Abstract
Although several epidemiological studies have investigated the association between dietary calcium intake and the risk of esophageal cancer, the results are inconsistent. This study aimed to make a comprehensive evaluation regarding the association between calcium intake and risk of esophageal cancer through a meta-analysis approach. We searched for all relevant articles from the inception to April 2017, using PUBMED, EMBASE, and Web of Knowledge. The pooled odds ratio (ORs) with the 95% confidence interval (95% CI) for the highest versus the lowest categories of calcium intake was calculated using a Mantel–Haenszel fixed-effect model. In total, 15 articles reporting 17 studies including 3396 esophageal cancer cases and 346,815 controls were selected for the meta-analysis. By comparing the highest vs. the lowest levels of dietary calcium intake, we found that dietary calcium intake was inversely associated with the risk of esophageal cancer (OR = 0.80, 95% CI: 0.71–0.91, I2 = 33.6%). The subgroup analysis indicated that the protective function of dietary calcium intake were observed in esophageal squamous cell cancer, but not in esophageal adenocarcinoma in the studies conducted in Asia, but not those in Europe and America. In conclusion, our results suggest that higher dietary calcium intake is associated with a lower risk of esophageal cancer—especially esophageal squamous cell cancer—in Asian populations, though more data from prospective cohort studies are needed.
Keywords: esophageal cancer, dietary calcium, meta-analysis
1. Introduction
Esophageal cancer (EC) is one of the most common cancers in the world. With estimated 455,800 new cases and 400,200 deaths in 2012, esophageal cancer has been the tenth most common malignancy and the eighth leading cause of cancer-related deaths [1]. In fact, the overall 5-year survival is less than 20%, due to the diagnosis made at advanced stage [2]. Therefore, a better understanding of the etiology is especially important for esophageal cancer prevention and control.
Epidemiologic evidence demonstrated a number of risk factors for esophageal cancer, such as age, gender, alcohol drinking, tobacco smoking, obesity, chronic gastroesophageal reflux disease, dietary carcinogens, and insufficiencies of micronutrient consumption [3,4,5], among which dietary factors may play a more important role [6]. Accordingly, chemopreventive agents for esophageal cancer have attracted great attention, including vitamin C, vitamin E, carotenoids, and various minerals [7,8]. Calcium is an essential element, and is only available to the human body through dietary sources. It plays a critical role in skeletal mineralization, and presents a wide range of biological functions in soft tissues [9], including antitumor properties. Animal studies have suggested that high calcium intake could suppress the cell cycle, promote apoptosis, and reduce the formation of colonic tumors [10]. Compared to oral supplements, dietary calcium intake is relatively safe. Thus, many prior meta-analyses have focused on the association between dietary calcium intake and risk of colorectal cancer, breast cancer, and prostate cancer [11,12,13]. However, there is no systemic analysis carried out regarding the relationship between calcium intake and the risk of esophageal cancer. For esophageal cancer, the published results in epidemiological studies are controversial. Therefore, we conducted a meta-analysis to assess the association of dietary calcium intake and the risk of esophageal cancer.
2. Materials and Methods
2.1. Search Strategy
Three electronic databases (PUBMED, EMBASE, and Web of Knowledge) were searched aiming to assess the association between dietary calcium intake and esophageal cancer up to April 2017. The following search terms were used: (calcium OR dairy products OR dairy OR milk OR cheese OR yogurt OR cream) AND (esophagus OR esophageal OR oesophageal) AND (cancer OR tumor OR tumour OR carcinoma OR neoplasm). Besides, the references cited within the related articles were also searched for additional eligible publications. Only full-text original journal articles with a cohort or case–control study design were included.
2.2. Study Selection
To be included in this meta-analysis, the studies had to meet the following inclusion criteria: (1) the study design was a cohort or case–control study; (2) the exposure of interest was dietary calcium intake; (3) the outcome was esophageal cancer; (4) relative risk (RR), hazard ratio (HR), or odds ratio (OR), and corresponding 95% confidence intervals (95% CI) for the highest versus the lowest calcium intake were reported or could be calculated. If data were duplicated in more than one study, the one with the largest number of cases or the longest follow-up period was selected.
2.3. Data Extraction
Two reviewers independently assessed the articles with the inclusion criteria and extracted data with a standardized form [14], and any discrepancies were resolved by a third investigator. Information extracted from each article included the first author’s last name, publication year, country, study design (cohort or case–control study), pathological type (esophageal adenocarcinoma (EAC); esophageal squamous cell carcinoma (ESCC); and mix type, which represents undefined pathological type), numbers of case and control, dietary assessment method, the reported ORs (RRs) and 95% CIs with the most adjustment for the highest versus the lowest calcium intake. Besides, quality assessment was performed according to the Newcastle–Ottawa scale [15]. The scores of 0–3, 4–6, and 7–9 were regarded as low, moderate, and high quality, respectively.
2.4. Statistical Analysis
The pooled ORs with corresponding 95% CI (highest versus lowest categories of calcium intake) were calculated to measure the association across studies. The heterogeneity among studies was examined by Q-test and I2 statistics. Generally, for the Q-test, heterogeneity with a value of p < 0.05 was considered as statistically significant. For the I2 statistic, the following cut-off points were used: <25% (low heterogeneity), 25–50% (moderate heterogeneity), and >75% (severe heterogeneity). If p < 0.05 and I2 > 50%, a DerSimonian and Laird random-effect model was used. Otherwise, a Mantel–Haenszel fixed-effect model was applied [16].
Meta-regression and subgroup analyses were performed to explore the possible source of heterogeneity based on geographic location (America, Europe, and Asia), study design (population-based case–control (PBCC), hospital-based case-control (HBCC), and cohort), pathological type (EAC, ESCC, and Mix type), dietary assessment (validated method or not validated method), publication year (before/in 2000 or after 2000), and adjustment for energy intake/body mass index (yes or no) [17]. The “leave-one-out” sensitive analysis was applied to test the stability of the meta-analysis results [18]. Both Begg’s rank correlation test and Egger’s linear regression test were performed to investigate potential publication bias (p < 0.10) [19]. The statistical analyses were performed using STATA version 11.0 (Stata Corporation, College Station, TX, USA). All the p-values were two-tailed, and p < 0.05 was considered as significant, unless explicitly stated.
