Abstract
Background:
Endotoxin is a lipopolysaccharide (LPS) constituent of the outer membrane of most gram negative bacteria. Ubiquitous in the environment, it has been implicated as a cause or con-tributing factor in several disparate disorders from sepsis to heatstroke and Type II diabetes mellitus. Starting at birth, the innate immune system develops cellular defense mechanisms against environmen-tal microbes that are in part modulated through a series of receptors known as toll-like receptors. Endo-toxin, often referred to as LPS, binds to toll-like receptor 4 (TLR4)/ myeloid differentiation protein 2 (MD2) complexes on various tissues including cells of the innate immune system, smooth muscle and endothelial cells of blood vessels including coronary arteries, and adipose tissue. Entry of LPS into the systemic circulation ultimately leads to intracellular transcription of several inflammatory mediators. The subsequent inflammation has been implicated in the development and progression atherosclerosis and subsequent coronary artery disease and heart failure.
Objective:
The potential roles of endotoxin and TLR4 are reviewed regarding their role in the pathogen-esis of atherosclerotic heart disease.
Conclusion:
Atherosclerosis is initiated by inflammation in arterial endothelial and subendothelial cells, and inflammatory processes are implicated in its progression to clinical heart disease. Endotoxin and TLR4 play a central role in the inflammatory process, and represent potential targets for therapeutic intervention. Therapy with HMG-CoA inhibitors may reduce the expression of TLR4 on monocytes. Other therapeutic interventions targeting TLR4 expression or function may prove beneficial in athero-sclerotic disease prevention and treatment.
Keywords: Endotoxin, lipopolysaccharide, atherosclerosis, toll-like receptor 4, coronary heart disease, heart failure
1. INTRODUCTION
Atherosclerosis, coronary heart disease, and subsequent chronic heart failure have been associated with many risk factors, including age, gender, dyslipidemia, hypertension, smoking, family history, diabetes mellitus, obesity, physical inactivity, and metabolic syndrome. The multifactorial nature of atherosclerotic disease has led to an increasing global burden of prevalence and expense of treatment [1]. The role of inflammation in the development of atherosclerotic disease has been associated with many of these risk factors [2-4]. The immune system may play a central role in modulating atherogenesis along with cholesterol [5]. This review focuses on the possible roles that endotoxin and toll-like receptors may play in the initiation and progression of atherosclerotic heart disease (AHD) to chronic heart failure (CHF).
Models of disease pathogenesis involving microorganisms or their toxins often include the presumption that the host-microbe interaction is harmful. Yet, an evolutionary perspective suggests that a symbiotic or at least commensal relationship may be necessary. The innate host defense against potential pathogens is one example of this symbiosis, which provides for immediate recognition and response to pathogenic invasion. Endotoxin stimulation of the innate immune system is important in the ontogeny of the normal immune system of individuals [6]. Endotoxins are ubiquitous in the environment and are main constituents of organic dust [7]. Without the innate immunity, newborns would not survive. While pathogenic bacteria have evolved invasive properties, commensal intestinal bacteria aid the human host in digestion and vitamin synthesis [8]. The immune response does not require prior exposure to a pathogen, but rather is based on recognition of conserved molecular structures such as Gram-negative lipid A (lipopolysaccharide or LPS) of endotoxin. These structures have been called pathogen-associated molecular patterns (PAMPs), a subset of pattern recognition receptors (PRRs) which also include danger-associated molecular patterns (DAMPs). Bacterial or fungal infections may lead to a range of immune-mediated inflammatory responses from a self-limiting process to an overwhelming response including sepsis and multiple organ failure [9]. Endotoxin is the PAMP that initiates the inflammatory cascade in gram negative sepsis. Activation of toll-like receptors (TLRs) on cell membranes by PAMPs and DAMPs leads to an inflammatory response. For example, cardiac myocyte cell death following myocardial infarction engages the DAMP response to stimulate repair, but the inflammatory response may also extend the damage [10].
Coronary artery disease and hypertension account for most cases of chronic heart failure, yet the pathogeneses of atherosclerosis and hypertension are not completely understood, in part because many individuals who develop heart disease have normal blood pressure and cholesterol levels [11]. For a number of years, inflammation and infection were considered to have atherogenic effects. Interest was renewed in the 1970s and later when high levels of antibodies to Chlamydophila pneumoniae were found in persons with chronic stable coronary artery disease and acute myocardial infarction [12, 13]. Subsequent studies have implicated Helicobacter pylori, cytomegalovirus (CMV) and periodontal pathogens as well [14-16]. Endotoxin is elevated in severe periodontitis and macrophage activation occurs [17]. More recently, endotoxin has been suggested as a mediator of the inflammatory response in atheromas and in other diseases, particularly in regard to chronic low grade endotoxemia [18].
In 1997, Anker and associates hypothesized that in patients with chronic heart failure, mesenteric venous congestion leads to increased bowel permeability, bacterial translocation, endotoxin release from bacteria, and then immune system activation with increased production of inflammatory cytokines, particularly TNF-α, as well as increased soluble CD14, the cell membrane receptor for endotoxin [19].
Subsequently, a number of inflammatory factors have been found to be elevated in patients who have CHF including tumor necrosis factor α (TNF-α), interleukin-6 (IL-6) and other members of the interleukin family, acute phase reactants including C-reactive protein (CRP), intracellular adhesion molecule-1 (ICAM-1), procalcitonin, vascular-cell adhesion molecule-1 (VCAM-1, P-selectin, and lipopolysaccharide (LPS or endotoxin), as well as a host of other factors [20]. Whether some of these are biomarkers of disease progression or part of the pathophysiologic causative chain of events that leads to chronic inflammation and CHF remains to be completely elucidated. Of these inflammatory factors, TNF-α has been considered “the prototypic pro-inflam-matory cytokine” in CHF due to the large number of studies published since 1990 [20]. Large studies of TNF-α inhibitors in CHF patients showed neutral or negative results and dampened enthusiasm for the treatment of chronic inflammation in CHF [21]. Nonetheless, there are many other avenues that can be investigated. Endotoxin is of particular interest because it is one of the strongest inducers of TNF-α, procalcitonin, and other pro-inflammatory substances [20]. Although a number of other contributors to endothelial inflammation have been studied, the focus of this review is endotoxin.
1.1. Structural Characteristics of and Inflammatory Responses to Endotoxin
Endotoxin is a lipopolysaccharide (LPS) constituent of the outer membrane of most gram negative bacteria. Its basic chemical structure is best described as hydrophilic sugar moieties bound to a hydrophobic region known as lipid A. The overall composition is generally heterogeneous with some focal pattern uniformity among the genetically diverse bacterial species. The hydrophilic or carbohydrate region of LPS is composed of the inner and outer cores and the O-specific chain (O antigen). The larger O-specific chain is characterized by as many as 40 to 50 repeating oligosaccharide subunits and is species-specific. This is the most structurally diverse part of the molecule and may contain more than 60 monosaccharides and 30 different noncarbohydrate components. The more homogenous inner core contains unusual carbohydrate residues, such as 2-keto-3-desoxyoc-tulosonic acid (KDO) and heptose, which are not usually found in host cells. The outer core consists of branched oligosaccharides. Removal of the hydrophilic region has only a minimal effect on the inflammatory activity of LPS, demonstrating that the core and O-specific chain play little role in host immune cell recognition. The hydrophobic lipid A portion of the molecule contains a phosphorylated diglucosamine backbone with four to seven attached acyl residues. The lipid A region is structurally conserved among various gram-negative bacteria. This uniformity provides a readily available target for the innate immune system especially cells of the monocyte-macrophage lineage [22-25].
