Abstract
Despite recent advances in clinical imaging, echocardiography remains as the most accessi-ble and reliable noninvasive. Since knowledge of left ventricular systolic function remains so critically important in determining prognosis; every effort should be made to prevent subjective estimations. The advent of strain imaging echocardiography now offers a readily available and portable imaging tool that not only offers an objective characterization of myocardial dynamics; but also allows for early detection of subclinical left ventricular dysfunction. This review outlines the basic concepts of strain imaging to better understand the mechanism of myocardial function as well their applicability in the least common cardiac diagnosis among current clinical practice.
Keywords: Echocardiography, speckle tracking, strain imaging, left ventricular mechanics, left ventricular function, clinical imaging
INTRODUCTION
For many years, conventional echocardiography has been the standard noninvasive imaging modality to assess left ventricular systolic function based on measures of left ventricular ejection fraction (LVEF). Unfortunately, a great majority of routine echocardiographic reports simply describe LVEF based on subjective visual estimates. This has prompted the American Society of Echocardiography to recommend the use of objective measures not only to standardize echocardiographic reading [1] but also to correctly identify serial changes in LVEF that are so critically related to early diagnosis and prognosis [2].
The newly recognized helical arrangement of left ventricle (LV) fibers as demonstrated by Torrent Guasp and further elaborated by Streeter is responsible for the torsional or wringing motion of the LV during contraction and further complicates accurate interpretations of LVEF even if objective conventional echocardiographic measures are used [3].
Strain and strain rate (SR) imaging have been recently shown not only to be readily available when performing advanced routine echocardiography but also invaluable in detecting subclinical LV systolic dysfunction in different clinical scenarios [4]. Therefore, in order to truly highlight the overall potential diagnostic value of strain and strain rate imaging as well as its impact on the management of specific cardiac conditions; not only it is crucial to have a better understanding of the physics of these imaging modalities, but also of the clinical applicability in routine practice. Hence, this work aims to review basic concepts and general applications in the least common cardiac entities, so that imaging cardiologist and any interested clinician may have a better understanding as to when these imaging tools might be clinically applicable.
STRAIN IMAGING BASICS
Basic Mechanical Definitions
The strain was first defined in isolated heart muscle and intact hearts in 1973 and it simply represents systolic deformation that occurs after the application of stress [5]. As a simple mathematical principle, strain is given by the following formula:
Where strain (ε), Lo the baseline length of the myocardium and L the length after systolic deformation [6]. Hence, given this relationship strain represents the fractional length change in one dimension. It is expressed in (%) units and it is considered positive when there is lengthening; however, negative with shortening [7].
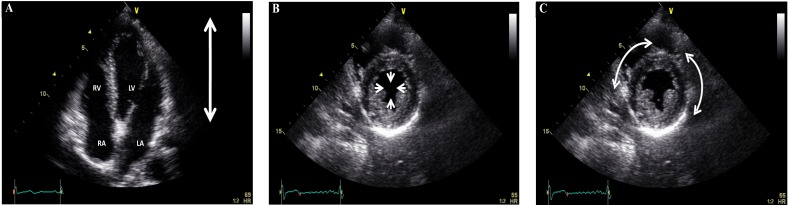
It is important to remember that the heart is a three-dimensional organ with a complex fiber arrangement whose structure/function has been now well established based on the unfolding of the helical ventricular myocardial band with oblique fibers mainly found in the left ventricle arising from both descending and ascending segments of the myocardial band apical loop [8-10]. Consequently, when applying strain imaging it is important that there are three main spatial orientations of myocardial contraction associated with the left ventricle (Fig. 1). First, longitudinal that occurs from base to apex as the mitral annulus contracts towards the left ventricular apex, denoted by negative strain as systolic contraction is occurring towards the ensonifying transducer located at the apex from the traditional four-chamber apical view. In contrast, when the oblique myocardial fibers are seen from the short axis view, based on the myocardial band arrangement, then two forms of contraction would be seen to result in myocardial thickening or the newly characterized “twisting motion” Second, radial contraction or relative thickening of the left ventricular wall towards the center that results in positive strain. Third, counterclockwise motion of the myocardial fibers as seen from base to apex with opposite clockwise rotation from apex to base to cause the “wringing motion” and effectively reduce the systolic left ventricular cavity size, known as circumferential shortening (seen as negative strain) (Figs. 2 and 3).
Fig. (1).
Four-chamber apical view showing longitudinal displacement from base to apex (A), and short axis view at the left ventricular papillary muscle level showing radial displacement towards the center (B) and circumferential displacement along side the cardiac structure (C). RV = right ventricle, LV = left ventricle, RA = right atrium, and LA = left atrium.
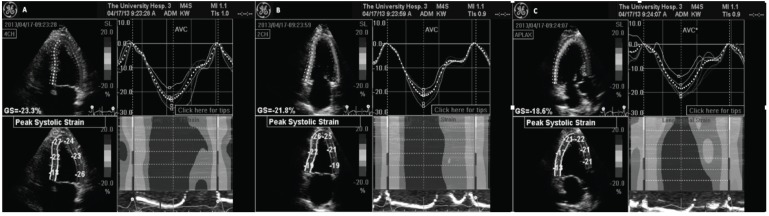
Fig. (2).
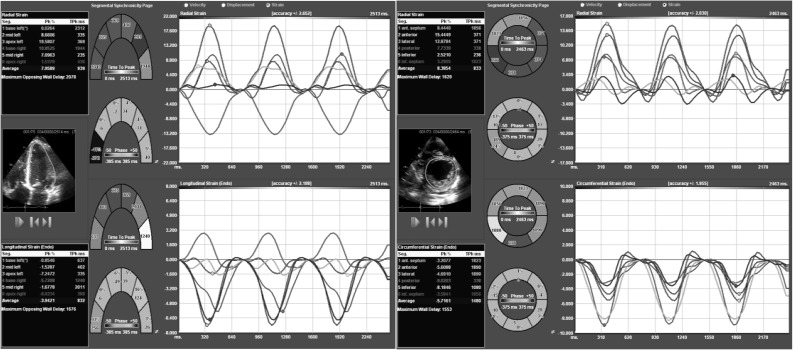
Representative GE Automated Functional Imaging using speckle-tracking algorithm. (A): Four-chamber view. Upper left hand panel shows tracking of the 6-individual color-coded segments assigned by the computer to identify each specific left ventricular region of interest. Bottom left hand panel identifies the individual peak systolic strain for each left ventricular myocardial region. Right hand upper panel displays all the individual curves of the color coordinated myocardial segments demonstrating a temporal correlation. The lower right hand panel displays a color-M-Mode representation of the myocardial deformation and as called “the red carpet” shows normal and synchronous strain of all interrogated segments. (B) and (C) are the two-chamber and apical long axis views respectively different myocardial segments for a more comprehensive evaluation.
Fig. (3).
Representative TomTec speckle-tracking algorithm computer system used for off line analysis. (A): Short axis view showing six-colored regional myocardial segment. The upper panel shows radial strain; while circumferential strain is seen in the bottom panel. (B): Four-chamber apical axis view. The upper panel shows radial strain; while longitudinal strain is seen in the bottom panel.
Obviously, in diastole, as the left ventricle relaxes and then assumes it's original resting state, myocardial motion not only would normally be in the opposite direction as that seen during systole; but also strain will be represented by an opposite strain designation. That is if the systolic strain was positive, then diastolic strain will be negative.
The difference between basal and apical segments strain is known as rotation and it is expressed in degrees per second [11].
On the other hand, strain rate (SR) simply represents the rate of deformation or stretch occurring over time and it is defined by the following formula [12]:
Where ΔV is the velocity gradient of the segment.
Both, strain and SR have the same direction. It is important to emphasize that deformation is load dependent; therefore, they are not direct measurements of myocardial contractility. Contractility represents the active state of the myocardial rather than loading conditions and is reflected by the stress/strain relationship [13]. Peak systolic SR is an early systolic event more closely related to myocardial contractility when compared with ejection fraction measured by conventional echocardiography. The reason behind this is the
fact that the final component of ejection fraction occurs by inertial effects once the myocyte contraction is finished [14].
Ultimately, an adequate understanding of these definitions not only is crucial to differentiate basic concepts of myocardial mechanics, but also to identify the potential utility as well as plausible limitations of echocardiography when imaging myocardial structures.
Advanced Strain Imaging Echocardiography
Three main echocardiographic modalities are currently available today, from different vendors, to perform advanced strain imaging assessment of myocardial function and basically include tissue Doppler imaging (TDI) and 2-D as well as 3-D speckle tracking imaging (STI).
Tissue Doppler Imaging
The initial concept of TDI was first applied to measure velocity displacement of both tricuspid and mitral annuli later found to be surrogate markers of the right ventricular and left ventricular systolic function, respectively [15-18].
This same Doppler technique was then applied to examine individual myocardial segments. Using TDI not only individual regions of myocardial tissue velocity were measured; but also myocardial strain or deformation analysis of those same segments was then possible.
Several studies have shown the utility of TDI not only in diagnosis subclinical cardiac disease but also in predicting adverse events [19-21]. However, TDI as a Doppler-based modality it’s bound by the same imaging limitations including angle dependence of the ultrasound beam. Therefore, when myocardial segments move out of the ensonifying beam line, inaccurate measurements are then obtained. In addition, the TDI-derived strain and SR is a time-consuming process and requires expert readers. Moreover, TDI parameters are very susceptible to signal noise [7]. Other important weakness of TDI is the influence of the tethering effect in getting the true strain or SR in hypokinetic myocardium. These limitations contribute to the measured 10-15% interobserver variability seen with TDI-derived strain and SR measurements [22].