3. Results
3.1. Characteristics of the Included Studies
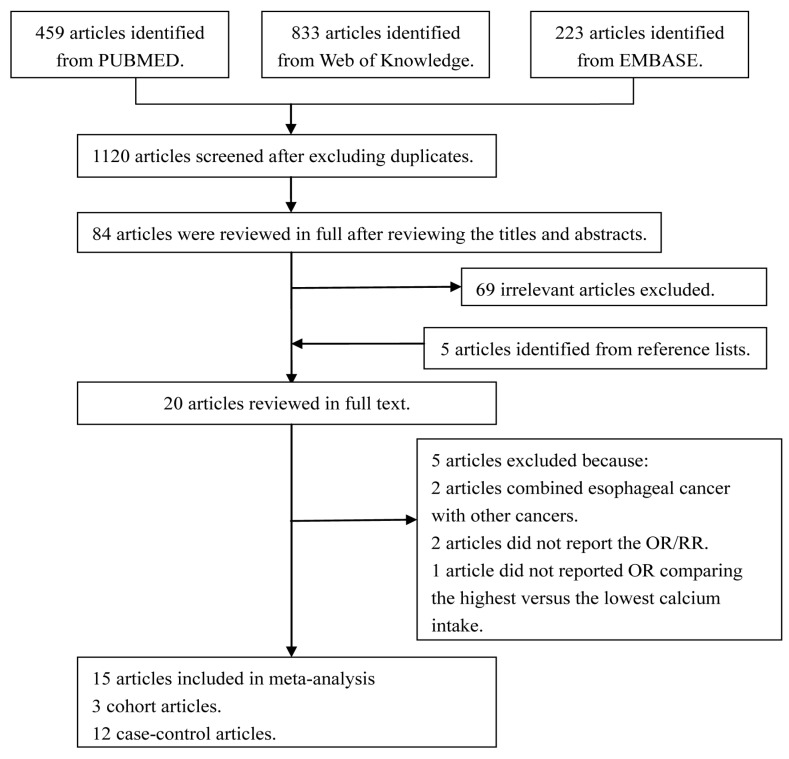
The study selection process and the results are shown in Figure 1. With our search strategy, a total of 459 articles from PUBMED, 236 articles from EMBASE, and 833 articles from Web of Knowledge were identified. After removing duplicates and studies that did not meet the inclusion criteria and adding articles identified through references review, 20 articles were reviewed in full. Among them, two articles did not report the OR/RR, two articles reported the association between calcium and cancer of upper aerodigestive tract, and one article only provided the OR comparing the 75th versus the 25th percentile of calcium intake to estimate the risk of esophageal cancer. As a result, 15 articles reporting 17 studies including 3396 esophageal cancer cases and 346,815 controls were selected for the meta-analysis [8,20,21,22,23,24,25,26,27,28,29,30,31,32,33]. Detailed characteristics of the included studies are presented in Table 1.
Figure 1.
The flow diagram of screened, excluded, and analyzed publications.
Table 1.
Characteristics of the included studies on dietary calcium intake and risk of esophageal cancer.
| Author, Year | Country | Study-Design | Pathological Type | Source of Control | Dietary Assessment | Participants (Cases) | Comparison | OR or RR (95% CI) | NOS Score | Adjustment for Covariates |
|---|---|---|---|---|---|---|---|---|---|---|
| Hashemian, 2015 [8] | Iran | Cohort | ESCC | PB | FFQ-116 items, validated | 47,204 (201) | ≥1048.0 vs. <409 (mg/day) | 0.49 (0.29–0.82) | 8 | Age, sex, total energy, place of residence, smoking, wealth score, ethnicity, opiate use, BMI, education, marital status, physical activity score, and fruit and vegetable intakes |
| Mulholland, 2011 [20] | Ireland | Case–control | EAC | PB | FFQ-101 items, validated | 252 (218) | ≥1262.0 vs. <929.3 (mg/day) | 0.88 (0.38–2.03) | 6 | Age, sex, energy intake, smoking status, BMI, education, occupation, alcohol, regular non-steroidal anti-inflammatory drug use, Helicobacter pylori infection, energy-adjusted glycemic index intake, energy-adjusted saturated fat intake, and location |
| Wolfgarten, 2001 [21] | Germany | Case–control | EAC | PB | DHQ, NA | 100 (40) | >1590 vs. <986 (mg/day) | 0.4 (0.1–1.3) | 7 | Age, residence, and nationality |
| Wolfgarten, 2001 [21] | Germany | Case–control | ESCC | PB | DHQ, NA | 100 (45) | >1590 vs. <986 (mg/day) | 0.4 (0.1–1.1) | 7 | Age, residence, and nationality |
| Graham, 1990 [22] | United states | Case–control | Mix type | PB | FFQ, NA | 174 (178) | >1028.5 vs. <543.5 (mg/day) | 2.15 (1.14–4.06) | 4 | Sex, age, education, smoking, and alcohol ingestion |
| Franceschi, 2000 [23] | Italy | Case–control | ESCC | HB | FFQ-78 items, validated | 743 (304) | Q5 vs. Q1 | 1.0 (0.6–1.7) | 8 | Age, gender, area of residence, education, physical activity, BMI, tobacco smoking, alcohol drinking, and non-alcohol energy |
| Jessri, 2011 [24] | Iran | Case–control | ESCC | HB | FFQ-125 items, validated | 96 (47) | T3 vs. T1 | 0.49 (0.15–0.87) | 7 | Age, sex, gastroesophageal reflux disease symptoms, BMI, smoking status, smoking intensity and duration (pack-years), physical activity, and education level |
