Abstract
Chordomas are rare, malignant bone tumors of the skull-base and axial skeleton. Until recently, there was no consensus among experts regarding appropriate clinical management of chordoma, resulting in inconsistent care and suboptimal outcomes for many patients. To address this shortcoming, the European Society of Medical Oncology (ESMO) and the Chordoma Foundation, the global chordoma patient advocacy group, convened a multi-disciplinary group of chordoma specialists to define by consensus evidence-based best practices for the optimal approach to chordoma. In January 2015, the first recommendations of this group were published, covering the management of primary and metastatic chordomas. Additional evidence and further discussion were needed to develop recommendations about the management of local-regional failures. Thus, ESMO and CF convened a second consensus group meeting in November 2015 to address the treatment of locally relapsed chordoma. This meeting involved over 60 specialists from Europe, the United States and Japan with expertise in treatment of patients with chordoma. The consensus achieved during that meeting is the subject of the present publication and complements the recommendations of the first position paper.
Keywords: chordoma, sarcoma, relapse, radiotherapy, surgery, chemotherapy
Introduction
Chordomas are rare, malignant bone tumors of the skull-base and axial skeleton [1]. Loco-regional recurrence is a common event following initial treatment of chordoma patients, and represents a major clinical challenge, which these recommendations seek to address. Loco-regional recurrence is defined as tumor relapse or progression after surgery and/or RT of the primary tumor at the same site and/or contiguous spreading of tumor from the primary site to adjacent areas. This includes progression of treated primary lesions, lesions recurring usually at, or near surgical margins, lesions that develop as a result of iatrogenic seeding along a biopsy or surgical tract, as well as skip metastases in the immediate vicinity of the tumor. In most cases, spread of the tumor is mediated by direct physical contact rather than dissemination via lymphatic, circulatory or subarachnoid routes. As such, cases with lymph node involvement are considered to have metastatic disease and are thus not addressed in these recommendations.
Published case series reporting post-surgical outcomes for chordoma indicate that loco-regional recurrence affects >50% of patients treated with macroscopic complete resection with or without RT (Tables 1–3). Notably, a high proportion of recurrences occur late (after 5 and 10 years), requiring long-term follow-up [2, 3]. Limited data are available about long-term recurrence-free survival (RFS), but all available long-term survival projections do not plateau, even after optimal local therapy. In particular, RFS or local control (LC) of skull-base chordomas at 5 and 10 years is 47–76% and 42–71% [3, 4], respectively, while 5- and 10-year estimated RFS for mobile spine chordomas is 58% and 32% [5]. Similarly, the 5-, 10- and 15-year local relapse (LR) incidence is reported to be 30%, 46% and 57%, respectively, in a recently published series of primary and completely resected sacral chordomas [6]. In this study, a plateau in RFS was not observed even at 15 years.
Table 2.
Outcome of primary and locally recurrent mobile spine and sacrum chordoma patients treated with surgery plus or minus RT
| Series (REF) | Year | No. pts | Sacrum/mobile spine | Quality of margins | No. pts. receiving RT/surgery | Median FU (years) | OS rate primary | OS rate recurrent | LR rate primary | LR rate recurrent | Prognosticators for LR |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Park [55] | 2006 | 27 |
|
|
27 (100%)/21(78%) | 8.8 (mean) | 93% at 10 years | 44% at 10 years | 9% at 10 years | 81% at 10 years | R1/R2 marginsRecurrent tumor |
| Boriani [56] | 2006 | 48 |
|
|
34 (65%)/48(100%) | NR | 23% overall | 44% overall | 53% overall | 89% overall | R1/R2 marginsRecurrent tumor |
| Stacchiotti [9] | 2010 | 130 |
|
|
42 (32%)/130(100%) | 11.8 | 54% at 10 years | 26% at 10 years | 67% at 10 years | 69% at 10 years | Large tumor sizeR1/R2 marginsRecurrent tumor |
| Zabel Du Bois [57] | 2010 | 34 | Sacrum=34 |
|
34 (100%)/24(71%) | 4.5 | 76% at 5 years | 76% at 5 years | 53% at 5 years | 76% at 5 years | No radiation therapyR1/R2 marginsRecurrent tumor |
| Staab [58] | 2011 | 40 |
|
|
40(100%)/40(100%) | 3.6 | 80% at 5 years | NR | 38% at 5 years | NR | No pre-RT surgical stabilization |
| DeLaney [59] | 2014 | 29 | NR |
|
29(100%)/20(69%) | 7.3 | NR | NR | 0% at 5 years | 50% at 5 years | Recurrent tumor |
| Xie [8] | 2015 | 54 | Sacrum=54Mobile spine=0 |
|
NR/54(100%) | 7.8 | 82% at 5 years | 56% at 5 years | 51% at 5 years | NR | R1/R2 marginsRecurrent tumor |
| Rotondo [60] | 2015 | 126a |
|
|
126 (100%)/126(100%) | 3.4 | 81% at 5 years | 78% at 5 years | 32% at 5 years | 51% at 5 years | R1/R2 marginsRecurrent tumor |
| Uhl [61] | 2015 | 56 |
|
|
56(100%)/32(57%) | 2.1 | 100% at 2 years | 100% at 2 years | 100% at 2 years | 53% at 2 years | Recurrent tumorFemale patients |
| Imai [62] | 2016 | 188 | Sacrum=188 | NA | 188(100%)/0(0%) | 5.1 | 69% at 10 years | 52% at 5 years | 52% at 10 years | NR | R1/R2 margins |
| Radaelli [6] | 2016 | 99 |
|
|
19 (19%)/99(100%) | 8.7 | 92% at 5 years | 30% at 5 years | 56% at 15 years | NR | Large tumor sizeR1/R2 marginsRecurrent tumor |
One patient had synchronous lumbar and sacrococcygeal chordoma.
pts, patients; RT, radiotherapy; FU, follow-up; OS, overall survival; LR, local recurrence; NR, not reported; R0, wide resection; R1, marginal resection; R2, intralesional resection.
Table 1.
Outcome of patients with recurrent skull-base and cervical spine chordoma and treated with surgical re-resection
| Series (REF) | Year | N. of patientsa | Location | Resection rate (%) | Complications % | Median follow-up (years) | 1 | 2 | 5 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Recurrence-free survival (year-survival) (%) | ||||||||||
| Colli [45] | 2001 | 19 | Skull-base |
|
NA | 3.2 | 74 | NA | 32 | NA |
| Crockard [46] | 2001 | 18 (21) | Skull-base and Cranio-vertebral junction |
|
NA | NA | NA | NA | NA | |
| Tzortzidis [4] | 2006 | 27 | Skull-base |
|
|
8 | 77 | 52 | 39 | 26 |
| Samii [47] | 2007 | 23 | Skull-base | NA | NA | NA | NA | NA | NA | |
| Takahashi [48] | 2009 | 13 | Skull-base |
|
NA | 3 | NA | NA | NA | – |
| Sen [49] | 2010 | 12 | Craniovertebral junction | GT: 33 | NA | 5 | NA | NA | NA | – |
| Koutourousiou [50] | 2012 | 25 | Skull-base |
|
NA | NA | NA | NA | NA | – |
| Yasuda [51] | 2012 | 17 |
|
|
CSF, meningitis, hydrocephalus, CN worsening: 23% | 4.7 | NA | NA | (77; 82 PFS) | NA |
| Chibbaro [52] | 2014 | 22 | Skull-base |
|
NA | 2.8 | NA | NA | NA | – |
| Boari [53] | 2016 | 13 | Clivus | GT: 40 | NA | 6.3 | NA | NA | NA | – |
| Gui [54] | 2016 | 91 | Skull-base |
|
NA | NA | NA | NA | NA | – |
Data on the outcome at a longer follow-up are frequently not available since primary and recurrent cases are not analysed separately.
