Abstract
In the last twenty years, our comprehension of the molecular mechanisms involved in the formation, progression and complication of atherosclerotic plaque has advanced significantly and the main role of inflammation and immunity in this phenomenon is now largely accepted. Accumulating evidence highlight the crucial role of different inflammatory and immune cells, such as monocytes and T-lymphocytes, in the pathophysiology of atherosclerotic lesion, particularly in contributing to its com-plications, such as rupture or ulceration. According to the new terminology, “vulnerable plaque” identi-fies an inflamed atherosclerotic lesion that is particularly prone to rupture. Once disrupted, prothrom-botic material is exposed to the flowing blood, thus activating coagulation cascade and platelet aggrega-tion, ultimately leading to acute thrombus formation within the coronary vessel. To date this is the key event underlying the clinical manifestations of acute coronary syndromes (ACS).
The degree of vessel occlusion (complete vs. incomplete) and the time of blood flow cessation will define the severity of clinical picture. This phenomenon seems to be the final effect of a complex inter-action between different local and systemic factors, involving the degree of inflammation, type of cells infiltration and the rheological characteristics of blood flow at the site of plaque rupture, thrombogenic substrates within the atherosclerotic lesion and different soluble mediators, already present or acutely released in the circulating blood. This article will review currently available data on the pathophysiology of ACS, emphasizing the immunological and inflammatory aspects of vulnerable plaque. We may pos-tulate that intraplaque antigens and local microenvironment will define the immune-inflammatory re-sponse and cells phenotype, thus determining the severity of clinical manifestations.
Keywords: Atherosclerosis, inflammation, immunity, coagulation, thrombosis
INTRODUCTION
Coronary atherosclerosis is the anatomopathological substrate recognized in nearly all cases of acute coronary syndrome (ACS) [1-3]. The term atherosclerosis describes a chronic inflammatory process involving almost all vascular districts. Deposition of lipids and blood borne materials (also including white cells) within the intima of the vessels is essential for atherosclerosis formation [1]. Almost a century after the formulation of the “lipid hypothesis” of Virchow and the “incrustation hypothesis” of Rokitansky [4], Ross defined atherosclerosis an inflammatory disease, characterized by specific cellular and molecular responses to an undefined “noxa patogena” [5]. Accumulating evidence highlight the crucial role of local and systemic inflammation in plaque rupture and the subsequent thrombus formation. Therefore, to better describe these phenomena, the term athero-thrombosis has been proposed [6]. One of the main characteristics of the atherosclerotic disease is the silent progression. Clinical manifestations appear when vessel stenosis becomes severe to reduce coronary reserve. At this time symptoms of effort angina develop. Paradoxically, a complete coronary artery occlusion may occur in absence of significant myocardial necrosis if the occlusion progresses slowly and collateral vessels develop. This finding may be not infrequent in patients affected by chronic angina undergoing coronary angiography. However, there are patients who suddenly develop a clinical episode of ACS. In the vast majority of these cases, plaque disruption, acute intracoronary thrombus formation and abrupt coronary blood flow reduction occur. This represents the pathophysiological substrate responsible for the conversion of coronary atherosclerosis from a chronic disease to an acute medical emergency [2].
The biological mechanisms underlying plaque complication are diverse and complex. Different local and systemic factors have been identified [7-9]. Inflammatory mediators and related pathways, immune system activation and T-cell derived effects [10] within the atherosclerotic lesion play a pivotal role in determining plaque vulnerability [11]. Disruption of the atherosclerotic plaque induces thrombogenic substances exposure to the flowing blood, mainly Tissue Factor (TF) that induces the activation of coagulation cascade and, as final event, platelet aggregation, leading to an intracoronary thrombus formation [3]. The severity (complete vs. incomplete occlusion) and the timing (persistent vs. transient occlusion) of this phenomenon are modulated by different local factors, such as plaque morphology and composition, and systemic factors already present or newly released in circulating blood [2, 12, 13]. The following cascade, plaque-related tissue factor (TF) exposure, thrombin formation, platelet aggregation, fibrin deposition, inflammatory cytokines production, circulating procoagulant microparticles, and soluble tissue factor will define thrombus formation and propagation [9, 12, 14, 15].
The relationship between major cardiovascular risk factors (i.e. hypertension, diabetes, hypercholesterolemia, and smoking) and atherosclerotic disease has been reported by several epidemiological studies. However, the basic mechanisms by which these risk factors induce lesion formation and lead to cardiovascular events have not been entirely elucidated. Based on the data reported by our group and others in the last two decades, we believe that one of the potentially important mechanisms underlying the inflammation-atherosclerosis-thrombosis trilogy is the degree of inflammation and the infiltration of immune cells, mainly macrophages and T-lymphocytes. Within the atherosclerotic lesion, antigen-presenting cells (APC) modulate the immune response, thus defining plaque evolution.
This article will review currently available studies on the pathophysiology of ACS. Particular emphasis will be placed on the immunological and inflammatory aspects underlying vulnerable plaque, trying to explain the effects of inflammation and immunity in determining clinical manifestations of ACS.
MORPHOLOGICAL SIDE OF ATHEROSCLEROSIS:ROLE OF LIPIDS
As already reported, early atherosclerotic lesions may develop even in childhood and adolescence. These observations came from several autoptical studies performed on individuals who died of non-cardiac causes in which fatty streaks were frequently seen during the histological examination [16]. Specifically, these data concluded that even in young adults (mean age, 36±14 years) who died of non-natural causes, coronary atherosclerosis was reported in 82% of the individuals with 8% of them having significant obstructive disease [17]. However, despite the evidence of coronary atherosclerosis at pathology in the most of middle-aged individuals in developed countries, it should be noted that the annual incidence of acute coronary events for individuals ≥40 years of age is relatively small (between 0.2% and 1%) [18]. The interaction between the genetic background and traditional risk factors, will define the fast or slow progression of atherosclerotic lesions. Other factors may be involved in the switch from chronic disease to acute event as suggested by the fact that the severity of coronary lesions does not correlate with the clinical occurrence of ACS: indeed, >70% of patients with ACS show a culprit coronary lesion <50% of luminal diameter at angiography [19, 20]. Thus, the available data suggest that the mere presence of a coronary atherosclerotic lesion is not enough for an acute coronary event to occur.