| Chen, 2002 [25] | United states | Case–control | EAC | PB | DHQ, validated | 449 (124) | Q4 vs. Q1 | 0.5 (0.2–0.9) | 7 | Age, age squared, gender, respondent type, BMI, alcohol use, tobacco use, education level, family history of respective cancers, and vitamin supplement use |
| Park, 2009 [26] | United states | Cohort | Mix type | PB | FFQ-124 items, validated | 29,3439 (468) | >1247 vs. <478 (mg/day) | 0.66 (0.49–0.90) | 9 | Smoking status, time since quitting smoking, smoking dose, antacid use, personal history of diabetes, and hypertension |
| Lu, 2006 [27] | China | Case–control | ESCC | PB | FFQ-97 items, NA | 415 (218) | ≥344 vs. <157 (mg/day) | 0.82 (0.38–1.75) | 8 | Age, gender, educational level, income, BMI, total energy intake, smoking, and drinking. |
| Rogers, 1993 [32] | United states | Case–control | Mix type | PB | FFQ, NA | 593 (127) | >1419 vs. <571 (mg/day) | 0.6 (0.3–1.5) | 6 | Age, sex, pack-years of cigarette use, drink-years of alcohol, energy intake, β-carotene intake, and ascorbic acid intake |
| Tuyns, 1987 [28] | France | Cohort | mix type | PB | DHQ, validate | 2788 (743) | >1000 vs. <600 (mg/day) | 0.84 (0.56–1.25) | 3 | Age, alcohol consumption, and tobacco smoking |
| Tzonou A 1996 [31] | Greece | Case–control | ESCC | HB | FFQ-115 items, validated | 243 (43) | Q5 vs. Q1 | 0.92 (0.64–1.32) | 7 | Gender, age, birthplace, schooling, height, analgesics, coffee drinking, alcohol intake, tobacco smoking, and energy intake. |
| Tzonou A 1996 [31] | Greece | Case–control | EAC | HB | FFQ-115 items, validated | 256 (56) | Q5 vs. Q1 | 1 (0.72–1.4) | 7 | Gender, age, birthplace, schooling, height, analgesics, coffee drinking, alcohol intake, tobacco smoking, and energy intake, though not mutually |
| Zhang, 1997 [29] | United States | Case–control | EAC | HB | HHHQ, validated | 189 (29) | Q4 vs. Q1 | 1.3 (0.5–3.3) | 5 | Age, sex, race, education, total dietary intake of calories. Smoking, alcohol use, and BMI |
| Tang, 2014 [30] | China | Case–control | mix type | HB | FFQ-137 items, validated | 739 (359) | >470 vs. <260 (mg/day) | 0.75 (0.52–1.1) | 8 | Age, gender, education level, BMI, total energy intake, smoking status, alcohol drinking, and family history of cancer in first-degree relatives. |
| Hu,1994 [33] | China | Case–control | mix type | HB | FFQ-32 items, NA | 588 (196) | Q4 vs. Q1 | 0.8 (0.4–1.4) | 7 | Alcohol intake, smoking. and family income |
Abbreviations: EAC, esophageal adenocarcinoma; ESCC, esophageal squamous cell cancer; HB, hospital-based; PB, population-based; DHQ, Dietary History Questionnaire; FFQ, Food Frequency Questionnaire; HHHQ, Health Habits and History Questionnaire; OR, odds ratio; CI, confidence interval; N/A, not available.
3.2. Meta-Analysis of Calcium Intake and Esophageal Cancer Risk
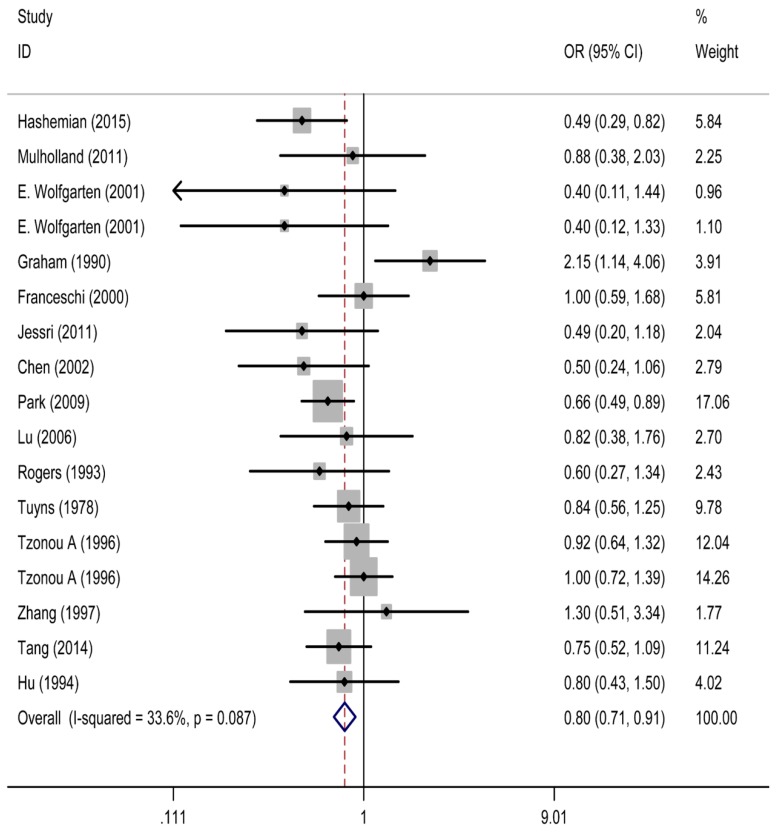
As shown in Figure 2, the pooled OR of esophageal cancer for the highest versus lowest category of calcium intake was 0.80 (95% CI: 0.71, 0.91), with moderate heterogeneity (I2 = 33.6%), suggesting that calcium intake was inversely associated with the risk of esophageal cancer.
Figure 2.
