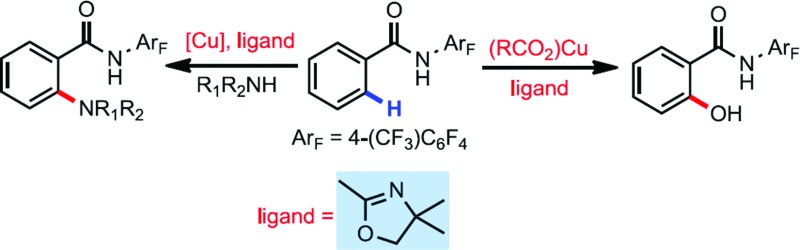
 The use of a weakly coordinating monodentate directing group for copper-mediated ortho-hydroxylation and amination reactions allows for the identification of an external oxazoline ligand as a promoter.
The use of a weakly coordinating monodentate directing group for copper-mediated ortho-hydroxylation and amination reactions allows for the identification of an external oxazoline ligand as a promoter.
Abstract
The use of a weakly coordinating monodentate directing group for copper mediated ortho-hydroxylation and amination reactions allows for the identification of an external oxazoline ligand as a promoter.
Introduction
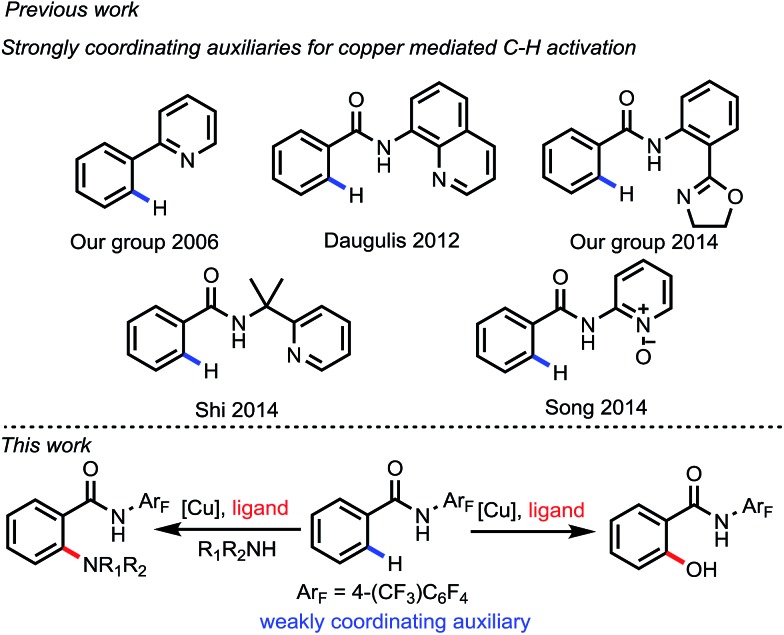
Over the past decade, directed C–H activation has emerged as a useful tool for organic synthesis.1 Diverse carbon–carbon bond and carbon–heteroatom bond forming reactions have been developed with various transition metals. In this regard, precious metals such as Pd, Rh and Ir have shown superior catalytic reactivity. Notably, Pd catalysts have demonstrated versatility in both C(sp2)–H activation and C(sp3)–H activation via different manifolds including Pd(0)/Pd(ii), Pd(ii)/Pd(0), Pd(ii)/Pd(iv) and Pd(ii)/Pd(ii) catalysis.2 However, replacing these precious metals with first-row transition metals such as Fe and Cu is more desirable due to their high abundance and low toxicity.3,4 In particular, copper-mediated C–H functionalization has made rapid progress in recent years, with various C–H transformations developed via different redox manifolds corresponding to the oxidants employed. However, owing to their low reactivity, nearly all of the inexpensive metals, especially in copper mediated C–H activation reactions, rely on strongly coordinating directing groups, for example, pyridine or bidentate pyridine-based auxiliaries (Fig. 1).4,5 The advantage of weakly coordinating directing groups to form the less thermodynamically stable metallacycle, thereby kinetically facilitating the subsequent functionalization step, has been demonstrated with only precious metal catalysts.6 The use of a weakly coordinating monodentate directing group in combination with ligand acceleration remains to be demonstrated with Cu catalysts.
Fig. 1. Auxiliaries used for copper-mediated C–H activation.
Phenols are a class of important structural motifs prevalent both in natural products and pharmaceuticals.7 Direct C–H hydroxylation is an appealing method for the synthesis of functionalized phenols. Pd-mediated hydroxylation of excess benzene at 180 °C was found to give phenol in less than 5% yield in an early study.8a Recently, directed ortho-C–H hydroxylation of simple substrates has reached synthetically useful yields with Pd and Ru catalysts.8,9 However, inexpensive metal-catalyzed or -mediated C–H hydroxylation reactions remain rare. In 2006, our group reported a pyridine-directed hydroxylation of inert C–H bonds using Cu(OAc)2 as a promoter, which involved the formation of acetoxylated products and subsequent hydrolysis.10 Recently, copper-mediated C–H hydroxylation of benzoic acid derivatives with the assistance of bidentate auxiliaries has also been disclosed.11 Guided by the development of Pd-catalyzed C–H activation reactions enabled by ligands, we set out to explore the combination of weakly coordinating monodentate directing groups and ligands for Cu-catalyzed C–H activation reactions. Herein, we report the first example of copper-mediated C–H hydroxylation and amination using a weakly coordinating directing group under the assistance of a monodentate ligand.