The initial step of atherosclerosis is endothelial dysfunction [8]. The endothelium exerts several biological activities. A dynamic autocrine and paracrine organ with anti-inflammatory and mitogenic properties, contractile activities, as well as regulatory effects of the hemostatic process, by synthesis and release of nitric oxide (NO), the main mediators of these regulatory functions [21] have been considered. The traditional risk factors such as hypercholesterolemia, hypertension, advanced glycation end-products in diabetes mellitus, chemical irritants in tobacco smoke and the loss of shear stress at bending points and near bifurcations (probably for loss of tangential flow) are the principal factors of endothelial dysfunction [22, 23]. The deposition of lipid material within the arterial wall is the consequence of an imbalance between the mechanisms responsible for the ‘influx’ and ‘efflux’ of cholesterol [24]. The overtime accumulation of these materials with the subsequent thickening of the wall progressively reduces the residual lumen vessel, seriously compromising the blood flow, thus leading to ischemic events distal to the arterial stenosis [1]. At the initial stage, the disease progresses silently (“pre-clinical atherosclerosis”). Retention and modification of LDL particles and subsequent accumulation of LDL derived lipids in the intima are the central pathogenetic events that promote atherosclerosis lesion formation [1]. Several reports suggest that oxidized LDLs (oxLDLs) represent the main trigger for the inflammatory response in the arterial wall and it is traditionally thought to inhibit endothelial function. More recent data also suggest that oxLDLs may also have proangiogenic effects thus, linking hyperlipidemia, inflammation, and angiogenesis in atherosclerosis [25].
Circulating high plasma levels of LDL cholesterol are thought to be the major determinants for the influx and on-site retention of LDL particles within the sub-endothelial layer [26]. The major protein component of LDLs is apoprotein B (Apo B). It seems to mediate the binding with the endothelial cells surface via an ionic interaction with matrix protein including proteoglycan, collagen and fibronectin [27]. Proteoglycans are located between the basement membrane of the endothelial cell and internal elastic membrane. The interaction between oxLDLs and proteoglycans (especially with those that contain side chains of chondroitin sulphate such as versican or byglican) is crucial in early phase of atherosclerosis, because of lipoprotein retention, intravascular aggregation of LDLs leading to chemical modification, and induction of inflammation [27]. LDLs sequestered in the intimal microenvironment became susceptible to modification (including oxidation by lipoxygenase, myeloperoxidase and free radicals, or their enzymatic cleavage with proteolitic, lipolytic and hydrolytic enzyme). The endothelium damaged by LDLs may change its gene expression profile, probably through the activation of a common transcription factor (NF-kappa B), resulting in up-regulation of cell adhesive molecules (CAMs,), particularly VCAM1 and a series of selectin (E-selectin – endhotelial- and P-selectin). These proteins interact with other proteic molecules on the surface of white cells (i.e. very late antigen 4 – VLA4) and will facilitate their adhesion [27-30].
Formation of an atherosclerotic plaque is considered an almost mandatory step in the occurrence of ACS [2]. However, the mechanisms by which a stable lesion becomes unstable are complex and remain partly elusive. Based on the several reports by our group and others, the contribution of local and systemic inflammation and involvement of immune-competent cells seems to play a pivotal role in this conversion, with both natural and adaptive immunity being magna pars of this phenomenon [3, 4, 11, 31]. This hypothesis is supported by many functional as well as morphological studies. On this matter, it has been shown that ruptured plaques contain more inflammatory and immune cells than intact plaques [11, 31]; these cells often infiltrate the atherosclerotic lesion adjacent to the site of fibrous cap rupture and around the lipid core, as well as in the adventitia around areas of neovascularization, suggesting a causative role in plaque complication [15]. By the morphological point of view, vulnerable plaques (i.e. prone to rupture lesion) are often large in size with associated expansive arterial remodelling. Most frequently they show a thin-cap fibroatheroma (TCFA), characterized by a large lipid or necrotic core separated from the coronary arterial lumen by a thin membrane cap. Infiltration of immuno-inflammatory cells in the fibrous cap and adventitia, increases plaque neovascularity, thus intraplaque haemorrhage may occur [14, 15]. Inflammation and immune cell activation may be crucial in plaque complication. First, because of its involvement in the fibrous cap rupture through release of collagen degrading enzymes, such as metalloproteinases (MMPs), thus inducing loss of collagen. Second, inflammation seems to be also responsible for the death of collagen-synthesizing smooth muscle cells, further contributing to loss of fibrous cap integrity [11, 14, 32].
INFLAMMATORY SIDE OF ATHEROSCLEROSIS:SYSTEMIC VS. LOCAL INFLAMMATORY PATHWAYS.
Since the first report by Ross et al. highlighting the inflammatory “face” of atherosclerosis [5], different epidemiological evidence exploring this aspect, have clearly demonstrated that systemic levels of inflammatory markers, such as C-reactive protein (CRP), may be useful prognostic tools for the prediction of major cardiovascular events (MACE) in patients with known cardiovascular disease as well as in apparently healthy subjects [33-37]. However, despite the emerging role of CRP and other inflammatory mediators as systemic prognostic markers, more recent studies have focused on the “inflammatory status” at the site of atherosclerotic plaque as major determinant in plaque destabilization [8, 38]. These data have been confirmed by the discovery that CRP may be released not only from hepatocytes but also from other different cell types [39-41]. Taken together with previous observation from our group and others [38, 42, 43], regarding the active production of CRP within the coronary circulation of ACS patients and in cerebrovascular patients, the main question raised was related to the possible local effects exerted by CRP on the different cellular components of the atherosclerotic lesion. By biological and molecular point of view, CRP may promote endothelial function inducing adhesion molecules [44-47], favouring a pro-atherogenic phenotype via expression of TF [48], releasing MCP-1 [49] and other inflammatory cytokines [50, 51], increasing iNOS and superoxide production and decreasing eNOS, prostacyclin and tPA expression in endothelial cells [45, 52]. The impact of CRP levels on plaque rupture seems also to be related to the expression and release of MMPs, mainly the isoforms 2 and 9. In this regard, our group has reported a direct effect of CRP in releasing MMPs in cellular components of atherosclerotic plaque in vitro and in ACS patients in vivo [53], highlighting the linear correlation between CRP and MMP-9 intracoronary levels. Interestingly, the release of enzymes-degrading extracellular matrix may be amplified by platelets. As reported by our group, in ACS patients platelets may release MMP-2 at the site of lesion destabilitazion [54]. CRP circulates in a pentameric form that mainly sustains an anti-inflammatory innate immune response. Once dissociated in monomeric form, CRP exerts potent proinflammatory and prothrombotic actions. This conversion seems to be induced by activated platelets at the site of plaque rupture, thus favouring local inflammation [55, 56].