The forest plot between highest vs. lowest categories of dietary calcium intake and esophageal cancer. OR, relative risk; CI, confidence interval.
3.3. Heterogeneity Analysis
Next, we conducted meta-regression analysis and subgroup analyses by geographic location, study design, pathological type, dietary assessment, publication year, as well as adjustment for energy intake and BMI. Meta-regression analysis showed that the inverse association between calcium intake and esophageal cancer was not significantly affected by these factors (p > 0.05).
As shown in Table 2, when stratified by geographic location, publication year, and adjustment for energy intake, the statistically inverse association between dietary calcium intake and risk of esophageal cancer remained in studies conducted in Asia (OR = 0.67, 95% CI: 0.52, 0.86, I2 = 0.0%), published after 2000 (OR = 0.64, 95% CI: 0.53, 0.77, I2 = 0.0%), and adjusted for dietary energy intake (OR = 0.83, 95% CI: 0.70, 0.98, I2 = 3.6%), with no statistically significant heterogeneity. Likewise, negative association was also found in studies of ESCC (OR = 0.76, 95% CI: 0.60, 0.96, I2 = 28.3%), cohort studies (OR = 0.67, 95% CI: 0.54, 0.84, I2 = 23.6%), and studies with high quality score (OR = 0.76, 95% CI: 0.66, 0.87, I2 = 12.7%), as well as in studies using validated method (OR = 0.81, 95% CI: 0.48, 1.35, I2 = 15.5%). The heterogeneity alleviated in all four subgroups. The inverse association was not altered in either subgroup when stratified by adjustment for BMI. Heterogeneity decreased in the subgroup adjusted for BMI, while it increased in the other subgroup.
Table 2.
Subgroup analysis of dietary calcium intake and risk of esophageal cancer.
| Subgroups | No. of Studies | No. of Cases | Pooled ORs (95% CI) | p | Heterogeneity Test | ||
|---|---|---|---|---|---|---|---|
| Chi-Square | I2 | Phet | |||||
| All studies | 17 | 3396 | 0.80 (0.71, 0.91) | 0.001 | 24.11 | 33.6% | 0.087 |
| Location | |||||||
| Europe | 7 | 1449 | 0.90 (0.75, 1.08) | 0.262 | 3.97 | 0.0% | 0.681 |
| America | 5 | 926 | 0.88 (0.51, 1.49) | 0.625 | 13.83 | 71.1% | 0.008 |
| Asia | 5 | 1021 | 0.67 (0.52, 0.86) | 0.002 | 2.80 | 0.0% | 0.591 |
| Study design | |||||||
| Cohort | 3 | 1412 | 0.67 (0.54, 0.84) | 0.000 | 2.62 | 23.6% | 0.270 |
| PBCC | 7 | 950 | 0.84 (0.61, 1.14) | 0.261 | 13.68 | 56.2% | 0.033 |
| HBCC | 7 | 1034 | 0.89 (0.74, 1.06) | 0.182 | 4.00 | 0.0% | 0.677 |
| Pathological type | |||||||
| ESCC | 6 | 858 | 0.76 (0.60, 0.96) | 0.019 | 6.97 | 28.3% | 0.223 |
| EAC | 5 | 467 | 0.89 (0.68, 1.16) | 0.381 | 4.84 | 17.4% | 0.304 |
| Mixed type | 6 | 2071 | 0.84 (0.63, 1.13) | 0.252 | 11.50 | 56.5% | 0.042 |
| Dietary assessment | |||||||
| Validated method | 11 | 2592 | 0.79 (0.69, 0.90) | 0.001 | 11.84 | 15.5% | 0.296 |
| Not Validated method | 6 | 804 | 0.81 (0.48, 1.35) | 0.413 | 11.68 | 57.2% | 0.039 |
| NOS score | |||||||
| Low quality | 1 | 743 | 0.84 (0.56, 1.25) | 0.395 | 0.00 | N/A | N/A |
| Moderate quality | 4 | 552 | 1.14 (0.63, 2.05) | 0.671 | 6.64 | 54.8% | 0.084 |
| High quality | 12 | 2101 | 0.76 (0.66, 0.87) | 0.000 | 12.60 | 12.7% | 0.320 |
| Adjustment for energy intake | |||||||
| Yes | 8 | 1251 | 0.83 (0.70, 0.98) | 0.031 | 7.26 | 3.6% | 0.402 |
| No | 9 | 2145 | 0.78 (0.58, 1.04) | 0.093 | 16.61 | 51.8% | 0.034 |
| Adjustment forBMI | |||||||
| Yes | 8 | 1500 | 0.72 (0.58, 0.90) | 0.003 | 7.16 | 2.2% | 0.413 |
| No | 9 | 1896 | 0.85 (0.73, 0.99) | 0.037 | 15.57 | 48.6% | 0.049 |
| Publication year | |||||||
| Before/in 2000 | 8 | 1676 | 0.97 (0.82, 1.16) | 0.767 | 8.75 | 20.0% | 0.271 |
| After 2000 | 9 | 1720 | 0.64 (0.53, 0.77) | 0.000 | 4.58 | 0.0% | 0.802 |
Abbreviations: ESCC, esophageal squamous cell cancer; EAC, esophageal adenocarcinoma; PBCC, population-based case–control; HBCC, hospital-based case–control; NOS, Newcastle–Ottawa scale; BMI, body mass index; OR, odds ratio; CI, confidence interval; N/A, not available.
