Abstract
Colchicine is a well-established drug approved by the Food and Drug Administration (FDA) for the prevention and treatment of gout. It possesses unique anti-inflammatory properties. Interests in the usage of colchicine in cardiovascular medicine have been rekindled recently with several large trials been carried out to investigate its efficacy in treatment of various cardiac conditions including pericarditis, postpericardiotomy syndrome, atrial fibrillation and coronary artery disease. In this review, the basic pharmacological properties of colchicine will be discussed, and the evidences of its benefits for different applications in cardiovascular medicine will be reviewed.
Keywords: Colchicine, pericarditis, postpericardiotomy syndrome, atrial fibrillation, atherosclerosis
BACKGROUND
Colchicine is one of the oldest drugs used by human. Its medicinal usage was first described by the ancient Egyptian in 1500 BC as a remedy for rheumatism and swelling [1]. The active components of colchicine come from the plant Colchicum Autumnale (autumn crocus), and were first isolated by two French Chemists Pelletier and Caventon in 18201. Colchicine is approved by the FDA for the prophylaxis and treatment of gout flares in adults, and for the treatment of Familial Mediterranean Fever [2]. Given its relatively low cost and lack of long term adverse side effects, numerous clinical trials were conducted to investigate its efficacy in the treatment of diseases outside the FDA indication. The commonest usage of colchicine is in the treatment of acute and recurrent pericarditis, and this indication is endorsed by the European Society of Cardiology (ESC) 2015 guideline on the management of pericardial disease [3]. Other areas of potential benefit of colchicine in cardiovascular medicine include the prevention of atrial fibrillation, and primary and secondary prevention of adverse cardiovascular events in high risk patients. In this review, the basic pharmacological properties of colchicine will be discussed, and the evidence of its benefits for different applications in cardiovascular medicine will be reviewed and summarized.
PHARMACOLOGICAL PROPERTIES
Colchicine is a liquid soluble alkaloid that reaches its peak plasma concentration 1 hour after oral administration
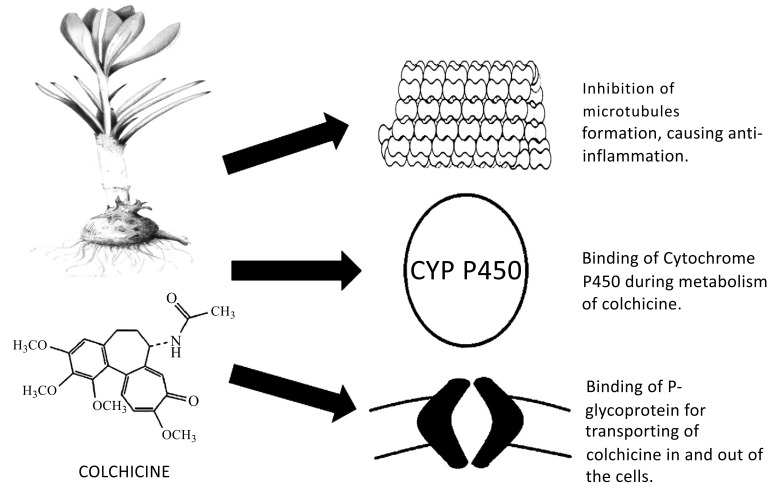
and the steady-state plasma colchicine level can be achieved on average with doses between 0.5 to 2 mg per day. The bioavailability of colchicine ranges from 24% to 88% [4]. The difference in colchicine bioavailability may be partly explained by the variation of the ATPase efflux pump P-glycoprotein level in different individuals. These proteins which are situated at the enterocytes extrude colchicine into the gastrointestinal tract when colchicine binds to them [5]. The concentration of colchicine tends to be higher in the neutrophils than in the plasma, and this might explain its prolonged anti-inflammatory effect after discontinuation [6]. Colchicine is metabolized mainly by the P450 cytochrome (CYP) pathway, specifically CYP 3A4. Biliary excretion is the chief route via which colchicine is eliminated, while renal excretion account for around 5-20% of total colchicine clearance [7]. Due to its dependence on the P450 CYP pathway, the metabolism of colchicine is affected with the concurrent administration of CYP3A4 substrate, such as Cimetidine, proton pump inhibitors, statin, macrolides and cyclosporine [7].
The anti-inflammatory action of colchicine is unique and is different from that of non-steroidal anti-inflammatory drug (NSAID) in that it does not involve the arachidonic acid pathway. The main effect of colchicine comes from its ability to bind tubulin which is a ubiquitous intracellular protein that polymerizes into microtubules. By blocking the polymerization of the microtubules, any process that requires cytoskeletal changes such as cellular migration, division, degranulation, and phagocytosis is inhibited [8]. Other effects of colchicine include inhibiting intracellular signaling by blocking of tyrosine kinase and phospholipase, impeding neutrophil adhesion and extravasation by modulating E-selectin adhesion molecules on the endothelial cells, and reducing the level of inflammatory cytokines such as tumor necrosis factor alpha (TNF-a) and interleukin (IL)-6 by suppressing the activation of the caspase-1 which convert pro-IL to active IL [9]. The key pharmacological actions of colchicine are illustrated in Fig. 1.
Fig. (1).
Key binding sites of Colchicine and their effects.
The commonest adverse effect of colchicine is gastrointestinal upset such as nausea, vomiting and diarrhea. This occurs in up to 10% of patient taking colchicine even at the recommended dose of 1.2mg per day, and causes high drop-out rate in the clinical trials [10]. It might be caused by the extrusion of colchicine into the gastrointestinal tract by the ATPase efflux pump P-glycoprotein at the enterocytes as previously mentioned. Dosage reduction or temporarily withdrawal of colchicine is advised if the symptoms are severe. Drugs that interact with the P-glycoprotein such as proton pump inhibitors, anti-diarrheal agent Loperamide, and macrolide antibiotics might increase the risk of the gastrointestinal side effects. Other potential adverse side effects of colchicine include marrow failure, myopathy and hepatotoxicity. The risk myopathy is increased especially when used in combination with statin [11].