Results and discussion
Initial studies and reaction optimization
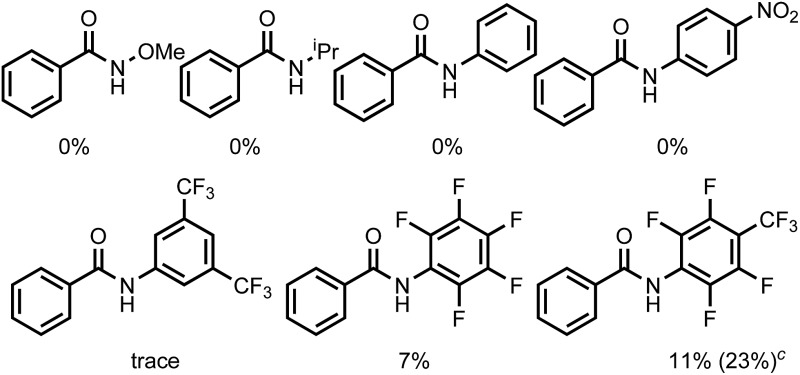
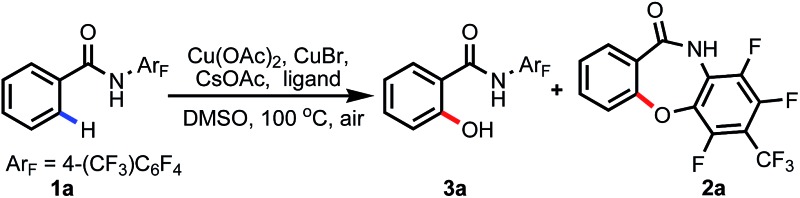
We commenced our studies with screening of a series of weakly coordinating amides (Table 1). Although displaying good reactivity in Pd-catalyzed C–H activation reactions, the N–OMe substituted amide gave no product. Other simple amides, such as N-isopropyl, N-phenyl and N-(4-NO2)-phenyl substituted amides, were also unreactive, with starting material fully recovered. Encouragingly, when N-(3,5-di-trifluoromethyl)-phenyl amide was employed as the substrate, trace product was observed. Further decreasing the electron density of the N-aryl group by using a 2,3,5,6-tetrafluoro-4-(trifluoromethyl) substituent increased the yield to 11%.12 When using 0.8 equiv. CuBr as an additive, the yield was improved to 23%. However, a side product (2a) arising from nucleophilic attack of the hydroxyl group to the C–F bond on the directing group was observed in 10% yield.
Table 1. Screening of weakly coordinating directing groups a , b .
|
|
aReaction conditions: 1a (0.1 mmol), Cu(OAc)2 (0.2 mmol), CsOAc (0.2 mmol), DMSO (1.0 mL), 100 °C, air, 6 h.
bYield determined by 1H NMR analysis of crude reaction mixture using CH2Br2 as an internal standard.
cCuBr (0.8 eq.) as an additive.
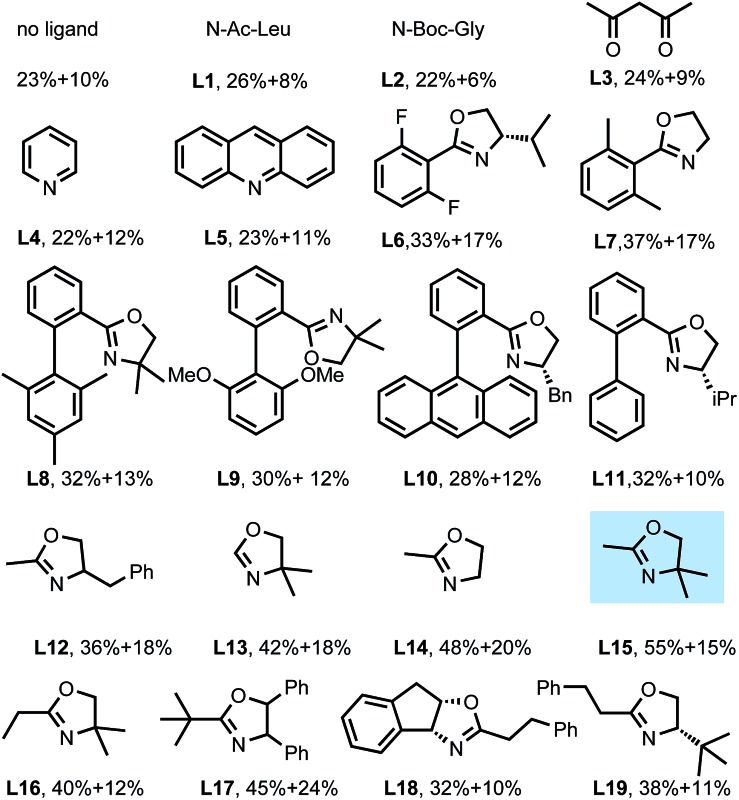
During the past several years, we have developed two kinds of ligand, the N-protected amino acid ligand and pyridine- or quinoline-based ligand, for accelerating or promoting Pd catalyzed C(sp2)–H and C(sp3)–H bond activation.13 Based on these results, we next tested the ligand effect on this reaction (Table 2). N-protected amino acids, such as N-Ac-Leu (L1) and N-Boc-Gly (L2), and pyridine- (L4) or quinoline-based (L5) ligands are found to have negligible effect on the yield. Considering that oxazoline ligands have been demonstrated to be effective in various copper-mediated reactions, we subsequently focused on investigating theses ligands. To our delight, with ligand L6 as an additive, the yield of 3a could be increased to 33%, though it also promoted the nucleophilic attack process, correspondingly affording 2a in 17% yield. Modifying the phenyl moiety on the oxazoline ligand with a biaryl framework (L8–L11) provided no improvement. Interestingly, when employing readily available oxazoline L15 as a ligand, the yield was improved to 55%. Further optimization by changing the steric bulk and electron-donating ability of the ligands proved ineffective (L16–L19, and see ESI†).