3.4. Sensitivity Analysis and Publication Bias
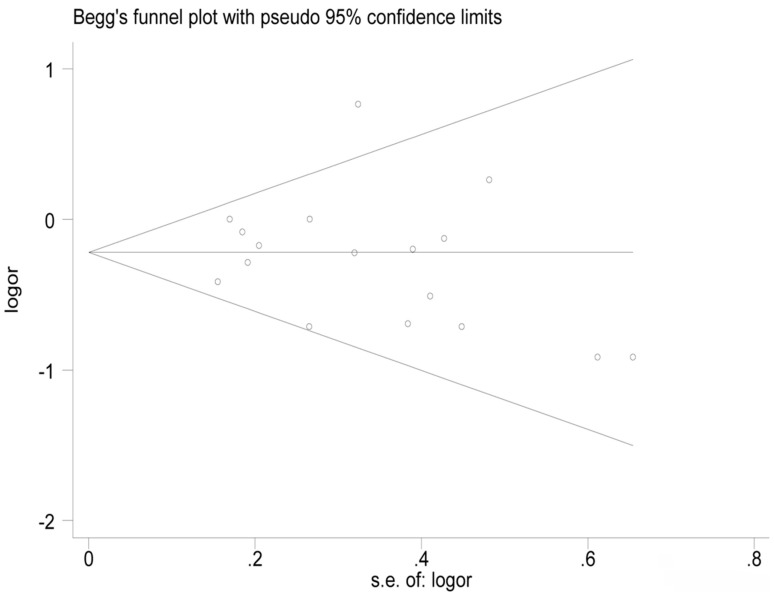
Sensitivity analysis was conducted by leaving one study out in turn and pooling the ORs of the remaining studies. The summary ORs did not substantially change, which indicated that our results were statistically robust. However, after excluding the study conducted by Graham [22], there was no statistically significant heterogeneity in the remaining studies (I2 = 0.0%, p = 0.489). Egger’s test showed no evidence of significant publication bias in this meta-analysis (t = −0.65, p = 0.523). The funnel plot was provided in Figure 3.
Figure 3.
Funnel plots of dietary calcium intake and the risk of esophageal cancer.
4. Discussion
This study is the first systemic meta-analysis regarding the association between dietary calcium intake and the risk of esophageal cancer, which is based on 15 articles reporting 17 studies (3 cohort studies and 14 case–control studies), including 3396 cases and 346,815 controls. The sample size is large enough to evaluate the effect of calcium intake on esophageal cancer.
In addition to its bone formation role, calcium is also a ubiquitous second messenger and plays a critical role in human health [34]. It was hypothesized that calcium intake could reduce the risk of cancer by promoting the activation of transcription factors CREB (cAMP response element binding protein) [35] and oncogenic Ras [36], downregulating the synthesis of 1,25-dihydroxyvitamin D [37], inducing cell cycle arrest, and promoting cell differentiation and tumor cell apoptosis. The previous studies have shown that a high calcium diet could induce cell differentiation and suppress cell proliferation and carcinogenesis production underlying the expression of p120-catenin and the formation of p120-dependent E-cadherin-β-catenin-p120-catenin complex in epithelial tissues in mice [38,39]. In addition, an in vitro model of esophageal squamous cell differentiation proposed that extracellular calcium could induce esophagin expression by upregulating the activity of esophagin promoter, which is silenced at the transcription level in esophageal tumors [40,41]. To date, studies of chemopreventive agents for esophageal cancer have attracted great attention. For example, in our previous meta-analysis, it was found that intakes of anthocyanidins, flavanones, and flavones could significantly reduce the risk of esophageal cancer by 40%, 35%, and 22%, respectively [42]. Likewise, the meta-analysis conducted by Bo and her colleagues showed that vitamin C intake was associated with a 42% reduction in esophageal cancer risk [43]. In the current meta-analysis, we found that calcium intake was associated with a 33% reduction in esophageal cancer risk in Asian populations, which supports another important piece of information for the chemoprevention of esophageal cancer. Numerous studies have investigated the association between dietary calcium intake and various cancer risks. Previous meta-analyses have found that dietary calcium intake might have a protective effect on colorectal cancer (RR = 0.86, 95% CI = 0.78–0.95) [44] and breast cancer (RR = 0.92, 95% CI = 0.85–0.99) [45], but have an opposite influence on the risk of prostate cancer (RR = 1.18, 95% CI = 1.08–1.30) [46], while having no significant relationship with the risk of lung cancer (RR = 0.85, 95% CI = 0.63–1.13) [47]. The findings presented in this meta-analysis add new information regarding the association between calcium intake and cancer risk.
However, some information should be considered in the subgroup analyses. First and foremost, in the subgroup analysis by geographic locations, we found the inverse association between dietary calcium intake and esophageal cancer in studies of Asia, but not in studies of America and Europe, which indicated that the results are acceptable to Asian populations, but cannot be extended to Americans and Europeans. According to the information about calcium intake quantity presented in Table 1, we found that the average of highest dietary calcium intake in Asian populations (621 mg/day) was much lower than in Americans (1284 mg/day) and Europeans (1232 mg/day). Similar findings were reported before [48]. In addition, Dai and colleagues recently reported that high calcium intake may decrease the risk of colorectal adenoma only in the context of the lower dietary calcium: magnesium intake ratio [49]. However, epidemiological evidence reported that the ratio of Ca: Mg intake is much higher in the US (2.8) [50] and Irish populations (3.0) [51] compared with the East Asian population (1.6) [52], which might be another explanation for the heterogeneity in different geographic locations. Second, when stratified by pathological type, a significant inverse association was found in studies of ESCC, but not in studies of EAC, which indicated that the protective effect of dietary calcium intake on esophageal cancer might be pathological type-selective. Third, the inverse association still existed in prospective cohort studies, but not in case–control subgroups, which might be explained by the limitation of sample size and the influence of recall bias in case–control studies. In addition, the inverse association persisted in studies using validated dietary assessment methods for calcium intake estimation, but not in those using non-validated ones. Misclassification of exposure may be introduced, which could lead to an inaccurate estimation of the association between calcium intake and esophageal cancer risk [13]. Moreover, the results also differed when stratified by publication year. We found a strong inverse correlation in studies published after 2000, but no statistical association in studies published before or in 2000. Similarly, we also found an inverse association in studies with high quality scores, but no statistical association in studies with low or moderate quality scores. Thus, we could partially attribute the inconsistent result in studies published before or in 2000 to the lower average quality score (5.9), compared with studies published after 2000 (7.6). Finally, we found that the significant inverse association persisted in studies adjusted by dietary energy intake, but not in the other subgroup, which suggested that the status of dietary energy intake might confound the association between dietary calcium intake and esophageal cancer.
