small cell lung cancer; ODD, orphan drug designation; OL, open label; PD, pharmacodynamics; PD-L1+, positive for expression of programmed death ligand 1; PD-L1–, negative for expression of programmed death ligand 1; PK, pharmacokinetic; pts, patients; Q2W, every 2 weeks; Q3W, every 3 weeks; RCC, renal cell carcinoma; RP2D, recommended phase 2 dose; US FDA, United States Food and Drug Administration. aFDA granted ODD for stage IIB-IV malignant melanoma [24]. bFDA granted BTD for advanced melanoma and a pediatric waiver based on ODD status [25]. cFDA granted accelerated approval for unresectable or metastatic melanoma and disease progression after ipilimumab and, if BRAFV600 mutation positive, a BRAF inhibitor; approved dose 2 mg/kg Q3W [23]. dFDA granted BTD for treatment of EGFR mutation negative and ALK rearrangement-negative NSCLC with disease progression on or after platinum-based chemotherapy [28]. eFDA granted accelerated approval for metastatic NSCLC with tumors expressing PD-L1 (as determined by an FDA-approved test) and with disease progression on or after platinum-containing chemotherapy (EGFR or ALK genomic tumor aberrations should have disease progression on FDA-approved therapy for these aberrations before receiving pembrolizumab); approved dose 2 mg/kg Q3W [29].
The clinical evaluation of pembrolizumab in the KEYNOTE-001 trial in patients with metastatic NSCLC led to breakthrough therapy designation in that indication in 2014 [28] and subsequent accelerated FDA approval in 2015 [29] for the treatment of patients with PD-L1-expressing metastatic NSCLC with disease progression on or after platinum-containing therapy. The approval for NSCLC was accompanied by approval of a companion diagnostic (PD-L1 immunohistochemistry [IHC] 22C3 pharmDx, Dako, Carpinteria, CA) for PD-L1 expression status [30, 31]. This review describes the unique design and evolution of the pembrolizumab KEYNOTE-001 study and the resulting unprecedented regulatory outcomes.
Design and evolution of the KEYNOTE-001 study
Clinical development
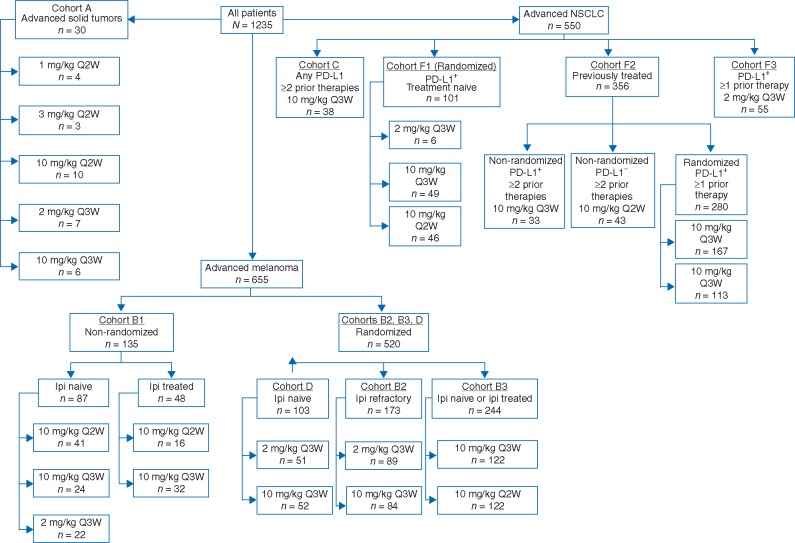
At the time of the pembrolizumab investigational new drug application, there was a substantial unmet need for new treatments in both melanoma and NSCLC [32, 33]. It was hypothesized that with its novel mechanism of action, pembrolizumab might be of clinical benefit in patients with these tumor types. This hypothesis was supported by the finding of PD-L1 expression in a proportion of both melanoma and NSCLC tumors [15, 19, 20] and the reported correlation between PD-L1 expression, poor prognosis, and high invasiveness in NSCLC [19, 34–37]. Preclinical studies suggesting that pembrolizumab had antitumor properties in multiple cancers [38, 39] led to initiation of the phase 1 KEYNOTE-001 first-in-human study in January 2011 (Figure 2). Initially designed as a dose-finding study, the primary objective of KEYNOTE-001 was to explore the safety and tolerability of pembrolizumab and to determine whether it conferred antitumor activity in patients with advanced solid tumors. Primary efficacy endpoints were objective response rate (ORR) per Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v1.1) and disease control rate (DCR) per RECIST v1.1; progression-free survival (PFS) and overall survival (OS) were included as secondary efficacy objectives. Through its unique evolution based on interim findings and the addition of melanoma- and NSCLC-specific expansion cohorts that included three dose-finding, randomized experiments with pre-specified statistical analyses, this study eventually culminated in the recruitment and treatment of 1235 patients; enrollment was complete in July 2014 (Figure 3) [40].
Figure 3.
KEYNOTE-001 treatment cohorts. Ipi, ipilimumab; PD-L1, programmed dealth ligand 1; Q2W, every 2 weeks; Q3W, every 3 weeks. Figure adapted with permission from Khoja et al. [40].
Pembrolizumab in advanced solid tumors
The initial aims of the first cohorts in KEYNOTE-001 were to define dose-limiting toxicities (DLTs), to characterize the pharmacokinetic properties, and to establish a recommended phase 2 dose (RP2D) for pembrolizumab in patients with advanced solid tumors. Therefore, the first part of the study was composed of a 3 + 3 dose-escalation design (cohort A), the main aim of which was to establish the safety and tolerability of pembrolizumab and to identify the RP2D, with doses ranging up to a maximum of 10 mg/kg every 2 weeks (Q2W) [41]. Confirmed ORR as assessed by investigator review and duration of response (DOR) were also evaluated. Based on the findings of cohort A, additional patients were enrolled in two additional cohorts: one to be treated at the maximum-tolerated dose (MTD) or the maximum administered dose (MAD) defined in cohort A (cohort A1, n = 7) and the second (cohort A2, n = 13) in which patients were randomly assigned to one of the three parallel intra-patient dose-escalation schedules (starting at 0.005, 0.02, and 0.06 mg/kg titrating 8 days into the first cycle), followed by treatment with either 2 or 10 mg/kg every 3 weeks (Q3W), to further define the pharmacokinetic and pharmacodynamic properties of the drug [41].