Table 2. Ligand effect on C(sp2)–H hydroxylation a , b .
|
|
aReaction conditions: 1a (0.1 mmol), Cu(OAc)2 (0.2 mmol), CuBr (0.08 mmol), CsOAc (0.2 mmol), ligand (0.1 mmol), DMSO (1.0 mL), 100 °C, air, 6 h.
bYield determined by 1H NMR analysis of crude reaction mixture using CH2Br2 as an internal standard.
Having identified the optimal ligand, we next performed further optimization of the reaction conditions to suppress the undesired product 2a (Table 3). By using Cs2CO3 and PivOH as a combined additive to generate CsOPiv in situ, the yield of 3a could be increased to 59% while decreasing the yield of 2a to 12% (Table 3, entry 1). 1-Ad-COOH proved to be a better acid, which gave the product 3a in 61% yield (Table 3, entry 2). The yield could be slightly improved to 63% when increasing the amount of 1-Ad-COOH to 1.8 equiv. (Table 3, entry 3). The reaction proceeded in the presence of 1.5 equiv. Cu(OAc)2, 0.4 equiv. ligand and 0.5 mL DMSO to provide the product in 67% yield (Table 3, entry 4–6). After a brief survey of Cu(ii) salts, we found that using Cu(OPiv)2 could inhibit the undesired product completely and increase the product yield to 75% (Table 3, entry 7–9). Finally, slightly increasing the temperature to 105 °C improved the yield to 80%.
Table 3. Further optimization of C(sp2)–H hydroxylation a , b .
| |||||
| Entry | Copper salts | Base | Acid | Yield |
|
| 3a | 2a | ||||
| 1 | Cu(OAc)2 + CuBr | Cs2CO3 | PivOH | 59 | 12 |
| 2 | Cu(OAc)2 + CuBr | Cs2CO3 | 1-Ad-COOH | 61 | 13 |
| 3 c | Cu(OAc)2 + CuBr | Cs2CO3 | 1-Ad-COOH | 63 | 12 |
| 4 c , d | Cu(OAc)2 + CuBr | Cs2CO3 | 1-Ad-COOH | 63 | 11 |
| 5 c , d , e | Cu(OAc)2 + CuBr | Cs2CO3 | 1-Ad-COOH | 66 | 8 |
| 6 c , d , e , f | Cu(OAc)2 + CuBr | Cs2CO3 | 1-Ad-COOH | 67 | 8 |
| 7 c , d , e , f | Cu(OPiv) 2 + CuBr | Cs 2 CO 3 | 1-Ad-COOH | 75(80) g | 0 |
| 8 c , d , e , f | Cu(OCOiPr)2 + CuBr | Cs2CO3 | 1-Ad-COOH | 68 | 0 |
| 9 c , d , e , f | Cu(OCO)2 + CuBr | Cs2CO3 | 1-Ad-COOH | 45 | 0 |
aReaction conditions: 1 (0.1 mmol), Cu(OAc)2 (0.2 mmol), CuBr (0.08 mmol), base (0.15 mmol), acid (0.15 mmol), ligand (0.1 mmol), DMSO (1.0 mL), 100 °C, air, 6 h.
bYield determined by 1H NMR analysis of crude reaction mixture using CH2Br2 as an internal standard.
cAcid (0.18 mmol).
dCuX2 (0.15 mmol).
eDMSO (0.5 mL).
fLigand (0.04 mmol).
g105 °C.
Substrate scope
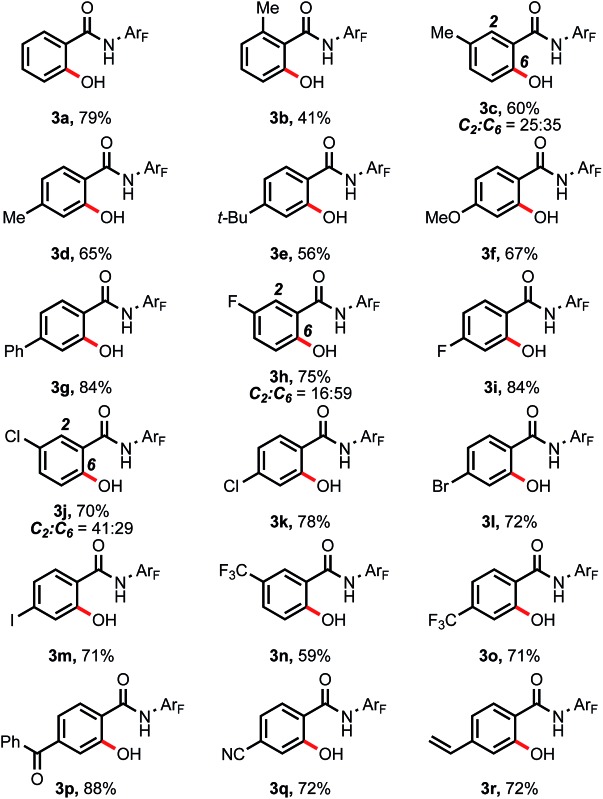
With the optimal conditions in hand, we next investigated the substrate scope (Table 4).‡ Arenes containing o-methyl and p-methyl substitutions gave yields of 41% and 65% respectively (3b and 3d), whereas an m-methyl-substituted arene afforded two regioisomers in a total of 60% yield (3c). Other electron-donating groups such as tert-butyl, methoxyl and phenyl were also well tolerated to give the corresponding products in 56–84% yields (3e–3g). An arene with m-fluoro substitution afforded a mixture of hydroxylation products at the C-2 and C-6 positions, with the sterically less hindered C-6 position as the major product (3h), while the reaction with p-fluoroarene proceeded smoothly to give the product in 84% yield (3i). Importantly, this reaction was also compatible with Cl-, Br-, and I-substituted arenes, which left synthetic handles for further functionalization (3j–3m). Arenes bearing strong electron-withdrawing functional groups underwent C–H activation efficiently, thus providing the hydroxylated products in good to excellent yields (3n–3q). Notably, this hydroxylation protocol was also compatible with a vinyl-substituted arene, which is rare in noble metal-catalyzed C–H activation reactions.