The present meta-analysis has several advantages. First, our analysis is the first comprehensive meta-analysis to reveal the possible associations between dietary calcium intake and risk of esophageal cancer. Second, it enables a reliable conclusion by using meta-regression analyses and sub-group analyses to explore the sources of heterogeneity. However, several limitations should also be noted. First, most of the eligible studies were case–control studies, which were difficult to rule out the influence of recall bias. Second, the levels of dietary calcium intake ranged widely among the studies included in our meta-analysis, and the definitions of the intake categories were different. Another limitation is that this meta-analysis is study-based, but not individual patient-based. Finally, we did not perform a dose–response effect analysis due to the incomplete data of dietary calcium intake.
5. Conclusions
The present study suggests that a higher intake of dietary calcium might have protective effect against esophageal cancer—especially esophageal squamous cell cancer—in Asian populations. To further solidify the association of dietary calcium intake with the risk of esophageal cancer, well-designed studies—especially prospective cohort studies with validated FFQ and adjusted for dietary energy intake—should be conducted.
Acknowledgments
This work was supported by National Natural Science Foundation of China (81402670, Lingling Cui). The funding sponsors had no role in the study design, data collection and analysis; in the writing of the manuscript, and in the decision to publish the results.
Author Contributions
Qianwen Li and Ling Wang designed the study; Qianwen Li, Lingling Cui and Yalan Tian assessed studies for inclusion, extracted data, and assessed validity; Qianwen Li, Lingling Cui and Han Cui conducted meta-analyses; Li Li, Weifeng Dou, Haixia Li tabulated data; Qianwen Li wrote the first draft of the manuscript; Ling Wang and Lingling Cui provided critical review of the manuscript.
Conflict of Interest
The authors declared that they had no conflict of interest.
References
- 1.Torre L.A., Bray F., Siegel R.L., Ferlay J., Lortet-Tieulent J., Jemal A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Palladino-Davis A.G., Mendez B.M., Fisichella P.M., Davis C.S. Dietary habits and esophageal cancer. Dis. Esophagus. 2015;28:59–67. doi: 10.1111/dote.12097. [DOI] [PubMed] [Google Scholar]
- 3.Islami F., Kamangar F., Nasrollahzadeh D., Moller H., Boffetta P., Malekzadeh R. Oesophageal cancer in golestan province, a high-incidence area in northern iran—A review. Eur. J. Cancer. 2009;45:3156–3165. doi: 10.1016/j.ejca.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 4.Oze I., Matsuo K., Wakai K., Nagata C., Mizoue T., Tanaka K., Tsuji I., Sasazuki S., Inoue M., Tsugane S. Alcohol drinking and esophageal cancer risk: An evaluation based on a systematic review of epidemiologic evidence among the japanese population. Jpn. J. Clin. Oncol. 2011;41:677–692. doi: 10.1093/jjco/hyr026. [DOI] [PubMed] [Google Scholar]
- 5.Mlombe Y.B., Rosenberg N.E., Wolf L.L., Dzamalala C.P., Chalulu K., Chisi J., Shaheen N.J., Hosseinipour M.C., Shores C.G. Environmental risk factors for oesophageal cancer in malawi: A case-control study. Malawi Med. J. 2015;27:88–92. doi: 10.4314/mmj.v27i3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang C.S., Chen X., Tu S. Etiology and prevention of esophageal cancer. Gastrointest. Tumors. 2016;3:3–16. doi: 10.1159/000443155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ibiebele T.I., Hughes M.C., Nagle C.M., Bain C.J., Whiteman D.C., Webb P.M. Dietary antioxidants and risk of barrett’s esophagus and adenocarcinoma of the esophagus in an australian population. Int. J. Cancer. 2013;133:214–224. doi: 10.1002/ijc.28016. [DOI] [PubMed] [Google Scholar]
- 8.Hashemian M., Poustchi H., Abnet C.C., Boffetta P., Dawsey S.M., Brennan P.J., Pharoah P., Etemadi A., Kamangar F., Sharafkhah M., et al. Dietary intake of minerals and risk of esophageal squamous cell carcinoma: Results from the golestan cohort study. Am. J. Clin. Nutr. 2015;102:102–108. doi: 10.3945/ajcn.115.107847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peacock M. Calcium metabolism in health and disease. Clin. J. Am. Soc. Nephrol. 2010;5:05910809. doi: 10.2215/CJN.05910809. [DOI] [PubMed] [Google Scholar]
- 10.Yang K., Lamprecht S.A., Shinozaki H., Fan K., Yang W., Newmark H.L., Kopelovich L., Edelmann W., Jin B., Gravaghi C., et al. Dietary calcium and cholecalciferol modulate cyclin d1 expression, apoptosis, and tumorigenesis in intestine of adenomatous polyposis coli1638n/+ mice. J. Nutr. 2008;138:1658–1663. doi: 10.1093/jn/138.9.1658. [DOI] [PubMed] [Google Scholar]
- 11.Keum N., Lee D.H., Greenwood D.C., Zhang X., Giovannucci E.L. Calcium intake and colorectal adenoma risk: Dose-response meta-analysis of prospective observational studies. Int. J. Cancer. 2015;136:1680–1687. doi: 10.1002/ijc.29164. [DOI] [PubMed] [Google Scholar]