COLCHICINE IN ACUTE AND RECURRENT PERICARDITIS
The most well-known usage of colchicine in cardiovascular medicine is probably for the treatment of acute and recurrent pericarditis. This was extrapolated from the observation of the efficacy of colchicine in the treatment of recurrent polyserositis seen in Familial Mediterranean Fever [12]. Rodriguezet first reported in 1980 the initial success of addition of colchicine in patients with steroid dependent recurrent pericarditis [13]. Several retrospective series subsequently reported benefit of colchicine in the treatment of acute and recurrent pericarditis [14-22]. This has led to the drafting of the 2004 ESC guideline recommendation, in which the usage of colchicine was graded Class I in treatment of recurrent pericarditis, and Class IIa in the treatment of acute pericarditis [18]. From there onwards, several randomized controlled trials (RCT) were conducted by Imazio to evaluate the efficacy of colchicine in the treatment of acute as well as in recurrent pericarditis [20-24]. The results of these earlier retrospective studies as well as the recent RCTs are summarized in Table 1 and 2. In the setting of acute pericarditis, colchicine on top of aspirin or NSAID therapy significantly reduces the rate of pericarditis recurrence and shortens the duration of incessant symptoms. The number needed to treat was 4-5 [23, 24]. Similarly, in the setting of recurrent pericarditis, colchicine in addition to aspirin or NSAID significantly reduced the rate of pericarditis recurrence as well as symptoms persistence [20, 21]. The reported side effect of colchicine is comparable to placebo in the ICAP trial [24]. In the setting of acute pericarditis, the use of corticosteroid was shown to be an independent risk factor for pericarditis recurrence [23]. In preference over corticosteroid, colchicine should be considered standard therapy on top of NSAID of aspirin in treatment of acute as well as recurrent pericarditis.
Table 1.
Colchicine in acute pericarditis.
| First author | (study name) | Study type | Patients number | Clinical setting | Colchicine dosage | |
|---|---|---|---|---|---|---|
| Adverse event | Summary of findings | Millaire A [22], 1991 | Case series | 19 | Acute 1st episode pericarditis | 3mg 1st day, then 2mg for two days, then 1mg x 3-6 months. |
| 5%. | - 100% Acute phase symptoms control, 10% recurrence | Imazio M [23], 2005 | (COPE trial) | prospective, randomized, open-label trial. | 120 | |
| 1st episode of acute pericarditis, on top of aspirin | 1.0 to 2.0 mg for the first day and then 0.5 to 1.0 mg/d for 3 months | 8.3% | - Recurrence rate: colchicine 10.7% vs control 32.3%, p=0.009 NNT 5. | - Incessant symptom at 72 hours: Colchicine 11.7% versus control 36.7%, p=0.003. |
Imazio M [24], 2013
(ICAP trial)
Prospective, multicenter, double-blind randomized trial
240
1st episode of acute pericarditis, on top of aspirin or ibuprofen
0.5 mg twice daily for 3 months for patients weighing >70 kg or 0.5 mg once daily for patients weighing ≤70 kg, for total of 3 months.
11.7%,
NS, Diarrhoea 9.2% NS.
- Recurrence rate: colchicine 9.2% vs placebo 20.8%, p=0.02,NNT 9.
- Incessant symptoms at 72 hours: colchicine 19.2% vs placebo 40.0%, p=0.001.
NS=non-significant, NNT=number needed to treat.
Table 2.
Clinical studies on colchicine in treatment of recurrent pericarditis.
| First author (study name) | Study type | Patient number | Clinical setting | Colchicine dosage and duration | Adverse events | Summary and Comment |
|---|---|---|---|---|---|---|
| Rodriguez De La Serna [13], 1987 | Case series | 3 | Steroid dependent recurrent pericarditis | 1mg/day for 36 months then 0.5mg/day | 0% | - Recurrence rate 0%. |
| Guindo J [14], 1990 | Case series | 9 | Recurrent pericarditis | 1mg/day | 0% | - Recurrent rate 0%. |
| Millaire A [15], 1994 | Case series | 19 | Recurrent pericarditis | Loading of 3mg then 1mg/day for 37 months. | 0% | - Recurrence rate 21%, |
| Adler Y [16], 1995 | Case series | 8 | Recurrent pericarditis | 1-1.5mg/day for 3-9 months. | 50% | - Recurrence rate 0%. |
| Artom G [17], 2005 | Case series | 119 | Recurrent pericariditis | Variable, 9-48 months | Not mentioned | - Recurrence rate 18%, vs 30% after colchicine discontinued. |
| Imazio M [19], 2005 (CORE trial) | Prospective, randomized, open-label. | 84 | 1st recurrence pericarditis, colchicine on top of aspirin | 1.0-2.0 mg the first day and then 0.5-1.0 mg/d for 6 months (weight adjusted) | Colchicine 7% vs control 14%, NS | |
| - Recurrence rate: colchicine 24% vs control 50%, p=0.02, NNT 4. | - Prior steroid use increase risk of recurrence. | Imazio M [20], 2011 (CORP trial) | Prospective, randomized, double-blind, placebo-controlled multicenter study | 120 | 1st recurrence of pericarditis, on top of Aspirin or NSAID | 1.0-2.0 mg the first day and then 0.5-1.0 mg/d for 6 months |
| Colchicine 7% vs control 7%, NS | - Recurrence rate: colchicine 24% vs placebo 55%, p<0.001 NNT 3. | - Persistent symptoms at 72 hours: colchicine 23% vs placebo 53%, p<0.001. | Imazio M [21], 2014 | (CORP-2 trial) |
Prospective, randomized, double-blind, placebo-controlled, multicenter study
240
≥two episodes of pericarditis, on top of Aspirin or NSAID
0·5 or 1·0 mg daily for 6 months without a loading dose
Colchicine 11.7% vs control 8·3%, NS
- Recurrence rate: Colchicine 21·6% vs placebo 42·5%, p=0·0009, NNT5. – Persistent symptom at 72 h: Colchicine 19·2% vs placebo 44·2%, p=0.0001.
