Abstract
Objectives
Serum carcinoembryonic antigen (CEA) has been widely used for postoperative surveillance for colorectal cancer. However, serum CEA has a poor diagnostic accuracy for detecting recurrence. We tested the hypothesis that determining cutoff values according to the preoperative serum CEA levels would enhance the diagnostic accuracy.
Methods
Serum CEA was measured before and 1–6 months after surgery in 783 patients with curatively resected colon cancer from 2005 through 2013. The cutoff values during surveillance were determined separately according to preoperative serum CEA levels.
Results
In patients with negative preoperative serum CEA, the diagnostic accuracy for recurrence was 89.1% when a postoperative cutoff value was set at 5 ng/mL. However, in patients with positive preoperative serum CEA, the diagnostic accuracy was 58.4% when a postoperative cutoff value was set at 5 ng/mL, and was 75.6% when a cutoff value was set at 8 ng/mL. Among patients with positive preoperative serum CEA, the recurrence-free survival rate was significantly lower in patients with a serum CEA of ≥8 ng/mL than those with a serum CEA of <8 ng/mL (p = 0.0018).
Conclusions
The diagnostic accuracy of serum CEA for recurrence is enhanced by separately setting cutoff values according to preoperative serum CEA.
Keywords: Colon cancer, Carcinoembryonic antigen, Surveillance
Introduction
Carcinoembryonic antigen (CEA) is a glycoprotein that was extracted from colorectal cancer tissue. Its name is derived from the fact that CEA was also found in extracts of human embryonic intestine [1]. The serum CEA level is a representative tumor marker for colorectal cancer, and the preoperative serum CEA level has been reported to be positive in 40–60% of the patients who undergo surgery for colorectal cancer [2]. The rate of positive serum CEA levels increases in parallel to the clinical stage of colorectal cancer. Patients with metastasis often have particularly high serum CEA levels [3, 4, 5, 6, 7, 8, 9]. Serum CEA has a half-life of 3–5 days, and serum CEA levels have been reported to decrease to the normal range from 2 weeks to 1 month after curative resection [5, 10, 11, 12, 13].
When serum CEA levels are measured over time after surgery, the serum CEA level often increases several months before recurrence is detected on conventional imaging studies and clinical examinations. Serum CEA levels have therefore been widely used for postoperative surveillance in patients with colorectal cancer [14, 15, 16]. However, serum CEA levels fluctuate over time even in the same individuals. In some patients, the serum CEA level becomes positive in the absence of recurrence. In other patients who have positive serum CEA levels at initial surgery, the level becomes negative at the time of recurrence. Therefore, the sensitivity and specificity of serum CEA levels for the detection of recurrence are not high [12, 17, 18, 19]. The proportion of patients with a high serum CEA level at recurrence is higher among patients with a high serum CEA level than among those with a normal serum CEA level at initial diagnosis [20].
The present study was designed to test the hypothesis that determining cutoff values according to whether the serum CEA level is negative or positive before surgery would enhance the diagnostic accuracy for the detection of recurrence during postoperative surveillance. We separately determined the cutoff values of serum CEA levels during postoperative surveillance according to whether the preoperative serum CEA was negative or positive in patients who underwent curative resection for colon cancer and thereby estimated the sensitivity and specificity for the diagnosis of recurrence.
Methods
From January 2005 through December 2013, a total of 877 patients with pathological stage I, II, or III colon cancer underwent curative resection at Tokai University Hospital. We studied 783 patients whose serum CEA levels were measured before and 1–6 months after surgery. Serum CEA levels were assayed with the E-test “TOSOH” II CEA system (TOSOH Inc., Tokyo, Japan), using a high-affinity anti-CEA monoclonal antibody. The cutoff value of the serum CEA level was set at 5 ng/mL. The median follow-up period of the survivors was 41.8 months.
In patients without recurrence, serum CEA levels were measured several times during 1–6 months after surgery, and the highest serum CEA level was used as the postoperative serum CEA level. In patients with recurrence, the serum CEA level when recurrence was suspected on imaging studies was recorded as the serum CEA level at recurrence.
Screening of postoperative recurrence was performed according to a previously reported surveillance program [21]. In short, serum CEA levels were measured and abdominal ultrasonography and plain chest radiography were performed postoperatively every 3 months during the first 2 years and every 4–6 months for the next 3 years. Computed tomography of the chest and the abdomen was performed every 6 months, starting 6 months after surgery.
