Abstract
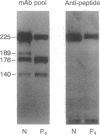
Proteolytic cleavage of the von Willebrand factor subunit may be important for processing and/or function of the molecule and is altered in certain subtypes of von Willebrand disease. It results in the generation of two main fragments with apparent molecular masses of 140 kDa and 176 kDa from the 225-kDa subunit. We have now obtained chemical evidence to locate the protease-sensitive bond between residues Tyr-842 and Met-843, a site that appears to reflect the specificity of calcium-dependent neutral proteases (calpains). Antibodies were raised against four synthetic peptides that represented sequences immediately preceding or following or including the cleavage site. One antibody (against the fragment from Ala-837 through Asp-851) reacted only with the intact subunit, and its epitope included the cleavage site. All others reacted specifically with either the 140-kDa or the 176-kDa fragment, demonstrating their origin from a single cleavage. In samples of purified von Willebrand factor from four of five patients with type IIA von Willebrand disease, the anti-peptide antibodies showed markedly decreased reactivity with either the 140-kDa or the 176-kDa fragment, suggesting the existence of distinct molecular abnormalities clustered around the cleavage site. Thus, in the majority of type IIA patients, a common pathogenetic mechanism may lead to the disappearance of the larger multimers as a consequence of structural changes that may expose a sensitive bond to the action of specific proteases. These studies demonstrate the use of anti-peptide antibodies directed at a relevant structural domain for the immunochemical differentiation of normal and mutant molecules.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batlle J., Lopez Fernandez M. F., Campos M., Justica B., Berges C., Navarro J. L., Diaz Cremades J. M., Kasper C. K., Dent J. A., Ruggeri Z. M. The heterogeneity of type IIA von Willebrand's disease: studies with protease inhibitors. Blood. 1986 Dec;68(6):1207–1212. [PubMed] [Google Scholar]
- Berkowitz S. D., Dent J., Roberts J., Fujimura Y., Plow E. F., Titani K., Ruggeri Z. M., Zimmerman T. S. Epitope mapping of the von Willebrand factor subunit distinguishes fragments present in normal and type IIA von Willebrand disease from those generated by plasmin. J Clin Invest. 1987 Feb;79(2):524–531. doi: 10.1172/JCI112843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz S. D., Nozaki H., Titani K., Murachi T., Plow E. F., Zimmerman T. S. Evidence that calpains and elastase do not produce the von Willebrand factor fragments present in normal plasma and IIA von Willebrand disease. Blood. 1988 Aug;72(2):721–727. [PubMed] [Google Scholar]
- Berliner S., Niiya K., Roberts J. R., Houghten R. A., Ruggeri Z. M. Generation and characterization of peptide-specific antibodies that inhibit von Willebrand factor binding to glycoprotein IIb-IIIa without interacting with other adhesive molecules. Selectivity is conferred by Pro1743 and other amino acid residues adjacent to the sequence Arg1744-Gly1745-Asp1746. J Biol Chem. 1988 Jun 5;263(16):7500–7505. [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom A. L., Giddings J. C., Wilks C. J. Factor 8 on the vascular intima: possible importance in haemostasis and thrombosis. Nat New Biol. 1973 Feb 14;241(111):217–219. doi: 10.1038/newbio241217a0. [DOI] [PubMed] [Google Scholar]
- Bonthron D., Orr E. C., Mitsock L. M., Ginsburg D., Handin R. I., Orkin S. H. Nucleotide sequence of pre-pro-von Willebrand factor cDNA. Nucleic Acids Res. 1986 Sep 11;14(17):7125–7127. doi: 10.1093/nar/14.17.7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde U., Dent J. A., Berkowitz S. D., Ruggeri Z. M., Zimmerman T. S. Subunit composition of plasma von Willebrand factor in patients with the myeloproliferative syndrome. Blood. 1986 Dec;68(6):1213–1217. [PubMed] [Google Scholar]
- Federici A. B., Elder J. H., De Marco L., Ruggeri Z. M., Zimmerman T. S. Carbohydrate moiety of von Willebrand factor is not necessary for maintaining multimeric structure and ristocetin cofactor activity but protects from proteolytic degradation. J Clin Invest. 1984 Dec;74(6):2049–2055. doi: 10.1172/JCI111628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura Y., Titani K., Holland L. Z., Russell S. R., Roberts J. R., Elder J. H., Ruggeri Z. M., Zimmerman T. S. von Willebrand factor. A reduced and alkylated 52/48-kDa fragment beginning at amino acid residue 449 contains the domain interacting with platelet glycoprotein Ib. J Biol Chem. 1986 Jan 5;261(1):381–385. [PubMed] [Google Scholar]