- Subgroup analysis showed significant reduction of recurrent pericarditis only in idiopathic pericarditis.
- presence of pericariditis independently predicts future recurrence.
NS = non-significant, NNT = number needed to treat.
COLCHICINE IN POST PERICARDIOTOMY SYNDROME AND PERICARDIAL EFFUSION
Post pericardiotomy syndrome (PPS) is characterized by the presence of fever, pleuritic chest pain and the development of pericardial effusion after cardiac surgery [25]. It can affect as many as 30% of patient undergoing open heart surgery [26]. Inflammatory component plays a part in its pathogenesis as evidenced by the raised C-reactive protein (CRP) level [26]. Several RCTs have been conducted to evaluate the effectiveness of colchicine in preventing PPS as well as pericardial effusion after cardiac surgery, and they yielded conflicting result. The largest ones among them were the CORRP and CORRP-2 trials conducted by Imazio et al. [27-29]. Although a reduction in the incidence of PPS and post-op atrial fibrillation (POAF) were demonstrated, the efficacy of colchicine in the prevention of pericardial effusion was equivocal. In a subgroup analysis of the CORRP-2 trial, colchicine was effective in reducing pericardial effusion in patient with raised CRP, raising the postulation that colchicine might be effective in highly selective patients with heightened inflammation [30, 31]. This selective effect of colchicine on suppressing inflammation might also explain why its administration did not reduce persistent effusion that was present for more than 7 days after surgery where inflammation might have already subsided, and the persistent of effusion might be resulted more from mechanical causes such as failure of reabsorption than inflammatory. The results of these clinical trials are summarized in Table 3.
Table 3.
Colchicine in post pericardiotomy syndrome (PPS) and pericardial effusion.
| First author (study name) | Study type | Patient number | Clinical setting | Colchicine dosage and duration | Adverse events | Summary and Comment | ||
|---|---|---|---|---|---|---|---|---|
| Finkelstein Y [27], 2002 | Prospective, randomized, double-blind trial | 111 | primary prevention of PPS following cardiac surgery | 1.5mg/day for 1 months starting from the 3rd postoperative day. | 11.7%, NS | - incidence of PPS: colchicine 10.6% vs placebo 21.9%, p=0.135, NS. | ||
| Imazio M [28], 2010 (COPPS trial) |
Prospective, randomized, double-blind, placebo-controlled, multicenter trial | 360 | primary prevention of PPS following cardiac surgery | 1.0 mg BD for the 1st day, then 0.5 mg BD daily for 1 month starting from the 3rd post-operative day. | 8.9%, NS | - incidence of PPS: colchicine 8.9% vs placebo 21.1%, p=0.002, NNT 8. – incidence of pericardial effusion: colchicine 12.8% vs placebo 22.8%, p = 0.019. | ||
| Imazio M [29], 2014 (COPPS-2 trial) |
Prospective, double-blind, placebo-controlled, randomized multicenter trial | 360 | Primary prevention of PPS, postoperative AF, and postoperative pericardial/pleural effusions. | 0.5mg BD for patient >70kg, or 0.5mg daily for patient <70kg, starting 48 and 72 hours before surgery and continued for 1 month after surgery. | Colchicine 20%, vs placebo 11.7%, NNH 12. | - Incidence of PPS: colchicine 19.4% vs placebo 29.4%, NNT 10. - Incidence of post-operative AF colchicine 33.9% vs placebo 41.7%, NS. - incidence of pericardial/pleural effusion: Colchicine 57.2% vs placebo 58.9%, NS. |
||
| Meurin P [30], 2015 (POPE-2 trial) |
Randomized, double-blinded, placebo-controlled, parallel-group study |
197 | Pericardial effusion persisting >7 days after heart surgery. | 1mg daily for 14 days. | 10.2% | - mean change of pericardial effusion: colchicine -1.3 grade vs placebo -1.1 grade, p=0.23, NS. | ||
| Izadi Amoli A [31], 2015 | Prospective, randomized, triple-blind, placebo-controlled single-center trial | 149 | Pericardial effusion persisting >3 weeks after CABG. | 1mg daily for 2 weeks | 0% | - mean change of pericardial effusion: colchicine -5mm vs placebo -5mm, p=0.932, NS. | ||
NS = non-significant, NNH = number needed to harm, NNT = number needed to treat.
COLCHICINE IN ATRIAL FIBRILLATION
POAF is common after cardiac surgery and is part of the complication of PPS. Its occurrence is associated with significant morbidity and prolonged hospital stay. Post-operative inflammatory process plays an important role in its pathogenesis. With its anti-inflammatory property, colchicine was studied for its efficacy in reducing POAF. In the COPPS and COPPS-2 study conducted by Imazio, Colchicine at the dosage of 0.5mg BD for 1 month after cardiac surgery was shown to be effective in reducing the incidence POAF [29, 32]. Besides lowering POAF after cardiac surgery, colchicine was also evaluated in preventing the recurrence of AF after catheter ablation as it was postulated that local inflammation after ablation therapy from the direct injury to the atrium and pulmonary artery area might play a role in causing AF recurrence [33]. This was tested by Deftereo in a RCT that used Colchicine 0.5mg BD for 3 months after ablation. AF recurrence was shown to be significantly reduced in that trial, along with a reduction of inflammatory markers such as IL-6 and CRP level [34]. The longterm effect of colchicine in post ablation might be even longer lasting as freedom from post ablation AF recurrence was shown to be a protective factor for long term AF recurrence [33]. These clinical trials of colchicine in AF are summarized in Table 4.
Table 4.