Statistical analyses were performed with χ2 tests, Fisher exact tests, the Kaplan-Meier method, and log-rank tests. A receiver-operating characteristic curve was used to calculate the optimal cutoff value of the postoperative CEA level for the detection of recurrence. The optimal cutoff value was based on the Youden index (maximum [sensitivity + specificity − 1]). p values of <0.05 were considered to indicate significant difference. JMP® 11 software (SAS Institute Inc., Cary, NC, USA) was used for statistical analysis. This study was approved by the Institutional Review Board of Tokai University (13R-206).
Results
Table 1 shows the relations between serum CEA levels at initial surgery and clinicopathological factors. Overall, the preoperative serum CEA level was negative (<5 ng/mL) in 386 patients (49.3%) and positive (≥5 ng/mL) in 397 patients (50.7%) (Table 1). The rate of positive serum CEA levels at initial surgery was significantly higher in patients who were smokers or had well- or moderately differentiated adenocarcinoma, venous invasion, lymphatic invasion, or advanced-stage disease.
Table 1.
Patient characteristics according to preoperative serum CEA levels
| Preoperative serum CEA levels |
|||
|---|---|---|---|
| <5 ng/mL (n = 386) | ≥5 ng/mL (n = 397) | p | |
| Age, years | 67 (27 – 90) | 69 (22 – 93) | <0.001 |
| Gender | 0.772 | ||
| Male | 221 (57.3) | 232 (58.4) | |
| Female | 165 (42.7) | 165 (41.6) | |
| Smoking status | <0.001 | ||
| Never smoked | 220 (57.0) | 174 (43.8) | |
| Smoker | 131 (33.9) | 197 (49.6) | |
| Missing | 35 (9.1) | 26 (6.6) | |
| Tumor locationa | 0.47 | ||
| Right colon | 171 (44.3) | 165 (41.6) | |
| Left colon | 215 (55.7) | 232 (58.4) | |
| Histologic grade | <0.001 | ||
| Well | 216 (56.0) | 184 (46.3) | |
| Mod | 157 (40.7) | 180 (5.3) | |
| Por | 4 (1.0) | 5 (1.3) | |
| Others | 9 (2.3) | 28 (7.1) | |
| Lymphatic invasion | <0.001 | ||
| Positive | 221 (57.3) | 299 (75.3) | |
| Negative | 165 (42.7) | 98 (24.7) | |
| Vascular invasion | <0.001 | ||
| Positive | 210 (54.4) | 287 (72.3) | |
| Negative | 176 (45.6) | 110 (27.7) | |
| Stage | <0.001 | ||
| I | 158 (40.9) | 56 (14.1) | |
| II | 133 (4.5) | 194 (48.9) | |
| III | 95 (24.6) | 147 (37.0) | |
Values are median (ranges) or n (%). CEA, carcinoembryonic antigen; well, well differentiated adenocarcinoma; mod, moderately differentiated adenocarcinoma; por, poorly differentiated adenocarcinoma.
Right colon: cecum, ascending colon, transverse colon; left colon: descending colon, sigmoid colon, rectosigmoid.
Among the 783 patients, 106 (13.4%) had recurrence. Recurrence was detected in 34 patients (8.8%) with a preoperative serum CEA level of <5 ng/mL and 72 patients (18.1%) with a preoperative serum CEA level of ≥5 ng/mL. The preoperative serum CEA level was positive among a higher proportion of patients with recurrence than among those without recurrence (p < 0.001). The relations between the preoperative serum CEA level and the serum CEA level at the time of recurrence are shown for the 106 patients with recurrence and the 677 patients without recurrence in Table 2. Among patients with recurrence who had a preoperative serum CEA level of <5 ng/mL, the serum CEA level at the time of recurrence was <5 ng/mL in 55.9% of the patients and was <10 ng/mL in 73.5%. Among the patients who had a preoperative serum CEA level of ≥5 ng/mL, the serum CEA level at recurrence was ≥5 ng/mL in 75.0% of the patients and ≥10 ng/mL in 56.9% of the patients. The proportion of patients with a high serum CEA level at recurrence after surgery was thus significantly higher among patients who had a positive serum CEA level before surgery (p < 0.001). Among patients without recurrence who had a preoperative serum CEA level of <5 ng/mL, the postoperative serum CEA level was ≥5 ng/mL in 2.6% of the patients in the absence of recurrence, and no patient had a postoperative serum CEA level of ≥10 ng/mL. On the other hand, among patients who had a preoperative serum CEA level of ≥5 ng/mL, the postoperative serum CEA level was ≥5 ng/mL in 38.2% of the patients and ≥10 ng/mL in 6.8% of the patients despite the absence of recurrence. Patients with positive preoperative serum CEA levels thus frequently had serum CEA levels of ≥5 ng/mL after surgery despite no recurrence.