IMMUNOLOGICAL SIDE OF ATHEROSCLEROSIS:ROLE OF IMMUNE CELLS
A highly dynamic and coordinated immune response occurs in atherosclerosis as well as in ischemic cardiac tissue [57], which is dependent not only on macrophages but also on leukocytes, both resident and newly recruited from the blood flow. Infiltration of immune-competent cells within coronary lesions has been reported by our group [58] and others [59, 60], thus indicating their contribution to plaque vulnerability [11, 61]. Macrophages are the prototypical cells of innate immunity and they progressively accumulate since the initial stage of plaque formation, ingesting lipids and releasing inflammatory mediators that act orchestrating the local and systemic immunological response [62]. Blood-borne monocytes are recruited upon endothelial activation following stimulation by modified lipids and other factors in the intima. The importance of these effects is supported by animal models of atherosclerosis, in which prevention of monocytes entry by blocking receptors or their chemokines retards atherosclerotic plaque formation [63]. Once recruited in the sub-endothelium, monocytes become macrophages or dendritic-like cells [11, 62]. Different subtypes of monocytes have been identified: 1) the “classic” proinflammatory CD14++/CD16− monocytes, or Mon1, characterized by the expression of CCR2 (receptor for MCP-1), CD62L, CD64, andCD115; 2) the “nonclassic” or resident CD14+/ CD16++ monocytes, or Mon3, who express the receptor for fractalkine (CX3CR1) and are involved in reparative processes; 3) the recently described “intermediate” CD14++/CD16+ monocytes, or Mon2, who express high levels of CCR2, CX3CR1, and CD115, and possible involvement in inflammation [64]. In the last few years some reports have correlated the monocytes phenotype with cardiovascular outcomes. One study suggested that CD14++/CD16- monocytes predict adverse future cardiovascular events [65], while another report highlights only the role of CD14++/CD16+ subpopulation [66].
Moreover, the effects of local factors may influence macrophages heterogeneity, particularly the proinflammatory phenotype (M1) versus those involved in resolution and/or repair (M2), also called alternatively activated [62]. Both of these phenotypes may be found during the atherosclerotic process [67]. In this regard, macrophages-loaded cholesterol becomes proinflammatory cells most probably for the enrichment of free cholesterol in plasma membrane inducing an intracellular inflammatory signalling [68], or for a direct action of oxidized lipoproteins [62]. This kind of stimulation induces M1 polarization. Because of production and release of a wide range of cytokines, reactive oxygen species and toxic nitric oxide, M1 macrophages promote atherosclerotic progression and complication [69]. Beyond M1/M2 polarization, other macrophages subtypes have been identified in the atherosclerotic lesion, such as M4, HA-mac, M(Hb) and Mhem with distinct functions and properties [70].
The rescue from global and local inflammation as well as the reduction in macrophages infiltration in the arterial wall during lipid lowering therapy or HDL raising treatment has been reported [71-73], confirming the role of cholesterol in this effect. Another prominent feature of atherosclerotic plaques seems to be macrophage apoptosis. The available data indicate that in the early stage of atherosclerosis, macrophages apoptosis exerts an inhibitory effect, while in advance stage it supports vascular inflammation by promoting further monocytes recruitment and cytokines release, thus favouring disease progression [74].
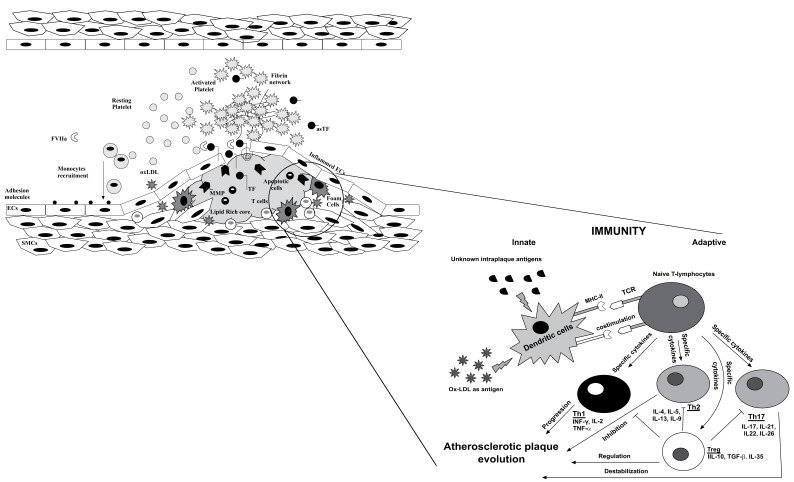
Once activated within the atherosclerotic lesion, macrophages become a source of inflammatory mediators that amplify the inflammatory cycle by recruiting more macrophages as well as B and T-lymphocytes [31, 75]. Although numerically T-cells represent a minority of the leukocytes present in plaques, several reports support their decisive regulatory role within atherosclerotic lesion [76]. Specifically, as master cells of the adaptive immune response, they act like as the director of an orchestra, instructing the more abundant monocytic effectors of the innate immune response [11, 77]. On the other hand, recruitment of T-cells perpetuates the inflammatory process via release of other cytokines, thus amplifying local inflammation. The antigen presenting cells infiltrating atherosclerotic lesions plus the soluble mediators released upon in loco activation of macrophages may act simultaneously inducing T-cells polarization in different subsets [78]. This differentiation appears to be of great importance in determining plaque destiny, because some lineages exert more pro-inflammatory effects (Th1 and Th17 cells), while others act as negative regulators of inflammation (Treg and Th2 cells) [11, 79]. Thus, based on the available evidence, T-cell activation and the balance between the different subsets are thought to be a crucial mechanism in the pathophysiology of plaque vulnerability [14, 32], thus showing a causative role in ACS as schematically illustrated in the Fig. 1 [80]. In this regard, a report from Liuzzo et al. showed that patients presenting with unstable angina displayed an increased number of T-cells producing large amounts of IFN-γ in peripheral blood, whereas patients with stable angina did not [59, 60]. Moreover, the authors stated that the presence of some circulating clonotypes in the tissue was not random but a selective recruitment. This was one of the first studies supporting the hypothesis of selective expansion of T- lymphocytes in patients with ACS most probably for a continuous antigenic stimulation [60]. Other studies were proposed to characterize specific T-cell subsets in different settings, such as investigating cytotoxic T-cells function in peripheral blood of ACS patients, [81], culturing T-cells stimulated with plaque homogenates supplemented with different growth factors [80], or evaluating T-lymphocytes infiltration of plaque at autopsy [59] However, although those reports have been considered a milestone in improving our knowledge in the role of immune system in atherosclerosis, as they have shifted the focus on T-cell activation and, overall, on immunological response, as potentially crucial mechanisms of coronary plaque vulnerability, they have the important limitation that no direct evaluation of T-cells dynamics within atherosclerotic lesion can be derived.
Fig. (1).