Pembrolizumab was generally well tolerated, with no DLTs reported in these cohorts. There was no MTD, and the MAD was 10 mg/kg Q2W. Treatment-related AEs were observed in 70% of all patients in cohorts A, A1, and A2, but none were grade 3 or 4; however, the death of one patient because of disseminated cryptococcal infection was considered to be indirectly related to treatment because it was probably caused by prolonged use of corticosteroids to treat grade 2 gastritis, which was itself considered related to treatment. Three patients discontinued therapy because of treatment-related AEs (grade 2 fatigue, pneumonitis, and decreased weight, n = 1 each) [41]. Although these cohorts were not powered for efficacy, substantial antitumor activity was observed. Across all doses and schedules, two patients had complete response (CR; melanoma and Merkel cell carcinoma, n = 1 each), 3 had partial response (PR; all melanoma), and 15 had stable disease (10 solid tumor types, including melanoma and NSCLC) [41].
Non-compartmental analysis of the data from cohorts A and A1 revealed a pembrolizumab half-life of 14–22 days, and the findings of ex vivo experiments suggested that complete peripheral target engagement commenced at 1 mg/kg across doses and was durable for at least 21 days. Further analysis of data from cohort A2, with intra-patient dose escalation from 0.005 to 10 mg/kg over a 3-week period demonstrated a linear serum exposure to pembrolizumab over the range of 0.1–10.0 mg/kg; lower doses were associated with a non-linear clearance component. Translational modeling predicted robust responses at doses ≥2 mg/kg Q3W, with no (or limited) activity at <1.0 mg/kg Q3W. These findings provided the rationale for studying a dose range of 2 mg/kg Q3W to 10 mg/kg Q2W in subsequent KEYNOTE-001 cohorts and clinical trials [41].
Pembrolizumab in melanoma
Several melanoma expansion cohorts were initiated based on the findings from cohort A. The first was a non-randomized cohort in which 135 ipilimumab-naive (ipi-N; n = 87) or ipilimumab-treated (ipi-T; n = 48) patients were enrolled and administered pembrolizumab at doses of 10 mg/kg every Q3W or Q2W, or 2 mg/kg Q3W (cohort B1; Table 1). The aim was to more fully characterize the safety and tolerability of pembrolizumab at different doses and schedules and to assess preliminary antitumor activity in both ipi-N and ipi-T patients [42]. A sample size of 61 for ipi-N patients provided >99% power to detect an overall ORR of 30% or DCR of 55% in ipi-N patients versus an ORR of 10% and DCR of 30% (one-sided P = 0.05; based on the Hochberg procedure). The confirmed ORR across all doses was 38% and was not different between ipi-N and ipi-T patients; the highest confirmed ORR (52%) was observed with the 10 mg/kg Q2W dose regimen. Responses seemed to be durable in the majority of patients, with DORs in the range of 2–11 and 3–8 months for ipi-N and ipi-T patients, respectively. Treatment was ongoing for 81% of patients who had a response at the time of the analysis in March 2013 (median follow-up time, 11 months) [42].
Table 1.
Primary efficacy data for the non-randomized (n = 135) and randomized melanoma cohorts (n = 520) from KEYNOTE-001 [41–43, 45, 46]
| Cohort | n | ORR |
|---|---|---|
| % (95% CI) | ||
| B1 (non-randomized)a | ||
| Ipi-N | ||
| 10 mg/kg Q2W | 39 | 49 (32–65) |
| 10 mg/kg Q3W | 19 | 26 (9–51) |
| 2 mg/kg Q3W | 20 | 25 (9–49) |
| Ipi-T | ||
| 10 mg/kg Q2W | 13 | 62 (32–86) |
| 10 mg/kg Q3W | 26 | 27 (12–48) |
| B2 (randomized)b | ||
| Ipi-R | ||
| 2 mg/kg Q3W | 81 | 26 (17–37) |
| 10 mg/kg Q3W | 76 | 26 (17–38) |
| D (randomized)b | ||
| Ipi-N | ||
| 2 mg/kg Q3W | 51 | 33 (20–49) |
| 10 mg/kg Q3W | 52 | 40 (26–56) |
| B3 (randomized)c | ||
| Ipi-N | ||
| 10 mg/kg Q3W | 57 | 35 (23–49) |
| 10 mg/kg Q2W | 56 | 38 (25–52) |
| Ipi-T | ||
| 10 mg/kg Q3W | 50 | 26 (15–40) |
| 10 mg/kg Q2W | 61 | 33 (21–46) |
| Pooled analysis of cohorts B1, B2, D, and B3 (N = 655)d | 581 | 33 (30–37) |
| Ipi-N | 277 | 39 (33–45) |
| Ipi-T | 304 | 29 (24–34) |
| Treatment-naive | 133 | 45 (36–54) |
Data cutoff, March, 2013.
Data cutoff, October 18, 2013.
Data cutoff, April 18, 2014.
Data cutoff, October 18, 2014.
CI, confidence interval; Ipi-N, ipilimumab naive; Ipi-R, ipilimumab refractory; Ipi-T, ipilimumab treated; ORR, overall response rate; Q2W, every 2 weeks; Q3W, every 3 weeks.
This preliminary evidence of activity in both ipi-N and ipi-T patients [42] led to breakthrough therapy designation, and a cohort of patients with ipilimumab-refractory (ipi-R) melanoma was added to evaluate the safety and tolerability of pembrolizumab in a strictly defined population who had unequivocal or confirmed disease progression per immune-related response criteria after at least two ipilimumab doses (cohort B2; randomized 1:1 to receive either 2 or 10 mg/kg Q3W until disease progression, intolerable toxicity, or withdrawal of consent) [43, 44]. Pembrolizumab was similarly well tolerated between the two dose groups; grade 3 fatigue was the only grade 3 or 4 treatment-related AE reported in more than one patient, and there were no drug-related deaths. There was no difference in ORR between the two dose groups (26% in both; P = 0.96), and 73% and 68% of the 2 and 10 mg/kg groups, respectively, experienced a reduction from baseline in target lesion size. In terms of secondary end points, median PFS was 22 weeks [95% confidence interval (CI) 12–36] for the 2 mg/kg group and 14 weeks (95% CI 12–24) for the 10 mg/kg group [hazard ratio (HR) 0.84; 95% CI 0.57–1.23], and the 1-year OS rate (analysis date May 2014) was 58% (95% CI 47–68) and 63% (95% CI 51–72), respectively [43].