Total number of patients with evidence of recurrent chordoma, including those not operated at latest follow-up or dead for disease progression.
Reported for reoperations (first and subsequent).
In brackets, complication at second and third reoperation.
N of patients, number of patients with chordoma undergoing surgery after initial treatment; REF, reference; N, number; GT, gross total; ST, sub-total; D, death; NA, not available; CS, cavernous sinus.
Table 3.
Local control of primary and locally recurrent chordoma patients of all sites treated with surgery plus RT or definitive RT
| Series (REF) | Year | N. pts | Type of RT | Dose and fractionation | Mean FU (years) | Oncological outcome |
|---|---|---|---|---|---|---|
| Skull base | ||||||
| Munzenrider [63] | 1999 | 290 | Surgery+protontherapy (passive fields)+photon RT |
|
3.4 | 5-year LRFS 73% chordoma |
| Noel [64] | 2005 | 88 | Surgery+protontherapy (passive fields) | Median dose 67 CGE with standard fractionation | 2.6 | 2-year LC 86% |
| Ares [65] | 2009 | 41 | Surgery+protontherapy (active spot scanning) | Median total dose 73.5 Gy (RBE) with standard fractionation | 3.2 | 5-year LC—81% |
| Mizoe [66] | 2009 | 33 | Surgery+carbon ions (passive fields) | Dose escalation 48.0, 52.8, 57.6, and 60.8 Gy in 16 fractions | 4.4 |
|
| Uhl [61] | 2014 | 155 | Surgery+carbon ions (active spot scanning) |
|
6 |
|
| Choy [67] | 2016 | 57 | Surgery+stereotactic radio surgery (SRS) or stereotactic radiotherapy (SRT) |
|
4.8 | Overall LC 48%5-year PFS 35.2% |
| Bugoci [68] | 2013 | 12 | Surgery+fractionated stereotactic radiotherapy | Median dose 66.6 Gy with standard fractionation | 3.5 | 5-year PFS 37.5% |
| Kano [69] | 2011 | 71 | Surgery+Gamma Knife stereotactic radiosurgery (SRS) | Median margin dose 15.0 Gy (range 9–25 Gy) | 5 | 5-year LC 66% |
| Chang [70] | 2001 | 10 (8 skull base, 2 cervical spine) | Surgery+LINAC stereotactic radiosurgery | Mean radiation dose 19.4 Gy | 4 | Gross LC 80% |
| Zorlu [71] | 2000 | 18 | Surgery+3D photons RT | Median 60 Gy with standard fractionation | 3.6 | 5-year PFS 23% |
| Foweraker [72] | 2007 | 12 (10 clivus, 2 cervical spine) | Surgery+photons radiotherapy | 65 Gy in 39 fractions | 3.2 | Gross LC 92% |
| Sacrum and spine | ||||||
| Imai [62] | 2016 | 188 | Exclusive carbon ions (passive fields) | Median 67.2 GyE in 16 fractions | 5.2 (median) |
|
| Uhl [61] | 2015 | 56 (41 primary tumors, 15 recurrent tumors) | Carbon ions (active scanning) or photons RT and carbon ions (active scanning)±surgery (10 R0/R1 resection 11 R2 resections 20 biopsy only, 15 recurrences) | Median 66 GyE | 2.1 |
|
| Mima [73] | 2014 | 23 | Exclusive carbon ions or exclusive protontherapy | 70.4 GyE in 16 fractionsor in 32 fractions | 3.2 | 3-year LC—94% |
| Rotondo [60] | 2015 | 126 (71 sacrococcygeal, 40 lumbar, 16 thoracic) | Surgery+protontherapy | Median 72.4 Gy RBE with standard fractionation | 3.5 |
|
| Holliday [74] | 2015 | 19 | Surgery+protontherapy | Median 70 Gy RBE with standard fractionation | 32.9 | 2-year LC—58% |
| DeLaney [59] | 2014 | 29 (23 primary, 6 recurrent) | Surgery+protontherapy | 77.4 Gy RBE with standard fractionation | 7.3 |
|
| Chen [75] | 2013 | 24 (19 sacrum, 2 cervical, 1 thoracic, and 2 lumbar spine) | Exclusive protontherapy (passive fields) | 77.4 Gy RBE (range 71.6–79.2 Gy RBE) with standard fractionation | 4.7 | 5-year LPFS 79.8% |
| Staab [58] | 2011 | 40 (32 primary, 8 recurrent) (21 adjuvant RT, 19 macroscopic disease) | Protontherapy spot scanning±radical surgery | 72.5 Gy RBE with standard fractionation | 3.6 |
|
| Dhawale [76] | 2014 | 21 (sacrum) | Surgery + (18) − (3) 3D conformal RT or IMRT | Mean dose 56 Gy with conventional fractionation | 5.8 | Gross LC 60% |
| Zabel-du Bois [57] | 2010 | 34 |
|
Mean dose 66 Gy with conventional fractionation | 4.5 |
|
There is no paper specifically reporting the outcome of relapsed chordoma treated with RT. Most series include both first line RT and salvage treatments.
N, number; pts, patients; RT, radiotherapy; FU, follow-up; CGE, cobalt gray equivalent; LRFS, local recurrence-free survival; LC, local control; RBE, relative biological effectiveness; Gy, Gray; PFS, progression-free survival; GyE, Gray equivalent; OS, overall survival; IMRT, intensity-modulated radiation therapy; LINAC, linear accelerator; R0, wide resection; R1, marginal resection; R2, intralesional resection.
Major determinants of local control in primary chordomas at all sites include tumor size, extent of resection, quality of surgery, quality of RT (e.g. dose, volume, timing and dose inhomogeneity) and patient age [2, 7–9]. The experience of the treatment center may also play a role in the likelihood of recurrence.
Patients whose tumors recur/progress locally are challenging to control in the long-term and only a minority can be cured. Hence, every effort is needed to maximize the chances for long-term control of tumor with optimal management of the patient at the time of initial treatment. Nevertheless, with optimal treatment, long-term disease control and good quality of life (QOL) may still be possible for some patients. Thus, defining evidence-based best practice to manage this disease state is of utmost importance in order to improve patient outcomes.