Within the atherosclerotic plaque, antigen-presenting cells modulate the immune response, which may play a key role in the inflammation-atherosclerosis-thrombosis trilogy. In detail, dendritic cells polarize naive T-lymphocytes into different effector T cells through antigen presentation to the T-cell receptor (TCR), secretion of cytokines, and costimulation. Th1, Th2, or Th17 cells are categorized by the transcription factors they express and the cytokines they release. The interaction between the various T-cell subsets involved in atherosclerosis remains a complex phenomenon. Th1 and Th17 seem to be associated to a progression/destabilization of atherosclerotic lesion. Th2 and Treg are linked to regulatory/inhibitory effects.
Probably, the most compelling evidence of T-cell involvement in plaque complication derives from our study [58] in which using directional coronary atherectomy (DCA), we collected specimens of atherosclerotic plaques from living patients during a coronary acute event. This approach has offered the opportunity to study directly the “culprit lesion” documenting that a specific, immune-driven response and inflammatory pathways occur within the unstable plaque of patients presenting with ACS, as indicated by the following results: first, unstable plaques show much greater T-cell content than plaques from stable patients; secondly, TCR rearrangement occurs at higher extent in unstable plaques compared to stable lesion; finally, and perhaps most importantly, contemporaneous evaluation of T-cell repertoire in coronary plaques as well as in peripheral blood gave us the opportunity to specifically demonstrate that in the majority of patients a specific oligoclonal T-cell expansion occur exclusively within the culprit lesion, and not in the peripheral blood. This study has been the first direct observation of the existence of specific local immune-inflammatory response, which may occur independently of the systemic immunological activation. Moreover, the evidence that in some ACS patients similar patterns of T cell repertoire were found, might indicate a selective expansion driven by a given intraplaque antigen. Lessons from malignancy studies have revealed that the TCR repertoire reflects the clonality of the malignant cells and is therefore monoclonal [82], thus translating these findings to our data, we may postulate that the specific T-cells selection within the vulnerable plaque because of TCR rearrangement is associated to an “aggressive” disease.
To date, the putative antigens responsible for this local activation remain areas of active investigation [31]. Modified lipoproteins, such as oxLDLs, have been proposed as major determinant in T-cells activation [83-85]. Once processed within atherosclerotic lesion, oxLDLs may be presented to naïve T-lymphocytes by dendritic cells, thus inducing differentiation via MCH-TCR interaction based on the costimuli already present in the lesion environment. According to this polarization, plaque stability or progression will be defined in Fig. 1.
In the last few years, intensive research has focused on a novel subset of Th-cells known as Th-17. Once activated and differentiated in this lineage, T-cells exclusively produce large amounts of IL-17s and other small soluble factors such as IL-21, granulocyte–macrophage colony-stimulating factor, and IL-6 [86]. In vivo, Th-17 cells originate from a small subset of CD161+ T-cell precursors, which polarize into Th-17 cells upon IL-1β plus IL-23 stimulation. To date the role of Th-17 cells has been proven in certain human autoimmune diseases and other chronic inflammatory disorders, but their involvement in the pathogenesis of atherosclerosis is still a matter of debate. Controversial issue has been published in this field. Some studies have reported that blocking IL-17 pathway may attenuate atherosclerosis progression thus stabilizing vulnerable plaque [87-89], other reports have correlated IL-17 signalling with lesion complication and acute event [90, 91]. In this regard, a report from our group has shown that increased levels of transcoronary IL-17 and a higher number of Th-17 cells were found in the blood collected from the coronary sinus of an ACS patients subgroup presenting with a more severe disease at admission as demonstrated by the higher levels of troponin, as compared to patients with stable angina. This data supported the other published studies on the possible contribution of Th-17 cells in determining a more severe disease [90].
More interestingly, we have recently linked the activity of T-lymphocytes to the athero-thrombotic process. Specifically, we have shown that in vitro activation of T-cells by a combination of IL-17 plus INF-γ may induce the expression of functional TF. These findings may be of a clinical importance because this effect has been observed also in vivo in circulating T-cells isolated from the coronary circulation as well as in thrombotic material aspirated from the occluded vessel of patients presenting with ACS [92].
As summary of the most recent advances in the involvement of the immune-inflammatory pathways during acute myocardial infarction, an “inverted pyramid” model has been proposed [93]. Although it is a simplistic view, this theory tries to explain the damaging effects of an uncontrolled inflammatory response putting together basic research, pre-clinical and clinical data, as well as findings derived from non cardiac studies. At the “inverted apex” of pyramid the Treg lymphocytes are included [94]. On the next level the proinflammatory Th1, the antiinflammatory Th2, and the autoaggressive CD4+CD28null cells are positioned [95]. Based on this model, Treg exerts a suppressing effect on Th-1, an enhancing function on Th2 and inhibitory properties on the autoaggressive cells. Thus, failure of these activities will result in a proinflammatory disequilibrium of the Th1/ Th2 state [96] and expansion of CD4+CD28null cells [95]. As reported by our group [7, 58, 90] and others [59, 60, 80], the acute event exacerbates further the proinflammatory state, bringing to the next level of the model in which the cytokines storm will induce pleiotropic inflammatory response. At this step, modulation of monocytes and neutrophils function, as well as downstream products will occur, generating an uncontrolled immune response [93]. As final event at the highest level of the pyramid, plaque complication, vascular obstruction, myocardial ischemia, and clinical events will take place. Based on this theory, homeostasis of Treg is essential [94]. Conventional and non conventional cardiovascular risk factors might reduce Treg activity that during acute myocardial infarction will be further demodulated, thus worsening the outcome.
INTERPLAY INFECTION-INFLAMMATIONAGGREGATION
Some epidemiological observations have linked respiratory tract infections with an increased risk of acute cardiovascular events [97]. Specifically, in patients presenting mainly with pneumonia infection, elevation of platelet activation biomarkers may occur, suggesting that platelet overactivation may be responsible for the cardiovascular events recurrence [98]. By the mechanistic point of view, it may postulate that platelets interact directly with bacteria or with lipopolysaccharide (LPS) on Gram-negative bacteria surface, resulting in platelet activation and aggregation and, eventually, thrombus formation [99].
CONCLUSION
Involvement of immunity in all stages of atherosclerosis is now largely accepted by the scientific community. Contribution of macrophages is defined while the role of T-lymphocytes is still under investigation. T-cells have a crucial role in plaque progression and complication. Based on the plaque environment (i.e. activating antigens), local release of cytokines and other soluble factors, they become polarized, thus defining the destiny of atherosclerotic lesion. Further studies are required to better understand the molecular mechanisms underlying antigen processing, local recruitment of T-lymphocytes and modulation of the immune response. Shedding more light on this field will be of great importance to develop future specific therapy, such as an anti atherosclerotic vaccine.
ACKNOWLEDGEMENTS
Declared none.