Table 4. Scope of C–H hydroxylation a , b .
|
|
aReaction conditions: 1a–1r (0.1 mmol), Cu(OPiv)2 (0.15 mmol), CuBr (0.08 mmol), Cs2CO3 (0.15 mmol), 1-Ad-COOH (0.18 mmol), ligand (0.04 mmol), DMSO (0.5 mL), 105 °C, air, 6 h.
bIsolated yield.
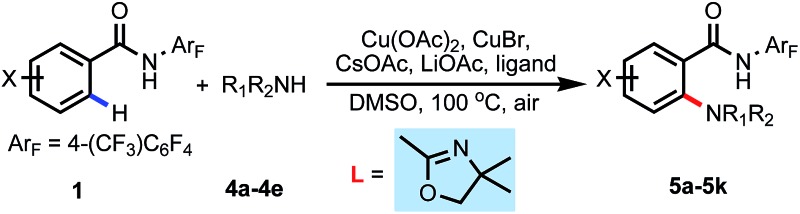
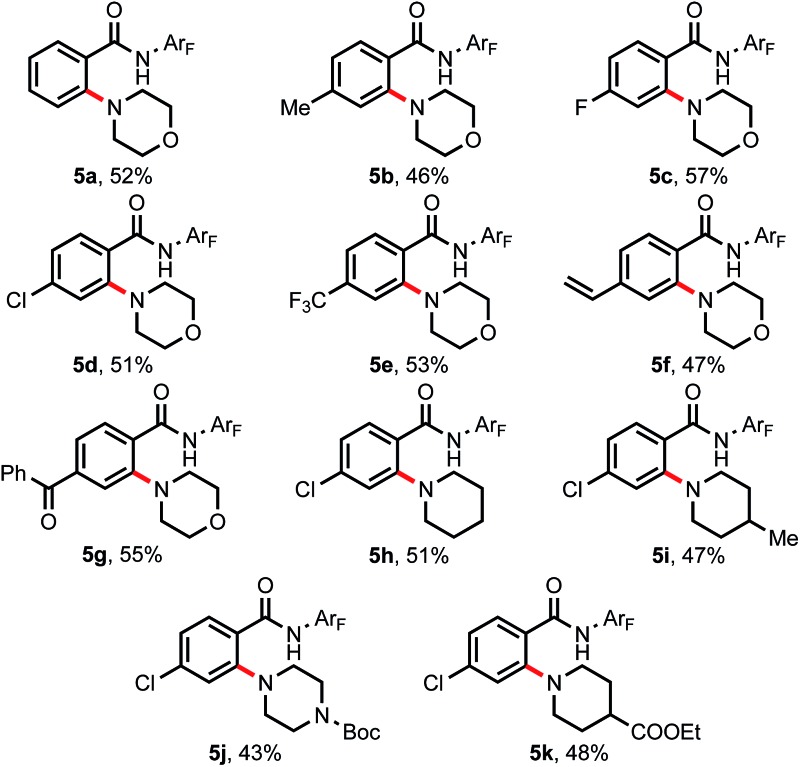
With the success of achieving C–H hydroxylation of weakly coordinating amides, we wondered whether this newly developed external ligand-promoted C–H activation protocol could be compatible with C–H amination reactions using a free alkyl amine donor, which is still an unsolved problem with weakly coordinating auxiliaries.14 To our delight, using unprotected morpholine as the amine coupling partner, the C–H amination reaction proceeded smoothly providing the desired products in moderate yields (Table 5). A variety of substituted arenes containing electron donating and electron withdrawing groups gave acceptable yields (5b–5g). Different cyclic alkyl amines also showed good compatibility with this reaction (5h–5k).
Table 5. Scope of C–H amination a , b .
|
|
aReaction conditions: 1 (0.1 mmol), 4a–4e (0.3 mmol), Cu(OAc)2 (0.2 mmol), CuBr (0.1 mmol), CsOAc (0.2 mmol), LiOAc (0.1 mmol), ligand (0.05 mmol), DMSO (1.0 mL), 100 °C, air, 6 h.
bIsolated yield.
Conclusions
In conclusion, we have developed a Cu-promoted C–H hydroxylation and amination of weakly coordinating amides with the assistance of an external oxazoline ligand. This finding provides guidance for further ligand development in promoting copper-catalyzed C–H activation reactions in the future.
Acknowledgments
We gratefully acknowledge the Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, the CAS/SAFEA International Partnership Program for Creative Research Teams, NSFC-21121062, NSFC-21472211, NSFC-21421091, Youth Innovation Promotion Association CAS (No.2014229), and The Recruitment Program of Global Experts for financial support. We gratefully acknowledge The Scripps Research Institute for financial support. This work was supported by NSF under the CCI Center for Selective C–H Functionalization, CHE-1205646, and Novartis for an unrestricted grant.