Two-Dimensional Speckle Tracking
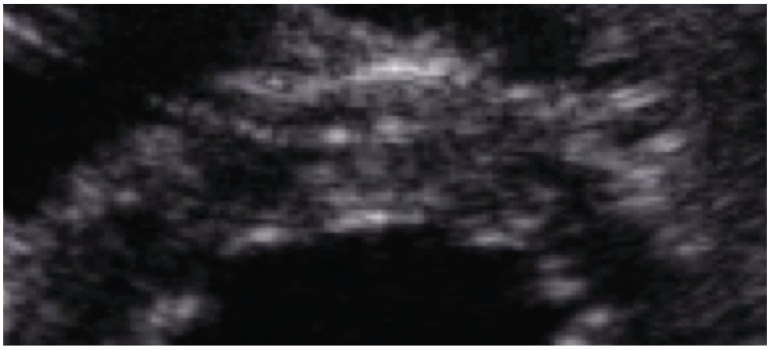
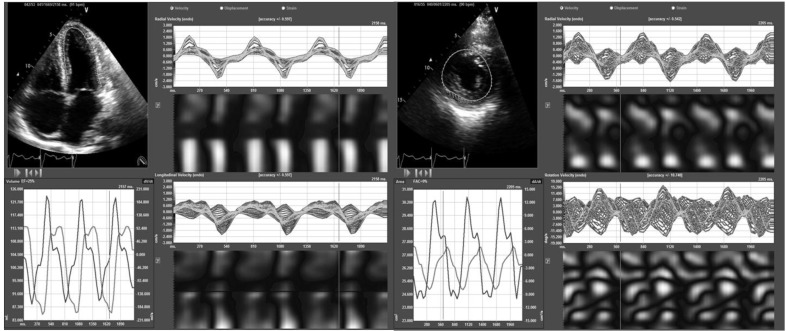
Two-dimensional speckle tracking echocardiography (2-D-STE) tracks frame-to-frame movements of the natural acoustic markers or speckles observed on myocardial tissue as a result of high-resolution frame rate imaging [23]. Unlike TDI, this modality tracks in two dimensions along the direction of the wall, thus is relatively angle independent and appears to be more reproducible [24]. This 2-D-STE-derived SR is calculated through temporal derivation of 2-D strain data and its spatial integration will show 2-D myocardial velocities [7]. Another important limitation of 2-D-STE is the need of high-resolution imaging quality; consequently, measurements are significantly limited with tachycardia (Fig. 4).
Fig. (4).
Representative high zoom image of the myocardium demonstrating the different white-and-gray color intensity gradation of the different myocardial speckles for that particular cardiac region.
Three-Dimensional Speckle Tracking
Three-dimensional speckle tracking (3-D-STE) is a novel echocardiographic modality based on acquiring a 3D volumetric echocardiographic rendition of the left ventricle, generally from the apical widow [25]. This 3D full-volume LV data acquisition should overcome the limitation of plane dependency of 2D imaging [26].
Even though 3-D-STE has been validated against sonomicrometry, current guidelines are still not widely available and its use in routine clinical practice is still limited to some major centers or for research purposes [27].
Some limitations of 3-D-STE are the high vendor and imaging quality dependency [25, 28]. However, area strain appears to be a highly accurate parameter to assess myocardial deformation, as combined data for 2 directional deformations can reduce the tracking error because of higher signal-noise ratio compared with that of longitudinal and circumferential strain.
CLINICAL APPLICATIONS
Strain or strain imaging has shown to be valuable in several clinical scenarios in cardiology. Although many applications have been reported in the most frequent cardiac entities seen in the clinical practice (e.g., hypertensive heart disease, coronary artery disease, LV dysfunction caused by valvular heart disease or heart failure); some applications for the least common cardiac diagnosis remain unknown to the imaging cardiologist.
Cardiac Dyssynchrony
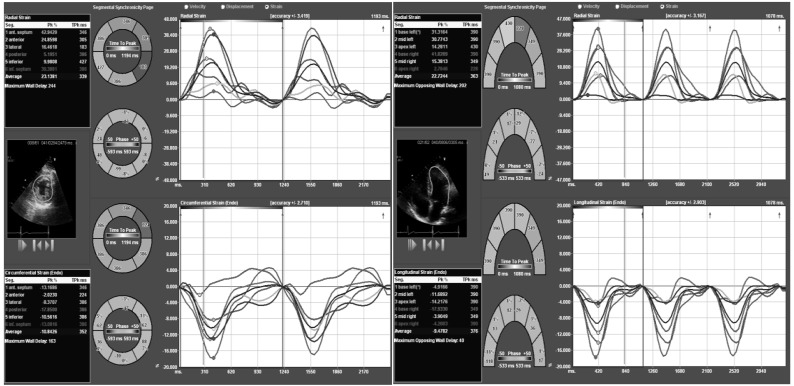
One of the first clinical applications of TDI and STE were to assess temporal differences in the generation of maximal systolic deformation among different myocardial segments in patients with dilated left ventricles (Fig. 5). The concept of cardiac resynchronization therapy (CRT) was then introduced to find if echocardiography would be useful in identifying these segments and using pacing therapy resynchronize those myocardial segments with the largest temporal difference with regards to strain generation in order to reduce symptomatology and improves survival [29, 30]. The American Heart Association/American College of Cardiology/ European Society of Cardiology and the Heart Rhythm Society had agreed that CRT should be undoubtedly offered to patients with the following profile including those with continuing symptoms of heart failure despite optimal therapy, a left bundle branch block morphology with a QRS duration ≥ 120 ms, a left ventricular ejection fraction < 35% and patients have to be in normal sinus rhythm [31-33]. Despite these indications, there is a low responder rate among these patients and still remains uncertain the influences of biventricular pacing over myocardial mechanics [34], mainly because even though dyssynchronous myocardial segments might be identified, placement of a pacing wire on those segments might be technically challenging.
Figure 5.
(A): Four-chamber apical axis view from a patient with dilated cardiomyopathy. Please note that not only there is the presence of different temporal curves, suggestive of dyssynchrony; but also the opposite direction of curves, the latter showing abnormal relation at times of contraction that could be secondary to wall motion abnormalities. (B): Short axis view of the same patient. Please note the presence of different temporal curves, also suggestive of dyssynchrony.
Thus, this field continues to evolve despite the unquestionably proven benefit of STE in the assessment of both dyssynchrony and myocardial function. Suffoleto et al. reported that baseline radial STE dyssynchrony (time difference in peak septal wall-to-posterior wall strain > or =130 ms) predicted an immediate and long-term significant increase in ejection fraction after CRT placement with a 91% sensitivity/ 75% specificity and 89% sensitivity/ 83% specificity respectively [35]. Later on, several other studies have validated these same findings and at present it is clear that radial strain is the best directional strain parameters to identify CRT responders [36-38].
More recently, apical transverse motion (ATM), to quantify “apical rocking”, has been proposed as a novel parameter for assessing LV dyssynchrony and predicting response to CRT. In a study of 35 patients with non-ischemic dilated cardiomyopathy, ATM revealed a significant correlation with radial dyssynchrony (r=0.78, p<0.001). In addition, a cut-off value of 2.5 mm for ATMloop was recommended to discriminate between patients with and without radial dyssynchrony [39].
On the other hand, there is some evidence that LV lead position is optimal at the site of latest mechanical activation and away from possible areas of a scar, which it is known that may affect the response to CRT [40]. Becker et al. proved that an adequate lead position defined by circumferential strain analysis results in significant improvement in LV function and more LV reverse remodeling [41]. The same results were recently found in the TARGET (Targeted Left Ventricular Lead Placement to Guide Cardiac Resynchronization Therapy) Trial were 250 patients were evaluated. An optimal LV lead position at the site of latest mechanical activation, avoiding low strain amplitude (scar), was correlated with a better CRT response and survival. Furthermore, a suboptimal LV lead placement independently predicted all-cause mortality (hazard ratio: 1.8; p = 0.024) [42].
Three-dimensional STE imaging has emerged as a useful modality when compared to either 2-D-STE or TDI for the ongoing evaluation of LV dyssynchrony [43]; however, prediction of both responses to CRT and LV reverse remodeling requires more studies with prospective data [44, 45].
Athlete’s Heart
Regular intense exercise training is known to result in increased left ventricular wall thickness and mass as well as cavity dilatation known as athlete’s heart [46]. Athletes participating in high endurance sports are usually affected with cardiac hypertrophy. This particular finding of LV hypertrophy could be particularly troublesome when LV hypertrophy is also the hallmark of hypertrophic cardiomyopathy (HCM), the most common of sudden cardiac death. Therefore, it would be critically important to differentiate both conditions in order to allow participation of young athletes in competitive sports. In addition, young patients with unrecognized hypertension might also have left ventricular hypertrophy and have similar echocardiographic findings. However, this form of hypertensive hypertrophy is also associated with increased morbidity and mortality.