Methods, level of evidence and grade of recommendation
To generate the recommendations summarized herein, a consensus group meeting was organized in Milan in November 2015 by ESMO and the Chordoma Foundation (CF). Representatives from the all the disciplines involved in care and treatment of patients with chordoma participated, including specialists in pathology, radiology, neurosurgery, ENT surgery, orthopedic surgery, general surgery, radiotherapy (RT), medical oncology, and palliative care (PC). A representative from main European, United States and Japanese RT centers with protons/carbon ions facilities and with experience in chordoma joined the meeting. Additional participants included patient representatives, statisticians, and molecular biologists. Prior to the meeting a literature search was conducted (details in the supplementary Appendix 1, available at Annals of Oncology online) to elucidate data upon which to base consensus recommendations. During the meeting, representatives from 14 of the participating institutions presented unpublished clinical data on patients treated with surgery and/or RT for recurrent chordoma from 2005. Based on these data and the literature review, the group reached consensus about key aspects of the management of patients with loco-regional recurrence, reported in this position paper. The present article is aimed at complementing the recommendations of the first position paper, published in 2015. To avoid repetition, this text contains several cross references to it [2].
We chose to grade level of evidence (LOE) from I to V and use grades of recommendation from A to D adapted from the system used by the Infectious Diseases Society of America-US Public Health Service Grading System 2 (Table 4). When published evidence was scarce but a strong consensus was present, we recorded the LOE as V. Points for which consensus among participating experts was not achieved are acknowledged an noted herein. While stronger evidence would be desirable in many areas, we recognize the inherent difficulty of generating such data for rare cancers like chordoma, and, thus, accept that a higher degree of uncertainty must be tolerated for purposes of guideline development to avoid depriving rare cancer patients and those who care for them of much needed guidance [10].
Table 4.
Level of evidence and grade of recommendation
| Adapted from the Infectious Diseases Society of American-United States Public Health Service Grading System. |
| Level of evidence |
| I. Evidence from at least one large randomized controlled trial of good methodological quality (low potential for bias) or meta-analyses of well conducted randomized trials without heterogeneity |
| II. Small randomized trials or large randomized trials with a suspicion of bias (lower methodological quality) or meta-analyses of such trials or of trials with demonstrated heterogeneity |
| III. Prospective cohort studies |
| IV. Retrospective cohort studies or case-control studies |
| V. Studies without control group, case reports, and experts’ opinions |
| Grade of recommendation: |
| A. Strong evidence for efficacy with a substantial clinical benefit, strongly recommended |
| B. Strong or moderate evidence for efficacy but with a limited clinical benefit, generally recommended |
| C. Insufficient evidence for efficacy or benefit does not outweigh the risk or the disadvantages (including adverse events and costs), optional |
| D. Moderate evidence against efficacy or for adverse outcome, generally not recommended |
| E. Strong evidence against efficacy or for adverse outcome, never recommended |
| To distinguish prospectively planned studies from retrospective case series, we assigned the level of evidence V followed by ‘*’ to single-group prospective trials |
| The guidelines were adapted from the Infectious Diseases Society of America-US Public Health Service Grading System 2 |
Treatment strategy
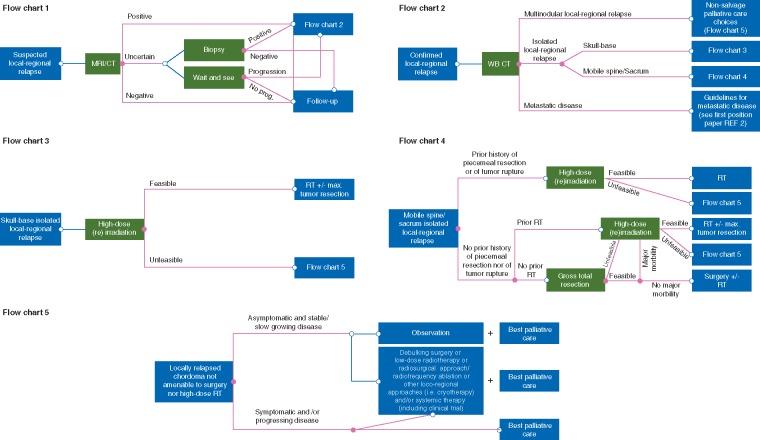
Figure 1 summarizes the recommended treatment strategy for patients with loco-regional recurrence.
Figure 1.
Flow-charts summarizing the recommended treatment strategy for patients with local-regional recurrence. MR, magnetic resonance; CT, computerized tomography; prog., progressioni; WB, whole body; REF, reference; RT, radiotherapy.
Patients who experience LR should be evaluated by a multidisciplinary team including at least a medical oncologist, radiotherapist, surgeon, pathologist, radiologist and PC specialist with expertise in chordoma. This recommendation is consistent with best practice for managing musculoskeletal neoplasms [11].
The presence/absence of symptoms should be factored in the decision-making algorithm. It is important to involve the patient when deciding which treatment to pursue.
The ‘extent of local disease’ should be determined using intra-venous contrast-enhanced MRI. In addition, restaging with total body computed tomography scan (CT) and whole spine MRI with a thorough clinical examination should complement loco-regional assessment to rule out distant metastases and/or subarachnoid spread.
‘Histological confirmation’ of recurrent disease is needed when there is diagnostic uncertainty, or when there is the suspicion of tumor dedifferentiation (e.g. unusually fast growth), or of a secondary malignancy. In cases where a tumor relapse is uncertain, a period of observation and re-imaging is an appropriate alternative to histologic assessment. A biopsy can be considered in selected cases for directing medical therapy.
‘Salvage treatment choices with curative intent’ can include surgery and/or RT, balancing morbidity, QOL and expected disease control. Surgical and RT strategy should be guided by the nature and extent of the previous procedure(s), the location of the recurrence, tumor resectability, deliverability of RT and the expected added morbidity of each procedure. Other relevant factors to consider include age, comorbidity, performance status (PS), and status of the surrounding tissues including the skin. The choice between surgery alone, surgery + RT, and RT alone must be based on individual case assessment; to date, there are no specific data to back generalized recommendations. A period of observation and re-imaging may help select best candidates for resection/RT or both. In particular, postponing active therapy can be considered in case of stable disease and/or no progression of symptoms.
The goal of ‘salvage re-resection with curative intent’ should be to achieve gross total resection, and, when feasible, en-bloc resection with negative surgical margins (IV-B). The best candidates for a complete re-resection are patients with isolated disease, a long disease-free interval, good PS (i.e. Eastern Cooperative Oncology Group-ECOG PS ≤ 2) and with a reasonable likelihood of acceptable morbidity. In cases of multifocal disease, a cure is virtually impossible so re-resection with curative intent should not be performed (IV-B); in these cases, only a limited resection should be considered with the goal of preventing the ill effects associated with disease progression whilst preserving function. A prior history of piecemeal resection (except for skull-base tumors where resection may be necessarily piecemeal), prior high-dose RT (in case of mobile spine and sacral chordoma), and/or tumor rupture are obvious exclusion criteria for re-resection with curative intent (IV-B). There is no consensus on how to treat intracanalar disease. In patients who have not previously received high-dose RT at the time of primary treatment, pre- and/or post-operative treatment with RT may also be appropriate [12, 13]. This approach is currently the standard treatment strategy in primary disease at some referral centers [14] and may be particularly well suited for treating local recurrences as the chance of achieving a true R0 resection after prior surgical procedures is low. It is currently not possible to make any recommendations regarding the role of adjuvant re-irradiation after macroscopically complete resection of recurrent chordoma.