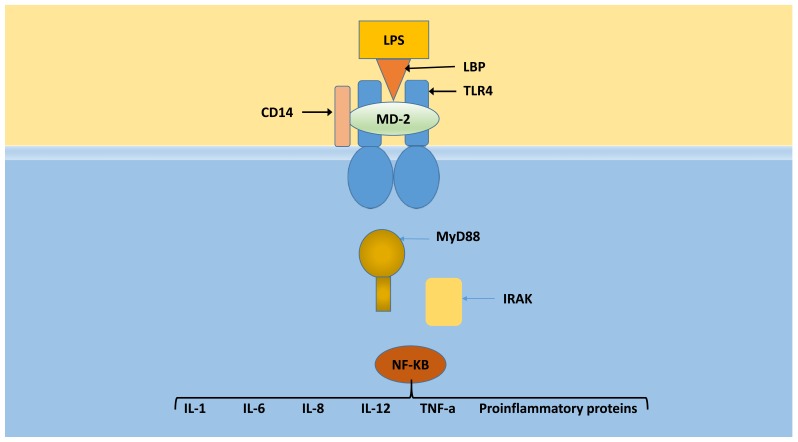
In the event that gut or oral endotoxin “leaks” or translocates into the systemic circulation, it generally binds to a carrier molecule: either lipopolysaccharide-binding protein (LBP), bactericidal/permeability-increasing protein (BPI), soluble CD14, or serum lipoproteins (HDL, VLDL) (See Fig. 1). LBP is believed to be responsible for extracting LPS from bacterial membranes and removing aggregations of free endotoxin in circulation [26]. As noted above, interaction with LBP enhances binding or transfer to membrane-bound CD14 receptors on the surface of innate immune cells of monocyte/macrophage lineage (including Kupffer cells of the liver) as well as smooth muscle and endothelial cells of blood vessels including coronary arteries. The interaction enables monocytes to respond to concentrations of LPS as low as 10 pg/mL [27]. CD14 receptors in turn, present endotoxin to the TLR4/MD2 complex (toll-like receptor-4/myeloid differentiation factor-2). The transmembrane domain of TLR4 transmits a signal that is facilitated by a series of adapter proteins and kinases such as myeloid differentiation factor (MyD88), IL-1 receptor-associated kinase (IRAK), tumor necrosis factor (TNF) receptor-associated factor 6 (TRAF6), NF-kB-inducing kinase (NIK), inhibitor kappa B (IқB), and ultimately to nuclear factor-kappa B (NF-қB) [28]. NF-қB binds to multiple gene promoter regions in the nucleus resulting in transcription of several hundred genes and synthesis of clotting elements, complement, other acute phase proteins, cytokines, chemokines and nitric oxide synthase. Among the proinflammatory cytokines/chemokines synthesized are interleukins (IL-1α, -1β, -6, -8, and -12) as well as TNF-α [9, 27, 29, 30]. Adipocytes are other cytokine producing cells that possess TLR4/MD2 receptor complexes but lack membrane-bound CD14 receptors. Binding of LPS to these complexes requires the assistance of soluble CD14 [31]. Park and Lee provided a detailed review of the structural basis of LPS recognition by TLR4 complexes [25]. It should be noted here that although TLR4-deficient cells lose all responses to LPS, recent studies have noted host responses that do not require TLR4 [32].
Fig. (1).
Overview of LPS recognition by TLR4-MD-2. LPS binding induces dimerization of the TLR4-MD-2 complex, whose intracellular domains are termed Toll/IL-1R homology (TIR) domains due to structural similarity to the interleukin-1 receptor (IL-1R) family. Dimerization results in recruitment of adapter proteins such as MyD88. Aggregation of the death domains (DD) of MyD88 stacks four IRAK4 and four IRAK2 molecules together into a structure called the “Myddosome.” (Source: 14).
NF-қB is the primary intracellular transcription factor and is up-regulated in leukocytes of CHF patients [33]. Similarly, TLR2 and TLR4 expression on circulating monocytes of CHF patients was found to be elevated compared with normal controls [34, 35]. TLR4-regulated TNF-α plasma levels increased in relation to deteriorating functional classes in the Studies of Left Ventricular Dysfunction (SOLVD) trials [36]. These clinical observations support the roles of LPS, NF-қB, and TLR4 in the pathogenesis of chronic heart failure with endotoxin being a putative upstream stimulus for subsequent inflammatory responses.
The “toxin” portion of endotoxin is primarily the lipid A constituent. A partial structure characterized by a disaccharide with two hexosamine residues (D-gluco-configured hexosamine), two phosphoryl groups, and six fatty acids is all that is necessary for optimal toxicologic activity in vitro and in vivo [24]. The hetero-polysaccharide component helps maintain hydrophilicity.
The “toxicity” of LPS is best described as the intense provocation of the immune system rather than any intrinsic toxic effect [24, 25]. Exogenous endotoxin in nanogram amounts can induce symptoms in humans similar to the septic shock syndrome [37]. Unfortunately, there is currently no diagnostic biomarker with high accuracy to predict or monitor sepsis in humans, and it is likewise difficult to assess a “dose-response” relationship between circulating endotoxin and biological response [38]. The distinction between “toxic” levels of endotoxin and “healthy” or immunogenic levels of endotoxin has not been established. Liposaccharide-binding protein has been proposed as a means of monitoring sepsis, but seems less useful than other acute-phase reactants [38].
1.2. Endotoxemia and Disease
Endotoxemia has been reported to at least partially contribute to the inflammatory manifestations of several heterogeneous pathologies including heat stroke, inflammatory bowel disease and other autoimmune disorders, severe pancreatitis, ethanol-induced liver disease, HIV infection, insulin resistance, obesity, diabetes mellitus, chronic heart failure, atherosclerosis, memory impairment, multiple trauma, hemorrhagic shock, burns, chronic kidney disease, and asthma exacerbation [26, 31, 39-53]. Circulating levels have also been found in patients with a ruptured abdominal aortic aneurysm [54]. Pain in sickle cell disease has been related to endotoxin elevations [55]. The severity of the response seems to be related to the amount of endotoxin absorbed as well as the sensitivity of the immune system and the presence of inhibitory factors; however, as noted earlier, accurate and reliable assays for LPS serum levels have not been developed. Environmental sources of LPS are believed to contribute to many asthma and COPD exacerbations and could also be involved in the development of atherosclerosis and Parkinsonism [7, 56-59]. Translocation of endotoxin from the mouth in patients with periodontal disease may also be associated with atherosclerosis [52, 57, 60]. Chronically increased circulating LPS has been associated with low-grade inflammation and insulin resistance and thereby related to metabolic syndrome [61].
The various reports of endotoxin-associated diseases are difficult to interpret due to the use of different assays, the variation in correlation of plasma endotoxin levels with cytokine levels and inflammatory biomarkers, the pharmacokinetic differences in routes of entry of endotoxin into the circulation, and the degree of inactivation in vivo. These factors have been reviewed in detail elsewhere [62].