Along with the B2 cohort of patients with refractory melanoma, additional randomized dose cohorts were evaluated for ipi-N and ipi-T patients: 10 mg/kg Q2W and Q3W in ipi-T and ipi-N patients (cohort B3, n = 244), and 2 and 10 mg/kg Q3W in ipi-N patients (cohort D, n = 103) (Table 1) [44, 45]. As with previous KEYNOTE-001 cohorts, pembrolizumab was well tolerated and demonstrated efficacy among both ipi-T and ipi-N patients [44, 45]. The ORR was not significantly different between the 2 and 10 mg/kg ipi-N arms in cohort D (33% and 40%, respectively; P = 0.48) [44]. There were no significant differences between schedules for either ORR (31% for Q3W and 35% for Q2W; P = 0.51) or DCR (48% for Q3W and 51% for treated with pembrolizumab 10 mg/kg Q3W or Q2W; P = 0.59) among ipi-T + ipi-N patients in cohort B3, and results were similar between the ipi-N and ipi-T arms [46]. Responses were durable in both patient cohorts, from 6+ weeks to 39+ weeks for cohort D (all ipi-N and 47% of ipi-T patients had ≥36 weeks of follow-up, with 90% of responses ongoing at the analysis cutoff date of 18 October 2013), and from 6+ to 36+ weeks for cohort B3 (with 91% of responses ongoing at the analysis cutoff date; median duration of follow-up, 42.3 weeks) [44, 46]. Preliminary survival findings demonstrated a 24-week PFS rate of 43% and 47% for the Q3W and Q2W dosing schedules, respectively, in cohort B3 [not significant (NS); P < 0.3], [45] and 51% and 48% for the 2 and 10 mg/kg schedules, respectively, in cohort D [44]. Taken together, the results from cohorts B2, D, and B3 led to the conclusion that the pembrolizumab RP2D should be 2 mg/kg Q3W [46].
Pooled analysis of the data from the entire melanoma population of KEYNOTE-001 (cohorts B1 + B2 + B3 + D; N = 655), regardless of ipilimumab treatment history or pembrolizumab dose or schedule, revealed an ORR of 33% (Table 1), a 12-month PFS rate of 35%, and a median OS of 23 months (median duration of follow-up, 21 months). Response lasted >1 year in 44% of responders, and the estimated median DOR was 28 months. Pooled sub-analysis of ipi-N patients yielded an ORR of 45% and a median OS of 31 months [47]. These data suggested that a substantial proportion of patients with advanced melanoma treated with pembrolizumab will achieve a durable objective response and supported accelerated approval of the 2 mg/kg Q3W dose [23].
Pembrolizumab in NSCLC
A cohort of 38 previously treated patients with NSCLC (cohort C) was included in KEYNOTE-001 based on the observation that four of seven patients with NSCLC enrolled in cohort A (i.e. cohorts A, A1 and A2) experienced stable disease [41, 48]. Pembrolizumab had an acceptable and manageable toxicity profile in this group of patients, and antitumor activity was demonstrated in patients who had received two previous NSCLC treatment regimens, with an ORR of 21% per RECIST v1.1 by independent review. Preliminary data suggested that the level of PD-L1 expression was associated with increased antitumor activity of pembrolizumab [48].
Three cohorts in NSCLC (n = 512), which included previously treated and treatment-naive patients (cohorts F1–F3), were added to further investigate dose using a mainly randomized approach. Breakthrough designation for pembrolizumab in NSCLC was based on the findings from treated patients in cohorts F1, F2, and C irrespective of their line of therapy who had tumors evaluable for PD-L1 expression (n = 146). The findings from cohorts F1 (n = 101, treatment-naive, initially 2 mg/kg Q3W versus 10 mg/kg Q3W and then 10 mg/kg Q3W versus 10 mg/kg Q2W), F2 (n = 356, previously treated, 10 mg/kg Q3W versus Q2W), and F3 (n = 55, previously treated, 2 mg/kg Q3W) contributed to the approval of pembrolizumab in NSCLC (Figure 3).
Randomized comparisons between the 10 mg/kg Q2W and Q3W dosing schedules and between the 10 mg/kg Q3W and 2 mg/kg Q3W dosing schedules in patients with PD-L1-positive NSCLC (from cohorts F1 and F2) revealed similar ORRs and safety profiles [49, 50]. Furthermore, a pooled analysis demonstrated that pembrolizumab was well tolerated and conferred durable antitumor activity across all patient cohorts (i.e. cohorts C, F1, and F2; n = 495) regardless of prior treatment status, with no significant difference in either efficacy or safety among the three doses/schedules tested: ORR was 19.4% (95% CI 16.0–23.2), median PFS was 3.7 months (95% CI 2.9–4.1), and median OS was 12.0 months (95% CI 9.3–14.7). ORR was 18.0% (95% CI 14.4–22.2) in previously treated patients (n = 394; cohorts C and F2), and 24.8% (95% CI 16.7–34.3) in treatment-naive patients (n = 101; cohort F1) (Table 2) [51].
Table 2.
Primary efficacy data for the NSCLC cohorts (N = 495) from KEYNOTE-001 [50]
| Cohort | ORR |
|---|---|
| % (95% CI) | |
| All (N = 495)a | 19.4 (16.0–23.2) |
| Previously treated (n = 394)b | 18.0 (14.4–22.2) |
| Previously untreated (n = 101)c | 24.8 (16.7–34.3) |
| PD-L1+ TPS ≥50% (N = 73)d | 45.2 (33.5–57.3)* |
| Previously treated (n = 57) | 43.9 (30.7–57.6) |
| Previously untreated (n = 16) | 50.0 (24.7–75.3) |
| PD-L1+ TPS 1–49% (n = 103) | 16.5 (9.9–25.1) |
| PD-L1+ TPS <1% (n = 28) | 10.7 (2.3–28.2) |
Cohorts C, F1, and F2.
Cohorts C and F2.
Cohort F1.
Data for the validation set.