‘ Salvage radiotherapy with curative intent’ should be offered with the same dose and techniques as employed in first-line therapy [2]. Thus, in case of recurrence in patients not previously treated with RT, definitive RT alone (e.g. without debulking) is a reasonable alternative to surgery plus radiation, although neither is very effective (V-C). Comparative effectiveness data for these approaches are limited and additional research is needed to determine which approach is superior. Patients considering definitive RT need to be informed about the risk of late toxicities from high-dose radiation (IV-B).
In the case of recurrent disease after previous RT, a new course of RT is indicated only when (i) this can be delivered without exceeding the estimated dose constraints on organs at risk (OARs) and (ii) adequate coverage of target volumes can be achieved. If this is not feasible, other treatment modalities are preferable (V-C). Currently, the cumulative dose tolerance for key OARs and the potentially protective role of partial damage repair after the first course of RT are still largely unknown. When complete resection of a recurrent lesion is not feasible, and proximity to critical structures precludes adequate RT coverage of target volumes, debulking surgery may be an appropriate option in order to separate critical structures from the residual tumor, thereby allowing delivery of a tolerable radiation dose.
‘ Salvage palliative/supportive treatment choices’ include debulking surgery, low-dose RT, stereotactic body RT (SBRT), including radiosurgery to small volume, radiofrequency ablation (RFA) and other loco-regional approaches (i.e. cryotherapy), systemic therapy, PC and observation. The patient’s symptom burden should guide the selection of an appropriate therapeutic approach as the potential for cure is nil. Care should be taken to avoid aggressive therapies that could cause unnecessary additional morbidity. Maximal debulking surgery should only be considered to alleviate or prevent symptoms related to nerve/cord/brain compression or for separating vital structures from the tumor to allow for radiation of the residual disease (V-C). This type of surgery is indeed only a temporizing measure, as local disease that remains after surgery will regrow in the region. Particular caution should be exercised in performing surgery near prior high-dose RT (IV-C) as the risk of surgical complications is dramatically greater in this setting. Additionally, the oncologic outcome generally deteriorates, and the chance of mortality and serious morbidity increases, with each serial resection (IV-B). Low-dose re-irradiation with palliative intent can be considered in selected case if it can be performed with negligible risk of toxicity (V-C).
‘PC’ should be considered as part of the active management of all patients and should include pain and symptom control, discussion about a patient’s concerns and wishes, a conversation about advanced directives, and evaluation of patient and family psychosocial needs.
‘ Salvage palliative anticancer medical therapy’ should be considered to attempt to stop tumor growth and/or alleviate symptoms in cases not amenable to local treatment or when symptomatic relief is needed, taking into consideration the PS, co-morbidities, expected treatment-related side effects, and the patient’s preferences (V*-B).
Technical aspects of treatment
Pre-treatment assessment
Imaging
Any relevant imaging studies performed prior to and after treatment of the primary chordoma should be obtained and reviewed. The first post-operative baseline imaging should be evaluated to confirm the initial extent of resection. Likewise, the post-radiation imaging at best response should be evaluated to assess the extent of residual disease. A comparative analysis of the imaging from first diagnosis to recurrence is important to distinguish recurrent disease from treatment sequelae and for assessing areas at high-risk of microscopic infiltration.
Although MRI is the modality of choice, CT may be a useful ancillary imaging modality, particularly to assess the bone involvement and when surgical implants or hardware limit MRI reliability. Myelo-CT can be useful to visualize peridural spaces when chordoma tissue invades the spinal canal. Furthermore, CT is a helpful tool in assessing stability of the spinal column.
For patients with skull-base tumors, assessment of internal carotid artery (ICA) and/or vertebral arteries with angio-CT can be needed for surgical planning. If curative surgery is considered, formal angiography with balloon test occlusion can be considered if ICA involvement is a limiting factor for tumor resection.
FDG-PET may be used in combination with other modalities in certain cases to exclude distant relapse and/or to evaluate tumor activity when tumor dedifferentiation is suspected or if a lesion is not clearly recurrent tumor.
Pathology
At the time of recurrence, the primary excised chordoma sample, including immunohistochemistry for brachyury and cytokeratin, should be reviewed and confirmed by an expert pathologist. The diagnosis should be based on the World Health Organization (WHO) Classification [1].
Tumor biopsy of recurrent disease, when warranted, must be performed with every attempt to limit the risk of tumor seeding [2]. A percutaneous core-needle biopsy is the preferable approach.
If a biopsy is obtained, it should be compared with the primary tumor to assess whether the tumor has changed or dedifferentiated over time. Dedifferentiated chordoma can show a deletion of INI1, which is a potentially targetable molecular alteration [15, 16].
Baseline patient evaluation
Prior to treatment, a complete physical examination and neurological assessment should be performed. For skull-base chordomas, endocrinological, ophthalmological and audiological examination are suggested. The patient’s symptoms and pace of symptom progression should be noted. Pain assessment should be performed using a 0–10 pain assessment scale [17]. Chronic pain secondary to RT or surgery should be distinguished from acute symptoms related to tumor progression for purposes of considering treatment approaches.
The evaluation should also include a detailed review of notes describing prior resections and/or RT, including but not limited to fields, dose and type of RT. The location of previous incisions or biopsies should be noted in relationship to new tumor lesion(s) for purposes of surgical planning.
Resection of recurrent or progressive disease
For mobile spine and sacral tumors, the goal of salvage surgery with curative intent should be to achieve en-bloc resection with negative surgical margins (IV-B). Particular attention should be paid to avoid tumor rupture, as this is associated with significant risk of tumor seeding. Recurrences in the skull-base or neck, as well as in the intrathoracic, intra-abdominal or intra-pelvic areas, are usually not amenable to margin negative/R0 resections, and therefore surgery should be aimed at a gross total resection (IV-B). For skull-base tumors R1 resection should be the goal of surgical treatment in all cases, in order to reduce tumor volume and increase the effectiveness of subsequent RT (V-A).
Debulking surgery should be cautiously considered only in certain rare cases, as it is unlikely to prolong survival. When subtotal resection is performed, every effort should be made to minimize contamination of the surrounding tissues (V-B).
When no prior RT had been delivered, post-operative RT should be considered, especially when microscopic margins were positive/R1. A component of preoperative RT can also be considered [18].
Radiotherapy of recurrence
RT can be delivered both with curative or palliative intent. To achieve local control in recurrent chordoma it is necessary to give a biologically high-dose while limiting the cumulative dose delivered to the critical structures near the target volume (IV-B). The feasibility and utility of RT for patients with recurrent chordoma depends primarily on whether or not the patient received RT to the same area as part of primary management. Thus, recommendations are presented below for two scenarios: patients without and with previous irradiation.
RT in patients without previous irradiation
Salvage RT with curative intent should be offered with the same modality employed for first line therapy (V-C) [2]. Since chordomas are radioresistant, a dose of at least 74 GyE should be delivered, using conventional fractionation (1.8–2 GyE) for photon and proton therapy (V*-A); moderately hypofractionated schedules can be used with carbon ions with dose per fractions ranging between 3 and 4.4 Gy RBE and total doses ranging from 60 and 70.4 Gy RBE [2]. Prior to RT, surgical re-resection should be discussed in all cases. Target volumes should be delineated considering the primary tumor location and its recurrence. The high-dose volume should include any macroscopic disease as well as surgical margins, while the low-dose volume should encompass areas at risk of microscopic spread, skip metastases, or seeding due to surgical procedures. In selected cases, a radio-surgical approach to gross disease may be appropriate, although there is no consensus as of yet about the criteria for recommending it.