STE has been particularly useful in differentiating pathologic from physiologic hypertrophy in top-level athletes. In study by Butz and associates STE was used to study deformation differences between top-level athletes and HCM as well as sedentary normal subjects. In this study, these investigators found that all strain components in HCM patients were significantly reduced. In this study, a cut-off value of < -10% for peak global longitudinal strain (GLS) identified pathologic hypertrophy due to HCM with a sensitivity of 80.0% and a specificity of 95.0% [47].
In another study assessing long-term cardiac remodeling, marathon runners and wrestlers who had quit sports after an extensive period of time were evaluated using standard echocardiographic and STE parameters and their data was then compared to that of healthy subjects. In this study, wrestlers maintained both structural and functional properties despite having stopped the sports more than 10 years earlier. In wrestlers, LV global longitudinal strain and systolic strain rate values - although within normal limits- were significantly higher when compared with marathon runners and control group. The reason for these differences when compared with runners is still unknown. However, they could not detect the magnitude of such changes due to the lack of data during their active sports periods [48]. In another study by Simsek et al. STE was found more useful than conventional echocardiography in characterizing LV systolic and diastolic function that both should be normal in young elite athletes when eccentric and concentric hypertrophy was present in contrast to pathological hypertrophy that both might be abnormal [49].
In addition, since the right ventricle (RV) also determines exercise tolerance by using STE preserved RV systolic function is also a normal criteria found among athletes when performing high-intensity chronic exertion [50].
Finally, left atrial enlargement with normal left diastolic function is a common finding in athletes. Using STE, both left atrial global peak atrial longitudinal strain (43.9 ± 9.5% versus 39.8 ± 6.5%; P<0.05) and peak atrial contraction strain (15.5 ± 4.0% versus 13.9 ± 4.0%; P<0.05) were found to be significantly decreased after 16 weeks of intensive training. However, no changes were observed in biventricular E/e’ ratio and atrial stiffness, suggesting that left atrial remodeling had a strong volume rather than a pressure overload component [51].
When comparing 2D to 3D STE in characterizing athlete’s heart, so far no significant differences have been identified [52]. However, studies are limited to a small number of patients; hence, differences between these two images modalities might be noted when more studies become available.
Acute Rejection in Cardiac Transplantation
Acute allograft rejection still remains a significant cause of mortality in post heart transplant recipients [53]. At the moment, there is no reliable noninvasive method to detect cardiac transplant rejection (CTR) aside from endomyocardial biopsy that remains as the “gold standard” invasive method for definitive diagnosis [54]. The advent of STE has been proposed as a possible alternative tool to monitor cardiac allograft function and define the best time to perform myocardial biopsies as well as coronary angiography [22]. Significant data is currently available showing significant advantages of STE over conventional echocardiography in identifying acute-subclinical rejection (including asymptomatic patients with histological evidence of rejection); hence it has now become an essential imaging tool in the follow-up of patients after cardiac transplant [55].
Buddhe et al., in a study of 50 children with heart transplant undergoing routine cardiac catheterization found a better correlation between diastolic function parameters by STE and pulmonary capillary wedge pressure, when compared with conventional echocardiography [56]. Furthermore, 24% of children were found having an abnormal global longitudinal peak systolic strain despite normal parameters reported on conventional echocardiography. Thus, changes in global longitudinal peak systolic strain appear to be more common than previously thought in pediatric heart transplant recipients with no acute heart rejection. In support of this theory, a recent study reported by Clemmensen et al. recognized the importance of global longitudinal strain in detecting moderate rejection in adults, providing solid evidence to consider GLS as a marker of graft function involvement during acute rejection [57]. Moreover, other strain parameters such as peak systolic longitudinal, radial and circumferential strain have been proposed to be useful in detecting acute allograft rejection in children [54].
Finally, LV torsion (LV-tor) measured by STE has been preliminarily shown of clinical value for monitoring acute rejection cardiac transplant recipients. Specifically, a 25% reduction in LV-tor value from baseline was noted to be a good predictor of Grade 2 or higher rejection with an accuracy of 92.2% [58]. Furthermore, despite being not only a retrospective but also small study neither the individual use of longitudinal or radial strain nor torsion were useful in detecting vasculopathy among heart transplant recipients [59]. However, more data is certainly needed before any definitive conclusions can be reached.
Chemotherapy-Induced Cardiotoxicity
Since antitumor drugs have significantly decreased mortality and consequently enhanced survival rates of many cancer patients; it has now become apparent that these same survivors are experiencing serious cardiotoxic effects, clinically recognized as irreversible cardiac injury and symptomatic heart failure. In fact, cardiotoxicity induced by certain chemotherapeutic agents commonly used for certain cancers represents the leading cause of morbidity and mortality in cancer survivors [60].
Therefore, baseline determination of LVEF and most importantly close surveillance remain critically important in the management of cancer patients. Traditionally, imaging modalities such as nuclear-based multigated acquisition (MUGA) scans and conventional echocardiography were recommended for assessment of LVEF. Unfortunately, subtle identification of a reduction in LVEF by any of these two imaging tools to conclusively determine that myocardial damage has occurred as a result of chemotherapy might not occur and when it does it may be too late. It has been estimated that progression of cardiac injury has already occurred in up to 58% of patients and even though aggressive heart failure therapy might be initiated, most of these patients not only their systolic function fails to recover, but also they continue to deteriorate [61, 62].
Since STE has been recently shown not only to be readily available when performing advanced routine echocardiography but also its value in detecting subclinical ventricular dysfunction, as well as its prognostic value in several clinical scenarios [63]; its use has also been investigated in cancer patients. In a recent literature review, involving a total of 1,504 patients, the overall utility of STE in measuring myocardial deformation in the diagnosis and prediction of cardiotoxicity in patients receiving potentially cardiotoxic chemotherapy was assessed and initial results seemed promising. Specifically, peak systolic longitudinal strain rate was consistently shown to detect early myocardial changes during therapy. In this analysis, a 10% to 15% early reduction in GLS by STE during therapy was the most useful parameter for cardiotoxicity prediction, defined as a drop in LVEF or development of heart failure symptoms. In late survivors of cancer, measures of global radial and circumferential strain were consistently abnormal, even when LVEF was normal [64]. Most recently, when peak left ventricular GLS was added to standard clinical variables used in the routine follow-up of cancer patients with normal ejection fractions receiving chemotherapy; incremental prognostic information was obtained regarding mortality in these patients [65].
In 2014, Florescu et al., studying the effects of Epirubicin in potentially inducing cardiotoxicity in 40 patients with breast cancer found that the systolic TDI velocity (S'), longitudinal strain, and longitudinal strain rate were all abnormally low only after the third cycle of epirubicin, even though radial and circumferential strain measures, as well as rotation parameters were still normal. Furthermore, a decrease in longitudinal strain after the third cycle of epirubicin was identified as the best independent and accurate predictor of cardiotoxicity after completion of epirubicin-induced cardiotoxicity [66].
To date, the St. Jude Lifetime Cohort Study represents the largest study focusing on the utility of GLS in identifying late cardiotoxicity in cancer survivors [67]. This cross-sectional study was performed in 1,807 childhood survivors diagnosed whose cancers were previously diagnosed and treated with anthracycline chemotherapy, chest radiotherapy or both more than 10 years prior to their follow-up data. These investigators found that 28% had preserved LV function while 8.7% of patients had abnormal GLS as well as evidence of left ventricular diastolic dysfunction. Obviously, these findings highlight the importance of identifying subtle abnormalities of systolic and diastolic function that might be present in asymptomatic cancer survivors when compared with the normal population that would otherwise not be treated if correctly identified with the appropriate imaging tool [68].
Hypertrophic Cardiomyopathy
HCM is an autosomal dominant disease in which part of the left ventricle (and sometimes of the right ventricle) is hypertrophied by an unclear cause with associated myocyte disarray and fibrosis [69]. It was first called a “disease of the sarcomere” when the first three disease genes to be identified were discovered to encode part of the myocardium [70]. Single-points missense mutations in one of nine sarcomeric genes encoding sarcomeric proteins have been proposed to cause the disease and have been described in up to 2/3 of patients with HCM [71]. The genes MYBPC3, encoding cardiac myosin-binding protein C (cMyBP-C) and MYH7, encoding the β-myosin heavy chain are often the most frequent target of mutations, each accounting for 1/4 to 1/3 of all cases diagnosed [72].
HCM is the most prevalent inheritable myocardial disease and cause of left ventricular outflow tract LVOT obstruction as the latter is present in the majority of patients with HCM (±70%) [73]. Not only is LVOT obstruction associated with a diversity of symptoms such as dyspnea on exertion, syncope, chest pain, and general fatigue, but previous studies have also shown an associated increase of all-cause mortality and sudden cardiac death in these patients [74, 75]. Consequently, the HCM Outcomes Investigators have incorporated the presence of obstruction as a risk factor in the novel clinical risk prediction model [76].