Table 2.
Relation between serum CEA levels before surgery and at recurrence/after surgery
| Before surgery | At recurrence |
|||||
|---|---|---|---|---|---|---|
| <5 ng/mL | ≥5 ng/mL | p | <10 ng/mL | ≥10 ng/mL | p | |
| Relation between serum CEA levels before surgery and those at recurrence in 106 patients with recurrence | ||||||
| <5 ng/mL (n = 34) | 19 (55.9%) | 15 (44.1%) | <0.001 | 25 (73.5%) | 9 (26.5%) | <0.001 |
| ≤5 ng/mL (n = 72) | 18 (25.0%) | 54 (75.0%) | 31 (43.1%) | 41 (56.9%) | ||
| Before surgery | After surgery |
|||||
|---|---|---|---|---|---|---|
| <5 ng/mL | ≥5 ng/mL | p | <10 ng/mL | ≥10 ng/mL | p | |
| Relation between serum CEA levels before surgery and those after surgery in 677 patients without recurrence | ||||||
| <5 ng/mL (n = 352) | 343 (97.4%) | 9 (2.6%) | <0.001 | 352 (100%) | 0 (0%) | <0.001 |
| ≥5 ng/mL (n = 325) | 201 (61.8%) | 124 (38.2%) | 303 (93.2%) | 22 (6.8%) | ||
In patients who had a preoperative serum CEA level of ≥5 ng/mL, the relation of the postoperative serum CEA level to recurrence was assessed by receiver-operating characteristic curve analysis. Because a cutoff value of 7.6 was obtained, a postoperative serum CEA level of 8.0 was used as a cutoff value in the subsequent analysis. Patients were divided into 2 groups according to whether the preoperative serum CEA level was <5 or ≥5 ng/mL. The cutoff value of the postoperative serum CEA level was set at 5.0 and 8.0 ng/mL. The sensitivity, specificity, positive predictive value, negative predictive value, and diagnostic accuracy for recurrence obtained with these values are summarized in Tables 3 and 4.
Table 3.
Prediction of recurrence on the basis of postoperative serum CEA levels in 386 patients with a preoperative serum CEA level of <5 ng/mL
| Serum CEA after surgery |
||||
|---|---|---|---|---|
| <5 ng/mL | ≥5 ng/mL | <8 ng/mL | ≥8 ng/mL | |
| No recurrence (n = 352) | 343 (97.4) | 9 (2.6) | 352 (100) | 0 (0) |
| Recurrence (n = 34) | 33 (97.1) | 1 (2.9) | 33 (97.1) | 1 (2.9) |
| Sensitivity | 2.9 | 2.9 | ||
| Specificity | 97.4 | 100 | ||
| Positive predictive value | 10.0 | 100 | ||
| Negative predictive value | 91.2 | 91.4 | ||
| Accuracy | 89.1 | 91.5 | ||
Values are presented as n (%) or percentages.
Table 4.
Prediction of recurrence on the basis of postoperative serum CEA levels in 397 patients with a preoperative serum CEA level of ≥5 ng/mL
| Serum CEA after surgery | ||||
|---|---|---|---|---|
| <5 ng/mL | ≥5 ng/mL | <8 ng/mL | ≥8 ng/mL | |
| No recurrence (n = 325) | 201 (61.8) | 124 (38.2) | 284 (87.4) | 41 (12.6) |
| Recurrence (n = 72) | 41 (56.9) | 31 (43.1) | 56 (77.8) | 16 (22.2) |
| Sensitivity | 43.1 | 22.2 | ||
| Specificity | 61.8 | 87.4 | ||
| Positive predictive value | 20.0 | 28.1 | ||
| Negative predictive value | 83.1 | 83.5 | ||
| Accuracy | 58.4 | 75.6 | ||
Values are presented as n (%) or percentages.