Significantly greater than PD-L1+ TPS 1–49% (P < 0.001) and PD-L1+ TPS <1% (P = 0.01).
NSCLC, non-small cell lung cancer; PD-L1+, positive for expression of programmed death ligand 1; TPS, tumor proportion score (percentage of PD-L1+ tumor cells).
These cohorts also provided pre-specified training and validation sets that were instrumental in the development of the first companion diagnostic for an immunotherapy and ultimately resulted in FDA approval of the Dako IHC 22C3 pharmDx PD-L1 expression assay (to identify PD-L1-positive tumors) in patients with NSCLC [31]. A tumor proportion score (TPS) cut point of ≥50% (i.e. PD-L1 expression on ≥50% of tumor cells) was selected by receiver operating characteristic curve analysis (and identified using the Youden index) for this assay based on clinical data from the NSCLC training set, which included patients from cohorts C (n = 27), F1 (n = 9), and F2 (n = 110) with at least one measurable lesion, adequate organ function, an Eastern Cooperative Oncology Group performance status of 0 or 1, and a tumor biopsy collected within 60 days of the first dose of pembrolizumab [51]. In patients with a tumor PD-L1 TPS ≥50%, the ORR for the validation set was 45.2% (95% CI 33.5–57.3), with a median PFS of 6.4 months (95% CI 4.2–not reached; median OS was not reached). ORR (Table 2) and median PFS for patients in the validation set with TPS 1%–49% were 16.5% (95% CI 9.9–25.1) and 4.1 months (95% CI 2.3–4.4), respectively, and were 10.7% (95% CI 2.3–28.2) and 4.0 months (95% CI 2.1–6.2), respectively, for those in the validation set with TPS <1% [51]. For the validation set, in patients with TPS ≥50%, the ORRs for all patients, those who were previously treated, and those who were treatment-naive were 45.2% (95% CI 33.5–57.3), 43.9% (95% CI 30.7–57.6), and 50.0% (95% CI 24.7–75.3), respectively, and the median PFS was 6.3 months (95% CI 2.9–12.5), 6.1 months (95% CI 2.1–12.5), and 12.5 months (95% CI 2.4–12.5), respectively. Median OS among those with TPS ≥50% was not reached for either previously treated or untreated patients [51].
The final cohort, F3, enrolled 55 patients with NSCLC who had at least one prior systemic therapy and whose tumors were PD-L1–positive (i.e. TPS ≥1%). Patients were administered pembrolizumab 2 mg/kg Q3W to further characterize the antitumor activity and safety profile of pembrolizumab monotherapy, and to evaluate the dose and antitumor activity in NSCLC. Together with cohorts F1 and F2, cohort F3 also evaluated the relationship between the degree of biomarker positivity and tumor response. The results were consistent with those of a subpopulation analysis of patients in cohort F2 with previously treated NSCLC and a TPS of ≥50%, and who were treated with pembrolizumab at 10 mg/kg Q2W or Q3W [52]. These findings provided supportive data for 2 mg/kg Q3W as the RP2D for NSCLC, and confirmation of that dose as the RP2D for melanoma. The overall safety profile of pembrolizumab in patients with NSCLC was similar, particularly with respect to immune- and drug-related AEs, to that observed in the melanoma cohorts [42, 43, 52].
The efficacy of pembrolizumab in patients with NSCLC enrolled in KEYNOTE-001 paved the way for further development in this indication and introduced the potential for prospective testing of PD-L1 expression to focus treatment on those patients most likely to benefit from pembrolizumab. These data supported accelerated approval of pembrolizumab 2 mg/kg Q3W for patients with metastatic NSCLC whose tumors express PD-L1 (TPS ≥50%) as determined by an FDA-approved test, and who have progressed on or after platinum-containing treatment, or for patients with PD-L1-expressing NSCLC tumors with genomic epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK) aberrations, and disease progression after FDA-approved therapy for those aberrations [29].
Study design and regulatory considerations
Drug development
Unlike the classic drug-development pathway of progressing from phase 1 (MTD, DLTs, safety, and tolerability) to phase 2 (dose-finding, efficacy assessment), and then to phase 3 (randomized controlled trials designed to demonstrate efficacy and safety in support of regulatory approval) for a single indication, KEYNOTE-001 was composed of nested phase-2-like studies in two oncologic indications—melanoma and NSCLC—with six randomized dose/schedule-comparison sub-studies involving both patient populations, as well as training and validation sets for the development of the PD-L1 IHC companion diagnostic assay. The use of multiple expansion cohorts conferred certain advantages. First, they allowed multiple hypotheses to be addressed (pertaining to populations, doses, and biomarker development) with appropriate type 1 error (false-positive) control and allowed for the simultaneous evaluation of multiple tumor types. In addition, they enabled the expedited development and approval of a drug that was considered transformative based on early and strong efficacy signals, with sufficient rigor to support regulatory filings, while being aligned with the single-arm trial design as one of the accepted approaches for seeking accelerated approval. The time from investigational new drug designation for pembrolizumab to first FDA approval in melanoma was approximately 4 years (Figure 2). Using the traditional development approach, this process would likely have taken more than twice as long, with the time from first testing of a potential therapeutic agent to regulatory approval generally being 10–15 years [53, 54].
However, there can also be inherent issues with this type of design. The rapid patient accrual in multiple separate cohorts, inclusion of additional tumor types, and multiple protocol amendments (nine in total) led to a high level of protocol complexity, which carries with it the potential to cause adherence issues at both the site and the patient levels. Testing of multiple hypotheses simultaneously rather than sequentially also increases the complexity of data analysis and interpretation. For example, dose hypotheses were evaluated in melanoma and NSCLC simultaneously, rather than waiting for the melanoma dose data to become available to inform the NSCLC studies. Finally, with complex protocols such as this, it is difficult to isolate single cohorts for submission purposes because multiple database locks are required during an ongoing study.