Footnotes
‡General procedure for copper-promoted ortho-C–H hydroxylation: to a 15 mL sealed tube was added substrate 1 (0.1 mmol, 1.0 equiv.), Cu(OPiv)2 (0.15 mmol), CuBr (0.08 mmol), Cs2CO3 (0.15 mmol), 1-Ad-COOH (0.18 mmol), ligand L15 (0.04 mmol) and DMSO (0.5 mL). The reaction tube was then placed into a pre-heated oil bath and stirred at 105 °C for 6 h under air. Upon completion, EtOAc was added to dilute the mixture, which was then washed with NH3·H2O and saturated NaCl(aq). The organic fraction was dried over Na2SO4, evaporated and purified by preparative TLC (EtOAc/hexane) to provide the corresponding products as white solids.
References
- For selected reviews on C–H functionalization using transition metals, see: ; (a) Kakiuchi F., Sekine S., Tanaka Y., Kamatani A., Sonoda M., Chatani N., Murai S. Bull. Chem. Soc. Jpn. 1995;68:62–83. [Google Scholar]; (b) Chen X., Engle K. M., Wang D.-H., Yu J.-Q. Angew. Chem., Int. Ed. 2009;48:5094–5115. doi: 10.1002/anie.200806273. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Giri R., Shi B.-F., Engle K. M., Maugel N., Yu J.-Q. Chem. Soc. Rev. 2009;38:3242–3272. doi: 10.1039/b816707a. [DOI] [PubMed] [Google Scholar]; (d) Lyons T. W., Sanford M. S. Chem. Rev. 2010;110:1147–1169. doi: 10.1021/cr900184e. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Colby D. A., Bergman R. G., Ellman J. A. Chem. Rev. 2010;110:624–655. doi: 10.1021/cr900005n. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Wencel-Delord J., Dröge T., Liu F., Glorius F. Chem. Soc. Rev. 2011;40:4740–4761. doi: 10.1039/c1cs15083a. [DOI] [PubMed] [Google Scholar]; (g) Arockiam P. B., Bruneau C., Dixneuf P. H. Chem. Rev. 2012;112:5879–5918. doi: 10.1021/cr300153j. [DOI] [PubMed] [Google Scholar]; (h) Ackermann L. Acc. Chem. Res. 2014;47:281–295. doi: 10.1021/ar3002798. [DOI] [PubMed] [Google Scholar]
- For Pd-catalyzed C–H functionalizations via various redox manifolds, see: ; (a) Baudoin O., Herrbach A., Guéritte F. Angew. Chem., Int. Ed. 2003;42:5736–5740. doi: 10.1002/anie.200352461. [DOI] [PubMed] [Google Scholar]; (b) Campeau L. S., Rousseaux S., Fagnou K. J. Am. Chem. Soc. 2005;127:18020–18021. doi: 10.1021/ja056800x. [DOI] [PubMed] [Google Scholar]; (c) Wasa M., Engle K. M., Yu J.-Q. J. Am. Chem. Soc. 2009;131:9886–9887. doi: 10.1021/ja903573p. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Jia C., Piao D., Oyamada J., Lu W., Kitamura T., Fujiwara Y. Science. 2000;287:1992–1995. doi: 10.1126/science.287.5460.1992. [DOI] [PubMed] [Google Scholar]; (e) Chen X., Goodhue C. E., Yu J.-Q. J. Am. Chem. Soc. 2006;128:12634–12635. doi: 10.1021/ja0646747. [DOI] [PubMed] [Google Scholar]; (f) Ball N. D., Kampf J. W., Sanford M. S. J. Am. Chem. Soc. 2010;132:2878–2879. doi: 10.1021/ja100955x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Ma S., Villa G., Thuy-Boun P. S., Homs A., Yu J.-Q. Angew. Chem., Int. Ed. 2014;53:734–737. doi: 10.1002/anie.201305388. [DOI] [PubMed] [Google Scholar]
- For iron catalyzed sp2 and sp3 C–H functionalizations, see: ; (a) Matsumoto A., Ilies L., Nakamura E. J. Am. Chem. Soc. 2011;133:6557–6559. doi: 10.1021/ja201931e. [DOI] [PubMed] [Google Scholar]; (b) Asako S., Ilies L., Nakamura E. J. Am. Chem. Soc. 2013;135:17755–17757. doi: 10.1021/ja4106368. [DOI] [PubMed] [Google Scholar]; (c) Shang R., Ilies L., Matsumoto A., Nakamura E. J. Am. Chem. Soc. 2013;135:6030–6032. doi: 10.1021/ja402806f. [DOI] [PubMed] [Google Scholar]; (d) Ilies L., Matsubara T., Ichikawa S., Asako S., Nakamura E. J. Am. Chem. Soc. 2014;136:13126–13129. doi: 10.1021/ja5066015. [DOI] [PubMed] [Google Scholar]; (e) Shang R., Ilies L., Asako S., Nakamura E. J. Am. Chem. Soc. 2014;136:14349–14352. doi: 10.1021/ja5070763. [DOI] [PubMed] [Google Scholar]; (f) Shang R., Ilies L., Nakamura E. J. Am. Chem. Soc. 2015;137:7660–7663. doi: 10.1021/jacs.5b04818. [DOI] [PubMed] [Google Scholar]