Colchicine in prevention of atrial fibrillation (AF).
| First author (study name) | Study type | Patient number | Clinical setting | Colchicine dosage and duration | Adverse events | Summary and Comment |
|---|---|---|---|---|---|---|
| Imazio [32], 2011 | (COPPS substudy) | Prospective, randomized, double-blind, placebo-controlled, multicenter trial | 336 | Prevention of POAF after cardiac surgery | 1.0 mg BD for the 1st day, then 0.5 mg BD daily for 1 month starting from the 3rd post-operative day. | |
| 9.5%, NS | - incidence of POAF: colchicine 12.0% vs placebo 22.0%, p=0.021, NNT 11. | - hospital stay: colchicine 9.4 days vs placebo 10.3 days, p =0.04 | Deftereos [33], 2012 | Prospective, randomized, double-blind, controlled trial | 161 | |
| Prevention of AF recurrence after pulmonary vein isolation in patients with paroxysmal AF | 0.5mg BD for 3 months, starting from day 1 post ablation. | Colchicine 8.6% vs placebo 1.3%, p=0.03 | - Incidence of AF recurrence at 3 months: colchicine 16% vs placebo 33.5%, p=0.01, NNT 5.6. | - IL-6 and CRP level significantly reduced in Colchicine group. |
Imazio M [29], 2014
(COPPS-2 trial)
Prospective, randomized, double-blind, placebo-controlled, multicenter trial
360
Primary prevention of PPS, POAF, and postoperative pericardial/pleural effusions.
0.5mg BD for patient >70kg, or 0.5mg daily for patient <70kg, starting 48 and 72 hours before surgery and continued for 1 month after surgery.
Colchicine 20%, vs placebo 11.7%, NNH 12
- Incidence of POAF colchicine 33.9% vs placebo 41.7%, NS.
POAF = post-operative atrial fibrillation, PPS = post pericardiotomy syndrome, NNT = number needed to treat, NNH = number needed to harm, NS = non-significant.
COLCHICINE IN CORONARY ARTERY DISEASE
Inflammation is implicated in the pathogenesis of atherosclerosis and the development of coronary artery disease (CAD) [35]. Heightened inflammation such as an elevated level of CRP is an independent predictor of CAD [36]. The role of inflammation is even more pronounced during acute vascular occlusion when a rupture plaque causing vascular inflammation is at its peak [35]. The effect of statin in reducing adverse events in atherosclerosis hails not only from its lipid lowering property but also involves some low-grade anti-inflammatory effect as well [37]. It is therefore reasonable to postulate that colchicine with its unique anti-inflammatory property which does not involve the thromboxane pathway or as pro-thrombotic like the NSAID or steroid, could be useful in reducing adverse event in patient with atherosclerotic disease. The clue that colchicine could be useful in preventing adverse cardiovascular events first came to light from a large retrospective analysis of patient receiving long term colchicine therapy for the treatment for gout [38]. In this study, the author observed that the usage of colchicine was associated with significantly lower rate of myocardial infarction. In view of this observation, Nidof conducted the LoDoCo study which prospectively looked at patients with established CAD treated with 2 years of colchicine [39, 40]. Although this study demonstrated a significant reduction of adverse cardiovascular events with the treatment of colchicine, issues such as relative small number of patients, lacking of blinding and placebo arm, no mentioning of specific drugs use, and additional recruitment of patients in the midst of the study period might have skewed the result.
In the setting of acute coronary syndrome (ACS), inflammasomes play even a greater role in triggering of adverse events and this is evidenced by the elevated inflammatory cytokines such as IL and CRP [41]. Martines conducted
a pilot study in this aspect to look at the effect of colchicine on the level of inflammasomes during ACS [42]. In this study, it was shown that the administration of colchicine significantly reduced the level of IL when compared with control. In another small study conducted on patients with ST elevation myocardial infarction conducted by Deftereos, colchicine was shown to significantly reduce the infarction size on magnetic resonance imaging [43]. The results of these clinical studies are summarized in Table 5.
Table 5.
Colchicine in coronary artery disease.
| First author (study name) | Study type | Patient number | Clinical setting | Colchicine dosage and duration | Adverse events | Summary and Comment |
|---|---|---|---|---|---|---|
| Crittenden [38], 2012 | Retrospective, cross-sectional observational study | 1288 | Reduction of MI in patient with gout treated with colchicine. | Unknown. | Unknown. | |
| - Incidence of MI: colchicine 1.2% vs no colchicine 2.6%, P=0.03. | - Mortality: colchicine 3.9% vs no colchicine 5.1%, P=0.18, NS. | Raju [39], 2012 | Prospective double-blind, randomized controlled trial | 80 | Reduction of hs-CRP and platelet aggregation in patient with ACS or stroke. | 1mg daily for 30 days. |
| Colchicine 38% vs placebo 14%, p=0.04 | - hs-CRP level: Colchicine 1.0mg/L vs placebo 1.5mg/L, p=0.22, NS. | - No difference in platelet function. | Nidof [40], 2013 (LoDoCo study) | Prospective, randomized, observer-blinded endpoint study. | 532 | |
| Reduction of cardiovascular events in patients with clinically stable coronary disease. | 0.5mg daily for a minimum of 2 years. | 4.9%. | - Combined incidence of ACS, out-of-hospital cardiac arrest, or ischemic stroke: Colchicine 5.3% vs placebo 16%, p<0.001, NNT 11) | - Incidence of ACS only: colchicine 4.6% vs placebo 13.4%, p<0.001. | Martínez [42], 2015 | |
| Prospective, randomized study | 83 | Effect of colchicine on the level of cytokines IL-1β, IL-18 and IL-6 in patient with ACS. | 1 mg, followed by 0.5 mg 1 hour later, 6 to 24 hours before cardiac catheterization | Not mentioned | - Colchicine significantly reduces intracoronary infalmmatomry cytokines level in patients with ACS by 40-80%. | Deftereos [43], 2015 |
Prospective, double-blinded, placebo-controlled study,
151
Reduction of infarct size in patients presenting with
STEMI treated with primary percutaneous coronary intervention.