To date, several studies have confirmed LV systolic dysfunction as measured by left ventricular torsion and strain imaging is present in HCM despite normal measurements by conventional echocardiography [77, 78]. Specifically, when STE was used to study HCM subjects with preserved LVEF, peak GLS was shown to be lower when compared with healthy group [79]. Furthermore, even though endocardial global circumferential strain (GCS) was no different; the ratio of endocardial GCS strain to epicardial GCS strain was significantly increased in the HCM group. The investigators proposed a possible initial involvement of subendocardial fibers as a plausible mechanism causing early LV dysfunction in HCM patients. In another study in which 41 patients with HCM were compared to 27 control subjects, not only peak GLS of all layers was reduced, but also peak GCS of the mid and outer layers were also decreased, presumably reflecting impaired myocardial function. In this other study, peak GCS of the inner layer was preserved, being associated with maintenance of chamber function [80]. Therefore, peak GLS has been proposed as a promising predictor of adverse cardiovascular outcomes which may provide additional benefit over conventional variables for risk stratification in HCM patients [81].
Furthermore, the use of left atrial STE strain analysis has been shown the ability to discriminate between HCM mutation carriers and healthy individuals, even though no significant differences in LV systolic deformation were identified between these two groups [82]. Hence, STE could be considered as an alternative family screening approach for early identification of HCM mutation carriers.
Finally, Zhang H et al. found that LV twist also had a high discriminatory power in detecting extent of myocardial fibrosis in HCM patients, pointing at LV twist as a potential additional marker not only for detection of myocardial fibrosis; but also as a predictor of major cardiac events in these patients [83].
Cardiac Amyloidosis
Amyloidosis is a rare disease characterized by extracellular accumulation of amyloid fibrils in organs and tissues [84]. Misfolded protein aggregates compose these insoluble rigid, nonbranching fibrils that end up depositing within the extracellular space, leading to an abnormal tissue architecture and ultimately, a loss of function [85, 86]. To date, twenty-seven different amyloid precursor proteins, have been pointed out to be involved in the amyloid fibril formation and tissue/organ deposition [87]. However, despite this diversity of precursors, it is almost impossible to distinguish the final resulting fibrils. The final diagnosis of amyloidosis is based on pathology specimens identification of amyloid fibril deposition with Congo red, thioflavin T, or Alcian blue staining [86].
Cardiac amyloidosis (CA) is characterized by heart amyloid deposition, including the atria, ventricles, perivascular space and in some cases, the valves and conduction system [88]. It might be present in up to 50% of patients with systemic amyloidosis and it represents the major cause of mortality in light-chain subtype disease [89] (Fig. 6). Amyloid infiltration can lead to loss of heart elasticity (impaired relaxation) due to the resulting biventricular wall thickening; which is clinically translated in restrictive cardiomyopathy.
Figure 6.
(A): Four-chamber apical view from a patient with cardiac amyloidosis. Please note the significant temporal disarray of all signals as seen in both panels. (B): This short axis view shows a significant temporal disarray of rotational signals.
Cardiac amyloidosis has been traditionally viewed as a rare or orphan disease, particularly compared with acquired cardiovascular diseases such as coronary artery disease and hypertensive heart disease. Ongoing efforts have suggested, though, that the rarity of cardiac amyloidosis may be more a reflection of its underdiagnosis, rather than true incidence. This may be particularly true in the transthyretin amyloidoses. In general, diagnosis of amyloid cardiomyopathy has proven to be quite challenging, requiring cardiac biopsy and pathological evaluation for a definitive histological diagnosis in many patients. Cardiac involvement is probably often misdiagnosed during the early stages of the disease when ventricular wall thickening due to amyloid infiltration may be misdiagnosed as left ventricular hypertrophy due to hypertension. In patients with confirmed amyloid deposition in other organ systems, wall thickening on echocardiography, particularly if associated with low voltage on the electrocardiogram, is highly suggestive of cardiac involvement, and cardiac biopsy is rarely needed in such cases [88].
In early subclinical disease, STE have shown to provide more detailed information on regional myocardial deformation when compared with conventional echocardiography [90]. In this context, Sun et al demonstrated that cardiac amyloid strongly alters all strain parameters (longitudinal, circumferential, and radial strain) and STE can differentiate cardiac Amyloidosis from other causes of LV hypertrophy, including HCM [91]. Baccouche et al. obtained similar results by 3D STE but emphasized in oppositional basoapical radial strain gradient as suggestive of a “function-pattern-based” differentiation of amyloidosis and HCM [92]. Furthermore, parametric polar maps of regional longitudinal strain have been proposed to detect regional variations in strain parameters which represent an accurate tool to discriminate cardiac amyloidosis and HCM from hypertensive heart disease [93].
In 2012, Phelan et al. compared 55 CA patients with 30 control diagnosed with LV hypertrophy. They found a variation pattern in longitudinal strain from base to apex among patients diagnosed with CA. This study demonstrated that relative “apical sparing” pattern of longitudinal strain represents an important parameter that should be considered in the diagnosis of cardiac amyloidosis, being sensitive (93%) and specific (82%) enough in discriminating case from controls. [94].
Moreover, selected strain parameters can predict outcomes in cardiac amyloidosis. A recent study shows that early diastolic strain rate was a powerful independent predictor of outcomes in cardiac Amyloidosis patients with preserved LVEF [95]. Buss et al. reported similar results in a study of 206 patients with light-chain amyloidosis where diastolic dysfunction and 2D global longitudinal strain served as independent predictors of survival in patients with primary amyloidosis and offered incremental information beyond standard clinical and serological parameters [96].
Cardiac Sarcoidosis
Sarcoidosis is a systemic granulomatous disease of unknown etiology with direct involvement of the heart in more than 20% of affected patients [97-100]. Cardiac Sarcoidosis (CS) is frequently recognized post-mortem, as sudden death may be the clinical presentation [101-104]. To date, the experts in the field have failed to fully agree with the best diagnosis criteria and choice of therapy [105, 106].
The incidence of CS is influenced by race and ethnicity. In Japan, 50-78% of patients with sarcoidosis shown cardiac involvement in necropsy studies [107-111]. Between 77 to 85% of deaths in these patients have been attributed to CS, which has defined cardiac involvement as the leading cause of death due to sarcoidosis [109, 112]. By contrast, in the United States, 13 to 50% of sarcoid deaths have been attributed to myocardial involvement [101, 113].
Overall, when CS is present, overall prognosis is adversely affected and mortality increases as a result of fatal arrhythmia, atrioventricular conduction disturbances, and congestive heart failure. Unfortunately, when these events occur, most of the patients haven’t shown typical cardiovascular symptoms or structural abnormalities by basic cardiac testing and there was no time to establish an adequate intervention [114]. Since steroid therapy plays a critical role in cardioprotection and LV systolic function preservation, prompt detection of subclinical myocardial dysfunction in cardiac Sarcoidosis is crucial [115].
Further insights of subclinical myocardial involvement by CS can be gained with the use of STE. In a recent study, 40 patients with sarcoidosis and normal EF had evidence of bi-ventricular and atrial mechanical dysfunction as seen by STE [116]. Other investigators also found significant reduction in peak GLS in 67 newly diagnosed patients without symptoms or signs of cardiac disease, when compared to healthy control patients [117]. Furthermore, peak global radial strain was also found to be useful in differentiating patients with cardiac sarcoidosis from those with dilated cardiomyopathy [118].
Despite the harmful effects of CS on patient survival, few studies have explored the usefulness of STE in detecting subclinical disease and predicting clinical outcomes. Therefore, additional studies are necessarily warranted to advance our understanding of cardiac mechanics and identify if STE has a significant role in diagnosis and follow-up of patients with sarcoidosis.
FUTURE DIRECTIONS
Although innumerable publications have appeared to date regarding strain and strain rate, STI has yet to gain great acceptance into routine clinical practice. STE by itself is a robust technique that enables assessment of the deformation of the myocardium noninvasively and quantitatively. Sure STI has allowed us to gain a better understanding of myocardial mechanics; however, there are other areas that remain to be validated. Specifically, care should be taken, for example, when comparing strain measurements from very dilated to small left ventricles; presence of severe valvular stenosis or regurgitation; uncontrolled hypertension at the time of the study; left ventricles with concentric versus eccentric hypertrophy, etc. Though these limitations need to be acknowledged; examination of the same individual over time might be useful, particularly when considering the clinical scenarios mentioned in this review. As previously suggested by many, if strain could be quantitatively normalized to the wall stress, strain would become by far the clinically relevant pathophysiologic parameter to access myocardial function. Moreover, the addition of other imaging techniques such as cardiac computed tomography and magnetic resonance imaging in combination with STI, represent a promising field in the diagnosis, prognosis and management of cardiac disease. Undoubtedly, we all need to continue participating in this type of research.
CONCLUSION
STE is a robust non-Doppler, and thereby angle-independent technique, for the assessment of regional and global deformation of the myocardium. Although, STE-derived strain and strain rate measurements have several limitations, implementation of these advanced imaging tools on a routine basis; particularly in certain clinical settings as mentioned in this review not only will be of great diagnostic and therapeutic value, but also advance of understanding of these clinical entities.
ACKNOWLEDGEMENTS
This publication was partially supported by the National Institute on Minority Health and Health Disparities of the National Institutes of Health Award Numbers CCTRECD-R25MD007607 and HiREC-S21MD001830. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
CONFLICT OF INTEREST
This article content has no conflict of interest.