AUTHOR CONTRIBUTIONS
All authors equally contributed to this paper with conception and design of the study, literature review and analysis, drafting and critical revision and editing, and final approval of the final version.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Badimon J.J., Ibanez B., Cimmino G. Genesis and dynamics of atherosclerotic lesions: implications for early detection. Cerebrovasc. Dis. 2009;27(Suppl. 1):38–47. doi: 10.1159/000200440. [DOI] [PubMed] [Google Scholar]
- 2.Cimmino G., Conte S., Morello A., et al. The complex puzzle underlying the pathophysiology of acute coronary syndromes: from molecular basis to clinical manifestations. Expert Rev. Cardiovasc. Ther. 2012;10(12):1533–1543. doi: 10.1586/erc.12.157. [DOI] [PubMed] [Google Scholar]
- 3.Weber C., Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat. Med. 2011;17(11):1410–1422. doi: 10.1038/nm.2538. [DOI] [PubMed] [Google Scholar]
- 4.Libby P., Ridker P.M., Hansson G.K. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473(7347):317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 5.Ross R. Atherosclerosis--an inflammatory disease. N. Engl. J. Med. 1999;340(2):115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 6.Meerarani P., Moreno P.R., Cimmino G., et al. Atherothrombosis: role of tissue factor; link between diabetes, obesity and inflammation. Indian J. Exp. Biol. 2007;45(1):103–110. [PubMed] [Google Scholar]
- 7.Cirillo P., Cimmino G., D'Aiuto E., et al. Local cytokine production in patients with Acute Coronary Syndromes: A look into the eye of the perfect (cytokine) storm. Int. J. Cardiol. 2014;176(1):227–229. doi: 10.1016/j.ijcard.2014.05.035. [DOI] [PubMed] [Google Scholar]
- 8.Lowe G.D. Local inflammation, endothelial dysfunction and fibrinolysis in coronary heart disease. Clin. Sci. 2006;110(3):327–328. doi: 10.1042/CS20060002. [DOI] [PubMed] [Google Scholar]
- 9.Arbab-Zadeh A., Fuster V. The myth of the “vulnerable plaque”: transitioning from a focus on individual lesions to atherosclerotic disease burden for coronary artery disease risk assessment. J. Am. Coll. Cardiol. 2015;65(8):846–855. doi: 10.1016/j.jacc.2014.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olson N.C., Sallam R., Doyle M.F., et al. T helper cell polarization in healthy people: implications for cardiovascular disease. J. Cardiovasc. Transl. Res. 2013;6(5):772–786. doi: 10.1007/s12265-013-9496-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansson G.K., Hermansson A. The immune system in atherosclerosis. Nat. Immunol. 2011;12(3):204–212. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 12.Cimmino G., D'Amico C., Vaccaro V., et al. The missing link between atherosclerosis, inflammation and thrombosis: is it tissue factor? Expert Rev. Cardiovasc. Ther. 2011;9(4):517–523. doi: 10.1586/erc.11.40. [DOI] [PubMed] [Google Scholar]
- 13.Cimmino G., Golino P., Badimon J.J. Pathophysiological role of blood-borne tissue factor: should the old paradigm be revisited? Intern. Emerg. Med. 2011;6(1):29–34. doi: 10.1007/s11739-010-0423-4. [DOI] [PubMed] [Google Scholar]
- 14.Hansson G.K., Libby P., Tabas I. Inflammation and plaque vulnerability. J. Intern. Med. 2015;278(5):483–493. doi: 10.1111/joim.12406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michel J.B., Martin-Ventura J.L., Nicoletti A., et al. Pathology of human plaque vulnerability: mechanisms and consequences of intraplaque haemorrhages. Atherosclerosis. 2014;234(2):311–319. doi: 10.1016/j.atherosclerosis.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 16.Perrone J., Hollander J.E., De Roos F. Cardiovascular risk factors and atherosclerosis in children and young adults. N. Engl. J. Med. 1998;339(15):1083–1084. doi: 10.1056/NEJM199810083391514. [DOI] [PubMed] [Google Scholar]
- 17.Nemetz P.N., Roger V.L., Ransom J.E., et al. Recent trends in the prevalence of coronary disease: a population-based autopsy study of nonnatural deaths. Arch. Intern. Med. 2008;168(3):264–270. doi: 10.1001/archinternmed.2007.79. [DOI] [PubMed] [Google Scholar]
- 18.Mozaffarian D., Benjamin E.J., Go A.S., et al. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 19.Smith S.C., Jr, Benjamin E.J., Bonow R.O., et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation endorsed by the World Heart Federation and the Preventive Cardiovascular Nurses Association. J. Am. Coll. Cardiol. 2011;58(23):2432–2446. doi: 10.1016/j.jacc.2011.10.824. [DOI] [PubMed] [Google Scholar]
- 20.Stone G.W., Maehara A., Lansky A.J., et al. A prospective natural-history study of coronary atherosclerosis. N. Engl. J. Med. 2011;364(3):226–235. doi: 10.1056/NEJMoa1002358. [DOI] [PubMed] [Google Scholar]
- 21.Grassi D., Desideri G., Ferri C. Cardiovascular risk and endothelial dysfunction: the preferential route for atherosclerosis. Curr. Pharm. Biotechnol. 2011;12(9):1343–1353. doi: 10.2174/138920111798281018. [DOI] [PubMed] [Google Scholar]
- 22.Grover-Paez F., Zavalza-Gomez A.B. Endothelial dysfunction and cardiovascular risk factors. Diabetes Res. Clin. Pract. 2009;84(1):1–10. doi: 10.1016/j.diabres.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 23.Jowett J.B. Interplay of genetic and environmental factors: Innate immunity genetic polymorphisms in MBL2 affect endothelial dysfunction and risk of atherosclerosis. Atherosclerosis. 2010;208(1):32–33. doi: 10.1016/j.atherosclerosis.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 24.Santos-Gallego C.G., Badimon J.J., Rosenson R.S. Beginning to understand high-density lipoproteins. Endocrinol. Metab. Clin. North Am. 2014;43(4):913–947. doi: 10.1016/j.ecl.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 25.Hutter R., Speidl W.S., Valdiviezo C., et al. Macrophages transmit potent proangiogenic effects of oxLDL in vitro and in vivo involving HIF-1alpha activation: a novel aspect of angiogenesis in atherosclerosis. J. Cardiovasc. Transl. Res. 2013;6(4):558–569. doi: 10.1007/s12265-013-9469-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Segers D., Weinberg P., Krams R. Atherosclerosis: cell biology and lipoproteins--shear stress and inflammation in plaque formation: new evidence. Curr. Opin. Lipidol. 2008;19(6):627–628. doi: 10.1097/MOL.0b013e328318db32. [DOI] [PubMed] [Google Scholar]