1.5 mg initially followed by 0.5 mg 1 hour later and continue with 0.5 mg BD for 5 days after cardiac catheterization.
Colchicine 26% vs control 4%, p<0.001.
- CK-MB level: Colchicine 3144 ng/h/mL–1 vs control 6184 ng/h/ml-1, p<0.001.
- post STEMI LVEF: colchicine 53% vs control 46%, p=0.003.
- Infarct volume on MRI: colchicine 18.8ml vs control 25.1ml, p=0.019
- CRP level: colchicine 42.9mg/L vs control 63.8ml/L, p=0.019.
ACS = acute coronary syndrome, CRP = C-reactive protein, IL = interleukin, LVEF = left ventricular ejection fraction, MI = myocardial infarction, NS = non-significant, NNT = number needed to treat, STEMI = ST elevation myocardial infarction.
COLCHICINE IN PERCUTANEOUS ANGIOPLASTY
Even in the era of new generation of drug eluting stent (DES), the search for a remedy to prevent in-stent restenosis (ISR) is still relevant as ISR or late luminal loss still plague almost 20% of all the stents implanted [44]. The pathophysiology underlying ISR can be due to vascular injury and inflammation during balloon angioplasty or stent placement. Colchicine given its unique anti-inflammatory property was postulated to retard the progression of restenosis by suppressing the vascular inflammation. Studies done in the pre-stent era however did not show significant benefit of colchicine over placebo in patients undergone balloon angioplasty [45, 46]. In patients implanted with bare metal stent (BMS), Deftereos showed that colchicine significantly reduced ISR by 60% [47]. This difference in efficacy might be explained by the different mechanism underlying restenosis in patient receiving just balloon angioplasty where elastic recoils plays a greater role in luminal loss, and patients receiving stent where neo-atherosclerosis is the main culprit. The results of these studies are summarized in Table 6. There is no trial published for the usage of colchicine in patient receiving DES.
Table 6.
Colchicine in percutaneous intervention.
| First author (study name) | Study type | Patient number | Clinical setting | Colchicine dosage and duration | Adverse events | Summary and Comment |
|---|---|---|---|---|---|---|
| O'Keefe [45], 1992 | Prospective double-blinded, randomized control trial | 197 | Prevention of restenosis After PTCA (balloon angioplasty). | 0.6mg/BD started within 24 h of angioplasty continued for 6 months | Colchicine 28% vs placebo 5%. | Restenosis: colchicine 41%, vs placebo 45%, p = NS |
| Freed M [46], 1995 | Open label trial | 50 | Prevention of neointimal | Hyperplasia after PTCA (balloon angioplasty) in combination of enalapril and statin. | 0.6mg BD 1 day before angiography and until follow up angiography. | |
| 18% | Restenosis: Colchicine 51%. | Deftereos [47], 2013 | Double-blind, prospective, placebo-controlled study | 196 | Prevention of restenosis in diabetic patients undergoing BMS implantation. | Colchicine 0.5mg BD was administered for 6 months from the day of the index PCI (within 24 h, until follow-up angiograph. |
Colchicine 17% vs placebo 7%, p=0.058
Restenosis: Colchicine 16% vs placebo 33%, p= 0.007, NNT 6.
BMS= bare metal stent, PTCA = percutaneous transcatheter angioplasty, NNT = number needed to treat, NS = non-significant.
COLCHICINE IN CONGESTIVE HEART FAILURE
Although not commonly known to be associated with inflammation, the cellular process underlying the development of congestive heart failure (CHF) such as fibrosis, apoptosis and cellular dysfunction has been shown to link with inflammatory processes [48]. Although results from studies with the use of immunomodulatory agents for CHF (mainly targeting TNF) have been discouraging [49], interests in this line of research remain strong. In a hamster model of congestive heart failure, colchicine was shown to reduce myocardial cell stiffness [50]. There was one RCT conducted by Deftereos to evaluate the efficacy of colchicine in patients with chronic stable heart failure [51]. No benefit in terms of improvement in functional class, echographical measurements, or mortality was demonstrated in that trial. It might be possible that the administration of any anti-inflammatory agent after the development of CHF symptoms might be already too late to prevent the progression of cellular fibrosis.
SIDE EFFECTS OF COLCHICINE IN CLINICAL TRIALS
The commonest side effect associated with colchicine in the clinical trials is gastrointestinal upset, which occurs variably in a range of 0-20% as summarized in the tables. This has led up to 20% of discontinuation. This might be caused by the extrusion of colchicine into the gastrointestinal tract by the ATPase efflux pump P-glycoprotein at the enterocytes as previously mentioned. Other rarer side effects reported in trails include hepatotoxicity, rash, myalgia and alopecia, which occur with a combined incidence of less than 5%. There was no mortality reported to be associated with colchicine usage in all the published trials. The risk of colchicine toxicity increases with worsening Creatitine clearance and presence of severe liver disease [7]. These conditions were used as the exclusion criteria in the aforementioned RCTs to minimize the risk of adverse side effects. Other factors that might increase the risk of colchicine toxicity include older age and reduced body surface area.
FUTURE PERSPECTIVES
Several areas of cardiovascular medicine in which the usage colchicine could be further explored. In the treatment of acute pericarditis, the combination of colchicine with either aspirin or NSAID is now well established. However, high dose aspirin or NSAID are often associated with significant side effects such as GI bleeding. Colchicine which has low incidence of adverse side effects is an attractive alternative as a first line monotherapy and this should be tested in future clinical trials. In the prevention of the recurrence of pericardial effusion, the role of colchicine could be further clarified in pericardial effusions of other etiologies such as infective pericarditis or malignant pericardial effusion in which inflammation might play a greater role than in post pericardiotomy. Following the finding of its potential efficacy in prevention of adverse cardiovascular events in patients with CAD, several large RCTs are already under way to evaluate colchicine in the secondary prevention of cardiovascular event and in reducing adverse events in ACS (Table 7). The results from these studies should establish the future roles of colchicine in cardiovascular medicine.