- Fulcher C. A., Zimmerman T. S. Characterization of the human factor VIII procoagulant protein with a heterologous precipitating antibody. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1648–1652. doi: 10.1073/pnas.79.5.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg D., Konkle B. A., Gill J. C., Montgomery R. R., Bockenstedt P. L., Johnson T. A., Yang A. Y. Molecular basis of human von Willebrand disease: analysis of platelet von Willebrand factor mRNA. Proc Natl Acad Sci U S A. 1989 May;86(10):3723–3727. doi: 10.1073/pnas.86.10.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralnick H. R., Williams S. B., McKeown L. P., Maisonneuve P., Jenneau C., Sultan Y., Rick M. E. In vitro correction of the abnormal multimeric structure of von Willebrand factor in type IIa von Willebrand's disease. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5968–5972. doi: 10.1073/pnas.82.17.5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton K. K., Fretto L. J., Grierson D. S., McKee P. A. Effects of plasmin on von Willebrand factor multimers. Degradation in vitro and stimulation of release in vivo. J Clin Invest. 1985 Jul;76(1):261–270. doi: 10.1172/JCI111956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghten R. A. General method for the rapid solid-phase synthesis of large numbers of peptides: specificity of antigen-antibody interaction at the level of individual amino acids. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5131–5135. doi: 10.1073/pnas.82.15.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A., Hoyer L. W., Nachman R. L. Synthesis of von Willebrand factor by cultured human endothelial cells. Proc Natl Acad Sci U S A. 1974 May;71(5):1906–1909. doi: 10.1073/pnas.71.5.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunicki T. J., Montgomery R. R., Schullek J. Cleavage of human von Willebrand factor by platelet calcium-activated protease. Blood. 1985 Feb;65(2):352–356. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liu F. T., Bohn J. W., Ferry E. L., Yamamoto H., Molinaro C. A., Sherman L. A., Klinman N. R., Katz D. H. Monoclonal dinitrophenyl-specific murine IgE antibody: preparation, isolation, and characterization. J Immunol. 1980 Jun;124(6):2728–2737. [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Pareti F. I., Niiya K., McPherson J. M., Ruggeri Z. M. Isolation and characterization of two domains of human von Willebrand factor that interact with fibrillar collagen types I and III. J Biol Chem. 1987 Oct 5;262(28):13835–13841. [PubMed] [Google Scholar]
- Ruggeri Z. M., Houghten R. A., Russell S. R., Zimmerman T. S. Inhibition of platelet function with synthetic peptides designed to be high-affinity antagonists of fibrinogen binding to platelets. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5708–5712. doi: 10.1073/pnas.83.15.5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggeri Z. M., Zimmerman T. S. The complex multimeric composition of factor VIII/von Willebrand factor. Blood. 1981 Jun;57(6):1140–1143. [PubMed] [Google Scholar]
- Ruggeri Z. M., Zimmerman T. S. Variant von Willebrand's disease: characterization of two subtypes by analysis of multimeric composition of factor VIII/von Willebrand factor in plasma and platelets. J Clin Invest. 1980 Jun;65(6):1318–1325. doi: 10.1172/JCI109795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggeri Z. M., Zimmerman T. S. von Willebrand factor and von Willebrand disease. Blood. 1987 Oct;70(4):895–904. [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sasaki T., Kikuchi T., Yumoto N., Yoshimura N., Murachi T. Comparative specificity and kinetic studies on porcine calpain I and calpain II with naturally occurring peptides and synthetic fluorogenic substrates. J Biol Chem. 1984 Oct 25;259(20):12489–12494. [PubMed] [Google Scholar]
- Sporn L. A., Chavin S. I., Marder V. J., Wagner D. D. Biosynthesis of von Willebrand protein by human megakaryocytes. J Clin Invest. 1985 Sep;76(3):1102–1106. doi: 10.1172/JCI112064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson E. A., Howard M. A. Proteolytic cleavage of human von Willebrand factor induced by enzyme(s) released from polymorphonuclear cells. Blood. 1986 May;67(5):1281–1285. [PubMed] [Google Scholar]
- Titani K., Kumar S., Takio K., Ericsson L. H., Wade R. D., Ashida K., Walsh K. A., Chopek M. W., Sadler J. E., Fujikawa K. Amino acid sequence of human von Willebrand factor. Biochemistry. 1986 Jun 3;25(11):3171–3184. doi: 10.1021/bi00359a015. [DOI] [PubMed] [Google Scholar]
- Zimmerman T. S., Dent J. A., Ruggeri Z. M., Nannini L. H. Subunit composition of plasma von Willebrand factor. Cleavage is present in normal individuals, increased in IIA and IIB von Willebrand disease, but minimal in variants with aberrant structure of individual oligomers (types IIC, IID, and IIE). J Clin Invest. 1986 Mar;77(3):947–951. doi: 10.1172/JCI112394. [DOI] [PMC free article] [PubMed] [Google Scholar]