RT in patients with previous irradiation
The radiation dose previously received by nearby OARs often limits the dose of radiation that can be safely delivered to the tumor, making local control of recurrences challenging. In general, the dose constraints for re-irradiation to OARs are not clearly established and the degree of recovery from initial radiation is difficult to estimate. However, preliminary data are available regarding tolerance to re-irradiation of the spinal cord, brain and aorta, which can help guide decision-making [7, 19–22]. If a new course of high-dose RT can be delivered without exceeding the estimated dose constraints on OARs, the patient should be treated with the same intent and approach as a RT naïve recurrence (V-C). Radiation plans must be based on an accurate reconstruction of the previous RT dose distribution, and taking into account expected morbidity of additional radiation (V-C). In case of tumor seeding in the surgical pathway, the site of relapse is often outside the previously irradiated volume and can be adequately treated by radiation [7]. The radiotherapist must exercise professional judgment in developing the radiation plan, as there is currently insufficient data to recommend an optimal dose and fractionation scheme for radiation in this setting. Regardless, particular caution is warranted in re-irradiating the carotid artery as severe, life-threatening complications such as carotid blowout syndrome have been reported in patients treated with re-irradiation for head and neck cancer [23]. If re-irradiation cannot achieve sufficiently high-dose or adequate coverage of target volumes without exceeding estimated dose constraints, then other treatment modalities are preferable. Low-dose re-irradiation with palliative intent can be appropriate in selected cases but only if it can be performed with negligible risk of toxicity. The use of high Linear Energy Transfer (LET) radiation such as carbon ions can be considered especially in case of re-irradiation after an initial course of low LET treatment as it may be more effective against the radio-resistant clones that may have been selected by the first treatment.
Metal implants (e.g. for spine stabilization) complicate RT delivery by creating artifacts in CT/MRI images. This can interfere with precise delineation of target and OAR, especially in the spinal canal. Additionally, these artifacts affect range calculation for particle therapy, and, therefore, may result in an additional uncertainty in delivered dose. Consequently, the presence of metal implants may be a key factor in deciding not to deliver curative RT or in deciding to deliver it with photons, which are less sensitive to artifacts, instead of particles (IV-B). If a debulking or a separating surgery is planned, the possibility of modifying, removing or substituting metal implants with carbon fiber devices should be considered to enable radiation with potentially curative intent; however, this is appropriate only in very well selected cases after thorough multidisciplinary assessment.
Other local therapies
Retrospective data suggest that cryoablation and RFA can be safe and useful palliative treatments in recurrent extracranial chordomas with a benefit in pain control [24–26]. However, prospective studies are needed before recommending these procedures in chordoma.
SBRT, including radiosurgery, has been described in retrospective and prospective series as safe and effective salvage strategy for spine tumors that have recurred after prior RT [27]. SBRT has been suggested as a palliative treatment option also in chordoma patients who suffer LR after prior RT [28], nevertheless prospective confirmatory data are necessary to make any definitive recommendations.
In principle, other local therapies such as local microwave hyperthermia and high-intensity focused ultrasound (HIFU) may also offer benefit in a palliative setting; however, currently there are no published data supporting their use.
Medical therapy
Medical therapy is an appropriate palliative option for patients whose disease is actively progressing or who are symptomatic. A brief observation period may be warranted before starting medical therapy to determine whether, and at what rate, the disease is progressing. If no progression is detected, it may be more appropriate to continue with active surveillance.
Currently, medical therapy options are limited and no drugs are approved for the treatment of advanced chordoma. However, several targeted therapies have shown modest activity in patients with recurrent disease. Imatinib and sorafenib are the agents with the greatest evidence of efficacy in advanced chordoma and represent reasonable palliative treatment options to slow disease progression or alleviate symptoms (V*-B) [29–32]. Access to these drugs varies widely among countries, posing a challenge for patients in some areas. In addition, several case reports have noted activity of sunitinib and EGFR inhibitors (cetuximab, erlotinib, gefitinib) [33–38].
Cytotoxic chemotherapy is generally inactive, and there is insufficient evidence to recommend it (V-D). However, there are anecdotal reports of responses to chemotherapy in high-grade/dedifferentiated chordoma and in some pediatric cases [39].
Although no predictors of response to targeted agents have been identified in chordoma, molecular profiling of tumors may help guide selection of experimental therapies. One potentially relevant biomarker is INI1 loss, which has been reported in dedifferentiated chordomas and may confer sensitivity to EZH2 inhibitors [15, 16].
A more detailed and up to date description of published data on medical therapy in chordoma is provided in supplementary Appendix 2, available at Annals of Oncology online.
Palliative, supportive and end-of-life care
PC is part of the active care of patients with advanced illness [40]. A comprehensive PC approach and access to specialized PC are both necessary (Table 5) [41].
Table 5.
General schema for palliative care application to advanced chordoma patients
Palliative care domains
|
Clinical care pathways and integration with oncology care
|
Most chordoma patients suffer from both somatic and neuropathic pain that can be difficult to treat. Worsening of pain and/or of neurologic symptoms can be the first sign of disease relapse/progression even when this cannot be yet detected radiologically [42].
First-line analgesic therapy should be provided by the oncology team according to available guidelines [43]. Pain due to the compression of nervous tissues via epidural compression or radiculopathy often benefits from steroids (dexamethasone or methylprednisolone). Difficult pain syndromes poorly responsive to analgesic pharmacotherapy can benefit from more invasive analgesic techniques such as spinal administration of opioids, ziconotide and adjuvant drugs [44].
In the terminal phase, the patient’s preferred setting of care should be identified. Hospice and home-care are valid options depending on the patient’s and family’s preferences.
Follow-up
Currently, there is insufficient data to recommend an optimal routine follow-up policy for patients with recurrent chordoma. Thus, follow-up is usually chosen based on the best judgment of the patient’s care team. However, experts agreed that MRI should be performed every 3–6 months at least for the first 3 years from treatment of LR/local progression. There is currently no consensus about whether routine scanning of the rest of the body is beneficial and for how long follow-up should be continued, though long-term vigilance is warranted as relapses often take place after several years.
Emerging approaches and future directions
For those patients who fail surgery and RT, there remains an urgent unmet need for new therapeutic options. To facilitate patient participation in clinical trials, the CF maintains an up to date list of trials open to chordoma patients (www.chordomafoundation.org/clinical-trials/) and a ‘target dashboard’ (www.chordomafoundation.org/targets/) summarizing published data about therapeutically-relevant targets.