- 27.Khalil M.F., Wagner W.D., Goldberg I.J. Molecular interactions leading to lipoprotein retention and the initiation of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2004;24(12):2211–2218. doi: 10.1161/01.ATV.0000147163.54024.70. [DOI] [PubMed] [Google Scholar]
- 28.Choi S.H., Harkewicz R., Lee J.H., et al. Lipoprotein accumulation in macrophages via toll-like receptor-4-dependent fluid phase uptake. Circ. Res. 2009;104(12):1355–1363. doi: 10.1161/CIRCRESAHA.108.192880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salisbury D., Bronas U. Inflammation and immune system contribution to the etiology of atherosclerosis: mechanisms and methods of assessment. Nurs. Res. 2014;63(5):375–385. doi: 10.1097/NNR.0000000000000053. [DOI] [PubMed] [Google Scholar]
- 30.Tabas I., Williams K.J., Boren J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 2007;116(16):1832–1844. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]
- 31.Ketelhuth D.F., Hansson G.K. Cellular immunity, low-density lipoprotein and atherosclerosis: break of tolerance in the artery wall. Thromb. Haemost. 2011;106(5):779–786. doi: 10.1160/TH11-05-0321. [DOI] [PubMed] [Google Scholar]
- 32.Libby P. Inflammation in atherosclerosis. Nature. 2002;420(6917):868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 33.Correia L.C., Andrade B.B., Borges V.M., et al. Prognostic value of cytokines and chemokines in addition to the GRACE Score in non-ST-elevation acute coronary syndromes. Clin. Chim. Acta. 2010;411(7-8):540–545. doi: 10.1016/j.cca.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 34.Li J.J., Jiang H., Huang C.X., et al. Elevated level of plasma C-reactive protein in patients with unstable angina: its relations with coronary stenosis and lipid profile. Angiology. 2002;53(3):265–272. doi: 10.1177/000331970205300303. [DOI] [PubMed] [Google Scholar]
- 35.Libby P., Ridker P.M. Inflammation and atherosclerosis: role of C-reactive protein in risk assessment. Am. J. Med. 2004;116(Suppl. 6A):9S–16S. doi: 10.1016/j.amjmed.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 36.Michowitz Y., Arbel Y., Wexler D., et al. Predictive value of high sensitivity CRP in patients with diastolic heart failure. Int. J. Cardiol. 2008;125(3):347–351. doi: 10.1016/j.ijcard.2007.02.037. [DOI] [PubMed] [Google Scholar]
- 37.Niccoli G., Biasucci L.M., Biscione C., et al. Independent prognostic value of C-reactive protein and coronary artery disease extent in patients affected by unstable angina. Atherosclerosis. 2008;196(2):779–785. doi: 10.1016/j.atherosclerosis.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 38.Forte L., Cimmino G., Loffredo F., et al. C-reactive protein is released in the coronary circulation and causes endothelial dysfunction in patients with acute coronary syndromes. Int. J. Cardiol. 2011;152(1):7–12. doi: 10.1016/j.ijcard.2011.05.062. [DOI] [PubMed] [Google Scholar]
- 39.Calabro P., Chang D.W., Willerson J.T., et al. Release of C-reactive protein in response to inflammatory cytokines by human adipocytes: linking obesity to vascular inflammation. J. Am. Coll. Cardiol. 2005;46(6):1112–1113. doi: 10.1016/j.jacc.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 40.Calabro P., Willerson J.T., Yeh E.T. Inflammatory cytokines stimulated C-reactive protein production by human coronary artery smooth muscle cells. Circulation. 2003;108(16):1930–1932. doi: 10.1161/01.CIR.0000096055.62724.C5. [DOI] [PubMed] [Google Scholar]
- 41.De Rosa S., Cirillo P., Pacileo M., et al. Leptin stimulated C-reactive protein production by human coronary artery endothelial cells. J. Vasc. Res. 2009;46(6):609–617. doi: 10.1159/000226229. [DOI] [PubMed] [Google Scholar]
- 42.Date H., Imamura T., Sumi T., et al. Effects of interleukin-6 produced in coronary circulation on production of C-reactive protein and coronary microvascular resistance. Am. J. Cardiol. 2005;95(7):849–852. doi: 10.1016/j.amjcard.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 43.Tsuda K. C-reactive protein and nitric oxide production in ischemic stroke. Stroke. 2009;40(6) doi: 10.1161/STROKEAHA.109.551150. e471; author reply e2. [DOI] [PubMed] [Google Scholar]
- 44.Montecucco F., Steffens S., Burger F., et al. C-reactive protein (CRP) induces chemokine secretion via CD11b/ICAM-1 interaction in human adherent monocytes. J. Leukoc. Biol. 2008;84(4):1109–1119. doi: 10.1189/jlb.0208123. [DOI] [PubMed] [Google Scholar]
- 45.Pasceri V., Willerson J.T., Yeh E.T. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation. 2000;102(18):2165–2168. doi: 10.1161/01.cir.102.18.2165. [DOI] [PubMed] [Google Scholar]
- 46.Portelinha A., Belo L., Tejera E., et al. Adhesion molecules (VCAM-1 and ICAM-1) and C-reactive protein in women with history of preeclampsia. Acta Obstet. Gynecol. Scand. 2008;87(9):969–971. doi: 10.1080/00016340802322265. [DOI] [PubMed] [Google Scholar]
- 47.Postadzhiyan A.S., Tzontcheva A.V., Kehayov I., et al. Circulating soluble adhesion molecules ICAM-1 and VCAM-1 and their association with clinical outcome, troponin T and C-reactive protein in patients with acute coronary syndromes. Clin. Biochem. 2008;41(3):126–133. doi: 10.1016/j.clinbiochem.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 48.Cirillo P., Golino P., Calabro P., et al. C-reactive protein induces tissue factor expression and promotes smooth muscle and endothelial cell proliferation. Cardiovasc. Res. 2005;68(1):47–55. doi: 10.1016/j.cardiores.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 49.Guo S., Meng S., Chen B., et al. C-reactive protein can influence the proliferation, apoptosis, and monocyte chemotactic protein-1 production of human umbilical vein endothelial cells. DNA Cell Biol. 2011;30(3):157–162. doi: 10.1089/dna.2010.1093. [DOI] [PubMed] [Google Scholar]