REFERENCES
- 1.Lang R.M., Bierig M., Devereux R.B., et al. American Society of Echocardiography’s Nomenclature and Standards Committee Task Force on Chamber Quantification; American College of Cardiology Echocardiography Committee; American Heart Association; European Association of Echocardiography, European Society of Cardiology, Recommendations for chamber quantification. Eur. J. Echocardiogr. 2006;7:79–108. doi: 10.1016/j.euje.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 2.Baker D.W., Bahler R.C., Finkelhor R.S., Lauer M.S. Screening for left ventricular systolic dysfunction among patients with risk factors for heart failure. Am. Heart J. 2003;146(4):736–740. doi: 10.1016/S0002-8703(03)00396-X. [DOI] [PubMed] [Google Scholar]
- 3.Streeter D.D., Jr, Spotnitz H.M., Patel D.P., et al. Fiber orientation in the canine left ventricle during diastole and systole. Circ. Res. 1969;24:339–347. doi: 10.1161/01.res.24.3.339. [DOI] [PubMed] [Google Scholar]
- 4.Poterucha J.T., Kutty S., Lindquist R.Q., Li L., Eidem B.W. Changes in left ventricular longitudinal strain with anthracycline chemotherapy in adolescents precede subsequent decreased left ventricular ejection fraction. J. Am. Soc. Echocardiogr. 2012;25:733–740. doi: 10.1016/j.echo.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Mirsky I., Parmley W.W. Assessment of passive elastic stiffness for isolated heart muscle and the intact heart. Circ. Res. 1973;33:233–243. doi: 10.1161/01.res.33.2.233. [DOI] [PubMed] [Google Scholar]
- 6.D’Hooge J., Heimdal A., Jamal F., et al. Regional strain and strain rate measurements by cardiac ultrasound: principles, implementation and limitations. Eur. J. Echocardiogr. 2000;1(3):154–170. doi: 10.1053/euje.2000.0031. [DOI] [PubMed] [Google Scholar]
- 7.Leung D.Y., Edin F., Ng A.C. Emerging Clinical Role of Strain Imaging in Echocardiography. Heart Lung Circ. 2010;19:161–174. doi: 10.1016/j.hlc.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Hristov N., Liakopoulos O., Trummer G., Buckberg G.D. Septal structure and function relationships parallel the left ventricular free wall ascending and descending segments of the helical heart. Eur. J. Cardiothorac. Surg. 2006;29S:S115–S125. doi: 10.1016/j.ejcts.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 9.Torrent-Guasp F., Ballester M., Buckberg G.D., et al. Spatial orientation of the ventricular muscle band: physiologic contribution and surgical implications. J. Thorac. Cardiovasc. Surg. 2001;122:389–392. doi: 10.1067/mtc.2001.113745. [DOI] [PubMed] [Google Scholar]
- 10.Buckberg G.D., Clemente C., Cox J.L., et al. The structure and function of the helical heart and its buttress wrapping. IV. Concepts of dynamic function from the normal macroscopic helical structure. Semin. Thorac. Cardiovasc. Surg. 2001;13:342–357. doi: 10.1053/stcs.2001.29956. [DOI] [PubMed] [Google Scholar]
- 11.Motoki H., Koyama J., Nakazawa H., et al. Torsion analysis in the early detection of anthracycline-mediated cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging. 2012;13:95–103. doi: 10.1093/ejechocard/jer172. [DOI] [PubMed] [Google Scholar]
- 12.Urheim S., Edvardsen T., Torp H., et al. Myocardial strain by Doppler echocardiography validation of a new method to quantify regional myocardial function. Circulation. 2000;102(10):1158–1164. doi: 10.1161/01.cir.102.10.1158. [DOI] [PubMed] [Google Scholar]
- 13.Aurigemma G.P., Douglas P.S., Gaasch H.W. Quantitative evaluation of left ventricular structure, wall stress and systolic function. In: Otto C.M., editor. The practice of clinical echocardiography. Philadelphia: WB Saunders Company; 2002. pp. 65–87. [Google Scholar]
- 14.Stoylen A., Heimdal A., Bjornstad K., et al. Strain rate imaging by ultrasound in the diagnosis of regional dysfunction of the left ventricle. Echocardiography. 1999;16(4):321–329. doi: 10.1111/j.1540-8175.1999.tb00821.x. [DOI] [PubMed] [Google Scholar]
- 15.Alam M., Wardell J., Andersson E., et al. Characteristics of mitral and tricuspid annular velocities determined by pulsed wave Doppler tissue imaging in healthy subjects. J. Am. Soc. Echocardiogr. 1999;12:618–628. doi: 10.1053/je.1999.v12.a99246. [DOI] [PubMed] [Google Scholar]
- 16.Meluzin J., Spiranov’a L., Bakala J., et al. Pulsed Doppler tissue imaging of the velocity of tricuspid annular systolic motion. A new rapid and non-invasive method of evaluation right ventricular systolic function. Eur. Heart J. 2001;22:340–348. doi: 10.1053/euhj.2000.2296. [DOI] [PubMed] [Google Scholar]
- 17.Gulati V.K., Katz W.E., Follansbee W.P., et al. Mitral annular descent velocity by tissue Doppler echocardiography as an index of global left ventricular function. Am. J. Cardiol. 1996;77:979–984. doi: 10.1016/s0002-9149(96)00033-1. [DOI] [PubMed] [Google Scholar]
- 18.Saxena N., Rajagopalan N., Edelman K., López-Candales A. Tricuspid annular systolic velocity: A useful measurement in determining right ventricular systolic function regardless of pulmonary artery pressures. Echocardiography. 2006;23:750–755. doi: 10.1111/j.1540-8175.2006.00305.x. [DOI] [PubMed] [Google Scholar]
- 19.Biering-Sørensen T., Mogelvang R., Jensen J.S. Prognostic value of cardiac time intervals measured by tissue Doppler imaging M-mode in the general population. Heart. 2015;101(12):954–960. doi: 10.1136/heartjnl-2014-307137. [DOI] [PubMed] [Google Scholar]
- 20.Cevik A., Kula S., Olgunturk R., et al. Doppler Tissue Imaging Provides an Estimate of Pulmonary Arterial Pressure in Children with Pulmonary Hypertension Due to Congenital Intracardiac Shunts. Congenit. Heart Dis. 2013;8(6):527–534. doi: 10.1111/chd.12030. [DOI] [PubMed] [Google Scholar]
- 21.Sugimoto K., Fujii Y., Sunahara H., Aoki T. Assessment of left ventricular longitudinal function in cats with subclinical hypertrophic cardiomyopathy using tissue Doppler imaging and speckle tracking echocardiography. J. Vet. Med. Sci. 2015;77(9):1101–1108. doi: 10.1292/jvms.14-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dandel M., Lehmkuhl H., Knosalla C., Suramelashvili N., Hetzer R. Strain and Strain Rate Imaging by Echocardiography-Basic Concepts and Clinical Applicability. Curr. Cardiol. Rev. 2009;•••:133–148. doi: 10.2174/157340309788166642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leitman M., Lysyansky P., Sidenko S., et al. Two-dimensional strain – a novel software for real-time quantitative echocardiographic assessment of myocardial function. J. Am. Soc. Echocardiogr. 2004;17:1021–1029. doi: 10.1016/j.echo.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 24.Argyle R.A., Ray S.G. Stress and strain: double trouble or useful tool? Eur. J. Echocardiogr. 2009;10(6):716–722. doi: 10.1093/ejechocard/jep066. [DOI] [PubMed] [Google Scholar]
- 25.Mele D., Rizzo P., Pollina A.V., Fiorencis A., Ferrari R. Cancer Therapy-Induced Cardiotoxicity: Role of Ultrasound Deformation Imaging as an Aid to Early Diagnosis. Ultrasound Med. Biol. 2015;41(3):627–643. doi: 10.1016/j.ultrasmedbio.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 26.Seo Y., Ishizu T., Atsumi A., Kawamura R., Aonuma K. Three-Dimensional Speckle Tracking Echocardiography. Circ. J. 2014;78(6):1290–1301. doi: 10.1253/circj.cj-14-0360. [DOI] [PubMed] [Google Scholar]
- 27.Seo Y., Ishizu T., Enomoto Y., et al. Validation of 3-dimensional speckle tracking imaging to quantify regional myocardial deformation. Circ Cardiovasc Imaging. 2009;2:451–459. doi: 10.1161/CIRCIMAGING.109.858480. [DOI] [PubMed] [Google Scholar]
- 28.Gayat E., Ahmad H., Weinert L., Lang R.M., Mor-Avi V. Reproducibility and inter-vendor variability of left ventricular deformation measurements by three-dimensional speckle-tracking echocardiography. J. Am. Soc. Echocardiogr. 2011;24:878–885. doi: 10.1016/j.echo.2011.04.016. [DOI] [PubMed] [Google Scholar]