1.3. Gut function and Endotoxin
Gut microbiota composition and LPS are linked with inflammation, obesity, and metabolic disorders [63, 64]. These reviews suggest that obesity and diet, particularly high carbohydrate and fat diets, alter gut flora. Studies of increased plasma endotoxin levels in humans have shown a direct correlation with increased waist circumference, waist–hip ratio, insulin levels, inflammatory cytokines and lipids, including total cholesterol, triglycerides, and LDL cholesterol. Elevated LPS concentrations have demonstrated an inverse relationship with HDL cholesterol [64]. Recently, Korpela et al. demonstrated that the composition of gut microbiota was predictive of responses to dietary intervention [65]. Baseline abundances of members of Clostridium clusters and Bacilli were modeled among three cohorts from different countries to predict overall responsiveness to tested dietary interventions. These gut microbial signatures permitted highly accurate predictions of microbiota changes, total blood cholesterol, HOMA (Homeostatic Model Assessment, insulin sensitivity indicator) and C-reactive protein. The emerging evidence suggests that gut microbiota and permeability may be associated with chronic inflammation, endotoxemia, and enhanced risk factors for CHF.
The role of gut-derived endotoxin in human atherosclerosis seems less clear than its relationship to metabolic syndrome. Other sources of LPS such as periodontal, environmental, or airways (especially from colonization in chronic lung disease) have been reported to contribute to atherosclerosis [66]. It is estimated that as many 40% of new atherosclerosis cases may result from common chronic infections [12].
Even with an intact mucosa and healthy gut, small amounts of LPS can leak or translocate to the splanchnic circulation. This situation can occur, for example, after vigorous exercise and high fat diets [67, 68]. The effect of low level leakage can be positive by producing an immunostimulatory response leading to enhanced resistance to infections. There is also conflicting data to suggest low level leakage may be protective against malignancy [69-71]. In other cases the effect seems to be negative and contribute to obesity, diabetes, and atherosclerosis [31].
1.4. Endotoxin, Toll-Like Receptors and Atherosclerosis
Several studies have shown a relationship between TLR4 and TLR2 activation, subsequent inflammation, and atherosclerosis [72-75]. Endothelial dysfunction and subsequent activation results in the recruitment of leukocytes to the subendothelium that is facilitated through activation of toll-like receptors [28]. Endothelial chemokine receptors bind with leukocyte receptors leading to integrin activation and strong adhesion of the leukocyte. Recruited monocytes become activated macrophages which results in foam cell formation. Not all ligands in this scenario are endotoxins. Endogenous substances such as oxidized LDL cholesterol, minimally oxidized LDL, oxidized phospholipids, extracellular domain A of fibronectin, hyaluronan, HSP60, HMGB1, serum amyloid A, and amyloid beta also bind to TLR4 or activate TLR4 mediated inflammatory pathways [76-78]. However, the response is generally more subdued as compared with endotoxin [77]. TLR2 is also believed to be intimately associated with atherosclerosis. However, this receptor binds “atypical” LPS (e.g., from Porphyromonas gingivalis, an oropharyngeal bacterium), peptidoglycan, oxidized LDL cholesterol, HMGB1, serum amyloid A, and amyloid beta. Current research suggests that blocking TLR2 and perhaps TLR4 may reduce lesion formation and inflammation, while TLR2 blockade also reduces infarct size following myocardial infarction [76]. The role of TLR2 may be independent of dietary lipids [79]. Additional evidence of TLRs contribution to atherosclerosis includes studies using mouse models that showed gene deletion of TLR2, TLR4, or MyD88 resulted in reduction in atherosclerosis [60, 80].
Similarly, MyD88 and ApoE-deficient mice experienced substantially decreased atheroma formation and macrophage infiltration [81]. TLR1, TLR2, and TLR4 expression was shown to be increased in human atherosclerotic plaques compared with normal coronary arteries, and that endothelial cells and macrophages were activated [82, 83]. Hypertension in a rat model increased TLR4 expression and activity, which might in part account for its role in the pathogenesis of atherosclerotic heart disease [84].
One group of investigators has suggested an autoimmune basis for the earliest changes of atherosclerosis, via protective immunity acquired through exposure to bacterial or endogenous HSP60 [85]. Under this viewpoint, atherosclerotic risk factors cause endothelial stress, thereby increasing expression of HSP60 and cell surface adhesion molecules to incite an inflammatory response.
Boekholdt et al. genotyped subjects in the REGRESS study of pravastatin and coronary atherosclerosis for two TLR4 polymorphisms [86]. Pravastatin recipients with the 299Gly TLR4 allele developed substantially fewer cardiovascular events than noncarriers with no difference in lipid profiles. This demonstrates that genetic polymorphism in the innate immune system is related to atherosclerotic clinical events, and that statin treatment reduced the risk of these events without affecting cholesterol parameters. Kiechl and colleagues similarly monitored subjects in the Bruneck (Italy) Study of carotid atherosclerosis for the 299Gly TLR4 allele and found a decreased risk of atherosclerosis and lower levels of circulating inflammatory cytokines [87]. Those carrying the TLR4 allele were also predisposed to systemic infections.
1.5. Endotoxin, Atherosclerosis, and Chronic Infections
One of the first human studies of endotoxemia in chronic infections was by Wiedermann et al. who examined baseline endotoxin levels in a random population of 466 middle-aged to elderly adults including many with chronic infections [56]. Chronic infections included participants with H. pylori infection, recurrent UTIs, and periodontal infection, but primarily chronic obstructive pulmonary disease (COPD). The median concentration was 14.3 pg/ml although 31 participants had levels in excess of 50ng/ml. The end points were the presence and axial diameter of plaques in the carotid arteries as assessed with high-resolution Duplex ultrasound and “incident cardiovascular disease” defined as myocardial infarction, transient ischemic attacks, stroke, or peripheral vascular disease. Endpoint assessments were performed at baseline and 5 years later by the same sonographer. The results showed high baseline endotoxin levels (>50ng/ml) were associated with a high incidence of atherosclerosis. This was particularly true of participants who smoked and had COPD. In subjects with baseline endotoxin levels ≤ 50ng/ml, those with chronic infection had the greatest risk. The same groups were associated with the highest risks of incident cardiovascular disease. They concluded by commenting “As an outstanding finding, baseline endotoxemia emerged as one of the strongest risk predictors of five-year incidence of carotid atherosclerosis and cardiovascular disease in our survey”. The authors did not comment on the possible contribution of periodontal disease which was present at baseline in 31 patients [56].
Porphyromonas gingivalis-associated periodontal disease has been widely reported to be associated with atherosclerosis [88]. This organism produces an atypical LPS that does not interact with TLR4 receptors. Hayashi et al. published a study examining the role of P. gingivalis on the progression of atherosclerosis in apolipoprotein E (ApoE) negative mice with or without TLR4 expression [60]. Infected ApoE negative mice demonstrated luminal narrowing and significantly more plaque than uninfected ApoE negative controls, while ApoE negative TLR4 negative mice exhibited further luminal narrowing and greater plaque development. TLR4 positive mice manifested only superficial fatty streaks rather than plaque infiltrated with macrophages. The authors speculated that increased expression of TLR2 in the infected TLR4 negative group may have been responsible for the results. Their results suggest a role for pathogen-specific TLR signaling in atherosclerosis, and also demonstrate an atheroprotective role for TLR4 in response to P. gingivalis infection [60].