Regulatory considerations
For pembrolizumab, the approvals for metastatic melanoma and NSCLC arose from a single, first-in-human protocol. This was made possible by a solid scientific base together with close and frequent interactions, facilitated by the breakthrough designations, between the FDA and Merck & Co., Inc., which helped to rapidly resolve any issues and ensured alignment on study design and data analyses. The FDA provided the regulatory oversight necessary to ensure patient protection, reviewed and responded to the data regularly, and ensured appropriate statistical rigor. The achievement of breakthrough therapy designations, which involved multiple disciplines of the FDA, enabled a proactive approach to the availability of pembrolizumab to patients in the clinic within a relatively short timeframe. In fact, it has been proposed that breakthrough therapy designation should be used as a method of distinguishing drugs for which early efficacy has been demonstrated that is sufficient to justify precisely this type of drug development program [55]. Thus, the clinical development of pembrolizumab, beginning with KEYNOTE-001, has set a new precedent for the way in which such drugs can be more swiftly brought to patients in need [55].
Further clinical development of pembrolizumab
In December 2015 pembrolizumab received full approval for the treatment of patients with unresectable or metastatic melanoma and an expanded indication to include first-line treatment of this patient population [56], based on data from the large phase 2 KEYNOTE-002 (NCT01704287) and phase 3 KEYNOTE-006 (NCT01866319) studies [57, 58]. The durability of response to pembrolizumab has also been demonstrated by longitudinal data from KEYNOTE-001 in advanced melanoma [59, 60]. In August 2016 pembrolizumab received accelerated approval from the FDA for the treatment of HNSCC with progression on or after platinum-containing therapy [61], based on data from the KEYNOTE-012 trial (NCT01848834) [62, 63]. Based on the recently published results of KEYNOTE-010 (NCT01905657) [64], pembrolizumab received full approval in October 2016 for the treatment of patients with metastatic NSCLC whose tumors express PD-L1 (TPS ≥1%) as determined by an FDA-approved test, and who have progressed on or after platinum-containing treatment, or for patients with PD-L1-expressing NSCLC tumors with genomic EGFR or ALK aberrations and disease progression after FDA-approved therapy for those aberrations [65]. An expanded indication was also granted for the first-line treatment of patients with metastatic NSCLC whose tumors have high PD-L1 expression (TPS ≥50%) as determined by an FDA-approved test, and no EGFR or ALK aberrations [65]. The FDA has also approved the PD-L1 IHC 22C3 pharmDX assay as a companion diagnostic for the identification of PD-L1-positive tumors with a cutoff TPS of ≥1% (with high PD-L1 expression being defined as TPS ≥50%) [66]. In March 2017, pembrolizumab received accelerated approval from the FDA for the treatment of adult and pediatric patients who have refractory classical Hodgkin lymphoma, or who have relapsed after 3 or more prior lines of therapy [67]. In May 2017, pembrolizumab in combination with pemetrexed and carboplatin received accelerated approval from the FDA as first-line treatment of patients with metastatic non-squamous NSCLC based on results of the KEYNOTE-021 trial (NCT02039674) [68].
Pembrolizumab continues to be explored for the treatment of specific melanoma and NSCLC subpopulations (e.g. patients with asymptomatic brain metastases) and different stages of disease (e.g. adjuvant and neoadjuvant for melanoma and first-line for NSCLC), both as a monotherapy and in combination with other therapies (Table 3). This immune-checkpoint inhibitor has also demonstrated efficacy in several other advanced solid tumors and hematologic cancers [67–72] and is currently under investigation (also as a monotherapy and in combination with other cancer therapies) for the treatment of more than 30 cancers across more than 320 clinical trials [73]. In fact, breakthrough therapy designation for pembrolizumab has recently been granted for classic Hodgkin’s lymphoma, microsatellite-instability-high metastatic colorectal cancer, and unresectable or metastatic microsatellite-instability-high non-colorectal cancer [74–76].
Table 3.
Selection of ongoing phase 2/3 trials of pembrolizumab in melanoma and NSCLC
| Trial | Phase | Indication | Estimated enrollment | Intervention/arms | Status |
|---|---|---|---|---|---|
| Melanoma | |||||
| NCT02362594 | 3 | Stage III, high-risk melanoma after complete resection | 900 |
|
Currently recruiting |
| KEYNOTE-054 | |||||
| NCT02752074 | 3 | Unresectable or metastatic melanoma | 600 |
|
Currently recruiting |
| KEYNOTE-252/ECHO-301 | |||||
| NCT02263508 | 3 | Unresected melanoma | 660 |
|
Currently recruiting |
| MasterKey-265 | |||||
| NCT02506153 | 3 | Stage III-IV, high-risk melanoma that has been removed by surgery | 1378 |
|
Currently recruiting |
| NSCLC | |||||
| NCT01905657 | 2/3 | NSCLC with disease progression after platinum-containing therapy | 1034 |
|
Active, not recruiting |
| KEYNOTE-010a | |||||
| NCT02142738 | 3 | Previously untreated, PD-L1+-strong NSCLC | 305 |
|
Active, not recruiting |
| KEYNOTE-024 | |||||
| NCT02039674 | 1/2 | Previously untreated, metastatic NSCLC | 308 |
|
Currently recruiting |
| KEYNOTE-021 | |||||
| NCT02578680 | 3 | Previously untreated non-squamous, metastatic NSCLC | 570 |
|
Currently recruiting |
| KEYNOTE-189 | |||||
| NCT02220894 KEYNOTE-042 | 3 | Previously untreated, PD-L1+ NSCLC | 1240 |
|
Currently recruiting |
| NCT02775435 KEYNOTE-407 | 3 | First-line, metastatic, squamous NSCLC | 560 |
|
Currently recruiting |
| NCT02504372 KEYNOTE-091 | 3 | NSCLC after resection | 1380 |
|
Currently recruiting |
| Melanoma or NSCLC | |||||
| NCT02085070 | 2 | Melanoma + NSCLC with at least 2 untreated brain metastases | 64 | Pembrolizumab (OL) | Currently recruiting |
Some study results have now been published [64].
Investigator’s choice of: paclitaxel + carboplatin, pemetrexed + carboplatin, pemetrexed + cisplatin, gemcitabine + carboplatin, gemcitabine + cisplatin.
IFN-α2b, interferon alpha 2b; ipi, ipilimumab; NSCLC, non-small cell lung cancer; OL, open label; SOC, standard of care.