- 12.Chen P., Hu P., Xie D., Qin Y., Wang F., Wang H. Meta-analysis of vitamin D, calcium and the prevention of breast cancer. Breast Cancer Res. Treat. 2010;121:469–477. doi: 10.1007/s10549-009-0593-9. [DOI] [PubMed] [Google Scholar]
- 13.Gao X., LaValley M.P., Tucker K.L. Prospective studies of dairy product and calcium intakes and prostate cancer risk: A meta-analysis. J. Natl. Cancer Inst. 2005;97:1768–1777. doi: 10.1093/jnci/dji402. [DOI] [PubMed] [Google Scholar]
- 14.Stroup D.F., Berlin J.A., Morton S.C., Olkin I., Williamson G.D., Rennie D., Moher D., Becker B.J., Sipe T.A., Thacker S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis of observational studies in epidemiology (moose) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 15.Stang A. Critical evaluation of the newcastle-ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 16.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins J.P., Thompson S.G. Controlling the risk of spurious findings from meta-regression. Stat. Med. 2004;23:1663–1682. doi: 10.1002/sim.1752. [DOI] [PubMed] [Google Scholar]
- 18.Patsopoulos N.A., Evangelou E., Ioannidis J.P. Sensitivity of between-study heterogeneity in meta-analysis: Proposed metrics and empirical evaluation. Int. J. Epidemiol. 2008;37:1148–1157. doi: 10.1093/ije/dyn065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mulholland H.G., Murray L.J., Anderson L.A., Cantwell M.M. Vitamin d, calcium and dairy intake, and risk of oesophageal adenocarcinoma and its precursor conditions. Br. J. Nutr. 2011;106:732–741. doi: 10.1017/S0007114511000742. [DOI] [PubMed] [Google Scholar]
- 21.Wolfgarten E., Rosendahl U., Nowroth T., Leers J., Metzger R., Holscher A.H., Bollschweiler E. Coincidence of nutritional habits and esophageal cancer in germany. Onkologie. 2001;24:546–551. doi: 10.1159/000055142. [DOI] [PubMed] [Google Scholar]
- 22.Graham S., Marshall J., Haughey B., Brasure J., Freudenheim J., Zielezny M., Wilkinson G., Nolan J. Nutritional epidemiology of cancer of the esophagus. Am. J. Epidemiol. 1990;131:454–467. doi: 10.1093/oxfordjournals.aje.a115520. [DOI] [PubMed] [Google Scholar]
- 23.Franceschi S., Bidoli E., Negri E., Zambon P., Talamini R., Ruol A., Parpinel M., Levi F., Simonato L., La Vecchia C. Role of macronutrients, vitamins and minerals in the aetiology of squamous-cell carcinoma of the oesophagus. Int. J. Cancer. 2000;86:626–631. doi: 10.1002/(SICI)1097-0215(20000601)86:5<626::AID-IJC4>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 24.Jessri M., Rashidkhani B., Hajizadeh B., Jessri M., Gotay C. Macronutrients, vitamins and minerals intake and risk of esophageal squamous cell carcinoma: A case-control study in iran. Nutr. J. 2011;10:1475–2891. doi: 10.1186/1475-2891-10-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen H., Tucker K.L., Graubard B.I., Heineman E.F., Markin R.S., Potischman N.A., Russell R.M., Weisenburger D.D., Ward M.H. Nutrient intakes and adenocarcinoma of the esophagus and distal stomach. Nutr. Cancer. 2002;42:33–40. doi: 10.1207/S15327914NC421_5. [DOI] [PubMed] [Google Scholar]
- 26.Park Y., Leitzmann M.F., Subar A.F., Hollenbeck A., Schatzkin A. Dairy food, calcium, and risk of cancer in the nih-aarp diet and health study. Arch. Intern. Med. 2009;169:391–401. doi: 10.1001/archinternmed.2008.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu H., Cai L., Mu L.N., Lu Q.Y., Zhao J., Cui Y., Sul J.H., Zhou X.F., Ding B.G., Elashoff R.M., et al. Dietary mineral and trace element intake and squamous cell carcinoma of the esophagus in a chinese population. Nutr. Cancer. 2006;55:63–70. doi: 10.1207/s15327914nc5501_8. [DOI] [PubMed] [Google Scholar]
- 28.Tuyns A.J., Riboli E., Doornbos G., Pequignot G. Diet and esophageal cancer in calvados (france) Nutr. Cancer. 1987;9:81–92. doi: 10.1080/01635588709513915. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Z.F., Kurtz R.C., Yu G.P., Sun M., Gargon N., Karpeh M., Jr., Fein J.S., Harlap S. Adenocarcinomas of the esophagus and gastric cardia: The role of diet. Nutr. Cancer. 1997;27:298–309. doi: 10.1080/01635589709514541. [DOI] [PubMed] [Google Scholar]
- 30.Tang L., Lee A.H., Xu F., Zhang T., Lei J., Binns C.W. Fruit and vegetable consumption and risk of esophageal cancer: A case-control study in north-west china. Dis. Esophagus. 2014;27:777–782. doi: 10.1111/dote.12157. [DOI] [PubMed] [Google Scholar]
- 31.Tzonou A., Lipworth L., Garidou A., Signorello L.B., Lagiou P., Hsieh C., Trichopoulos D. Diet and risk of esophageal cancer by histologic type in a low-risk population. Int. J. Cancer. 1996;68:300–304. doi: 10.1002/(SICI)1097-0215(19961104)68:3<300::AID-IJC6>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 32.Rogers M.A., Thomas D.B., Davis S., Vaughan T.L., Nevissi A.E. A case-control study of element levels and cancer of the upper aerodigestive tract. Cancer Epidemiol. Biomark. Prev. 1993;2:305–312. [PubMed] [Google Scholar]