There are several reports linking Chlamydophila pneumoniae (C. pneumoniae) (formerly Chlamydia pneumoniae) with atherosclerosis. These include epidemiologic studies comparing its seroprevalance to the presence of CHD as well as cytology studies showing the organism in atheromas [11, 13, 89]. C. pneumonia is a mesophilic intracellular gram-negative bacterium that produces a somewhat abbreviated version of LPS lacking the oligopolysaccharide side chain [22]. The role of the bacterium in atherosclerosis may be multifactorial with endotoxin as only a partial contributor. Microbes may play a role in the pathogenesis of chronic artery disease at several steps: (i) direct or indirect endothelial vascular injury; (ii) accelerating early atherosclerosis by cytokine stimulation leading to local increases in LDL and oxidized LDL; (iii) precipitating acute events by predisposing to vulnerable plaque or activating the coagulation cascade; (iv) infecting the atherosclerotic plaque [11, 13]. Other components of C. pneumoniae such as heat shock protein 60 (cHSP60) have also been reported to contribute to atherosclerosis [90, 91].
1.6. Endotoxin and Heart Failure
Higher LPS concentrations in patients with decompensated heart failure have been observed along with reduced active carrier mediated intestinal sugar transport [92]. The LPS concentrations decreased when patients were therapeutically recompensated. In addition, thickened bowel wall and large changes in passive carrier mediated intestinal transport and permeability were found in stable, compensated CHF patients [93]. The authors suggested a cause/effect relationship between edematous gut wall, epithelial dysfunction, and translocating LPS. However, endotoxemia induced in mice was shown to reduce splanchnic blood flow and disrupt the mucosal barrier function, so the relationship is still not clear [94].
Low-dose endotoxemia activates TLR4 signaling and serves as a model for human experimental inflammation-induced metabolic disturbances [95]. Mehta et al. obtained blood samples and adipose aspiration biopsies before and after a 3ng/kg intravenous bolus of endotoxin [40]. The primary outcome was the frequently sampled intravenous glucose tolerance insulin sensitivity index. Secondary outcomes included inflammatory and metabolic markers, whole-blood mRNA and protein expression, and adipose mRNA and protein expression. LPS induced a significant reduction in FSIGT insulin sensitivity index with no effect on pancreatic function. In adipose tissue, endotoxin suppressed insulin receptor substrate-1 and markedly induced suppressors of cytokine signaling proteins (1 and 3). The result was activation of innate (interleukin-6, tumor necrosis factor) and adaptive (monocyte chemoattractant protein-1 and CXCL10 chemokines) inflammation. The authors concluded that endotoxemia “evoked adipose inflammation and modulation of insulin signaling pathways” and induced insulin resistance without beta cell dysfunction [40].
Venous congestion leading to altered gut permeability may be one mechanism for the source of circulating endotoxin in heart failure. Its potential role was first established in a paper by Niebauer et al. in 1999 [96]. The authors compared 20 patients (mean age 64) with chronic heart failure and recent onset peripheral edema to 20 non-edematous patients (mean age 63) with chronic heart failure. They collected markers of endotoxemia, inflammation, and immune activation. The results were compared to a control group of 14 healthy volunteers (mean age 55). The mean endotoxin concentrations were higher in edematous patients than in stable patients with chronic heart failure (p=0·0009) as well as in the control patients (p=0·02). Edematous patients had the highest concentrations of cytokines and other intermediaries or markers of inflammation (TNF-α, IL-6, CD14, CRP, procalcitonin) After short-term diuretic treatment, endotoxin concentrations decreased significantly from baseline (p<0·05) but cytokines remained raised. The authors speculated that heart failure associated edema leads to endotoxin mediated inflammation [96].
Other support for the role of endotoxin in heart failure is based on myocyte dysfunction in sepsis. Echocardiographic, experimental cellular, isolated heart, and in vivo animal studies have all shown impaired contractility and myocardial compliance in sepsis [97]. Cytokines such as TNF-α, IL6 and IL1 (especially IL-1 mediated stimulation of nitric oxide synthetase) have been mentioned as contributors.
Sharma et al. found an inverse relationship between TNF-α release and serum cholesterol in chronic heart failure patients, but not in healthy controls [98]. The authors suggested that serum lipoproteins may regulate the response to LPS such that the low LPS activity observed in heart failure patients can induce significant TNF-α production. In a small crossover human study, Hudgins et al examined the effects of a single dose of intravenous LPS on lipoprotein levels in six healthy volunteers [95]. After treatment, subjects developed markedly increased serum levels of TNF-α and its soluble receptors, IL-6, serum amyloid A, and C-reactive protein. Triglyceride and very low-density lipoprotein-triglyceride increased, while cholesterol, LDL cholesterol, apolipoprotein B, and phospholipid levels declined. LPS binding protein increased 3-fold.
1.7. Role of Statins and Other Drugs on Toll-Like Receptor Function and Expression
Toll-like receptor signaling pathways may represent intriguing therapeutic targets for treatment and/or prevention of complications of atherosclerosis and chronic heart failure [28]. Foldes et al. studied the effects of the HMG-CoA reductase inhibitor fluvastatin on ex vivo monocytes from CHF and normal patients, and found a dose-dependent reduction in TLR4 expression in both groups [34]. In addition, fluvastatin pretreatment blunted the LPS-induced TLR4 and CD14 expression by half, while beta-adrenergic inhibitors had no effect. In a double-blind placebo-controlled study of 20 healthy subjects, subjects were randomized to receive simvastatin (80 mg/d) or placebo for 4 days before intravenous LPS was administered [99]. Pretreatment blunted TLR4 and TLR2 expression on monocytes by more than half after the LPS infusion. Atorvastatin-incubated human monocytes demonstrated decreased TLR4 and NF-қB expression following LPS treatment [100]. These and other studies suggest that the pleiotropic effects of statins on cardiovascular outcomes is in part due to inhibition of the innate immune system’s inflammatory responses via the TLR4/NF-қB signaling pathway [101-103].
Adenosine reduced TLR4 expression in macrophages from acute myocardial infarction patients compared with controls, suggesting a role for adenosine or an adenosine agonist in ischemic myocardial injury. Endogenous adenosine is released in large amounts after ischemia due to cell death and adenosine triphosphate breakdown [104]. Carbohydrate-based small molecules have been investigated for possible TLR-4 antagonist therapeutic potentials [105].
Animal studies have identified other potential therapeutic agents for modulating TLR4 function in disease. Chronic treatment with metformin, an antidiabetic agent, has been shown to reduce inflammatory responses and cardiac dysfunction following myocardial infarction by suppressing TLR4 signaling [106]. Ischemia/reperfusion injury following myocardial infarction was attenuated in mice by eritoran and follistatin [107, 108].
2. CONCLUSION
Endotoxin is a lipopolysaccharide (LPS) constituent of the outer membrane of most gram negative bacteria. Although ubiquitous in the natural environment, the largest reservoir is the gut of humans and animals due to colonization by gram-negative aerobic and anaerobic bacteria. The interaction of endotoxin with the TLR4-MD2-CD14 receptor complex ultimately leads to intracellular transcription of of several inflammatory mediators. Toll-like receptors including TLR4 and TLR2 have shown in various in vitro, animal, and human studies to be major contributors to the development and progression of atherosclerosis and coronary heart disease. The relative contribution of LPS in comparison to other ligands still needs further research. The role of both TLRs and endotoxin in atherosclerotic heart disease is intriguing but our current knowledge could best be described as only preliminary. The question of whether drugs affecting toll-like receptor expression and function will ultimately be useful in improving clinical outcomes needs further analysis.