- 50.Galve-de Rochemonteix B., Wiktorowicz K., Kushner I., et al. C-reactive protein increases production of IL-1 alpha, IL-1 beta, and TNF-alpha, and expression of mRNA by human alveolar macrophages. J. Leukoc. Biol. 1993;53(4):439–445. doi: 10.1002/jlb.53.4.439. [DOI] [PubMed] [Google Scholar]
- 51.Xie L., Chang L., Guan Y., et al. C-reactive protein augments interleukin-8 secretion in human peripheral blood monocytes. J. Cardiovasc. Pharmacol. 2005;46(5):690–696. doi: 10.1097/01.fjc.0000183568.48389.a1. [DOI] [PubMed] [Google Scholar]
- 52.Hattori Y., Matsumura M., Kasai K. Vascular smooth muscle cell activation by C-reactive protein. Cardiovasc. Res. 2003;58(1):186–195. doi: 10.1016/s0008-6363(02)00855-6. [DOI] [PubMed] [Google Scholar]
- 53.Cimmino G., Ragni M., Cirillo P., et al. C-reactive protein induces expression of matrix metalloproteinase-9: A possible link between inflammation and plaque rupture. Int. J. Cardiol. 2013;168(2):981–986. doi: 10.1016/j.ijcard.2012.10.040. [DOI] [PubMed] [Google Scholar]
- 54.Gresele P., Falcinelli E., Loffredo F., et al. Platelets release matrix metalloproteinase-2 in the coronary circulation of patients with acute coronary syndromes: possible role in sustained platelet activation. Eur. Heart J. 2011;32(3):316–325. doi: 10.1093/eurheartj/ehq390. [DOI] [PubMed] [Google Scholar]
- 55.Eisenhardt S.U., Habersberger J., Murphy A., et al. Dissociation of pentameric to monomeric C-reactive protein on activated platelets localizes inflammation to atherosclerotic plaques. Circ. Res. 2009;105(2):128–137. doi: 10.1161/CIRCRESAHA.108.190611. [DOI] [PubMed] [Google Scholar]
- 56.Thiele J.R., Habersberger J., Braig D., et al. Dissociation of pentameric to monomeric C-reactive protein localizes and aggravates inflammation: in vivo proof of a powerful proinflammatory mechanism and a new anti-inflammatory strategy. Circulation. 2014;130(1):35–50. doi: 10.1161/CIRCULATIONAHA.113.007124. [DOI] [PubMed] [Google Scholar]
- 57.Epelman S., Mann D.L. Communication in the heart: the role of the innate immune system in coordinating cellular responses to ischemic injury. J. Cardiovasc. Transl. Res. 2012;5(6):827–836. doi: 10.1007/s12265-012-9410-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.De Palma R., Del Galdo F., Abbate G., et al. Patients with acute coronary syndrome show oligoclonal T-cell recruitment within unstable plaque: evidence for a local, intracoronary immunologic mechanism. Circulation. 2006;113(5):640–646. doi: 10.1161/CIRCULATIONAHA.105.537712. [DOI] [PubMed] [Google Scholar]
- 59.Liuzzo G., Goronzy J.J., Yang H., et al. Monoclonal T-cell proliferation and plaque instability in acute coronary syndromes. Circulation. 2000;101(25):2883–2888. doi: 10.1161/01.cir.101.25.2883. [DOI] [PubMed] [Google Scholar]
- 60.Liuzzo G., Kopecky S.L., Frye R.L., et al. Perturbation of the T-cell repertoire in patients with unstable angina. Circulation. 1999;100(21):2135–2139. doi: 10.1161/01.cir.100.21.2135. [DOI] [PubMed] [Google Scholar]
- 61.Wang C., Jin R., Zhu X., et al. Function of CD147 in atherosclerosis and atherothrombosis. J. Cardiovasc. Transl. Res. 2015;8(1):59–66. doi: 10.1007/s12265-015-9608-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moore K.J., Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145(3):341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mestas J., Ley K. Monocyte-endothelial cell interactions in the development of atherosclerosis. Trends Cardiovasc. Med. 2008;18(6):228–232. doi: 10.1016/j.tcm.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Santos-Gallego C.G., Picatoste B., Badimon J.J. Pathophysiology of acute coronary syndrome. Curr. Atheroscler. Rep. 2014;16(4):401. doi: 10.1007/s11883-014-0401-9. [DOI] [PubMed] [Google Scholar]
- 65.Berg K.E., Ljungcrantz I., Andersson L., et al. Elevated CD14++CD16- monocytes predict cardiovascular events. Circ Cardiovasc Genet. 2012;5(1):122–131. doi: 10.1161/CIRCGENETICS.111.960385. [DOI] [PubMed] [Google Scholar]
- 66.Rogacev K.S., Cremers B., Zawada A.M., et al. CD14++CD16+ monocytes independently predict cardiovascular events: a cohort study of 951 patients referred for elective coronary angiography. J. Am. Coll. Cardiol. 2012;60(16):1512–1520. doi: 10.1016/j.jacc.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 67.Leitinger N., Schulman I.G. Phenotypic polarization of macrophages in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2013;33(6):1120–1126. doi: 10.1161/ATVBAHA.112.300173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhu X., Lee J.Y., Timmins J.M., et al. Increased cellular free cholesterol in macrophage-specific Abca1 knock-out mice enhances pro-inflammatory response of macrophages. J. Biol. Chem. 2008;283(34):22930–22941. doi: 10.1074/jbc.M801408200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Martinez F.O., Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chistiakov D.A., Bobryshev Y.V., Orekhov A.N. Changes in transcriptome of macrophages in atherosclerosis. J. Cell. Mol. Med. 2015;19(6):1163–1173. doi: 10.1111/jcmm.12591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ibanez B., Vilahur G., Cimmino G., et al. Rapid change in plaque size, composition, and molecular footprint after recombinant apolipoprotein A-I Milano (ETC-216) administration: magnetic resonance imaging study in an experimental model of atherosclerosis. J. Am. Coll. Cardiol. 2008;51(11):1104–1109. doi: 10.1016/j.jacc.2007.09.071. [DOI] [PubMed] [Google Scholar]
- 72.Cimmino G., Ibanez B., Vilahur G., et al. Up-regulation of reverse cholesterol transport key players and rescue from global inflammation by ApoA-I(Milano). J. Cell. Mol. Med. 2009;13(9B):3226–3235. doi: 10.1111/j.1582-4934.2008.00614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ibanez B., Giannarelli C., Cimmino G., et al. Recombinant HDL(Milano) exerts greater anti-inflammatory and plaque stabilizing properties than HDL(wild-type). Atherosclerosis. 2012;220(1):72–77. doi: 10.1016/j.atherosclerosis.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 74.Gautier E.L., Huby T., Witztum J.L., et al. Macrophage apoptosis exerts divergent effects on atherogenesis as a function of lesion stage. Circulation. 2009;119(13):1795–1804. doi: 10.1161/CIRCULATIONAHA.108.806158. [DOI] [PubMed] [Google Scholar]