In the 386 patients with a preoperative serum CEA level of <5 ng/mL, the sensitivity, specificity, positive predictive value, negative predictive value, and diagnostic accuracy for the diagnosis of recurrence were respectively 2.9, 97.4, 10.0, 91.2, and 89.1% when the cutoff value was set at 5 ng/mL and 2.9, 100, 100, 91.4, and 91.5% when the cutoff value was set at 8 ng/mL (Table 3). In the 397 patients with a preoperative serum CEA level of ≥5 ng/mL, the sensitivity, specificity, positive predictive value, negative predictive value, and diagnostic accuracy for detecting recurrence were respectively 43.1, 61.8, 20.0, 83.1, and 58.4% when the cutoff value was set at 5 ng/mL and 22.2, 87.4, 28.1, 83.5, and 75.6% when the cutoff value was set at 8 ng/mL (Table 4). In patients with a preoperative serum CEA of <5 ng/mL, there was no appreciable difference in the diagnostic accuracy for recurrence between when the cutoff value was set at 5 ng/mL (89.1%) and when the cutoff value was set at 8 ng/mL (91.5%). In patients with a preoperative serum CEA level of ≥5 ng/mL, however, the diagnostic accuracy for recurrence was relatively higher when the cutoff value was set at 8 ng/mL (75.6%) than when the cutoff value was set at 5 ng/mL (58.4%).
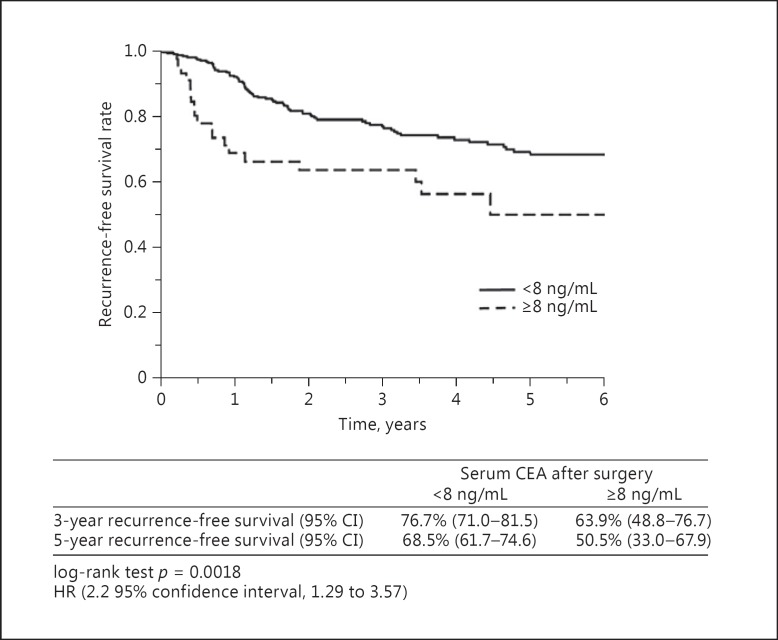
The patients were divided into 2 groups according to whether the preoperative serum CEA level was <5 or ≥5 ng/mL, and the recurrence-free survival was compared according to the serum CEA level 1–6 months after surgery. Among patients with a preoperative serum CEA level of <5 ng/mL, the recurrence-free survival rate did not differ significantly between patients with a postoperative serum CEA level of ≥5 ng/mL and those with a postoperative serum CEA level of <5 ng/mL; the hazard ratio was 0.9 (95% confidence interval, 0.05–4.13; p = 0.92). Among patients with a preoperative serum CEA of ≥5 ng/mL, the relapse-free survival rate was significantly lower in patients with a postoperative serum CEA level of ≥8 ng/mL than in those with a postoperative serum CEA level of <8 ng/mL; the hazard ratio was 2.2 (95% confidence interval, 1.29–3.57, p = 0.0018) (Fig. 1).
Fig. 1.
Recurrence-free survival rates when the cutoff value of the postoperative serum CEA was set at 8 ng/mL in patients with a preoperative serum CEA level of ≥5 ng/mL. HR, hazard ratio; CI, confidence interval.
Discussion
Serum CEA is a representative tumor marker for colorectal cancer that is widely used for postoperative surveillance in patients with colorectal cancer, as recommended by guidelines issued by the American Society of Clinical Oncology (ASCO), European Society of Medical Oncology (ESMO), and National Comprehensive Cancer Network (NCCN) [15, 16, 22, 23]. Serum CEA levels fluctuate over time in healthy individuals, and the intraindividual variation has been reported to be about 30% [19]. In clinical practice, we often encounter patients without recurrence in whom serum CEA levels do not become negative after surgery and those in whom serum CEA levels transiently increase after surgery and then subsequently decrease. An increase in the cutoff value to 3, 5, 10, and 15 ng/mL will increase the specificity for detecting recurrence, but decrease the sensitivity [24]. In the present study, we therefore tested the hypothesis that the use of cutoff values based on serum CEA levels before surgery would enhance the diagnostic accuracy for recurrence during postoperative surveillance.