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. The author(s) received no financial support for the research, authorship, and/or publication of this article.
REFERENCES
- 1.Nichols M., Townsend N., Scarborough P., Rayner M. Cardiovascular disease in Europe 2014: epidemiological update. Eur. Heart J. 2014;35(42):2950–2959. doi: 10.1093/eurheartj/ehu299. [DOI] [PubMed] [Google Scholar]
- 2.Kaptoge S., Di Angelantonio E., Lowe G., et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375(9709):132–140. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ross R. Atherosclerosis--an inflammatory disease. N. Engl. J. Med. 1999;340(2):115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 4.Libby P. Inflammation in atherosclerosis. Nature. 2002;420(6917):868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 5.Binder C.J., Chang M.K., Shaw P.X., et al. Innate and acquired immunity in atherogenesis. Nat. Med. 2002;8(11):1218–1226. doi: 10.1038/nm1102-1218. [DOI] [PubMed] [Google Scholar]
- 6.Vandenbulcke L., Bachert C., Van Cauwenberge P., Claeys S. The innate immune system and its role in allergic disorders. Int. Arch. Allergy Immunol. 2006;139(2):159–165. doi: 10.1159/000090393. [DOI] [PubMed] [Google Scholar]
- 7.Liebers V., Raulf-Heimsoth M., Bruning T. Health effects due to endotoxin inhalation. Arch. Toxicol. 2008;82(4):203–210. doi: 10.1007/s00204-008-0290-1. [review]. [DOI] [PubMed] [Google Scholar]
- 8.Bosshart H., Heinzelmann M. Targeting bacterial endotoxin: two sides of a coin. Ann. N. Y. Acad. Sci. 2007;1096:1–17. doi: 10.1196/annals.1397.064. [DOI] [PubMed] [Google Scholar]
- 9.Cinel I., Opal S.M. Molecular biology of inflammation and sepsis: a primer. Crit. Care Med. 2009;37(1):291–304. doi: 10.1097/CCM.0b013e31819267fb. [DOI] [PubMed] [Google Scholar]
- 10.Frangogiannis N.G. Inflammation in cardiac injury, repair and regeneration. Curr. Opin. Cardiol. 2015;30(3):240–245. doi: 10.1097/HCO.0000000000000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Honarmand H. Atherosclerosis Induced by Chlamydophila pneumoniae: A Controversial Theory.Interdiscip Perspect Infect Dis. 2013 doi: 10.1155/2013/941392. 941392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chatzidimitriou D., Kirmizis D., Gavriilaki E., Chatzidimitriou M., Malisiovas N. Atherosclerosis and infection: is the jury still not in? Future Microbiol. 2012;7(10):1217–1230. doi: 10.2217/fmb.12.87. [DOI] [PubMed] [Google Scholar]
- 13.Higgins J.P. Chlamydia pneumoniae and coronary artery disease: the antibiotic trials. Mayo Clin. Proc. 2003;78(3):321–332. doi: 10.4065/78.3.321. [DOI] [PubMed] [Google Scholar]
- 14.Hirschfeld J., Kawai T. Oral inflammation and bacteremia: implications for chronic and acute systemic diseases involving major organs. Cardiovasc. Hematol. Disord. Drug Targets. 2015;15(1):70–84. doi: 10.2174/1871529x15666150108115241. [DOI] [PubMed] [Google Scholar]
- 15.Vcev A., Nakic D., Mrden A., et al. Helicobacter pylori infection and coronary artery disease. Coll. Antropol. 2007;31(3):757–760. [PubMed] [Google Scholar]
- 16.Basinkevich A.B., Shakhnovich R.M., Martynova V.R., et al. Role of Chlamydia, mycoplasma and cytomegalovirus infection in the development of coronary artery disease. Kardiologiia. 2003;43(11):4–9. [PubMed] [Google Scholar]
- 17.Pussinen P.J., Vilkuna-Rautiainen T., Alfthan G., et al. Severe periodontitis enhances macrophage activation via increased serum lipopolysaccharide. Arterioscler. Thromb. Vasc. Biol. 2004;24(11):2174–2180. doi: 10.1161/01.ATV.0000145979.82184.9f. [DOI] [PubMed] [Google Scholar]
- 18.Glaros T.G., Chang S., Gilliam E.A., Maitra U., Deng H., Li L. Causes and consequences of low grade endotoxemia and inflammatory diseases. Front. Biosci. (Schol. Ed.) 2013;5:754–765. doi: 10.2741/s405. [DOI] [PubMed] [Google Scholar]
- 19.Anker S.D., Egerer K.R., Volk H.D., Kox W.J., Poole-Wilson P.A., Coats A.J. Elevated soluble CD14 receptors and altered cytokines in chronic heart failure. Am. J. Cardiol. 1997;79(10):1426–1430. doi: 10.1016/s0002-9149(97)00159-8. [DOI] [PubMed] [Google Scholar]
- 20.von Haehling S., Schefold J.C., Lainscak M., Doehner W., Anker S.D. Inflammatory biomarkers in heart failure revisited: much more than innocent bystanders. Heart Fail. Clin. 2009;5(4):549–560. doi: 10.1016/j.hfc.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Anker S.D., Coats A.J. How to RECOVER from RENAISSANCE? The significance of the results of RECOVER, RENAISSANCE, RENEWAL and ATTACH. Int. J. Cardiol. 2002;86(2-3):123–130. doi: 10.1016/s0167-5273(02)00470-9. [DOI] [PubMed] [Google Scholar]
- 22.Rietschel E.T., Kirikae T., Schade F.U., et al. The chemical structure of bacterial endotoxin in relation to bioactivity. Immunobiology. 1993;187(3-5):169–190. doi: 10.1016/S0171-2985(11)80338-4. [DOI] [PubMed] [Google Scholar]
- 23.Raetz C.R., Whitfield C. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caroff M., Karibian D. Structure of bacterial lipopolysaccharides. Carbohydr. Res. 2003;338(23):2431–2447. doi: 10.1016/j.carres.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 25.Park B.S., Lee J.O. Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp. Mol. Med. 2013;45:e66. doi: 10.1038/emm.2013.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stoll L.L., Denning G.M., Weintraub N.L. Potential role of endotoxin as a proinflammatory mediator of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2004;24(12):2227–2236. doi: 10.1161/01.ATV.0000147534.69062.dc. [DOI] [PubMed] [Google Scholar]
- 27.Marshall J.C. Lipopolysaccharide: an endotoxin or an exogenous hormone? Clin. Infect. Dis. 2005;41(Suppl. 7):S470–S480. doi: 10.1086/432000. [DOI] [PubMed] [Google Scholar]
- 28.Moghimpour Bijani F., Vallejo J.G., Rezaei N. Toll-like receptor signaling pathways in cardiovascular diseases: challenges and opportunities. Int. Rev. Immunol. 2012;31(5):379–395. doi: 10.3109/08830185.2012.706761. [DOI] [PubMed] [Google Scholar]
- 29.Opal S.M. Endotoxins and other sepsis triggers. Contrib. Nephrol. 2010;167:14–24. doi: 10.1159/000315915. [DOI] [PubMed] [Google Scholar]