- 29.Cleland J.G., Daubert J.C., Erdmann E., et al. Cardiac Resynchronization-Heart Failure (CARE-HF) Study Investigators The effect of cardiac re- synchronization on morbidity and mortality in heart failure. N. Engl. J. Med. 2005;352:1539–1549. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 30.Bristow M.R., Saxon L.A., Boehmer J., et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N. Engl. J. Med. 2004;350:2140–2150. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 31.Epstein A.E., DiMarco J.P., Ellenbogen K.A., et al. American college of cardiology/american heart association task force on practice guidelines (writing committee to revise the ACC/AHA/NASPE 2002 guideline update for implantation of cardiac pacemakers and antiarrhythmia devices); american association for thoracic surgery; society of thoracic surgeons. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the american college of cardiology/american heart association task force on practice guidelines (writing committee to revise the ACC/AHA/NASPE 2002 guideline update for implantation of cardiac pacemakers and antiarrhythmia devices): developed in collaboration with the american association for thoracic surgery and society of thoracic surgeons. Circulation. 2008;117:e350–e408. doi: 10.1161/CIRCUALTIONAHA.108.189742. [DOI] [PubMed] [Google Scholar]
- 32.Hunt S.A., Abraham W.T., Chin M.H. 2009 Focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults. A report of the american college of cardiology foundation/ american heart association task force on practice guidelines developed in collaboration with the international society for heart and lung transplantation. J. Am. Coll. Cardiol. 2009;53:e1–e90. doi: 10.1016/j.jacc.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 33.Dickstein K., Vardas P.E., Auricchio A. 2010 Focused Update of ESC Guidelines on device therapy in heart failure: an update of the 2008 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure and the 2007 ESC guidelines for cardiac and resynchronization therapy. Developed with the special contribution of the Heart Failure Association and the European Heart Rhythm Association. Eur. Heart J. 2010;31:2677–2687. doi: 10.1093/eurheartj/ehq337. [DOI] [PubMed] [Google Scholar]
- 34.Hasselberg N., Haugaa K., Edvardsen T., et al. Left ventricular markers of mortality and ventricular arrhythmias in heart failure patients with cardiac resynchronization therapy. Eur. Heart J. Cardiovasc. Imaging. 2016;17(3):343–350. doi: 10.1093/ehjci/jev173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suffoletto M.S., Dohi K., Cannesson M., Saba S., Gorcsan J., III Novel speckle-tracking radial strain from routine black-and-white echocardiographic images to quantify dyssynchrony and predict response to cardiac resynchronization therapy. Circulation. 2006;113:960–968. doi: 10.1161/CIRCULATIONAHA.105.571455. [DOI] [PubMed] [Google Scholar]
- 36.Bank A.J., Kaufman C.L., Kelly A.S., et al. PROMISE-CRT investigators Results of the prospective minnesota study of ECHO/TDI in cardiac resynchronization therapy (PROMISE- CRT) study. J. Card. Fail. 2009;15:401–409. doi: 10.1016/j.cardfail.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 37.Gorcsan J., III, Tanabe M., Bleeker G.B., et al. Combined longitudinal and radial dyssynchrony pre- dicts ventricular response after resynchronization therapy. J. Am. Coll. Cardiol. 2007;50:1476–1483. doi: 10.1016/j.jacc.2007.06.043. [DOI] [PubMed] [Google Scholar]
- 38.Tanaka H., Nesser H.J., Buck T., et al. Dyssynchrony by speckle-tracking echocardiography and response to cardiac resynchronization therapy: results of the Speckle Tracking and Resynchronization (STAR) study. Eur. Heart J. 2010;31:1690–1700. doi: 10.1093/eurheartj/ehq213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gürel E., Tigen K., Karaahmet T., Dündar C., Güler A., Başaran Y. Apical transverse motion is associated with speckle-tracking radial dyssynchrony in patients with non-ischemic dilated cardiomyopathy. Anatol J Cardiol. 2015;15(8):620–625. doi: 10.5152/akd.2014.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang X., Ha S., Wang X., Shi Y., Duan S., Li Z. Speckle tracking echocardiography: clinical applications in cardiac resynchronization therapy. Int. J. Clin. Exp. Med. 2015;8(5):6668–6676. [PMC free article] [PubMed] [Google Scholar]
- 41.Becker M., Kramann R., Franke A., et al. Impact of left ventricular lead position in cardiac resynchronization therapy on left ventricular remodelling. A circumferential strain analysis based on 2D echocardiography. Eur. Heart J. 2007;28:1211–1220. doi: 10.1093/eurheartj/ehm034. [DOI] [PubMed] [Google Scholar]
- 42.Khan F.Z., Virdee M.S., Palmer C.R., et al. Targeted left ventricular lead placement to guide cardiac resynchronization therapy: the TARGET study: a randomized, controlled trial. J. Am. Coll. Cardiol. 2012;59:1509–1518. doi: 10.1016/j.jacc.2011.12.030. [DOI] [PubMed] [Google Scholar]
- 43.Cai Q., Ahmad M. Left Ventricular Dyssynchrony by Three-Dimensional Echocardiography: Current Understanding and Potential Future Clinical Applications. Echocardiography. 2015;32(8):1299–1306. doi: 10.1111/echo.12965. [DOI] [PubMed] [Google Scholar]
- 44.Auger D., Bertini M., Marsan N.A., et al. Prediction of response to cardiac resynchronization therapy combining two different three-dimensional analyses of left ventricular dyssynchrony. Am. J. Cardiol. 2011;108:711–717. doi: 10.1016/j.amjcard.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 45.Straka F., Pirk J., Pindak M., et al. A pilot study of systolic dyssynchrony index by real time three-dimensional echocardiography and Doppler tissue imaging parameters predicting the hemodynamic response to biventricular pacing in the early postoperative period after cardiac surgery. Echocardiography. 2012;29:827–839. doi: 10.1111/j.1540-8175.2012.01694.x. [DOI] [PubMed] [Google Scholar]
- 46.Maron B.J. Structural features of the athlete heart as defined by echocardiography. J. Am. Coll. Cardiol. 1986;7(1):190–203. doi: 10.1016/s0735-1097(86)80282-0. [DOI] [PubMed] [Google Scholar]
- 47.Butz T., van Buuren F., Faber L., et al. Two-dimensional strain analysis of the global and regional myocardial function for the differentiation of pathologic and physiologic left ventricular hypertrophy: a study in athletes and in patients with hypertrophic cardiomyopathy. Int. J. Cardiovasc. Imaging. 2011;27(1):91–100. doi: 10.1007/s10554-010-9665-5. [DOI] [PubMed] [Google Scholar]
- 48.Demirelli S., Sam C., Simsek Z., et al. Long-Term Cardiac Remodeling in Elite Athletes: Assessment by Tissue Doppler and Speckle Tracking Echocardiography. Echocardiography. 2015;32(9):1367–1373. doi: 10.1111/echo.12860. [DOI] [PubMed] [Google Scholar]
- 49.Simsek Z., Hakan Tas M., Senocak H., et al. Speckle Tracking Echocardiographic Analysis of Left Ventricular Systolic and Diastolic Functions of Young Elite Athletes with Eccentric and Concentric Type of Cardiac Remodeling. Echocardiography. 2013;30(10):1202–1208. doi: 10.1111/echo.12263. [DOI] [PubMed] [Google Scholar]
- 50.Simsek Z., Tas M., Gunay E., Degirmenci H. Speckle-tracking echocardiographic imaging of the right ventricular systolic and diastolic parameters in chronic exercise. Int. J. Cardiovasc. Imaging. 2013;29(6):1265–1271. doi: 10.1007/s10554-013-0204-z. [DOI] [PubMed] [Google Scholar]
- 51.D’Ascenzi F., Pelliccia A., Mondillo S., et al. Morphological and functional adaptation of left and right atria induced by training in highly trained female athletes. Circ Cardiovasc Imaging. 2014;7(2):222–229. doi: 10.1161/CIRCIMAGING.113.001345. [DOI] [PubMed] [Google Scholar]
- 52.Stefani L., De Luca A., Toncelli L., Pedrizzetti G., Galanti G. 3D Strain helps relating LV function to LV and structure in athletes. Cardiovasc. Ultrasound. 2014;12(1):1–8. doi: 10.1186/1476-7120-12-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arias M., Rush D.N., Wiebe C., et al. Antibody-mediated rejection: analyzing the risk, proposing solutions. Transplantation. 2014;98:3–21. doi: 10.1097/TP.0000000000000218. [DOI] [PubMed] [Google Scholar]
- 54.Sehgal S., Blake J., Sommerfield J., Aggarwal S. Strain and strain rate imaging using speckle tracking in acute allograft rejection in children with heart transplantation. Pediatr. Transplant. 2015;19(2):188–195. doi: 10.1111/petr.12415. [DOI] [PubMed] [Google Scholar]
- 55.Pieper G., Shah A., Harmann L., Cooley B., Ionova I., Migrino R. Speckle-tracking 2-dimensional strain echocardiography: a new noninvasive imaging tool to evaluate acute rejection in cardiac transplantation. J. Heart Lung Transplant. 2010;29(9):1039–1046. doi: 10.1016/j.healun.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buddhe S., Richmond M., Gilbreth J., Lai W. Longitudinal Strain by Speckle Tracking Echocardiography in Pediatric Heart Transplant Recipients. Congenit. Heart Dis. 2015;10(4):362–370. doi: 10.1111/chd.12263. [DOI] [PubMed] [Google Scholar]