- 33.Hu J., Nyren O., Wolk A., Bergstrom R., Yuen J., Adami H.O., Guo L., Li H., Huang G., Xu X., et al. Risk factors for oesophageal cancer in northeast china. Int. J. Cancer. 1994;57:38–46. doi: 10.1002/ijc.2910570108. [DOI] [PubMed] [Google Scholar]
- 34.Berridge M.J., Lipp P., Bootman M.D. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 35.Lipskaia L., Lompre A.M. Alteration in temporal kinetics of Ca2+ signaling and control of growth and proliferation. Biol. Cell. 2004;96:55–68. doi: 10.1016/j.biolcel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 36.Cullen P.J., Lockyer P.J. Integration of calcium and ras signalling. Nat. Rev. Mol. Cell Biol. 2002;3:339–348. doi: 10.1038/nrm808. [DOI] [PubMed] [Google Scholar]
- 37.Rock C.L. Milk and the risk and progression of cancer. Nestle Nutr. Workshop Ser. Pediatr. Program. 2011;67:173–185. doi: 10.1159/000325583. [DOI] [PubMed] [Google Scholar]
- 38.Xie Z., Yuan Y., Jiang Y., Shrestha C., Chen Y., Liao L., Ji S., Deng X., Liao E., Bikle D.D. P120-catenin is required for dietary calcium suppression of oral carcinogenesis in mice. J. Cell Physiol. 2017;232:1360–1367. doi: 10.1002/jcp.25620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang Y., Liao L., Shrestha C., Li D., Li M., Mu Y., Crumrine D., Wang L., Xie Z. Inhibition of 4-nitroquinoline-1-oxide-induced oral carcinogenesis by dietary calcium. Int. J. Clin. Exp. Pathol. 2015;8:3529–3542. [PMC free article] [PubMed] [Google Scholar]
- 40.Smolinski K.N., Abraham J.M., Souza R.F., Yin J., Wang S., Xu Y., Zou T.T., Kong D., Fleisher A.S., Meltzer S.J. Activation of the esophagin promoter during esophageal epithelial cell differentiation. Genomics. 2002;79:875–880. doi: 10.1006/geno.2002.6775. [DOI] [PubMed] [Google Scholar]
- 41.Abraham J.M., Wang S., Suzuki H., Jiang H.Y., Rosenblum-Vos L.S., Yin J., Meltzer S.J. Esophagin cdna cloning and characterization: A tissue-specific member of the small proline-rich protein family that is not expressed in esophageal tumors. Cell Growth Differ. 1996;7:855–860. [PubMed] [Google Scholar]
- 42.Cui L., Liu X., Tian Y., Xie C., Li Q., Cui H., Sun C. Flavonoids, flavonoid subclasses, and esophageal cancer risk: A meta-analysis of epidemiologic studies. Nutrients. 2016;8:350. doi: 10.3390/nu8060350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bo Y., Lu Y., Zhao Y., Zhao E., Yuan L., Lu W., Cui L., Lu Q. Association between dietary vitamin c intake and risk of esophageal cancer: A dose-response meta-analysis. Int. J. Cancer. 2016;138:1843–1850. doi: 10.1002/ijc.29838. [DOI] [PubMed] [Google Scholar]
- 44.Cho E., Smith-Warner S.A., Spiegelman D., Beeson W.L., van den Brandt P.A., Colditz G.A., Folsom A.R., Fraser G.E., Freudenheim J.L., Giovannucci E., et al. Dairy foods, calcium, and colorectal cancer: A pooled analysis of 10 cohort studies. J. Natl. Cancer Inst. 2004;96:1015–1022. doi: 10.1093/jnci/djh185. [DOI] [PubMed] [Google Scholar]
- 45.Hidayat K., Chen G.C., Zhang R., Du X., Zou S.Y., Shi B.M., Qin L.Q. Calcium intake and breast cancer risk: Meta-analysis of prospective cohort studies. Br. J. Nutr. 2016;116:158–166. doi: 10.1017/S0007114516001768. [DOI] [PubMed] [Google Scholar]
- 46.Aune D., Navarro Rosenblatt D.A., Chan D.S., Vieira A.R., Vieira R., Greenwood D.C., Vatten L.J., Norat T. Dairy products, calcium, and prostate cancer risk: A systematic review and meta-analysis of cohort studies. Am. J. Clin. Nutr. 2015;101:87–117. doi: 10.3945/ajcn.113.067157. [DOI] [PubMed] [Google Scholar]
- 47.Yang Y., Wang X., Yao Q., Qin L., Xu C. Dairy product, calcium intake and lung cancer risk: A systematic review with meta-analysis. Sci. Rep. 2016;6:20624. doi: 10.1038/srep20624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Larsson S.C., Orsini N., Wolk A. Dietary calcium intake and risk of stroke: A dose-response meta-analysis. Am. J. Clin. Nutr. 2013;97:951–957. doi: 10.3945/ajcn.112.052449. [DOI] [PubMed] [Google Scholar]
- 49.Dai Q., Shrubsole M.J., Ness R.M., Schlundt D., Cai Q., Smalley W.E., Li M., Shyr Y., Zheng W. The relation of magnesium and calcium intakes and a genetic polymorphism in the magnesium transporter to colorectal neoplasia risk. Am. J. Clin. Nutr. 2007;86:743–751. doi: 10.1093/ajcn/86.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seelig M.S. The requirement of magnesium by the normal adult. Summary and analysis of published data. Am. J. Clin. Nutr. 1964;14:242–290. doi: 10.1093/ajcn/14.6.342. [DOI] [PubMed] [Google Scholar]
- 51.Dai Q., Cantwell M.M., Murray L.J., Zheng W., Anderson L.A., Coleman H.G. Dietary magnesium, calcium:Magnesium ratio and risk of reflux oesophagitis, barrett’s oesophagus and oesophageal adenocarcinoma: A population-based case-control study. Br. J. Nutr. 2016;115:342–350. doi: 10.1017/S0007114515004444. [DOI] [PubMed] [Google Scholar]
- 52.Cai H., Shu X.O., Hebert J.R., Jin F., Yang G., Liu D.K., Gao Y.T., Zheng W. Variation in nutrient intakes among women in Shanghai, China. Eur. J. Clin. Nutr. 2004;58:1604–1611. doi: 10.1038/sj.ejcn.1602013. [DOI] [PubMed] [Google Scholar]