- For recent development of Cu-catalyzed C–H functionalizations, see: ; (a) Brasche G., Buchwald S. L. Angew. Chem., Int. Ed. 2008;47:1932–1934. doi: 10.1002/anie.200705420. [DOI] [PubMed] [Google Scholar]; (b) Ueda S., Nagasawa H. Angew. Chem., Int. Ed. 2008;47:6411–6413. doi: 10.1002/anie.200801240. [DOI] [PubMed] [Google Scholar]; (c) Huffman L. M., Stahl S. S. J. Am. Chem. Soc. 2008;130:9196–9197. doi: 10.1021/ja802123p. [DOI] [PubMed] [Google Scholar]; (d) Ban I., Sudo T., Taniguchi T., Itami K. Org. Lett. 2008;10:3607–3609. doi: 10.1021/ol8013717. [DOI] [PubMed] [Google Scholar]; (e) Wang W., Luo F., Zhang S., Cheng J. J. Org. Chem. 2010;75:2415–2418. doi: 10.1021/jo1000719. [DOI] [PubMed] [Google Scholar]; (f) Suess A. M., Ertem M. Z., Cramer C. J., Stahl S. S. J. Am. Chem. Soc. 2013;135:9797–9804. doi: 10.1021/ja4026424. [DOI] [PubMed] [Google Scholar]; (g) Parsons A. T., Buchwald S. L. Angew. Chem., Int. Ed. 2011;50:9120–9123. doi: 10.1002/anie.201104053. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) John A., Nicholas K. M. J. Org. Chem. 2011;76:4158–4162. doi: 10.1021/jo200409h. [DOI] [PubMed] [Google Scholar]; (i) Tran L. D., Popov I., Daugulis O. J. Am. Chem. Soc. 2012;134:18237–18240. doi: 10.1021/ja3092278. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Ni Z.-K., Zhang Q., Xiong T., Zheng Y.-Y., Li Y., Zhang H.-W., Zhang J.-P., Liu Q. Angew. Chem., Int. Ed. 2012;51:1244–1247. doi: 10.1002/anie.201107427. [DOI] [PubMed] [Google Scholar]; (k) Roane J., Daugulis O. Org. Lett. 2013;15:5842–5845. doi: 10.1021/ol402904d. [DOI] [PMC free article] [PubMed] [Google Scholar]; (l) Gallardo-Donaire J., Martin R. J. Am. Chem. Soc. 2013;135:9350–9353. doi: 10.1021/ja4047894. [DOI] [PubMed] [Google Scholar]; (m) Bhadra S., Dzik W. I., Gooßen L. J. Angew. Chem., Int. Ed. 2013;52:2959–2962. doi: 10.1002/anie.201208755. [DOI] [PubMed] [Google Scholar]; (n) Nishino M., Hirano K., Satoh T., Miura M. Angew. Chem., Int. Ed. 2013;52:4457–4461. doi: 10.1002/anie.201300587. [DOI] [PubMed] [Google Scholar]; (o) Truong T., Klimovica K., Daugulis O. J. Am. Chem. Soc. 2013;135:9342–9345. doi: 10.1021/ja4047125. [DOI] [PMC free article] [PubMed] [Google Scholar]; (p) Shang M., Wang H.-L., Sun S.-Z., Dai H.-X., Yu J.-Q. J. Am. Chem. Soc. 2014;136:11590–11593. doi: 10.1021/ja507704b. [DOI] [PubMed] [Google Scholar]; (q) Shang M., Sun S.-Z., Wang H.-L., Laforteza B. N., Dai H.-X., Yu J.-Q. Angew. Chem., Int. Ed. 2014;53:10439–10442. doi: 10.1002/anie.201404822. [DOI] [PubMed] [Google Scholar]; (r) Shang M., Sun S.-Z., Dai H.-X., Yu J.-Q. Org. Lett. 2014;16:5666–5669. doi: 10.1021/ol5027377. [DOI] [PubMed] [Google Scholar]; (s) Dong J., Wang F., You J. Org. Lett. 2014;16:2884–2887. doi: 10.1021/ol501023n. [DOI] [PubMed] [Google Scholar]; (t) Wang S., Guo R., Wang G., Chen S.-Y., Yu X.-Q. Chem. Commun. 2014;50:12718–12721. doi: 10.1039/c4cc06246a. [DOI] [PubMed] [Google Scholar]; (u) Chen F.-J., Liao G., Li X., Wu J., Shi B.-F. Org. Lett. 2014;16:5644–5647. doi: 10.1021/ol5027156. [DOI] [PubMed] [Google Scholar]; (v) Hao X.-Q., Chen L.-J., Ren B., Li L.-Y., Yang X.-Y., Gong J.-F., Niu J.-L., Song M.-P. Org. Lett. 2014;16:1104–1107. doi: 10.1021/ol500166d. [DOI] [PubMed] [Google Scholar]; (w) McDonald S. L., Hendrick C. E., Wang Q. Angew. Chem., Int. Ed. 2014;53:4667–4670. doi: 10.1002/anie.201311029. [DOI] [PMC free article] [PubMed] [Google Scholar]; (x) Wang Z., Ni J., Ni Y., Kanai M. Angew. Chem., Int. Ed. 2014;53:3496–3499. doi: 10.1002/anie.201311105. [DOI] [PubMed] [Google Scholar]; (y) Wang Z., Kuninobu Y., Kanai M. Org. Lett. 2014;16:4790–4793. doi: 10.1021/ol5022542. [DOI] [PubMed] [Google Scholar]; (z) Takamatsu K., Hirano K., Miura M. Org. Lett. 2015;17:4066–4069. doi: 10.1021/acs.orglett.5b01986. [DOI] [PubMed] [Google Scholar]
- For a few examples of Fe- and Co-catalyzed C–H functionalizations with a weakly coordinating DG, see: ; (a) Chen Q., Ilies L., Yoshikai N., Nakamura E. Org. Lett. 2011;13:3232–3234. doi: 10.1021/ol2011264. [DOI] [PubMed] [Google Scholar]; (b) Chen Q., Ilies L., Nakamura E. J. Am. Chem. Soc. 2011;133:5221–5223. doi: 10.1021/ja200645w. [DOI] [PubMed] [Google Scholar]; (c) Ilies L., Konno E., Chen Q., Nakamura E. Asian J. Org. Chem. 2012;1:142–145. [Google Scholar]