Future clinical trials should be designed considering the rarity and distinctive natural history of chordoma. Due to its rarity, performing randomized trials may not be feasible. Additionally, due to its characteristically slow growth-rate and relatively long expected OS period, determining an OS benefit is likely impractical, thus necessitating the use of surrogate endpoints to assess efficacy. However, because patients often experience prolonged periods of symptomatic disease progression prior to end-stage disease, conventional surrogate endpoints based solely on dimensional response may miss improvements in QOL, and, thus, may be inadequate for inferring clinical benefit. New, and possibly unconventional, approaches are needed for assessing efficacy and facilitate the pathway to drug approval. Meanwhile, patients should be enrolled in prospective registries or observational studies to better understand the natural history of chordoma and identify relevant correlates of outcome that could aid in future trial design and help optimize clinical care.
Supplementary Material
Acknowledgements
We are deeply indebted with the Chordoma Foundation and with F. Longo, from the European Society for Medical Oncology (ESMO), for their invaluable support to the consensus building process, without which this effort would not have been possible. We are also deeply grateful to Chordoma Foundation European Liaison Hans Keulen, who, sadly, lost his battle with chordoma prior to completion of this article, for inspiring and helping to spearhead this international consensus-building effort.
Funding
None declared.
Disclosure
The authors have declared no conflicts of interest.
Outside this work:
SS: grants and personal fees from Novartis and Epizyme; grants from Bayer, GlaxoSmithKline, Pfizer; AG: personal fees from Bayer, Glaxo, Novartis and Pfizer; NH: nonfinancial support from Bayer; JMB: personal fees from Bayer, Glaxo and Novartis; CM: grants from Epizyme and Novartis; SP: personal fee from Bayer, Merk Serono; PR: grants and personal fees form Novartis, personal fees for Brystol-Meyrs Squibb, personal fees from Merk Serono and Pfizer; SS: personal fees and grant from Chordoma Foundation, nonfinancial support from Glaxo; PGC: grants and personal fees from Bayer, Glaxo, Novartis and Pfizer; personal fees from Merk Serono, grants from Epizyme; JS reported grant from Bristol-Myers Squibb; TA, CA, MB, JYB, SB, SB, RC, AC, RC, VC, JD, TD, AD, PD, SD, FD, AF, PF, SF, PAG, HG, ZLG, RH, CH, PH, FH, RI, LJ, RLJ, BK, AK, MK, AL, IL, DM, PN, OJN, NP, PP, SP, SR, FR, CS, ET, KAT, BT, VT, PUT, MU, YY, DCW, DV, PPV, CLAV-L declared no competing interests.
References
- 1. Flanagan AM, Yamaguchi T, Chordoma In Fletcher CDM, Bridge JA, Pancras CW, Mertens F (eds), World Health Organization (WHO) Classification of Tumours of Soft Tissue and Bone. Pathology and Genetics. Lyon: IARC Press; 2013; 328–329. [Google Scholar]
- 2. Stacchiotti S, Sommer J. on behalf of a Chordoma Global Consensus Group. Building a global consensus approach to chordoma: a position paper from the medical and patient community. Lancet Oncol 2015; 16: 71–83. [DOI] [PubMed] [Google Scholar]
- 3. Weber DC, Malyapa R, Albertini F. et al. Long term outcomes of patients with skull-base low-grade chondrosarcoma and chordoma patients treated with pencil beam scanning proton therapy. Radiother Oncol 2016; 120: 169–174. [DOI] [PubMed] [Google Scholar]
- 4. Tzortzidis F, Elahi F, Wright D. et al. Patient outcome at long-term follow-up after aggressive microsurgical resection of cranial base chordomas. Neurosurgery 2006; 59: 230–237. [DOI] [PubMed] [Google Scholar]
- 5. Cloyd JM, Acosta FL Jr, Polley MY. et al. En bloc resection for primary and metastatic tumors of the spine: a systematic review of the literature. Neurosurgery 2010; 67: 435–444. [DOI] [PubMed] [Google Scholar]
- 6. Radaelli S, Stacchiotti S, Ruggieri P. et al. Sacral chordoma: long-term outcome of a large series of patient surgically treated at two reference centers. Spine 2016; 41: 1049–1057. [DOI] [PubMed] [Google Scholar]
- 7. McDonald MW, Linton OR, Moore MG. et al. Influence of residual tumor volume and radiation dose coverage in outcomes for clival chordoma. Int J Radiat Oncol Biol Phys 2016; 95: 304–311. [DOI] [PubMed] [Google Scholar]
- 8. Xie C, Whalley N, Adasonla K. et al. Can local recurrence of a sacral chordoma be treated by further surgery?. Bone Joint J 2015; 97: 711–715. [DOI] [PubMed] [Google Scholar]
- 9. Stacchiotti S, Casali PG, Lo Vullo S. et al. Chordoma of the mobile spine and sacrum: a retrospective analysis of a series of patients surgically treated at two referral centers. Ann Surg Oncol 2010; 17: 211–219. [DOI] [PubMed] [Google Scholar]
- 10. Casali PG, Bruzzi P, Bogaerts J. et al. Rare Cancers Europe (RCE) methodological recommendations for clinical studies in rare cancers: a European consensus position paper. Ann Oncol 2015; 26(2): 300–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Randall RL, Bruckner JD, Papenhausen MD. et al. Errors in diagnosis and margin determination of soft-tissue sarcomas initially treated at non-tertiary centers. Orthopedics 2004; 27: 209–212. [DOI] [PubMed] [Google Scholar]
- 12. McDonald MW, Linton OR, Shah MV.. Proton therapy for reirradiation of progressive or recurrent chordoma. Int J Radiat Oncol Biol Phys 2013; 87: 1107–1114. [DOI] [PubMed] [Google Scholar]
- 13. Fagundes MA, Hug EB, Liebsch NJ. et al. Radiation therapy for chordomas of the base of skull and cervical spine: patterns of failure and outcome after relapse. Int J Radiat Oncol Biol Phys 1995; 33: 579–584. [DOI] [PubMed] [Google Scholar]
- 14. Wagner TD, Kobayashi W, Dean S. et al. Combination short-course preoperative irradiation, surgical resection, and reduced-field high-dose postoperative irradiation in the treatment of tumors involving the bone. Int J Radiat Oncol Biol Phys 2009; 73: 259–266. [DOI] [PubMed] [Google Scholar]
- 15. Mobley BC, McKenney JK, Bangs CD. et al. Loss of SMARCB1/INI1 expression in poorly differentiated chordomas. Acta Neuropathol 2010; 120: 745–753. [DOI] [PubMed] [Google Scholar]
- 16. Hasselblatt M, Thomas C, Hovestadt V. et al. Poorly differentiated chordoma with SMARCB1/INI1 loss: a distinct molecular entity with dismal prognosis. Acta Neuropathol 2016; 132: 149–151. [DOI] [PubMed] [Google Scholar]