- 30.Blomkalns A.L., Stoll L.L., Shaheen W., et al. Low level bacterial endotoxin activates two distinct signaling pathways in human peripheral blood mononuclear cells. J. Inflamm. (Lond.) 2011;8:4. doi: 10.1186/1476-9255-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cani P.D., Amar J., Iglesias M.A., et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 32.Tan Y., Kagan J.C. A cross-disciplinary perspective on the innate immune responses to bacterial lipopolysaccharide. Mol. Cell. 2014;54(2):212–223. doi: 10.1016/j.molcel.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jankowska E.A., von Haehling S., Czarny A., et al. Activation of the NF-kappaB system in peripheral blood leukocytes from patients with chronic heart failure. Eur. J. Heart Fail. 2005;7(6):984–990. doi: 10.1016/j.ejheart.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 34.Foldes G., von Haehling S., Okonko D.O., Jankowska E.A., Poole-Wilson P.A., Anker S.D. Fluvastatin reduces increased blood monocyte Toll-like receptor 4 expression in whole blood from patients with chronic heart failure. Int. J. Cardiol. 2008;124(1):80–85. doi: 10.1016/j.ijcard.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 35.Kuwahata S., Fujita S., Orihara K., et al. High expression level of Toll-like receptor 2 on monocytes is an important risk factor for arteriosclerotic disease. Atherosclerosis. 2010;209(1):248–254. doi: 10.1016/j.atherosclerosis.2009.08.046. [DOI] [PubMed] [Google Scholar]
- 36.Torre-Amione G., Kapadia S., Benedict C., Oral H., Young J.B., Mann D.L. Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: a report from the Studies of Left Ventricular Dysfunction (SOLVD). J. Am. Coll. Cardiol. 1996;27(5):1201–1206. doi: 10.1016/0735-1097(95)00589-7. [DOI] [PubMed] [Google Scholar]
- 37.Suffredini A.F., Fromm R.E., Parker M.M., et al. The cardiovascular response of normal humans to the administration of endotoxin. N. Engl. J. Med. 1989;321(5):280–287. doi: 10.1056/NEJM198908033210503. [DOI] [PubMed] [Google Scholar]
- 38.Riedel S., Carroll K.C. Laboratory detection of sepsis: biomarkers and molecular approaches. Clin. Lab. Med. 2013;33(3):413–437. doi: 10.1016/j.cll.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 39.Horseman M.A., Rather-Conally J., Saavedra C., Surani S. A case of severe heatstroke and review of pathophysiology, clinical presentation, and treatment. J. Intensive Care Med. 2013;28(6):334–340. doi: 10.1177/0885066611434000. [DOI] [PubMed] [Google Scholar]
- 40.Mehta N.N., McGillicuddy F.C., Anderson P.D., et al. Experimental endotoxemia induces adipose inflammation and insulin resistance in humans. Diabetes. 2010;59(1):172–181. doi: 10.2337/db09-0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sandek A., Valentova M., von Haehling S., Doehner W., Anker S.D. The small intestine: a critical linkage in pathophysiology of cardiac cachexia. Int. J. Cardiol. 2011;146(2):277–278. doi: 10.1016/j.ijcard.2010.10.083. [DOI] [PubMed] [Google Scholar]
- 42.Sandek A., Anker S.D., von Haehling S. The gut and intestinal bacteria in chronic heart failure. Curr. Drug Metab. 2009;10(1):22–28. doi: 10.2174/138920009787048374. [DOI] [PubMed] [Google Scholar]
- 43.Rao R. Endotoxemia and gut barrier dysfunction in alcoholic liver disease. Hepatology. 2009;50(2):638–644. doi: 10.1002/hep.23009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manthous C.A., Hall J.B., Samsel R.W. Endotoxin in human disease. Part 2: Biologic effects and clinical evaluations of anti-endotoxin therapies. Chest. 1993;104(6):1872–1881. doi: 10.1378/chest.104.6.1872. [DOI] [PubMed] [Google Scholar]
- 45.Krabbe K.S., Reichenberg A., Yirmiya R., Smed A., Pedersen B.K., Bruunsgaard H. Low-dose endotoxemia and human neuropsychological functions. Brain Behav. Immun. 2005;19(5):453–460. doi: 10.1016/j.bbi.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 46.Cicalese L., Sahai A., Sileri P., et al. Acute pancreatitis and bacterial translocation. Dig. Dis. Sci. 2001;46(5):1127–1132. doi: 10.1023/a:1010786701289. [DOI] [PubMed] [Google Scholar]
- 47.Grunfeld C., Feingold K.R. Endotoxin in the gut and chylomicrons: translocation or transportation? J. Lipid Res. 2009;50(1):1–2. doi: 10.1194/jlr.E800018-JLR200. [DOI] [PubMed] [Google Scholar]
- 48.Moore F.A., Moore E.E., Poggetti R., et al. Gut bacterial translocation via the portal vein: a clinical perspective with major torso trauma. J. Trauma. 1991;31(5):629–636. doi: 10.1097/00005373-199105000-00006. [DOI] [PubMed] [Google Scholar]
- 49.Saadia R., Schein M., MacFarlane C., Boffard K.D. Gut barrier function and the surgeon. Br. J. Surg. 1990;77(5):487–492. doi: 10.1002/bjs.1800770505. [DOI] [PubMed] [Google Scholar]
- 50.Lyons J.L., Uno H., Ancuta P., et al. Plasma sCD14 is a biomarker associated with impaired neurocognitive test performance in attention and learning domains in HIV infection. J. Acquir. Immune Defic. Syndr. 2011;57(5):371–379. doi: 10.1097/QAI.0b013e3182237e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peden D.B. The role of oxidative stress and innate immunity in O(3) and endotoxin-induced human allergic airway disease. Immunol. Rev. 2011;242(1):91–105. doi: 10.1111/j.1600-065X.2011.01035.x. [DOI] [PubMed] [Google Scholar]
- 52.Wiesner P., Choi S.H., Almazan F., et al. Low doses of lipopolysaccharide and minimally oxidized low-density lipoprotein cooperatively activate macrophages via nuclear factor kappa B and activator protein-1: possible mechanism for acceleration of atherosclerosis by subclinical endotoxemia. Circ. Res. 2010;107(1):56–65. doi: 10.1161/CIRCRESAHA.110.218420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kelly J.L., O'Sullivan C., O'Riordain M., et al. Is circulating endotoxin the trigger for the systemic inflammatory response syndrome seen after injury? Ann. Surg. 1997;225(5):530–541. doi: 10.1097/00000658-199705000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roumen R.M., Frieling J.T., van Tits H.W., van der Vliet J.A., Goris R.J. Endotoxemia after major vascular operations. J. Vasc. Surg. 1993;18(5):853–857. [PubMed] [Google Scholar]
- 55.Muehlberger T., Wong L., Redett R., Girotto J.A., Munster A.M. A temporal correlation of pain and endotoxin levels in sickle cell disease. J. Trauma. 1998;45(4):814–815. doi: 10.1097/00005373-199810000-00036. [DOI] [PubMed] [Google Scholar]
- 56.Wiedermann C.J., Kiechl S., Dunzendorfer S., et al. Association of endotoxemia with carotid atherosclerosis and cardiovascular disease: prospective results from the Bruneck Study. J. Am. Coll. Cardiol. 1999;34(7):1975–1981. doi: 10.1016/s0735-1097(99)00448-9. [DOI] [PubMed] [Google Scholar]