Conclusions
The pembrolizumab KEYNOTE-001 study is an innovative and groundbreaking study that has led to multiple regulatory achievements, including orphan drug designation, breakthrough therapy designations, accelerated approvals for the treatment of melanoma and NSCLC, and approval for a companion diagnostic for PD-L1 tumor expression in NSCLC. This was a phase 1 first in-human study with a single-arm, non-randomized design that was subsequently adapted on the basis of interim analyses and, through numerous amendments and addition of multiple expansion cohorts with carefully pre-specified statistical analysis plans, ultimately enrolled 1235 patients to address an unmet clinical need in melanoma and NSCLC. Its success is attributable to a strong scientific base, partnership with the FDA through frequent interactions, academic partners who quickly mobilized resources and efforts to facilitate execution of the study, and commitment from all parties involved to expedite the delivery of pembrolizumab to patients in need. This regulatory outcome is unprecedented and has undoubtedly altered the way in which clinical development of oncology therapeutics can be achieved.
Acknowledgements
The authors thank the patients and their families and all investigators and site personnel, and Roger Dansey, MD (Merck & Co., Inc., Kenilworth, NJ, USA) and Nageatte Ibrahim, MD (Merck & Co., Inc., Kenilworth, NJ, USA) for critical manuscript review. Editorial assistance was provided by Jacqueline Kolston, PhD, and Payal Gandhi, PhD, of the ApotheCom oncology team (Yardley, Pennsylvania, USA).
Funding
The study was funded by Merck & Co., Inc., Kenilworth, NJ, USA (no grant numbers apply).
Disclosure
All authors are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
References
- 1. Wu HC, Chang DK, Huang CT.. Targeted therapy for cancer. J Cancer Mol 2006; 2: 57–66. [Google Scholar]
- 2. Gerber DE. Targeted therapies: a new generation of cancer treatments. Am Fam Physician. 2008; 77: 311–319. [PubMed] [Google Scholar]
- 3. Miller MJ, Foy KC, Kaumaya PT.. Cancer immunotherapy: present status, future perspective, and a new paradigm of peptide immunotherapeutics. Discov Med 2013; 15: 166–176. [PubMed] [Google Scholar]
- 4. Almendro V, Marusyk A, Polyak K.. Cellular heterogeneity and molecular evolution in cancer. Annu Rev Pathol 2013; 8: 277–302. [DOI] [PubMed] [Google Scholar]
- 5. Ogino S, Fuchs CS, Giovannucci E.. How many molecular subtypes? Implications of the unique tumor principle in personalized medicine. Expert Rev Mol Diagn 2012; 12: 621–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12: 252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hanahan D, Weinberg RA.. Hallmarks of cancer: the next generation. Cell 2011; 144: 646–674. [DOI] [PubMed] [Google Scholar]
- 8. Cavallo F, De GC, Nanni P. et al. The immune hallmarks of cancer. Cancer Immunol Immunother 2011; 60: 319–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Leach DR, Krummel MF, Allison JP.. Enhancement of antitumor immunity by CTLA-4 blockade. Science 1996; 271: 1734–1736. [DOI] [PubMed] [Google Scholar]
- 10. O'Day SJ, Maio M, Chiarion-Sileni V. et al. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: a multicenter single-arm phase II study. Ann Oncol 2010; 21: 1712–1717. [DOI] [PubMed] [Google Scholar]
- 11. Weber J, Thompson JA, Hamid O. et al. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res 2009; 15: 5591–5598. [DOI] [PubMed] [Google Scholar]
- 12. Hodi FS, O'Day SJ, McDermott DF. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363: 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McDermott DF, Atkins MB.. PD-1 as a potential target in cancer therapy. Cancer Med 2013; 2: 662–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Greenwald RJ, Freeman GJ, Sharpe AH.. The B7 family revisited. Annu Rev Immunol 2005; 23: 515–548. [DOI] [PubMed] [Google Scholar]
- 15. Keir ME, Butte MJ, Freeman GJ, Sharpe AH.. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008; 26: 677–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Agata Y, Kawasaki A, Nishimura H. et al. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol 1996; 8: 765–772. [DOI] [PubMed] [Google Scholar]
- 17. Buchbinder EI, Desai A.. CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am J Clin Oncol 2016; 39: 98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Francisco LM, Sage PT, Sharpe AH.. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev 2010; 236: 219–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. He J, Hu Y, Hu M, Li B.. Development of PD-1/PD-L1 pathway in tumor immune microenvironment and treatment for non-small cell lung cancer. Sci Rep 2015; 5: 13110.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Topalian SL, Drake CG, Pardoll DM.. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol 2012; 24: 207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yearley J, Gibson G, Yu N. et al. PD-L2 expression in human tumors: relevance to anti-PD-1 therapy in cancer. Eur J Biochem FEBS 2016; 51(suppl 3): S718.. [DOI] [PubMed] [Google Scholar]
- 22. Keytruda [package insert]. Whitehouse Station, NJ, Merck: Sharp & Dohme Corp; 2015. [Google Scholar]
- 23. US Food and Drug Administration. FDA approves Keytruda for advanced melanoma [press release]. 2014. http://www.fiercebiotech.com /biotech/fda-approves-keytruda-for-advanced-melanoma (28 February 2017, date last accessed).
- 24. US Food and Drug Administration. US Department of Health and Human Services. FDA orphan drug designations and approvals. Pembrolizumab. https://www.accessdata.fda.gov/scripts/opdlisting/oopd/detailedIndex.cfm?cfgridkey=381012 (28 February 2017, date last accessed).
- 25. Merck announces breakthrough therapy designation for lambrolizumab an investigational antibody therapy for advanced melanoma [press release]. 4-24-2013. http://www.mercknewsroom.com/press-release/research-and-development-news/merck-announces-breakthrough-therapy-designation-lambrol (28 February 2017, date last accessed).
- 26. US Food and Drug Administration. Guidance for industry. Expedited programs for serious conditions-drugs and biologics. May 2014. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm358301.pdf (28 February 2017, date last accessed).
- 27. Keytruda [summary of product characteristics]. Belgium: Schering-Plough Lab NV; 2015.
- 28. Merck receives FDA breakthrough therapy designation for Keytruda (pembrolizumab) in advanced non-small cell lung cancer [press release]. 10-27-2014. http://www.mercknewsroom.com/news-release/oncology-newsroom/merck-receives-fda-breakthrough-therapy-designation-keytruda-pembroli (28 February 2017, date last accessed).