In our study, among patients who had a preoperative serum CEA level of <5 ng/mL, the postoperative serum CEA level was ≥5 ng/mL in 2.6% of the patients and ≥10 ng/mL in 0% of the patients despite no recurrence. Among patients with a preoperative serum CEA level of ≥5 ng/mL, the postoperative serum CEA level was ≥5 ng/mL in 38% of the patients and ≥8 ng/mL in 12.6% of the patients in the absence of recurrence. Our results thus showed that the use of postoperative cutoff values based on the preoperative serum CEA level decreased the false-positive rate of the diagnosis of recurrence.
In patients with a preoperative serum CEA level of <5 ng/mL, the use of a cutoff value of 5 ng/mL during postoperative surveillance resulted in a sensitivity for diagnosis of recurrence of only 2.9%, but a high specificity of 97.4% and a high diagnostic accuracy for recurrence of 89.2%. In patients with a preoperative serum CEA level of ≥5 ng/mL, setting the cutoff value during postoperative surveillance at 8 ng/mL resulted in a sensitivity of 22.2%, a specificity of 87.4%, and a diagnostic accuracy of 75.6%, which were relatively high. CEA levels are markedly higher in colorectal cancer tissue than in normal mucosal tissue [25]. However, the CEA level in colon cancer tissue has been reported to be unrelated to the plasma CEA level [26].
We previously reported that although immunohistologically evaluated CEA staining in primary colon cancer tissue is not significantly related to the serum CEA level, CEA staining of metastatic tissue is intimately related to the serum CEA level [9]. This is attributed to the facts that serum CEA levels are related to the secretional capacity of CEA into blood and that the tissue environment around cancer cells differs between primary lesions and metastatic lesions. The CEA production capacity of metastatic lesions at the time of recurrence has a greater impact on serum CEA levels. This most likely accounted for the fact that a high proportion of patients with a serum CEA of ≥5 ng/mL at initial surgery had a serum CEA level of ≥5 or ≥8 ng/mL at the time of recurrence. In patients with negative serum CEA levels before surgery for colon cancer, the false-negative rate at the time of recurrence was 50% or higher. In patients with positive serum CEA levels before surgery, the false-positive rate of the detection of recurrence markedly decreased when the postoperative cutoff level was set at 8 ng/mL. Given that patients with a serum CEA level of ≥8 ng/mL have significantly poorer outcomes, close follow-up is essential.
There are some potential limitations of this study because it is a retrospective single-center analysis. Further investigation of a large population of patients from multiple centers will be needed to clarify the optimal cutoff value among patients with positive preoperative serum CEA.
Conclusions
In surveillance after surgery for colorectal cancer, cutoff values should be determined according to whether the preoperative serum CEA level is negative or positive to enhance the diagnostic accuracy for detecting postoperative recurrence.
Disclosure Statement
G. Saito, S. Sadahiro, H. Kamata, H. Miyakita, K. Okada, A. Tanaka, and T. Suzuki have no potential conflicts of interest to disclose.