Table 7.
Upcoming trials.
| Clinical trial number | Study type | Target number | Clinical Setting |
|---|---|---|---|
| ACTRN12614000093684 | (LoDoCo 2 trial) | Randomized double-blind, placebo control trial | |
| 3000 | - To determine the effect of low dose [0.5mg/day] colchicine on the composite of clinical events including [1] acute coronary syndrome - troponin positive acute coronary syndrome [myocardial infarction] or unstable angina with angiographic evidence of disease progression, [2] sudden cardiac death or non-fatal out of hospital arrest and [3] non-cardio-embolic ischemic stroke in patients with documented but clinically stable coronary artery disease. | NCT02162303 | |
| (COPLET trial) | Randomized double-blind, placebo control trial | 106 | - To assess the effects of colchicine on vascular inflammation measured by metabolic imaging and plasma biomarkers in patients with atherosclerotic vascular disease. |
| NCT01906749 | (COACS trial) | Multicenter, Randomized double-blind, placebo control trial | |
| 500 | - To assess the effect of low-dose colchicine (0.5mg/day) on overall mortality, new coronary syndromes, and ischemic stroke at 2 years after an acute coronary syndrome. | NCT01709981 | Randomized double-blind, placebo control trial |
| 400 | - To characterize a potential mechanism of benefit in patients undergoing percutaneous coronary intervention (PCI) by evaluating the effects of colchicine on soluble and leukocyte surface markers after PCI. | - To determine the effects of colchicine on peri-procedural myonecrosis and myocardial infarction. |
Randomized double-blind, placebo control trial
60
- To access effect of colchicine on C-reactive protein in chronic atrial fibrillation and following atrial fibrillation ablation.
ACTRN12613001345774
Randomized double-blind, placebo control trial
520
- To evaluate the effect of colchicine in preventing postoperative atrial fibrillation after cardiac surgery.
CONCLUSION
Colchicine, although an ancient drug, has found many new usages and emerged as a novel therapy in cardiovascular medicine because of its low cost, unique anti-inflammatory actions, and relatively good longterm safety profile. Its efficacy in the treatment of acute and recurrent pericarditis, as well as in the prevention of PPS is well established in various RCTs. There are some evidences of benefits of colchicine in the secondary prevention of adverse cardiovascular events in high risk patients, and in reduction of adverse events in ACS patients. However, more evidence on these aspects is needed and should be available from upcoming RCTs. Until then, colchicine remained a promising addition to the armamentarium of cardiovascular medicines.
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Hartung E.F. History of the use of colchicum and related medicaments in gout; with suggestions for further research. Ann. Rheum. Dis. 1954;13:190–200. doi: 10.1136/ard.13.3.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Available at http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm174382.htm, http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm174382.htm,[Accessed 22 August 2015].
- 3.Adler Y., Charron P., Imazio M., et al. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: the task force for the diagnosis and management of pericardial diseases of the european society of cardiology (ESC) endorsed by: the european association for cardio-thoracic surgery (EACTS). Eur. Heart J. 2015;36(42):2921–2964. doi: 10.1093/eurheartj/ehv318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferron G.M., Rochdi M., Jusko W.J., Scherrmann J.M. Oral absorption characteristics and pharmacokinetics of colchicine in healthy volunteers after single and multiple doses. J. Clin. Pharmacol. 1996;36:874–883. doi: 10.1002/j.1552-4604.1996.tb04753.x. [DOI] [PubMed] [Google Scholar]
- 5.Sabouraud A., Chappey O., Dupin T., Scherrmann J.M. Binding of colchicine and thiocolchicoside to human serum proteins and blood cells. Int. J. Clin. Pharmacol. Ther. 1994;32(8):429–432. [PubMed] [Google Scholar]
- 6.Chappey O.N., Niel E., Wautier J.L., et al. Colchicine disposition in human leukocytes after single and multiple oral administration. Clin. Pharmacol. Ther. 1993;54(4):360–367. doi: 10.1038/clpt.1993.161. [DOI] [PubMed] [Google Scholar]
- 7.Niel E., Scherrmann J-M. Colchicine today. Joint Bone Spine. 2006;73(6):672–678. doi: 10.1016/j.jbspin.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Taylor E.W. The mechanism of colchicine inhibition of mitosis. I.Kinetics of inhibition and the binding of H3-Colchicine. J. Cell Biol. 1965;25(Suppl.):145–160. doi: 10.1083/jcb.25.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cronstein B.N., Sunkureddi P. Mechanistic aspects of inflammation and clinical management of inflammation in acute gouty arthritis. J. Clin. Rheumatol. 2013;19(1):19–29. doi: 10.1097/RHU.0b013e31827d8790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finkelstein Y., Aks S.E., Hutson J.R., et al. Colchicine poisoning: the dark side of an ancient drug. Clin. Toxicol. (Phila.) 2010;48(5):407–414. doi: 10.3109/15563650.2010.495348. [DOI] [PubMed] [Google Scholar]