- 29. FDA approves Keytruda (pembrolizumab) for the treatment of patients with metastatic non-small cell lung cancer whose tumors express PD-L1 with disease progression on or after platinum-containing chemotherapy [press release]. 10-2-2015. http://www.mercknewsroom.com/news-release/prescription-medicine-news/fda-approves-keytruda-pembrolizumab-treatment-patients-metas (28 February 2017, date last accessed).
- 30. US Food and Drug Administration. Premarket approval (PMA) PD-L1 IHC 22C3 pharmDX. 2016. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMA/pma.cfm?id=P150013 (28 February 2017, date last accessed).
- 31. Dako. Dako, an Agilent Technologies company, announces FDA approval of new companion diagnostic for lung cancer [press release]. 2015. http://www.agilent.com/about/newsroom/presrel/2015/02oct-dk 15004.html (28 February 2017, date last accessed).
- 32. Korn EL, Liu PY, Lee SJ. et al. Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin Oncol 2008; 26: 527–534. [DOI] [PubMed] [Google Scholar]
- 33. Temel JS, Greer JA, Muzikansky A. et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 2010; 363: 733–742. [DOI] [PubMed] [Google Scholar]
- 34. Mu CY, Huang JA, Chen Y. et al. High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Med Oncol 2011; 28: 682–688. [DOI] [PubMed] [Google Scholar]
- 35. Sun JM, Zhou W, Choi SJ. et al. PD-L1 expression and survival in patients with non-small cell lung cancer (NSCLC) in Korea. J Clin Oncol 2014; 32(suppl 5): Abstr 8066. [Google Scholar]
- 36. Gao Q, Wang XY, Qiu SJ. et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res 2009; 15: 971–979. [DOI] [PubMed] [Google Scholar]
- 37. Wu P, Wu D, Li L. et al. PD-L1 and survival in solid tumors: a meta-analysis. PLoS ONE 2015; 10: e0131403.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Deeks ED. Pembrolizumab: a review in advanced melanoma. Drugs 2016; 76: 375–386. [DOI] [PubMed] [Google Scholar]
- 39. McDermott J, Jimeno A.. Pembrolizumab: PD-1 inhibition as a therapeutic strategy in cancer. Drugs Today 2015; 51: 7–20. [DOI] [PubMed] [Google Scholar]
- 40. Khoja L, Butler MO, Kang SP. et al. Pembrolizumab. J Immunother Cancer 2015; 3: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Patnaik A, Kang SP, Rasco D. et al. Phase I study of pembrolizumab (MK-3475; anti-PD-1 monoclonal antibody) in patients with advanced solid tumors. Clin Cancer Res 2015; 21: 4286–4293. [DOI] [PubMed] [Google Scholar]
- 42. Hamid O, Robert C, Daud A. et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 2013; 369: 134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Robert C, Ribas A, Wolchok JD. et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet 2014; 384: 1109–1117. [DOI] [PubMed] [Google Scholar]
- 44. Hamid O, Robert C, Ribas A. et al. Randomized comparison of two doses of the anti-PD-1 monoclonal antibody MK-3475 for ipilimumab-refractory (IPI-R) and IPI-naive (IPI-N) melanoma (MEL). J Clin Oncol 2014; 32(suppl 15): Abstr 3000. [Google Scholar]
- 45. Robert C, Joshua AM, Weber JS. et al. Pembrolizumab (pembro; MK-3475) for advanced melanoma (MEL): randomized comparison of two dosing schedules. Ann Oncol 2014; 25: 1–41. [Google Scholar]
- 46. Robert C, Joshua AM, Weber JS. et al. Pembrolizumab (MK-3475) for advanced melanoma: randomized comparison of two dosing schedules Poster presented at: European Society for Medical Oncology 2014 Congress, 26–30 September 2014; Madrid, Spain. [Google Scholar]
- 47. Ribas A, Hamid O, Daud A. et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA 2016; 315: 1600–1609. [DOI] [PubMed] [Google Scholar]
- 48. Garon E, Balmanoukian A, Hamid O. et al. Preliminary clinical safety and activity of MK-3475 monotherapy for the treatment of previously treated patients with non-small cell lung cancer (NSCLC) Presented at: IASLC 15th World Conference on Lung Cancer, 27–30 October 2013, Sydney, Australia. [Google Scholar]
- 49. Garon EB, Leighl NA, Rizvi NA, Safety and clinical activity of MK-3475 in previously treated patients (pts) with non-small cell lung cancer (NSCLC) Poster presented at the ASCO Annual Meeting, 30 May–3 June 2014; Chicago, IL, USA. [Google Scholar]
- 50. Rizvi NA, Garon EB, Leighl N. et al. Optimizing PD-L1 as a biomarker of response with pembrolizumab (pembro; MK-3475) as first-line therapy for PD-L1-positive metastatic non-small cell lung cancer (NSCLC): Updated data from KEYNOTE-001 Poster presented at the ASCO Annual Meeting, 25 May–3 June 2015, Chicago, IL, USA. [Google Scholar]
- 51. Garon EB, Rizvi NA, Hui R. et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015; 372: 2018–2028. [DOI] [PubMed] [Google Scholar]
- 52. Sul J, Blumenthal GM, Jiang X. et al. FDA approval summary: pembrolizumab for the treatment of patients with metastatic non-small cell lung cancer whose tumors express programmed death-ligand 1. Oncologist 2016; 21: 643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Corr P, Williams DE, The Pathway from idea to regulatory approval: examples for drug development In Lo B, Field MJ (eds), Conflict of Interest in Medical Research, Education and Practice. Washington, DC: National Academies Press; 2009. [PubMed] [Google Scholar]
- 54. Independent Institute. The drug development and approval process. FDAReview.org website. http://www.fdareview.org/03_drug_development.php (28 February 2017, date last accessed).
- 55. Prowell TM, Theoret MR, Pazdur R.. Seamless oncology-drug development. N Engl J Med 2016; 374: 2001–2003. [DOI] [PubMed] [Google Scholar]
- 56. FDA approves expanded indication for Merck's Keytruda (pembrolizumab) for the treatment of patients with advanced melanoma [press release]. 12-18-2015. http://www.businesswire.com/news/home/20151218005 982/en (28 February 2017, date last accessed).