- 75.Hedrick C.C. Lymphocytes in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2015;35(2):253–257. doi: 10.1161/ATVBAHA.114.305144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tse K., Tse H., Sidney J., et al. T cells in atherosclerosis. Int. Immunol. 2013;25(11):615–622. doi: 10.1093/intimm/dxt043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Doherty T.M. T-cell regulation of macrophage function. Curr. Opin. Immunol. 1995;7(3):400–404. doi: 10.1016/0952-7915(95)80117-0. [DOI] [PubMed] [Google Scholar]
- 78.Profumo E., Buttari B., Tosti M.E., et al. Plaque-infiltrating T lymphocytes in patients with carotid atherosclerosis: an insight into the cellular mechanisms associated to plaque destabilization. J. Cardiovasc. Surg. (Torino) 2013;54(3):349–357. [PubMed] [Google Scholar]
- 79.Huber S.A., Sakkinen P., David C., et al. T helper-cell phenotype regulates atherosclerosis in mice under conditions of mild hypercholesterolemia. Circulation. 2001;103(21):2610–2616. doi: 10.1161/01.cir.103.21.2610. [DOI] [PubMed] [Google Scholar]
- 80.Caligiuri G., Paulsson G., Nicoletti A., et al. Evidence for antigen-driven T-cell response in unstable angina. Circulation. 2000;102(10):1114–1119. doi: 10.1161/01.cir.102.10.1114. [DOI] [PubMed] [Google Scholar]
- 81.Nakajima T., Goek O., Zhang X., et al. De novo expression of killer immunoglobulin-like receptors and signaling proteins regulates the cytotoxic function of CD4 T cells in acute coronary syndromes. Circ. Res. 2003;93(2):106–113. doi: 10.1161/01.RES.0000082333.58263.58. [DOI] [PubMed] [Google Scholar]
- 82.Pandolfi F., Cianci R., Casciano F., et al. Skewed T-cell receptor repertoire: more than a marker of malignancy, a tool to dissect the immunopathology of inflammatory diseases. J. Biol. Regul. Homeost. Agents. 2011;25(2):153–161. [PubMed] [Google Scholar]
- 83.Quinn M.T., Parthasarathy S., Fong L.G., et al. Oxidatively modified low density lipoproteins: a potential role in recruitment and retention of monocyte/macrophages during atherogenesis. Proc. Natl. Acad. Sci. USA. 1987;84(9):2995–2998. doi: 10.1073/pnas.84.9.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stemme S., Faber B., Holm J., et al. T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoprotein. Proc. Natl. Acad. Sci. USA. 1995;92(9):3893–3897. doi: 10.1073/pnas.92.9.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li D., Mehta J.L. Oxidized LDL, a critical factor in atherogenesis. Cardiovasc. Res. 2005;68(3):353–354. doi: 10.1016/j.cardiores.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 86.Taleb S., Tedgui A., Mallat Z. IL-17 and Th17 cells in atherosclerosis: subtle and contextual roles. Arterioscler. Thromb. Vasc. Biol. 2015;35(2):258–264. doi: 10.1161/ATVBAHA.114.303567. [DOI] [PubMed] [Google Scholar]
- 87.Erbel C., Chen L., Bea F., et al. Inhibition of IL-17A attenuates atherosclerotic lesion development in apoE-deficient mice. J. Immunol. 2009;183(12):8167–8175. doi: 10.4049/jimmunol.0901126. [DOI] [PubMed] [Google Scholar]
- 88.Taleb S., Romain M., Ramkhelawon B., et al. Loss of SOCS3 expression in T cells reveals a regulatory role for interleukin-17 in atherosclerosis. J. Exp. Med. 2009;206(10):2067–2077. doi: 10.1084/jem.20090545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gistera A, Robertson AK, Andersson J, et al. Transforming growth factor-beta signaling in T cells promotes stabilization of atherosclerotic plaques through an interleukin-17-dependent pathway. 2013. [DOI] [PubMed]
- 90.Cirillo P., Golino P., Piscione F., et al. Transcoronary Th-17 lymphocytes and acute coronary syndromes: new evidence from the crime scene? Int. J. Cardiol. 2011;153(2):215–216. doi: 10.1016/j.ijcard.2011.09.063. [DOI] [PubMed] [Google Scholar]
- 91.Erbel C., Dengler T.J., Wangler S., et al. Expression of IL-17A in human atherosclerotic lesions is associated with increased inflammation and plaque vulnerability. Basic Res. Cardiol. 2011;106(1):125–134. doi: 10.1007/s00395-010-0135-y. [DOI] [PubMed] [Google Scholar]
- 92.De Palma R., Cirillo P., Ciccarelli G., et al. Expression of functional tissue factor in activated T-lymphocytes in vitro and in vivo: A possible contribution of immunity to thrombosis? Int. J. Cardiol. 2016;218:188–195. doi: 10.1016/j.ijcard.2016.04.177. [DOI] [PubMed] [Google Scholar]
- 93.Bodi V., Sanchis J., Nunez J., et al. Uncontrolled immune response in acute myocardial infarction: unraveling the thread. Am. Heart J. 2008;156(6):1065–1073. doi: 10.1016/j.ahj.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 94.Caligiuri G., Nicoletti A. Lymphocyte responses in acute coronary syndromes: lack of regulation spawns deviant behaviour. Eur. Heart J. 2006;27(21):2485–2486. doi: 10.1093/eurheartj/ehl284. [DOI] [PubMed] [Google Scholar]
- 95.Liuzzo G., Biasucci L.M., Trotta G., et al. Unusual CD4+CD28null T lymphocytes and recurrence of acute coronary events. J. Am. Coll. Cardiol. 2007;50(15):1450–1458. doi: 10.1016/j.jacc.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 96.Cheng X., Liao Y.H., Ge H., et al. TH1/TH2 functional imbalance after acute myocardial infarction: coronary arterial inflammation or myocardial inflammation. J. Clin. Immunol. 2005;25(3):246–253. doi: 10.1007/s10875-005-4088-0. [DOI] [PubMed] [Google Scholar]
- 97.Corrales-Medina V.F., Musher D.M., Shachkina S., et al. Acute pneumonia and the cardiovascular system. Lancet. 2013;381(9865):496–505. doi: 10.1016/S0140-6736(12)61266-5. [DOI] [PubMed] [Google Scholar]
- 98.Cangemi R., Casciaro M., Rossi E., et al. Platelet activation is associated with myocardial infarction in patients with pneumonia. J. Am. Coll. Cardiol. 2014;64(18):1917–1925. doi: 10.1016/j.jacc.2014.07.985. [DOI] [PubMed] [Google Scholar]
- 99.Santos-Gallego C.G., Badimon J.J. The sum of two evils: pneumonia and myocardial infarction: is platelet activation the missing link? J. Am. Coll. Cardiol. 2014;64(18):1926–1928. doi: 10.1016/j.jacc.2014.08.023. [DOI] [PubMed] [Google Scholar]