- 57.Clemmensen T., Løgstrup B., Eiskjær H., Poulsen S. Changes in Longitudinal Myocardial Deformation during Acute Cardiac Rejection: The Clinical Role of Two-Dimensional Speckle-Tracking Echocardiography. J. Am. Soc. Echocardiogr. 2015;28(3):330–339. doi: 10.1016/j.echo.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 58.Sato T., Kato T., Kitakaze M., et al. Utility of left ventricular systolic torsion derived from 2-dimensional speckle-tracking echocardiography in monitoring acute cellular rejection in heart transplant recipients. J. Heart Lung Transplant. 2011;30(5):536–543. doi: 10.1016/j.healun.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 59.Zengin E., Westermann D., Sinning C., et al. Cardiac mechanics in heart transplant recipients with and without transplant vasculopathy. Int. J. Cardiovasc. Imaging. 2015;31(4):795–803. doi: 10.1007/s10554-015-0625-y. [DOI] [PubMed] [Google Scholar]
- 60.Hooning M.J., Botma A., Aleman B.M., et al. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J. Natl. Cancer Inst. 2007;99(5):365–375. doi: 10.1093/jnci/djk064. [DOI] [PubMed] [Google Scholar]
- 61.Cardinale D., Colombo A., Torrisi R., et al. Trastuzumab-induced cardiotoxicity: clinical and prognostic implications of troponin I evaluation. J. Clin. Oncol. 2010;28:3910–3916. doi: 10.1200/JCO.2009.27.3615. [DOI] [PubMed] [Google Scholar]
- 62.Wadhwa D., Fallah-Rad N., Grenier D., et al. Trastuzumab mediated cardiotoxicity in the setting of adjuvant chemotherapy for breast cancer: a retrospective study. Breast Cancer Res. Treat. 2009;117:357–364. doi: 10.1007/s10549-008-0260-6. [DOI] [PubMed] [Google Scholar]
- 63.Geyer H., Caracciolo G., Abe H., et al. Assessment of myocardial mechanics using speckle tracking echocardiography: fundamentals and clinical applications. J. Am. Soc. Echocardiogr. 2010;23:351–369. doi: 10.1016/j.echo.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 64.Thavendiranathan P., Poulin F., Lim K.D., Plana J.C., Woo A., Marwick T.H. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J. Am. Coll. Cardiol. 2014;63:2751–2768. doi: 10.1016/j.jacc.2014.01.073. [DOI] [PubMed] [Google Scholar]
- 65.Rhea I.B., Uppuluri S., Sawada S., Schneider B.P., Feigenbaum H. Incremental prognostic value of echocardiographic strain and its association with mortality in cancer patients. J. Am. Soc. Echocardiogr. 2015;28(6):667–673. doi: 10.1016/j.echo.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 66.Florescu M., Magda L.S., Enescu O.A., Jinga D., Vinereanu D. Early detection of epirubicin-induced cardiotoxicity in patients with breast cancer. J. Am. Soc. Echocardiogr. 2014;27(1):83–92. doi: 10.1016/j.echo.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 67.Armstrong G.T., Joshi V.M., Ness K.K., et al. Comprehensive echocardiographic detection of treatment-related cardiac dysfunction in adult survivors of childhood cancer: results from the St. Jude lifetime cohort study. J. Am. Coll. Cardiol. 2015;65:2511–2522. doi: 10.1016/j.jacc.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yeh E., Vejpongsa P. Subclinical Cardiotoxicity Associated With Cancer Therapy: Early Detection and Future Directions. J. Am. Coll. Cardiol. 2015;65(23):2523–2525. doi: 10.1016/j.jacc.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 69.Richardson P., McKenna W., Bristow M., et al. Report of the 1995 world health organization/international society and federation of cardiology task force on the definition and classification of cardiomyopathies. Circulation. 1996;93(5):841–842. doi: 10.1161/01.cir.93.5.841. [DOI] [PubMed] [Google Scholar]
- 70.Thierfelder L., Watkins H., MacRae C., et al. Alpha-tropomyosin and cardiac troponin T mutations cause familial hypertrophic cardiomyopathy: a disease of the sarcomere. Cell. 1994;77:701–712. doi: 10.1016/0092-8674(94)90054-x. [DOI] [PubMed] [Google Scholar]
- 71.Watkins H., Ashrafian H., Redwood C. Inherited Cardiomyopathies. N. Engl. J. Med. 2011;364:1643–1656. doi: 10.1056/NEJMra0902923. [DOI] [PubMed] [Google Scholar]
- 72.Richard P., Charron P., Carrier L., et al. Hypertrophic cardiomyopathy: distribution of disease genes, spectrum of mutations, and implications for a molecular diagnosis strategy. Circulation. 2003;107:2227–2232. doi: 10.1161/01.CIR.0000066323.15244.54. [DOI] [PubMed] [Google Scholar]
- 73.Maron M.S., Olivotto I., Zenovich A.G., et al. Hypertrophic cardiomyopathy is predominantly a disease of left ventricular outflow tract obstruction. Circulation. 2006;114:2232–2239. doi: 10.1161/CIRCULATIONAHA.106.644682. [DOI] [PubMed] [Google Scholar]
- 74.Maron M.S., Olivotto I., Betocchi S., et al. Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy. N. Engl. J. Med. 2003;348:295–303. doi: 10.1056/NEJMoa021332. [DOI] [PubMed] [Google Scholar]
- 75.Elliott P.M., Gimeno J.R., Tome M.T., et al. Left ventricular outflow tract obstruction and sudden death risk in patients with hypertrophic cardiomyopathy. Eur. Heart J. 2006;27:1933–1941. doi: 10.1093/eurheartj/ehl041. [DOI] [PubMed] [Google Scholar]
- 76.O’Mahony C., Jichi F., Pavlou M., et al. A novel clinical risk prediction model for sudden cardiac death in hypertrophic cardiomyopathy (HCM Risk-SCD). Eur. Heart J. 2014;35:2010–2020. doi: 10.1093/eurheartj/eht439. [DOI] [PubMed] [Google Scholar]
- 77.Kato T.S., Izawa H., Komamura K., et al. Heterogeneity of regional systolic function detected by tissue Doppler imaging is linked to impaired global left ventricular relaxation in hypertrophic cardiomyopathy. Heart. 2008;94:1302–1306. doi: 10.1136/hrt.2007.124453. [DOI] [PubMed] [Google Scholar]
- 78.Shetty R., Samanth J., Nayak K., Sarang A., Thakkar A. Evaluation of Subtle Left Ventricular Systolic Abnormalities in Adult Patients with Hypertrophic Cardiomyopathy. J. Clin. Diagn. Res. 2014;8(12):5–9. doi: 10.7860/JCDR/2014/10185.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ozawa K., Funabashi N., Kobayashi Y., et al. Characteristic myocardial strain identified in hypertrophic cardiomyopathy subjects with preserved left ventricular ejection fraction using a novel multi-layer transthoracic echocardiography technique. Int. J. Cardiol. 2015;184:237–243. doi: 10.1016/j.ijcard.2015.01.070. [DOI] [PubMed] [Google Scholar]
- 80.Okada K., Yamada S., Tsutsui H., et al. Myocardial Shortening in 3 Orthogonal Directions and Its Transmural Variation in Patients With Nonobstructive Hypertrophic Cardiomyopathy. Circ. J. 2015;79(11):2471–2479. doi: 10.1253/circj.CJ-15-0646. [DOI] [PubMed] [Google Scholar]
- 81.Hartlage G., Kim J., Williams B., et al. The prognostic value of standardized reference values for speckle-tracking global longitudinal strain in hypertrophic cardiomyopathy. Int. J. Cardiovasc. Imaging. 2015;31(3):557–565. doi: 10.1007/s10554-015-0590-5. [DOI] [PubMed] [Google Scholar]
- 82.Aly M.F., Brouwer W.P., Kleijn S.A., van Rossum A.C., Kamp O. Three-dimensional speckle tracking echocardiography for the preclinical diagnosis of hypertrophic cardiomyopathy. Int. J. Cardiovasc. Imaging. 2014;30(3):523–533. doi: 10.1007/s10554-014-0364-5. [DOI] [PubMed] [Google Scholar]
- 83.Zhang H., Wang H., Lin Q., et al. Assessment of left ventricular twist mechanics by speckle tracking echocardiography reveals association between LV twist and myocardial fibrosis in patients with hypertrophic cardiomyopathy. Int. J. Cardiovasc. Imaging. 2014;30(8):1539–1548. doi: 10.1007/s10554-014-0509-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Merlini G., Bellotti V. Molecular mechanisms of amyloidosis. N. Engl. J. Med. 2003;349:583–596. doi: 10.1056/NEJMra023144. [DOI] [PubMed] [Google Scholar]
- 85.Sipe J.D., Cohen A.S. Review: history of the amyloid fibril. J. Struct. Biol. 2000;130:88–98. doi: 10.1006/jsbi.2000.4221. [DOI] [PubMed] [Google Scholar]