- 57.Kiechl S., Egger G., Mayr M., et al. Chronic infections and the risk of carotid atherosclerosis: prospective results from a large population study. Circulation. 2001;103(8):1064–1070. doi: 10.1161/01.cir.103.8.1064. [DOI] [PubMed] [Google Scholar]
- 58.Niehaus I., Lange J.H. Endotoxin: is it an environmental factor in the cause of Parkinson's disease? Occup. Environ. Med. 2003;60(5):378. doi: 10.1136/oem.60.5.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Astrakianakis G., Murray E. Conflicting Effects of Occupational Endotoxin Exposure on Lung Health - A Hypothesis-Generating Review of Cancer and COPD Risk. J. Environ. Immunol. Toxicol. 2014;2(1):24–35. [Google Scholar]
- 60.Hayashi C., Papadopoulos G., Gudino C.V., et al. Protective role for TLR4 signaling in atherosclerosis progression as revealed by infection with a common oral pathogen. J. Immunol. 2012;189(7):3681–3688. doi: 10.4049/jimmunol.1201541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jialal I., Rajamani U. Endotoxemia of metabolic syndrome: a pivotal mediator of meta-inflammation. Metab. Syndr. Relat. Disord. 2014;12(9):454–456. doi: 10.1089/met.2014.1504. [DOI] [PubMed] [Google Scholar]
- 62.Munford R.S. Endotoxemia-menace, marker, or mistake? J. Leukoc. Biol. 2016 doi: 10.1189/jlb.3RU0316-151R. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Everard A., Cani P.D. Diabetes, obesity and gut microbiota. Best Pract. Res. Clin. Gastroenterol. 2013;27(1):73–83. doi: 10.1016/j.bpg.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 64.Piya M.K., Harte A.L., McTernan P.G. Metabolic endotoxaemia: is it more than just a gut feeling? Curr. Opin. Lipidol. 2013;24(1):78–85. doi: 10.1097/MOL.0b013e32835b4431. [DOI] [PubMed] [Google Scholar]
- 65.Korpela K., Flint H.J., Johnstone A.M., et al. Gut microbiota signatures predict host and microbiota responses to dietary interventions in obese individuals. PLoS One. 2014;9(3):e90702. doi: 10.1371/journal.pone.0090702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gibson F.C., III, Yumoto H., Takahashi Y., Chou H.H., Genco C.A. Innate immune signaling and Porphyromonas gingivalis-accelerated atherosclerosis. J. Dent. Res. 2006;85(2):106–121. doi: 10.1177/154405910608500202. [DOI] [PubMed] [Google Scholar]
- 67.Camus G., Poortmans J., Nys M., et al. Mild endotoxaemia and the inflammatory response induced by a marathon race. Clin. Sci. (Lond.) 1997;92(4):415–422. doi: 10.1042/cs0920415. [DOI] [PubMed] [Google Scholar]
- 68.Moreira A.P., Texeira T.F., Ferreira A.B., Peluzio Mdo C., Alfenas Rde C. Influence of a high-fat diet on gut microbiota, intestinal permeability and metabolic endotoxaemia. Br. J. Nutr. 2012;108(5):801–809. doi: 10.1017/S0007114512001213. [DOI] [PubMed] [Google Scholar]
- 69.Agalliu I., Costello S., Applebaum K.M., et al. Risk of lung cancer in relation to contiguous windows of endotoxin exposure among female textile workers in Shanghai. Cancer Causes Control. 2011;22(10):1397–1404. doi: 10.1007/s10552-011-9812-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Applebaum K.M., Ray R.M., Astrakianakis G., et al. Evidence of a paradoxical relationship between endotoxin and lung cancer after accounting for left truncation in a study of Chinese female textile workers. Occup. Environ. Med. 2013;70(10):709–715. doi: 10.1136/oemed-2012-101240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Heine H., Rietschel E.T., Ulmer A.J. The biology of endotoxin. Mol. Biotechnol. 2001;19(3):279–296. doi: 10.1385/MB:19:3:279. [DOI] [PubMed] [Google Scholar]
- 72.Mackman N. How do oxidized phospholipids inhibit LPS signaling? Arterioscler. Thromb. Vasc. Biol. 2003;23(7):1133–1136. doi: 10.1161/01.ATV.0000080641.10662.4C. [DOI] [PubMed] [Google Scholar]
- 73.Chavez-Sanchez L., Chavez-Rueda K., Legorreta-Haquet M.V., et al. The activation of CD14, TLR4, and TLR2 by mmLDL induces IL-1beta, IL-6, and IL-10 secretion in human monocytes and macrophages. Lipids Health Dis. 2010;9:117. doi: 10.1186/1476-511X-9-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kannan Y., Sundaram K., Aluganti Narasimhulu C., Parthasarathy S., Wewers M.D. Oxidatively modified low density lipoprotein (LDL) inhibits TLR2 and TLR4 cytokine responses in human monocytes but not in macrophages. J. Biol. Chem. 2012;287(28):23479–23488. doi: 10.1074/jbc.M111.320960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Curtiss L.K., Tobias P.S. Emerging role of Toll-like receptors in atherosclerosis. J. Lipid Res. 2009;50(Suppl.):S340–S345. doi: 10.1194/jlr.R800056-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Falck-Hansen M., Kassiteridi C., Monaco C. Toll-like receptors in atherosclerosis. Int. J. Mol. Sci. 2013;14(7):14008–14023. doi: 10.3390/ijms140714008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Miller Y.I., Choi S.H., Wiesner P., et al. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ. Res. 2011;108(2):235–248. doi: 10.1161/CIRCRESAHA.110.223875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wick M.C., Mayerl C., Backovic A., et al. In vivo imaging of the effect of LPS on arterial endothelial cells: molecular imaging of heat shock protein 60 expression. Cell Stress Chaperones. 2008;13(3):275–285. doi: 10.1007/s12192-008-0044-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu X., Ukai T., Yumoto H., et al. Toll-like receptor 2 plays a critical role in the progression of atherosclerosis that is independent of dietary lipids. Atherosclerosis. 2008;196(1):146–154. doi: 10.1016/j.atherosclerosis.2007.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shibata N., Glass C.K. Regulation of macrophage function in inflammation and atherosclerosis. J. Lipid Res. 2009;50(Suppl.):S277–S281. doi: 10.1194/jlr.R800063-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Michelsen K.S., Wong M.H., Shah P.K., et al. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc. Natl. Acad. Sci. USA. 2004;101(29):10679–10684. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Edfeldt K., Swedenborg J., Hansson G.K., Yan Z.Q. Expression of toll-like receptors in human atherosclerotic lesions: a possible pathway for plaque activation. Circulation. 2002;105(10):1158–1161. [PubMed] [Google Scholar]
- 83.Chang Z.L. Important aspects of Toll-like receptors, ligands and their signaling pathways. Inflamm. Res. 2010;59(10):791–808. doi: 10.1007/s00011-010-0208-2. [DOI] [PubMed] [Google Scholar]