- 17. Kaasa S, Apolone G, Klepstad P. et al. European Palliative Care Research Collaborative (EPCRC); European Association for Palliative Care Research Network (EAPCRNExpert conference on cancer pain assessment and classification–the need for international consensus: working proposals on international standards. BMJ Support Palliat Care 2011; 1: 281–287. [DOI] [PubMed] [Google Scholar]
- 18. DeLaney TF, Haas RLM.. Innovative radiotherapy of sarcoma: proton beam radiation. Eur J Cancer 2016; 62: 112–123. [DOI] [PubMed] [Google Scholar]
- 19. Evans JD, Gomez DR, Amini A. et al. Aortic dose constraints when reirradiating thoracic tumors. Radiother Oncol 2013; 106: 327–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nieder C, Grosu AL, Andratschke NH. et al. Update of human spinal cord reirradiation tolerance based on additional data from 38 patients. Int J Radiat Oncol Biol Phys 2006; 66: 1446–1449. [DOI] [PubMed] [Google Scholar]
- 21. Sminia P, Mayer R.. External beam radiotherapy of recurrent glioma: radiation tolerance of the human brain. Cancers (Basel) 2012; 4: 379–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Karam I, Huang SH, McNiven A. et al. Outcomes after reirradiation for recurrent nasopharyngeal carcinoma: North American experience. Head Neck 2016; 38(Suppl 1): E1102–E1109. [DOI] [PubMed] [Google Scholar]
- 23. Chen KC, Yen TT, Hsieh YL. et al. Postirradiated carotid blowout syndrome in patients with nasopharyngeal carcinoma: a case-control study. Head Neck 2015; 37: 794–799. [DOI] [PubMed] [Google Scholar]
- 24. Teichgräber V, Aubè C, Schmidt D. et al. Percutaneous MR-guided radiofrequency ablation of recurrent sacrococcygeal chordomas. AJR Am J Roentgenol 2006; 182: 571–574. [DOI] [PubMed] [Google Scholar]
- 25. Marchal F, Brunaud L, Bazin C. et al. Radiofrequency ablation in palliative supportive care: early clinical experience. Oncol Rep 2006; 15: 495–499. [PubMed] [Google Scholar]
- 26. Kurup AN, Woodrum DA, Morris JM. et al. Cryoablation of recurrent sacrococcygeal tumors. J Vasc Interv Radiol 2012; 23: 1070–1075. [DOI] [PubMed] [Google Scholar]
- 27. Brown JM, Koong AC.. High-dose single-fraction radiotherapy: exploiting a new biology?. Int J Radiat Oncol Biol Phys 2008; 71: 324–325. [DOI] [PubMed] [Google Scholar]
- 28. Yamada Y, Gounder M, Laufer I.. Multidisciplinary management of recurrent chordomas. Curr Treat Options Oncol 2013; 14: 442–453. [DOI] [PubMed] [Google Scholar]
- 29. Stacchiotti S, Longhi A, Ferraresi V. et al. A phase II study on imatinib in advanced chordoma. J Clin Oncol 2012; 30: 914–920. [DOI] [PubMed] [Google Scholar]
- 30. Hindi N, Casali PG, Morosi C. et al. Imatinib in advanced chordoma: a retrospective case series analysis. Eur J Cancer 2015; 51: 2609–2614. [DOI] [PubMed] [Google Scholar]
- 31. Bompas E, Le Cesne A, Tresch-Bruneel E. et al. Sorafenib in patients with locally advanced and metastatic chordomas: a phase II trial of the French Sarcoma Group (GSF/GETO). Ann Oncol 2015; 26: 2168–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lebellec L, Bertucci F, Tresch-Bruneel E. et al. Circulating vascular endothelial growth factor (VEGF) as predictive factor of progression-free survival in patients with advanced chordoma receiving sorafenib: an analysis from a phase II trial of the french sarcoma group (GSF/GETO). Oncotarget 2016; 7: 73984–73994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stacchiotti S, Marrari A, Tamborini E. et al. Response to imatinib plus sirolimus in advanced chordoma. Ann Oncol 2009; 20: 1886–1894. [DOI] [PubMed] [Google Scholar]
- 34. George S, Merriam P, Maki RG. et al. Multicenter phase II trial of sunitinib in the treatment of nongastrointestinal stromal tumor sarcomas. J Clin Oncol 2009; 27: 3154–3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hof H, Welzel T, Debus J.. Effectiveness of cetuximab/gefitinib in the therapy of a sacral chordoma. Onkologie 2006; 29: 572–7574. [DOI] [PubMed] [Google Scholar]
- 36. Singhal N, Kotasek D, Parnis FX.. Response to erlotinib in a patient with treatment refractory chordoma. Anticancer Drugs 2009; 20: 953–955. [DOI] [PubMed] [Google Scholar]
- 37. Linden O, Stenberg L, Kjellen E.. Regression of cervical spinal cord compression in a patient with chordoma following treatment with cetuximab and gefi tinib. Acta Oncol 2009; 48: 158–159. [DOI] [PubMed] [Google Scholar]
- 38. Stacchiotti S, Tamborini E, Lo Vullo S. et al. Phase II study on lapatinib in advanced EGFR-positive chordoma. Ann Oncol 2013; 24: 1931–1936. [DOI] [PubMed] [Google Scholar]
- 39. Walcott BP, Nahed BV, Mohyeldin A. et al. Chordoma: current concepts, management, and future directions. Lancet Oncol 2012; 13: e69–e76. [DOI] [PubMed] [Google Scholar]
- 40. World Health Organization. Strengthening of palliative care as a component of integrated treatment within the continuum of care; http://apps.who.int/gb/ebwha/pdf_files/EB134/B134_R7-en.pdf (23 February 2017, date last accessed).
- 41. Tassinari D, Drudi F, Monterubbianesi MC. et al. Early palliative care in advanced oncologic and non-oncologic chronic diseases: a systematic review of literature. Rev Recent Clin Trials 2016; 11: 63–71. [DOI] [PubMed] [Google Scholar]
- 42. Phimolsarnti R, Wiakaul S.. Prevalence of neuropathic pain after radical sacral Chordoma resection: a observational cohort study with 10 year-follow up. Eur J Orthop Surg Traumatol 2015; 25(Suppl 1): S225–S231. [DOI] [PubMed] [Google Scholar]
- 43. Caraceni A, Hanks G, Kaasa S. et al. European Palliative Care Research Collaborative (EPCRC); European Association for Palliative Care (EAPC). Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol 2012; 13: e58–e68. [DOI] [PubMed] [Google Scholar]
- 44. Tumber PS, Fitzgibbon DR.. The control of severe cancer pain by continuous intrathecal infusion and patient controlled intrathecal analgesia with morphine, bupivacaine and clonidine. Pain 1998; 78: 217–220. [DOI] [PubMed] [Google Scholar]
- 45. Colli BO, Al-Mefty O.. Chordomas of the skull base: follow-up review and prognostic factors. Neurosurg Focus 2001; 10: E1.. [DOI] [PubMed] [Google Scholar]
- 46. Crockard HA, Steel T, Plowman N. et al. A multidisciplinary team approach to skull base chordomas. J Neurosurg 2001; 95: 175–183. [DOI] [PubMed] [Google Scholar]
- 47. Samii A, Gerganov VM, Herold C. et al. Chordomas of the skull base: surgical management and outcome. J Neurosurg 2007; 107: 319–324. [DOI] [PubMed] [Google Scholar]
- 48. Takahashi S, Kawase T, Yoshida K. et al. Skull base chordomas: efficacy of surgery followed by carbon ion radiotherapy. Acta Neurochir (Wien). 2009; 151: 759–769. [DOI] [PubMed] [Google Scholar]