- (a) Engle K. M., Mei T.-S., Wasa M., Yu J.-Q. Acc. Chem. Res. 2012;45:788–802. doi: 10.1021/ar200185g. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Sarkar S. D., Liu W., Kozhushkov S. I., Ackermann L. Adv. Synth. Catal. 2014;356:1461–1479. [Google Scholar]; (c) Wang D.-H., Yu J.-Q. J. Am. Chem. Soc. 2011;133:5767–5769. doi: 10.1021/ja2010225. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Patureau F. W., Besset T., Glorius F. Angew. Chem., Int. Ed. 2011;50:1064–1067. doi: 10.1002/anie.201006222. [DOI] [PubMed] [Google Scholar]; (e) Engle K. M., Thuy-Boun P. S., Dang M., Yu J.-Q. J. Am. Chem. Soc. 2011;133:18183–18193. doi: 10.1021/ja203978r. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Leow D., Li G., Mei T.-S., Yu J.-Q. Nature. 2012;486:518–522. doi: 10.1038/nature11158. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Grohmann C., Wang H., Glorius F. Org. Lett. 2012;14:656–659. doi: 10.1021/ol203353a. [DOI] [PubMed] [Google Scholar]; (h) Chu L., Xiao K.-J., Yu J.-Q. Science. 2014;346:451–455. doi: 10.1126/science.1258538. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Liu Y.-J., Xu H., Kong W.-J., Shang M., Dai H.-X., Yu J.-Q. Nature. 2014;515:389–393. doi: 10.1038/nature13885. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Du Z.-J., Guan J., Wu G.-J., Xu P., Gao L.-X., Han F.-S. J. Am. Chem. Soc. 2015;137:632–635. doi: 10.1021/ja512029x. [DOI] [PubMed] [Google Scholar]; (k) Warratz S., Kornhaaß C., Cajaraville A., Niepötter B., Stalke D., Ackermann L. Angew. Chem., Int. Ed. 2015;54:5513–5517. doi: 10.1002/anie.201500600. [DOI] [PubMed] [Google Scholar]; (l) Bechtoldt A., Tirler C., Raghuvanshi K., Warratz S., Kornhaab C., Ackermann L. Angew. Chem., Int. Ed. 2016;55:264–267. doi: 10.1002/anie.201507801. [DOI] [PubMed] [Google Scholar]
- (a) Tyman J. H. P., Synthetic and Natural Phenols, Elsevier, New York, 1996. [Google Scholar]; (b) Rappoport Z., The Chemistry of Phenols, Wiley-VCH, Weinheim, 2003. [Google Scholar]; (c) Huang W.-Y., Cai Y.-Z., Zhang Y. Nutr. Cancer. 2010;62:1–20. doi: 10.1080/01635580903191585. [DOI] [PubMed] [Google Scholar]
- For palladium-catalyzed direct hydroxylation of arenes, see: ; (a) Jintoku T., Nishimura K., Takaki K., Fujiwara Y. Chem. Lett. 1991:193–194. [Google Scholar]; (b) Zhang Y.-H., Yu J.-Q. J. Am. Chem. Soc. 2009;131:14654–14655. doi: 10.1021/ja907198n. [DOI] [PubMed] [Google Scholar]; (c) Shan G., Yang X., Ma L., Rao Y. Angew. Chem., Int. Ed. 2012;51:13070–13074. doi: 10.1002/anie.201207458. [DOI] [PubMed] [Google Scholar]; (d) Mo F., Trzepkowski L. J., Dong G. Angew. Chem., Int. Ed. 2012;51:13075–13079. doi: 10.1002/anie.201207479. [DOI] [PubMed] [Google Scholar]; (e) Choy P. Y., Kwong F. Y. Org. Lett. 2013;15:270–273. doi: 10.1021/ol303088z. [DOI] [PubMed] [Google Scholar]; (f) Yan Y., Feng P., Zheng Q.-Z., Liang Y.-F., Lu J.-F., Cui Y., Jiao N. Angew. Chem., Int. Ed. 2013;52:5827–5831. doi: 10.1002/anie.201300957. [DOI] [PubMed] [Google Scholar]; (g) Zhang H.-Y., Yi H.-M., Wang G.-W., Yang B., Yang S.-D. Org. Lett. 2013;15:6186–6189. doi: 10.1021/ol403028a. [DOI] [PubMed] [Google Scholar]
- For ruthenium-catalyzed direct hydroxylation of arenes, see: ; (a) Yang Y., Lin Y., Rao Y. Org. Lett. 2012;14:2874–2877. doi: 10.1021/ol301104n. [DOI] [PubMed] [Google Scholar]; (b) Thirunavukkarasu V. S., Hubrich J., Ackermann L. Org. Lett. 2012;14:4210–4213. doi: 10.1021/ol3018819. [DOI] [PubMed] [Google Scholar]; (c) Thirunavukkarasu V. S., Ackermann L. Org. Lett. 2012;14:6206–6209. doi: 10.1021/ol302956s. [DOI] [PubMed] [Google Scholar]; (d) Yang F., Ackermann L. Org. Lett. 2013;15:718–720. doi: 10.1021/ol303520h. [DOI] [PubMed] [Google Scholar]; (e) Yang X., Shan G., Rao Y. Org. Lett. 2013;15:2334–2337. doi: 10.1021/ol400437a. [DOI] [PubMed] [Google Scholar]; (f) Liu W., Ackermann L. Org. Lett. 2013;15:3484–3486. doi: 10.1021/ol401535k. [DOI] [PubMed] [Google Scholar]; (g) Yang F., Rauch K., Kettelhoit K., Ackermann L. Angew. Chem., Int. Ed. 2014;53:11285–11288. doi: 10.1002/anie.201405647. [DOI] [PubMed] [Google Scholar]