- 11.Kuncl R.W., Duncan G., Watson D., Alderson K., Rogawski M.A., Peper M. Colchicine myopathy and neuropathy. N. Engl. J. Med. 1987;316:1562–1568. doi: 10.1056/NEJM198706183162502. [DOI] [PubMed] [Google Scholar]
- 12.Grattagliano I., Bonfrate L., Ruggiero V., Scaccianoce G., Palasciano G., Portincasa P. Novel therapeutics for the treatment of familial Mediterranean fever: from colchicine to biologics. Clin. Pharmacol. Ther. 2014;95:89–97. doi: 10.1038/clpt.2013.148. [DOI] [PubMed] [Google Scholar]
- 13.Rodríguez de la Serna A., Guindo Soldevila J., Martí Claramunt V., Bayés De Luna A. Colchicine for recurrent pericarditis. Lancet. 1987;2(8574):1517. doi: 10.1016/s0140-6736(87)92641-9. [DOI] [PubMed] [Google Scholar]
- 14.Guindo J., Rodriguez de la Serna A., Ramió J., et al. Recurrent pericarditis relief with colchicine. Circulation. 1990;82:1117–1120. doi: 10.1161/01.cir.82.4.1117. [DOI] [PubMed] [Google Scholar]
- 15.Millaire A., de Groote P., Decoulx E., Goullard L., Ducloux G. Treatment of recurrent pericarditis with colchicine. Eur. Heart J. 1994;15:120–124. doi: 10.1093/oxfordjournals.eurheartj.a060363. [DOI] [PubMed] [Google Scholar]
- 16.Adler Y., Zandman-Goddard G., Ravid M., et al. Usefulness of colchicine in preventing recurrences of pericarditis. Am. J. Cardiol. 1994;73:916–917. doi: 10.1016/0002-9149(94)90828-1. [DOI] [PubMed] [Google Scholar]
- 17.Artom G., Koren-Morag N., Spodick D.H., et al. Pretreatment with corticosteroids attenuates the efficacy of colchicine in preventing recurrent pericarditis: a multi-centre all-case analysis. Eur. Heart J. 2005;26:723–727. doi: 10.1093/eurheartj/ehi197. [DOI] [PubMed] [Google Scholar]
- 18.Maisch B., Seferović P.M., Ristić A.D., et al. Guidelines on the diagnosis and management of pericardial diseases executive summary; The Task force on the diagnosis and management of pericardial diseases of the European society of cardiology. Eur. Heart J. 2004;25(7):587–610. doi: 10.1016/j.ehj.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Imazio M., Bobbio M., Cecchi E., et al. Colchicine as first-choice therapy for recurrent pericarditis: results of the CORE (COlchicine for REcurrent pericarditis) trial. Arch. Intern. Med. 2005;165(17):1987–1991. doi: 10.1001/archinte.165.17.1987. [DOI] [PubMed] [Google Scholar]
- 20.Imazio M., Brucato A., Cemin R., et al. Colchicine for recurrent pericarditis (CORP): a randomized trial. Ann. Intern. Med. 2011;155(7):409–414. doi: 10.7326/0003-4819-155-7-201110040-00359. [DOI] [PubMed] [Google Scholar]
- 21.Imazio M., Belli R., Brucato A. Efficacy and safety of colchicine for treatment of multiple recurrences of pericarditis (CORP-2): a multicentre, double-blind, placebo-controlled, randomised trial. Lancet. 2014;383(9936):2232–2237. doi: 10.1016/S0140-6736(13)62709-9. [DOI] [PubMed] [Google Scholar]
- 22.Millaire A., Ducloux G. Treatment of acute or recurrent pericarditis with colchicine. Circulation. 1991;83(4):1458–1459. [PubMed] [Google Scholar]
- 23.Imazio M., Bobbio M., Cecchi E., et al. Colchicine in addition to conventional therapy for acute pericarditis: results of the Colchicine for acute PEricarditis (COPE) trial. Circulation. 2005;112(13):2012–2016. doi: 10.1161/CIRCULATIONAHA.105.542738. [DOI] [PubMed] [Google Scholar]
- 24.Imazio M., Brucato A., Cemin R., et al. A randomized trial of colchicine for acute pericarditis. N. Engl. J. Med. 2013;369(16):1522–1528. doi: 10.1056/NEJMoa1208536. [DOI] [PubMed] [Google Scholar]
- 25.Imazio M1 Brucato A, Ferrazzi P, Spodick DH, Adler Y. Postpericardiotomy syndrome: a proposal for diagnostic criteria. J. Cardiovasc. Med. (Hagerstown) 2013;14(5):351–353. doi: 10.2459/JCM.0b013e328353807d. [DOI] [PubMed] [Google Scholar]
- 26.Imazio M1 Contemporary features, risk factors, and prognosis of the post-pericardiotomy syndrome. Am. J. Cardiol. 2011;108(8):1183–1187. doi: 10.1016/j.amjcard.2011.06.025. [DOI] [PubMed] [Google Scholar]
- 27.Finkelstein Y., Shemesh J., Mahlab K., et al. Colchicine for the prevention of postpericardiotomy syndrome. Herz. 2002;27(8):791–794. doi: 10.1007/s00059-002-2376-5. [DOI] [PubMed] [Google Scholar]
- 28.Imazio M., Trinchero R., Brucato A., et al. Colchicine for the prevention of post-pericardiotomy Syndrome(COPPS): a multicentre, randomized, double-blind, placebo-controlled trial. Eur. Heart J. 2010;31:2749–2754. doi: 10.1093/eurheartj/ehq319. [DOI] [PubMed] [Google Scholar]
- 29.Imazio M., Brucato A., Ferrazzi P., et al. Colchicine for prevention of post pericardiotomy syndrome and postoperative atrial fibrillation: the COPPS-2 randomized clinical trial. JAMA. 2014;312(10):1016–1023. doi: 10.1001/jama.2014.11026. [DOI] [PubMed] [Google Scholar]
- 30.Meurin P., Lelay-Kubas S., Pierre B., et al. Colchicine for Post-Operative Pericardial Effusion: Preliminary Results of the POPE-2 Study. J. Am. Coll. Cardiol. 2015;66(10):1198–1199. doi: 10.1016/j.jacc.2015.05.078. [DOI] [PubMed] [Google Scholar]
- 31.Izadi Amoli A, Bozorgi A. Efficacy of colchicine versus placebo for the treatment of pericardial effusion after open-heart surgery: A randomized, placebo-controlled trial. Am. Heart J. 2015;170(6):1195–1201. doi: 10.1016/j.ahj.2015.09.020. [DOI] [PubMed] [Google Scholar]