- 84.Eissler R., Schmaderer C., Rusai K., et al. Hypertension augments cardiac Toll-like receptor 4 expression and activity. Hypertens. Res. 2011;34(5):551–558. doi: 10.1038/hr.2010.270. [DOI] [PubMed] [Google Scholar]
- 85.Grundtman C., Wick G. The autoimmune concept of atherosclerosis. Curr. Opin. Lipidol. 2011;22(5):327–334. doi: 10.1097/MOL.0b013e32834aa0c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Boekholdt S.M., Agema W.R., Peters R.J., et al. Variants of toll-like receptor 4 modify the efficacy of statin therapy and the risk of cardiovascular events. Circulation. 2003;107(19):2416–2421. doi: 10.1161/01.CIR.0000068311.40161.28. [DOI] [PubMed] [Google Scholar]
- 87.Kiechl S., Lorenz E., Reindl M., et al. Toll-like receptor 4 polymorphisms and atherogenesis. N. Engl. J. Med. 2002;347(3):185–192. doi: 10.1056/NEJMoa012673. [DOI] [PubMed] [Google Scholar]
- 88.Kurita-Ochiai T, Yamamoto M. Periodontal pathogens and atherosclerosis: implications of inflammation and oxidative modification of LDL. 2014. [DOI] [PMC free article] [PubMed]
- 89.Arroyo-Espliguero R., Avanzas P., Jeffery S., Kaski J.C. CD14 and toll-like receptor 4: a link between infection and acute coronary events? Heart. 2004;90(9):983–988. doi: 10.1136/hrt.2002.001297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Netea M.G., Kullberg B.J., Galama J.M., Stalenhoef A.F., Dinarello C.A., Van der Meer J.W. Non-LPS components of Chlamydia pneumoniae stimulate cytokine production through Toll-like receptor 2-dependent pathways. Eur. J. Immunol. 2002;32(4):1188–1195. doi: 10.1002/1521-4141(200204)32:4<1188::AID-IMMU1188>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 91.Di Pietro M., Filardo S., De Santis F., Sessa R. Chlamydia pneumoniae infection in atherosclerotic lesion development through oxidative stress: a brief overview. Int. J. Mol. Sci. 2013;14(7):15105–15120. doi: 10.3390/ijms140715105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sandek A., Bjarnason I., Volk H.D., et al. Studies on bacterial endotoxin and intestinal absorption function in patients with chronic heart failure. Int. J. Cardiol. 2012;157(1):80–85. doi: 10.1016/j.ijcard.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 93.Sandek A., Bauditz J., Swidsinski A., et al. Altered intestinal function in patients with chronic heart failure. J. Am. Coll. Cardiol. 2007;50(16):1561–1569. doi: 10.1016/j.jacc.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 94.Deitch E.A., Berg R., Specian R. Endotoxin promotes the translocation of bacteria from the gut. Arch. Surg. 1987;122(2):185–190. doi: 10.1001/archsurg.1987.01400140067008. [DOI] [PubMed] [Google Scholar]
- 95.Hudgins L.C., Parker T.S., Levine D.M., et al. A single intravenous dose of endotoxin rapidly alters serum lipoproteins and lipid transfer proteins in normal volunteers. J. Lipid Res. 2003;44(8):1489–1498. doi: 10.1194/jlr.M200440-JLR200. [DOI] [PubMed] [Google Scholar]
- 96.Niebauer J., Volk H.D., Kemp M., et al. Endotoxin and immune activation in chronic heart failure: a prospective cohort study. Lancet. 1999;353(9167):1838–1842. doi: 10.1016/S0140-6736(98)09286-1. [DOI] [PubMed] [Google Scholar]
- 97.Romero-Bermejo F.J., Ruiz-Bailen M., Gil-Cebrian J., Huertos-Ranchal M.J. Sepsis-induced cardiomyopathy. Curr. Cardiol. Rev. 2011;7(3):163–183. doi: 10.2174/157340311798220494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sharma R., von Haehling S., Rauchhaus M., et al. Whole blood endotoxin responsiveness in patients with chronic heart failure: the importance of serum lipoproteins. Eur. J. Heart Fail. 2005;7(4):479–484. doi: 10.1016/j.ejheart.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 99.Niessner A., Steiner S., Speidl W.S., et al. Simvastatin suppresses endotoxin-induced upregulation of toll-like receptors 4 and 2 in vivo. Atherosclerosis. 2006;189(2):408–413. doi: 10.1016/j.atherosclerosis.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 100.Yang S.S., Li R., Qu X., Fang W., Quan Z. Atorvastatin decreases Toll-like receptor 4 expression and downstream signaling in human monocytic leukemia cells. Cell. Immunol. 2012;279(1):96–102. doi: 10.1016/j.cellimm.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 101.Peng L., Luo Y.T., Liu J.L. The effects of atorvastatin on C-reactive protein induced Toll-like receptor 4 expression on CD14+ monocyte. Zhonghua. Xin Xue Guan Bing Za Zhi. 2011;39(7):664–669. [PubMed] [Google Scholar]
- 102.Yang J., Huang C., Yang J., Jiang H., Ding J. Statins attenuate high mobility group box-1 protein induced vascular endothelial activation: a key role for TLR4/NF-kappaB signaling pathway. Mol. Cell. Biochem. 2010;345(1-2):189–195. doi: 10.1007/s11010-010-0572-9. [DOI] [PubMed] [Google Scholar]
- 103.Moutzouri E., Tellis C.C., Rousouli K., et al. Effect of simvastatin or its combination with ezetimibe on Toll-like receptor expression and lipopolysaccharide - induced cytokine production in monocytes of hypercholesterolemic patients. Atherosclerosis. 2012;225(2):381–387. doi: 10.1016/j.atherosclerosis.2012.08.037. [DOI] [PubMed] [Google Scholar]
- 104.Haas B., Leonard F., Ernens I., et al. Adenosine reduces cell surface expression of toll-like receptor 4 and inflammation in response to lipopolysaccharide and matrix products. J. Cardiovasc. Transl. Res. 2011;4(6):790–800. doi: 10.1007/s12265-011-9279-x. [DOI] [PubMed] [Google Scholar]
- 105.Marzabadi C.H., Franck R.W. Small-Molecule Carbohydrate-Based Immunostimulants. Chemistry. 2017;32(8):1728–1742. doi: 10.1002/chem.201601539. [DOI] [PubMed] [Google Scholar]
- 106.Soraya H., Clanachan A.S., Rameshrad M., Maleki-Dizaji N., Ghazi-Khansari M., Garjani A. Chronic treatment with metformin suppresses toll-like receptor 4 signaling and attenuates left ventricular dysfunction following myocardial infarction. Eur. J. Pharmacol. 2014;737:77–84. doi: 10.1016/j.ejphar.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 107.Shimamoto A., Chong A.J., Yada M., et al. Inhibition of Toll-like receptor 4 with eritoran attenuates myocardial ischemia-reperfusion injury. Circulation. 2006;114(1) Suppl.:I270–I274. doi: 10.1161/CIRCULATIONAHA.105.000901. [DOI] [PubMed] [Google Scholar]
- 108.Chen Y., Rothnie C., Spring D., et al. Regulation and actions of activin A and follistatin in myocardial ischaemia-reperfusion injury. Cytokine. 2014;69(2):255–262. doi: 10.1016/j.cyto.2014.06.017. [DOI] [PubMed] [Google Scholar]