- (a) Chen X., Hao X.-S., Goodhue C. E., Yu J.-Q. J. Am. Chem. Soc. 2006;128:6790–6791. doi: 10.1021/ja061715q. [DOI] [PubMed] [Google Scholar]; (b) For a Cu-mediated C–H amidation see: Uemura T., Imoto S., Chatani N., Chem. Lett., 2006, 35 , 842 –843 . [Google Scholar]
- (a) Li X., Liu Y.-H., Gu W.-J., Li B., Chen F.-J., Shi B.-F. Org. Lett. 2014;16:3904–3907. doi: 10.1021/ol5016064. [DOI] [PubMed] [Google Scholar]; (b) Sun S.-Z., Shang M., Wang H.-L., Lin H.-X., Dai H.-X., Yu J.-Q. J. Org. Chem. 2015;80:8843–8848. doi: 10.1021/acs.joc.5b01351. [DOI] [PubMed] [Google Scholar]; (c) Singh B. K., Jana R. J. Org. Chem. 2016;81:831–841. doi: 10.1021/acs.joc.5b02302. [DOI] [PubMed] [Google Scholar]
- (a) Wasa M., Engle K. M., Yu J.-Q. J. Am. Chem. Soc. 2009;131:9886–9887. doi: 10.1021/ja903573p. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wasa M., Engle K. M., Yu J.-Q. J. Am. Chem. Soc. 2010;132:3680–3681. doi: 10.1021/ja1010866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For ligand-enabled or -promoted C–H activation reactions using Pd catalyst, see: ; (a) Wang D.-H., Engle K. M., Shi B.-F., Yu J.-Q. Science. 2010;327:315–319. doi: 10.1126/science.1182512. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Engle K. M., Wang D.-H., Yu J.-Q. J. Am. Chem. Soc. 2010;132:14137–14151. doi: 10.1021/ja105044s. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Wasa M., Chan K. S. L., Zhang X.-G., He J., Miura M., Yu J.-Q. J. Am. Chem. Soc. 2012;134:18570–18572. doi: 10.1021/ja309325e. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) He J., Li S., Deng Y., Fu H., Laforteza B. N., Spangler J. E., Homs A., Yu J.-Q. Science. 2014;343:1216–1220. doi: 10.1126/science.1249198. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Li S., Chen G., Feng C.-G., Gong W., Yu J.-Q. J. Am. Chem. Soc. 2014;136:5267–5270. doi: 10.1021/ja501689j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Zhu R.-Y., Tanaka K., Li G.-C., He J., Fu H.-Y., Li S.-H., Yu J.-Q. J. Am. Chem. Soc. 2015;137:7067–7070. doi: 10.1021/jacs.5b04088. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Li J., Warratz S., Zell D., Sarkar S. D., Ishikawa E. E., Ackermann L. J. Am. Chem. Soc. 2015;137:13894–13901. doi: 10.1021/jacs.5b08435. [DOI] [PubMed] [Google Scholar]; (h) Yang Y., Qiu X., Zhao Y., Mu Y., Shi Z. J. Am. Chem. Soc. 2016;138:495–498. doi: 10.1021/jacs.5b11569. [DOI] [PubMed] [Google Scholar]
- For selective examples of C–H amination reactions, see: ; (a) Thu H.-Y., Yu W.-Y., Che C.-M. J. Am. Chem. Soc. 2006;128:9048–9049. doi: 10.1021/ja062856v. [DOI] [PubMed] [Google Scholar]; (b) Ng K.-H., Chan A. S. C., Yu W.-Y. J. Am. Chem. Soc. 2010;132:12862–12864. doi: 10.1021/ja106364r. [DOI] [PubMed] [Google Scholar]; (c) Xiao B., Gong T.-J., Xu J., Liu Z.-J., Liu L. J. Am. Chem. Soc. 2011;133:1466–1470. doi: 10.1021/ja108450m. [DOI] [PubMed] [Google Scholar]; (d) Sun K., Li Y., Xiong T., Zhang J., Zhang Q. J. Am. Chem. Soc. 2011;133:1694–1697. doi: 10.1021/ja1101695. [DOI] [PubMed] [Google Scholar]; (e) Yoo E. J., Ma S., Mei T.-S., Chan K. S. L., Yu J.-Q. J. Am. Chem. Soc. 2011;133:7652–7655. doi: 10.1021/ja202563w. [DOI] [PubMed] [Google Scholar]; (f) Ng K.-H., Zhou Z., Yu W.-Y. Org. Lett. 2012;14:272–275. doi: 10.1021/ol203046n. [DOI] [PubMed] [Google Scholar]; (g) Grohmann C., Wang H., Glorius F. Org. Lett. 2012;14:656–659. doi: 10.1021/ol203353a. [DOI] [PubMed] [Google Scholar]; (h) Tran L. D., Roane J., Daugulis O. Angew. Chem., Int. Ed. 2013;52:6043–6046. doi: 10.1002/anie.201300135. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Shang M., Sun S.-Z., Dai H.-X., Yu J.-Q. J. Am. Chem. Soc. 2014;136:3354–3357. doi: 10.1021/ja412880r. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.