- 32.Imazio M., Brucato A., Ferrazzi P., et al. Colchicine reduces postoperative atrial fibrillation: results of the Colchicine for the Prevention of the Postpericardiotomy Syndrome (COPPS) atrial fibrillation substudy. Circulation. 2011;124(21):2290–2295. doi: 10.1161/CIRCULATIONAHA.111.026153. [DOI] [PubMed] [Google Scholar]
- 33.Lellouche N., Jais P., Nault I., et al. Early recurrences after atrial fibrillation ablation: prognostic value and effect of early reablation. J. Cardiovasc. Electrophysiol. 2008;19(6):599–605. doi: 10.1111/j.1540-8167.2008.01188.x. [DOI] [PubMed] [Google Scholar]
- 34.Deftereos S., Giannopoulos G., Kossyvakis C., et al. Colchicine for prevention of early atrial fibrillation recurrence after pulmonary vein isolation: a randomized controlled study. J. Am. Coll. Cardiol. 2012;60:1790–1796. doi: 10.1016/j.jacc.2012.07.031. [DOI] [PubMed] [Google Scholar]
- 35.Hansson G.K. Inflammation, atherosclerosis, and coronary artery disease. Engl J Med. 2005;352(16):1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 36.Danesh J., Wheeler J.G., Hirschfield G.M., et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N. Engl. J. Med. 2004;350:1387–1397. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 37.Sposito A.C., Chapman M.J. Statin therapy in acute coronary syndromes: mechanistic insight into clinical benefit. Arterioscler. Thromb. Vasc. Biol. 2002;22:1524–1534. doi: 10.1161/01.atv.0000032033.39301.6a. [DOI] [PubMed] [Google Scholar]
- 38.Crittenden D.B., Lehmann R.A., Schneck L., et al. Colchicine use is associated with decreased prevalence of myocardial infarction in patients with gout. J. Rheumatol. 2012;39:1458–1464. doi: 10.3899/jrheum.111533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raju N.C., Yi Q., Nidorf M., Fagel N.D., Hiralal R., Eikelboom J.W. Effect of colchicine compared with placebo on high sensitivity C-reactive protein in patients with acute coronary syndrome or acute stroke: a pilot randomized controlled trial. J. Thromb. Thrombolysis. 2012;1:88–94. doi: 10.1007/s11239-011-0637-y. [DOI] [PubMed] [Google Scholar]
- 40.Nidorf S.M., Eikelboom J.W., Budgeon C.A., Thompson P.L. Low-dose colchicine for secondary prevention of cardiovascular disease. J. Am. Coll. Cardiol. 2013;61:404–410. doi: 10.1016/j.jacc.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 41.Vogel R.A., Forrester J.S. Cooling off hot hearts: a specific therapy for vulnerable plaque? J. Am. Coll. Cardiol. 2013;61(4):411–412. doi: 10.1016/j.jacc.2012.10.026. [DOI] [PubMed] [Google Scholar]
- 42.Martínez G.J., Robertson S., Barraclough J. Colchicine Acutely Suppresses Local Cardiac Production of Inflammatory Cytokines in Patients With an Acute Coronary Syndrome. J. Am. Heart Assoc. 2015;4(8):e002128. doi: 10.1161/JAHA.115.002128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deftereos S., Giannopoulos G., Angelidis C., et al. Anti-Inflammatory Treatment With Colchicine in Acute Myocardial Infarction: A Pilot Study. Circulation. 2015;132(15):1395–1403. doi: 10.1161/CIRCULATIONAHA.115.017611. [DOI] [PubMed] [Google Scholar]
- 44.Dangas G.D., Claessen B.E., Caixeta A., Sanidas E.A., Mintz G.S., Mehran R. In-stent restenosis in the drug-eluting stent era. J. Am. Coll. Cardiol. 2010;56(23):1897–1907. doi: 10.1016/j.jacc.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 45.O’Keefe J.H., Jr, McCallister B.D., Bateman T.M., Kuhnlein D.L., Ligon R.W., Hartzler G.O. Ineffectiveness of colchicine for the prevention of restenosis after coronary angioplasty. J. Am. Coll. Cardiol. 1992;19:1597–1600. doi: 10.1016/0735-1097(92)90624-v. [DOI] [PubMed] [Google Scholar]
- 46.Freed M., Safian R.D., O’Neill W.W., Safian M., Jones D., Grines C.L. Combination of lovastatin, enalapril, and colchicine does not prevent restenosis after percutaneous transluminal coronary angioplasty. Am. J. Cardiol. 1995;76:1185–1188. doi: 10.1016/s0002-9149(99)80334-8. [DOI] [PubMed] [Google Scholar]
- 47.Deftereos S., Giannopoulos G., Raisakis K., et al. Colchicine treatment for prevention of bare-metal stent restenosis in diabetics. J. Am. Coll. Cardiol. 2013;61:1679–1685. doi: 10.1016/j.jacc.2013.01.055. [DOI] [PubMed] [Google Scholar]
- 48.Hofmann U., Frantz S. How can we cure a heart “in flame”? A translational view on inflammation in heart failure. Basic Res. Cardiol. 2013;108:356. doi: 10.1007/s00395-013-0356-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gullestad L., Aukrust P. Review of trials in chronic heart failure showing broad-spectrum anti-inflammatory approaches. Am. J. Cardiol. 2005;95:17C–23C. doi: 10.1016/j.amjcard.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 50.Fernandes F., Ramires F.J., Ianni B.M., et al. Effect of colchicine on myocardial injury induced by Trypanosoma cruzi in experimental Chagas disease. J. Card. Fail. 2012;18(8):654–659. doi: 10.1016/j.cardfail.2012.06.419. [DOI] [PubMed] [Google Scholar]
- 51.Deftereos S., Giannopoulos G., Panagopoulou V., et al. Anti-inflammatory treatment with colchicine in stable chronic heart failure: a prospective, randomized study. JACC Heart Fail. 2014;2(2):131–137. doi: 10.1016/j.jchf.2013.11.006. [DOI] [PubMed] [Google Scholar]