- 86.Guan J., Mishra S., Falk R.H., Liao R. Current perspectives on cardiac amyloidosis. Am. J. Physiol. Heart Circ. Physiol. 2012;302(3):H544–H552. doi: 10.1152/ajpheart.00815.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sipe J.D., Benson M.D., Buxbaum J.N., et al. Amyloid fibril protein nomenclature: 2010 recommendations from the nomenclature committee of the International Society of Amyloidosis. Amyloid. 2011;17:101–104. doi: 10.3109/13506129.2010.526812. [DOI] [PubMed] [Google Scholar]
- 88.Falk R.H., Dubrey S.W. Amyloid heart disease. Prog. Cardiovasc. Dis. 2010;52:347–361. doi: 10.1016/j.pcad.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 89.Kristen A.V., Perz J.B., Schonland S.O., et al. Non-invasive predictors of survival in cardiac amyloidosis. Eur. J. Heart Fail. 2007;9:617–624. doi: 10.1016/j.ejheart.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 90.Tsang W., Lang R.M. Echocardiographic evaluation of cardiac amyloid. Curr. Cardiol. Rep. 2010;12:272–276. doi: 10.1007/s11886-010-0108-7. [DOI] [PubMed] [Google Scholar]
- 91.Sun J.P., Stewart W.J., Yang X.S., et al. Differentiation of hypertrophic cardiomyopathy and cardiac amyloidosis from other causes of ventricular wall thickening by two-dimensional strain imaging echocardiography. Am. J. Cardiol. 2009;103:411–415. doi: 10.1016/j.amjcard.2008.09.102. [DOI] [PubMed] [Google Scholar]
- 92.Baccouche H., Maunz M., Beyer M., et al. Differentiating Cardiac Amyloidosis and Hypertrophic Cardiomyopathy by Use of Three-Dimensional Speckle Tracking Echocardiography. Echocardiography. 2012;29(6):668–677. doi: 10.1111/j.1540-8175.2012.01680.x. [DOI] [PubMed] [Google Scholar]
- 93.Phelan D., Thavendieanathan P., Popovic Z., et al. Application of parametric display of two-dimensional speckle-tracking longitudinal strain to improve the etiologic diagnosis of mild to moderate left ventricular hypertrophy. J. Am. Soc. Echocardiogr. 2014;27:888–895. doi: 10.1016/j.echo.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 94.Phelan D., Collier P., Thavendiranathan P., et al. Relative apical sparing of longitudinal strain using two-dimensional speckle-tracking echocardiography is both sensitive and specific for the diagnosis of cardiac amyloidosis. Heart. 2012;98(19):1442–1448. doi: 10.1136/heartjnl-2012-302353. [DOI] [PubMed] [Google Scholar]
- 95.Liu D., Hu K., Weidemann F., et al. Predictive Value of Assessing Diastolic Strain Rate on Survival in Cardiac Amyloidosis Patients with Preserved Ejection Fraction. PLoS One. 2014;9(12):1–17. doi: 10.1371/journal.pone.0115910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Buss S.J., Emami M., Mereles D., et al. Longitudinal left ventricular function for prediction of survival in systemic light-chain amyloidosis: Incremental value compared with clinical biochemical markers. J. Am. Coll. Cardiol. 2012;60:1067–1076. doi: 10.1016/j.jacc.2012.04.043. [DOI] [PubMed] [Google Scholar]
- 97.Fahy G.J., Marwick T., McCreery C.J., Quigley P.J., Maurer B.J. Doppler echocardiographic detection of left ventricular diastolic dysfunction in patients with pulmonary sarcoidosis. Chest. 1996;109(1):62–66. doi: 10.1378/chest.109.1.62. [DOI] [PubMed] [Google Scholar]
- 98.Burstow D.J., Tajik A.J., Bailey K.R., DeRemee R.A., Taliercio C.P. Two-dimensional echocardiographic findings in systemic sarcoidosis. Am. J. Cardiol. 1989;63(7):478–482. doi: 10.1016/0002-9149(89)90323-8. [DOI] [PubMed] [Google Scholar]
- 99.Tellier P., Paycha F., Antony I., et al. Reversibility by dipyridamole of thallium-201 myocardial scan defects in patients with sarcoidosis. Am. J. Med. 1988;85(2):189–193. doi: 10.1016/s0002-9343(88)80340-1. [DOI] [PubMed] [Google Scholar]
- 100.Bulkley B.H., Rouleau J.R., Whitaker J.Q., Strauss H.W., Pitt B. The use of 201thallium for myocardial perfusion imaging in sarcoid heart disease. Chest. 1977;72(1):27–32. doi: 10.1378/chest.72.1.27. [DOI] [PubMed] [Google Scholar]
- 101.Perry A., Vuitch F. Causes of death in patients with sarcoidosis. A morphologic study of 38 autopsies with clinicopathologic correlations. Arch. Pathol. Lab. Med. 1995;119(2):167–172. [PubMed] [Google Scholar]
- 102.Roberts W.C., McAllister H.A., Jr, Ferrans V.J. Sarcoidosis of the heart. A clinicopathologic study of 35 necropsy patients (group 1) and review of 78 previously described necropsy patients (group 11). Am. J. Med. 1977;63(1):86–108. doi: 10.1016/0002-9343(77)90121-8. [DOI] [PubMed] [Google Scholar]
- 103.Silverman K.J., Hutchins G.M., Bulkley B.H. Cardiac sarcoid: a clinicopathologic study of 84 unselected patients with systemic sarcoidosis. Circulation. 1978;58(6):1204–1211. doi: 10.1161/01.cir.58.6.1204. [DOI] [PubMed] [Google Scholar]
- 104.Bagwan I.N., Hooper L.V., Sheppard M.N. Cardiac sarcoidosis and sudden death. The heart may look normal or mimic other cardiomyopathies. Virchows Arch. 2011;458(6):671–678. doi: 10.1007/s00428-010-1003-8. [DOI] [PubMed] [Google Scholar]
- 105.Hamzeh N.Y., Wamboldt F.S., Weinberger H.D. Management of cardiac sarcoidosis in the United States: a Delphi study. Chest. 2012;141(1):154–162. doi: 10.1378/chest.11-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lynch J.P., Hwang J., Bradfield J., Fishbein M., Shivkumar K., Tung R. Cardiac Involvement in Sarcoidosis: Evolving Concepts in Diagnosis and Treatment. Semin. Respir. Crit. Care Med. 2014;35(3):372–390. doi: 10.1055/s-0034-1376889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yazaki Y., Isobe M., Hiramitsu S., et al. Comparison of clinical features and prognosis of cardiac sarcoidosis and idiopathic dilated cardiomyopathy. Am. J. Cardiol. 1998;82(4):537–540. doi: 10.1016/s0002-9149(98)00377-4. [DOI] [PubMed] [Google Scholar]
- 108.Sekiguchi M., Yazaki Y., Isobe M., Hiroe M. Cardiac sarcoidosis: diagnostic, prognostic, and therapeutic considerations. Cardiovasc. Drugs Ther. 1996;10(5):495–510. doi: 10.1007/BF00050989. [DOI] [PubMed] [Google Scholar]
- 109.Matsui Y., Iwai K., Tachibana T., et al. Clinicopathological study of fatal myocardial sarcoidosis. Ann. N. Y. Acad. Sci. 1976;278:455–469. doi: 10.1111/j.1749-6632.1976.tb47058.x. [DOI] [PubMed] [Google Scholar]
- 110.Iwai K., Sekiguti M., Hosoda Y., et al. Racial difference in cardiac sarcoidosis incidence observed at autopsy. Sarcoidosis. 1994;11(1):26–31. [PubMed] [Google Scholar]
- 111.Sekiguchi M., Numao Y., Imai M., Furuie T., Mikami R. Clinical and histopathological profile of sarcoidosis of the heart and acute idiopathic myocarditis. Concepts through a study employing endomyocardial biopsy. I. Sarcoidosis. Jpn. Circ. J. 1980;44(4):249–263. doi: 10.1253/jcj.44.249. [DOI] [PubMed] [Google Scholar]
- 112.Tachibana T., Ohmori F., Ueda E. Clinical study on cardiac sarcoidosis. Ann. N. Y. Acad. Sci. 1986;465:530–542. doi: 10.1111/j.1749-6632.1986.tb18530.x. [DOI] [PubMed] [Google Scholar]
- 113.Gideon N.M., Mannino D.M. Sarcoidosis mortality in the United States 1979-1991: an analysis of multiple-cause mortality data. Am. J. Med. 1996;100(4):423–427. doi: 10.1016/S0002-9343(97)89518-6. [DOI] [PubMed] [Google Scholar]
- 114.Joyce E., Ninaber M., Ajmone Marsan N., et al. Subclinical left ventricular dysfunction by echocardiographic speckle-tracking strain analysis relates to outcome in sarcoidosis. Eur. J. Heart Fail. 2015;17(1):51–62. doi: 10.1002/ejhf.205. [DOI] [PubMed] [Google Scholar]
- 115.Chiu C.Z., Nakatani S., Zhang G., et al. Prevention of left ventricular remodeling by long-term corticosteroid therapy in patients with cardiac sarcoidosis. Am. J. Cardiol. 2005;95(1):143–146. doi: 10.1016/j.amjcard.2004.08.083. [DOI] [PubMed] [Google Scholar]
- 116.Tigen K., Sunbul M., Akkaya E., et al. Early detection of bi-ventricular and atrial mechanical dysfunction using two-dimensional speckle tracking echocardiography in patients with sarcoidosis. Lung. 2015;193(5):669–675. doi: 10.1007/s00408-015-9748-0. [DOI] [PubMed] [Google Scholar]
- 117.Aggeli C., Felekos I., Tousoulis D., Gialafos E., Rapti A., Stefanadis C. Myocardial mechanics for the early detection of cardiac sarcoidosis. Int. J. Cardiol. 2013;168(5):4820–4821. doi: 10.1016/j.ijcard.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 118.Tsuji T., Tanaka H., Hirata K., et al. Capability of three-dimensional speckle tracking radial strain for identification of patients with cardiac sarcoidosis. Int. J. Cardiovasc. Imaging. 2013;29(2):317–324. doi: 10.1007/s10554-012-0104-7. [DOI] [PubMed] [Google Scholar]