- 57. Ribas A, Puzanov I, Dummer R. et al. Pembrolizumab versus investigator- choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol 2015; 16: 908–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Robert C, Schachter J, Long GV. et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 2015; 372: 2521–2532. [DOI] [PubMed] [Google Scholar]
- 59. Daud A, Ribas A, Robert C. et al. Long-term efficacy of pembrolizumab (pembro; MK-3475) in a pooled analysis of 655 patients (pts) with advanced melanoma (MEL) enrolled in KEYNOTE-001. J Clin Oncol 2015; 33(suppl 15): Abstr 9005. [Google Scholar]
- 60. Robert C, Ribas A, Hamid O. et al. Three year overall survival for patients with advanced melanoma treated with pembrolizumab in KEYNOTE001. Presented at 2016. ASCO Annual Meeting, June 3–7, Chicago, IL.
- 61. FDA approves Merck’s Keytruda (pembrolizumab) for patients with recurrent or metastatic head and neck squamous cell carcinoma with disease progression on or after platinum-containing chemotherapy [press release]. 8-5-2016. http://www.businesswire.com/news/home/2016080500 5807/en (28 February 2017, date last accessed).
- 62. Seiwert TY, Burtness B, Mehra R. et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol 2016; 17: 956–965. [DOI] [PubMed] [Google Scholar]
- 63. Keytruda [package insert]. Whitehouse Station, NJ, USA: Merck, Sharp & Dohme Corp.; August 2016.
- 64. Herbst RS, Baas P, Kim DW. et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016; 387: 1540–1550. [DOI] [PubMed] [Google Scholar]
- 65. Keytruda [package insert]. Whitehouse Station, NJ, USA; Merck, Sharp & Dohme Corp.; October 2016. [Google Scholar]
- 66. Agilent Technologies. Agilent Technologies receives expanded FDA approval for use of Dako PDL1 IHC 22C3 pharmDx companion diagnostic in nonsmall cell lung cancer [press release]. 10-24-2016. http://www.agilent. com /about /newsroom/ presrel/2016/24oct-ca16033.html (15 May 2017, date last accessed).
- 67. FDA approves Merck's Keytruda (pembrolizumab) for adult and pediatric patients with classical hodgkin lymphoma (cHL) refractory to treatment, or who have relapsed after three or more prior lines of therapy. [press release] 3-14-2017. http:// investors. merck. com/ news/ press- release- details/ 2017/ FDA- Approves- Mercks- KEYTRUDA- pembrolizumab- for- Adult- and - Pediatric - Patients- with- Classical- Hodgkin- Lymphoma- cHL- Refractory-to - Treatment- or-Who -Have -Relapsed- After- Three- or- More-Prior -Lines-of -Therapy /default.aspx (16 May 2017, date last accessed).
- 68. FDA approves Merck's Keytruda (pembrolizumab) as first-line combination therapy with pemetrexed and carboplatin for patients with metastatic nonsquamous non-small cell lung cancer (NSCLC), irrespective of PD-L1 expression. [press release] 5-10-2017. http:// www. mercknewsroom. com/ news- release/ prescription- medicine- news /fda- approves- mercks- keytruda- pembr o lizumab- first- line- combin (16 May 2017, date last accessed).
- 69. Armand P, Shipp MA, Ribrag V. et al. Programmed death-1 blockade with pembrolizumab in patients with classical Hodgkin lymphoma after brentuximab vedotin failure. J Clin Oncol 2016; 34: 3733–3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Armand P. Immune checkpoint blockade in hematologic malignancies. Blood 2015; 125: 3393–3400. [DOI] [PubMed] [Google Scholar]
- 71. Moskowitz CH, Ribrag V, Michot JM. et al. PD-1 blockade with the monoclonal antibody pembrolizumab (MK-3475) in patients with classical hodgkin lymphoma after brentuximab vedotin failure: preliminary results from a phase 1b study (KEYNOTE-013). 56th Annual Meeting of the American Society of Hematology. Blood 2014; 124: 290. [Google Scholar]
- 72. Nanda R, Chow LQ, Dees EC. et al. A phase Ib study of pembrolizumab (MK-3475) in patients with advanced triple-negative breast cancer. Presented at: 37th Annual CTRC-AACR San Antonio Breast Cancer Symposium, 9–13 December 2014, San Antonio, TX.
- 73. O'Donnell PH, Plimack ER, Bellmunt J. et al. Pembrolizumab (Pembro; MK-3475) for advanced urothelial cancer: results of a phase IB study. J Clin Oncol 2015; 33(suppl 7): Abstr 296. [Google Scholar]
- 74. Muro K, Bang YJ, Shankaran V. et al. Relationship between PD-L1 expression and clinical outcomes in patients with advanced gastric cancer treated with the anti-PD1 monoclonal antibody pembrolizumab (MK-3475) in KEYNOTE-012. J Clin Oncol 2015; 33(suppl 3): Abstr 03. [Google Scholar]
- 75. New Keytruda (pembrolizumab) data at 2016 ASCO annual meeting includes three-year overall survival data in melanoma and updated overall survival data in non-small cell lung cancer as well as updated findings in head and neck cancer [press release]. 5-16-2016. http://www.businesswire.com/news/home/20160516005363/en (28 February 2017, date last accessed).
- 76. Merck receives breakthrough therapy designation from US Food and Drug Administration for Keytruda (pembrolizumab) in advanced colorectal cancer [press release]. 11-2-2015. http://www.businesswire.com/news/home/20151102005577/en/ (28 February 2017, date last accessed).
- 77. Merck receives breakthrough therapy designation from US food and drug administration for Keytruda (pembrolizumab) in classical Hodgkin lymphoma (cHL) [press release]. 4-18-2016. http://www.businesswire.com/news/home/20160418005495/en/ (28 February 2017, date last accessed).
- 78. FDA grants priority review to Merck's supplemental biologics license application (sBLA) seeking approval for Keytruda (pembrolizumab) for new indication in microsatellite instability-high cancer [press release]. 11-28-2016. http://investors.merck.com/news/press-release-details/2016/FDA-Grants-Priority-Review-to-Mercks-Supplemental-Biologics-License-Application-sBLA-Seeking-Approval-for-KEYTRUDA-pembrolizumab-for-New-Indication-in-Microsatellite-Instability-High-Cancer/default.aspx (28 February 2017, date last accessed).