- 49. Sen C, Triana AI, Berglind N. et al. Clival chordomas: clinical management, results, and complications in 71 patients. J Neurosurg 2010; 113: 1059–1071. [DOI] [PubMed] [Google Scholar]
- 50. Koutourousiou M, Gardner PA, Tormenti MJ. et al. Endoscopic endonasal approach for resection of cranial base chordomas: outcomes and learning curve. Neurosurgery 2012; 71: 614–624. [DOI] [PubMed] [Google Scholar]
- 51. Yasuda M, Bresson D, Chibbaro S. et al. Chordomas of the skull base and cervical spine: clinical outcomes associated with a multimodal surgical resection combined with proton-beam radiation in 40 patients. Neurosurg Rev 2012; 35: 171–182. [DOI] [PubMed] [Google Scholar]
- 52. Chibbaro S, Cornelius JF, Froelich S. et al. Endoscopic endonasal approach in the management of skull base chordomas–clinical experience on a large series, technique, outcome, and pitfalls. Neurosurg Rev 2014; 37: 217–224. [DOI] [PubMed] [Google Scholar]
- 53. Boari N, Gagliardi F, Cavalli A. et al. Skull base chordomas: clinical outcome in a consecutive series of 45 patients with long-term follow-up and evaluation of clinical and biological prognostic factors. J Neurosurg 2016; 8: 1–11. [DOI] [PubMed] [Google Scholar]
- 54. Gui S, Zong X, Wang X. et al. Classification and surgical approaches for transnasal endoscopic skull base chordoma resection: a 6-year experience with 161 cases. Neurosurg Rev 2016; 39: 321–332. [DOI] [PubMed] [Google Scholar]
- 55. Park L, Delaney TF, Liebsch NJ. et al. Sacral chordomas: impact of high-dose proton/photon-beam radiation therapy combined with or without surgery for primary versus recurrent tumor. Int J Radiat Oncol Biol Phys 2006; 65: 1514–1521. [DOI] [PubMed] [Google Scholar]
- 56. Boriani S, Bandiera S, Biagini R. et al. Chordoma of the mobile spine: fifty years of experience. Spine 2006; 31: 493–503. [DOI] [PubMed] [Google Scholar]
- 57. Zabel-du Bois A, Nikoghosyan A, Schwahofer A. et al. Intensity modulated radiotherapy in the management of sacral chordoma in primary versus recurrent disease. Radiother Oncol 2010; 97: 408–412. [DOI] [PubMed] [Google Scholar]
- 58. Staab A, Rutz HP, Ares C. et al. Spot-scanning-based proton therapy for extracranial chordoma. Int J Radiat Oncol Biol Phys 2011; 81: e489–e496. [DOI] [PubMed] [Google Scholar]
- 59. DeLaney TF, Liebsch NJ, Pedlow FX. et al. Long-term results of Phase II study of high dose photon/proton radiotherapy in the management of spine chordomas, chondrosarcomas, and other sarcomas. J Surg Oncol 2014; 110: 115–122. [DOI] [PubMed] [Google Scholar]
- 60. Rotondo RL, Folkert W, Liebsch NJ. et al. High-dose proton-based radiation therapy in the management of spine chordomas: outcomes and clinicopathological prognostic factors. J Neurosurg Spine 2015; 23: 788–797. [DOI] [PubMed] [Google Scholar]
- 61. Uhl M, Welzel T, Jensen A. et al. Carbon ion beam treatment in patients with primary and recurrent sacrococcygeal chordoma. Strahlenther Onkol 2015; 191: 597–603. [DOI] [PubMed] [Google Scholar]
- 62. Imai R, Kamada T, Araki N.. Working group for bone and soft tissue sarcomas. Carbon ion radiation therapy for unresectable sacral chordoma: an analysis of 188 cases. Int J Radiat Oncol Biol Phys 2016; 95: 322–327. [DOI] [PubMed] [Google Scholar]
- 63. Munzenrider JE, Liebsch NJ.. Proton therapy for tumors of the skull base. Strahlenther Onkol 1999; 175(Suppl 2): 57–63. [DOI] [PubMed] [Google Scholar]
- 64. Noel G, Feuvret L, Calugaru V. et al. Chordomas of the base of the skull and upper cervical spine. One hundred patients irradiated by a 3D conformal technique combining photon and proton beams. Acta Oncol 2005; 44: 700–708. [DOI] [PubMed] [Google Scholar]
- 65. Ares C, Hug EB, Lomax AJ. et al. Effectiveness and safety of spot scanning proton radiation therapy for chordomas and chondrosarcomas of the skull base: first long-term report. Int J Radiat Oncol Biol Phys 2009; 75: 1111–1118. [DOI] [PubMed] [Google Scholar]
- 66. Mizoe JE, Hasegawa A, Takagi R. et al. Carbon ion radiotherapy for skull base chordoma. Skull Base 2009; 19: 219–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Choy W, Terterov S, Ung N. et al. Adjuvant stereotactic radiosurgery and radiation therapy for the treatment of intracranial chordomas. J Neurol Surg B 2016; 77: 38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bugoci DM, Girvigian MR, Chen JC. et al. Photon-based fractionated stereotactic radiotherapy for postoperative treatment of skull base chordomas. Am J Clin Oncol 2013; 36: 404–410. [DOI] [PubMed] [Google Scholar]
- 69. Kano H, Iqbal FO, Sheehan J. et al. Stereotactic radiosurgery for chordoma: a report from the North American Gamma Knife Consortium. Neurosurgery 2011; 68: 379–389. [DOI] [PubMed] [Google Scholar]
- 70. Chang SD, Martin DP, Lee E. et al. Stereotactic radiosurgery andhypofractionated stereotactic radiotherapy for residual or recurrent cranial base and cervical chordomas. Neurosurg Focus 2001;10(3): E5.. [DOI] [PubMed] [Google Scholar]
- 71. Zorlu F, Gürkaynak M, Yildiz F. et al. Conventional external radiotherapy in the management of clivus chordomas with overt residual disease. Neurol Sci 2000; 21: 203–207. [DOI] [PubMed] [Google Scholar]
- 72. Foweraker KL, Burton KE, Maynard SE. et al. High-dose radiotherapy in the management of chordoma and chondrosarcoma of the skull base and cervical spine: Part 1–Clinical outcomes. Clin Oncol (R Coll Radiol). 2007; 19: 509–516. [DOI] [PubMed] [Google Scholar]
- 73. Mima M, Demizu Y, Jin D. et al. Particle therapy using carbon ions or protons as a definitive therapy for patients with primary sacral chordoma. Br J Radiol 2014; 87(1033): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Holliday EB, Mitra HS, Somerson JS. et al. Postoperative proton therapy for chordomas and chondrosarcomas of the spine: adjuvant versus salvage radiation therapy. Spine 2015; 40: 544–549. [DOI] [PubMed] [Google Scholar]
- 75. Chen YL, Liebsch N, Kobayashi W. et al. Definitive high-dose photon/proton radiotherapy for unresected mobile spine and sacral chordomas. Spine 2013; 38: E930–E936. [DOI] [PubMed] [Google Scholar]
- 76. Dhawale AA, Gjolaj JP, Holmes L Jr. et al. Sacrectomy and adjuvant radiotherapy for the treatment of sacral chordomas: asingle-center experience over 27 years. Spine 2014; 39: E353–E359. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.