References
- 1.Gold P, Freedman SO. Specific carcinoembryonic antigens of the human digestive system. J Exp Med. 1965;122:467–481. doi: 10.1084/jem.122.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang JY, Lu CY, Chu KS, Ma CJ, Wu DC, Tsai HL, Yu FJ, Hsieh JS. Prognostic significance of pre- and postoperative serum carcinoembryonic antigen levels in patients with colorectal cancer. Eur Surg Res. 2007;39:245–250. doi: 10.1159/000101952. [DOI] [PubMed] [Google Scholar]
- 3.Dhar P, Moore T, Zamcheck N, Kupchik HZ. Carcinoembryonic antigen (CEA) in colonic cancer. Use in preoperative and postoperative diagnosis and prognosis. JAMA. 1972;221:31–35. [PubMed] [Google Scholar]
- 4.Livingstone AS, Hampson LG, Shuster J, Gold P, Hinchey EJ. Carcinoembryonic antigen in the diagnosis and management of colorectal carcinoma. Current status. Arch Surg. 1974;109:259–264. doi: 10.1001/archsurg.1974.01360020119023. [DOI] [PubMed] [Google Scholar]
- 5.Mach JP, Jaeger P, Bertholet MM, Ruegsegger CH, Loosli RM, Pettavel J. Detection of recurrence of large-bowel carcinoma by radioimmunoassay of circulating carcinoembryonic antigen (C.E.A.) Lancet. 1974;2:535–540. doi: 10.1016/s0140-6736(74)91872-8. [DOI] [PubMed] [Google Scholar]
- 6.Zamcheck N, Doos WG, Prudente R, Lurie BB, Gottlieb LS. Prognostic factors in colon carcinoma: correlation of serum carcinoembryonic antigen level and tumor histopathology. Hum Pathol. 1975;6:31–45. doi: 10.1016/s0046-8177(75)80108-0. [DOI] [PubMed] [Google Scholar]
- 7.Hirai H. A collaborative clinical study of carcinoembryonic antigen in Japan. Cancer Res. 1977;37:2267–2274. [PubMed] [Google Scholar]
- 8.Midiri G, Amanti C, Consorti F, Benedetti M, Del Buono S, Di Tondo U, Castagna G, Peronace L, Di Paola M. Usefulness of preoperative CEA levels in the assessment of colorectal cancer patient stage. J Surg Oncol. 1983;22:257–260. doi: 10.1002/jso.2930220410. [DOI] [PubMed] [Google Scholar]
- 9.Saito G, Sadahiro S, Okada K, Tanaka A, Suzuki T, Kamijo A. Relation between carcinoembryonic antigen levels in colon cancer tissue and serum carcinoembryonic antigen levels at initial surgery and recurrence. Oncology. 2016;91:85–89. doi: 10.1159/000447062. [DOI] [PubMed] [Google Scholar]
- 10.Rapellino M, Piantino P, Pecchio F, Ruffini E, Cavallo A, Scappaticci E, Baldi S, Ciocia E, Pivetti S. Disappearance curves of tumor markers after radical surgery. Int J Biol Markers. 1994;9:33–37. doi: 10.1177/172460089400900107. [DOI] [PubMed] [Google Scholar]
- 11.Holyoke D, Reynoso G, Chu TM. Carcinoembryonic antigen (CEA) in patients with carcinoma of the digestive tract. Ann Surg. 1972;176:559–564. doi: 10.1097/00000658-197217640-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holyoke ED, Chu TM, Murphy GP. CEA as a monitor of gastrointestinal malignancy. Cancer. 1975;35:830–836. doi: 10.1002/1097-0142(197503)35:3<830::aid-cncr2820350340>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 13.Fortner JG, Silva JS, Golbey RB, Cox EB, Maclean BJ. Multivariate analysis of a personal series of 247 consecutive patients with liver metastases from colorectal cancer. I. Treatment by hepatic resection. Ann Surg. 1984;199:306–316. doi: 10.1097/00000658-198403000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bruinvels DJ, Stiggelbout AM, Kievit J, van Houwelingen HC, Habbema JD, van de Velde CJ. Follow-up of patients with colorectal cancer. A meta-analysis. Ann Surg. 1994;219:174–182. doi: 10.1097/00000658-199402000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Locker GY, Hamilton S, Harris J, Jessup JM, Kemeny N, Macdonald JS, Somerfield MR, Hayes DF, Bast RC., Jr ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J Clin Oncol. 2006;24:5313–5327. doi: 10.1200/JCO.2006.08.2644. [DOI] [PubMed] [Google Scholar]
- 16.Labianca R, Nordlinger B, Beretta GD, Brouquet A, Cervantes A. Primary colon cancer: ESMO clinical practice guidelines for diagnosis, adjuvant treatment and follow-up. Ann Oncol. 2010;21(suppl 5):v70–v77. doi: 10.1093/annonc/mdq168. [DOI] [PubMed] [Google Scholar]
- 17.Mach JP, Vienny H, Jaeger P, Haldemann B, Egely R, Pettavel J. Long-term follow-up of colorectal carcinoma patients by repeated CEA radioimmunoassay. Cancer. 1978;42:1439–1447. doi: 10.1002/1097-0142(197809)42:3+<1439::aid-cncr2820420811>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 18.McCall JL, Black RB, Rich CA, Harvey JR, Baker RA, Watts JM, Toouli J. The value of serum carcinoembryonic antigen in predicting recurrent disease following curative resection of colorectal cancer. Dis Colon Rectum. 1994;37:875–881. doi: 10.1007/BF02052591. [DOI] [PubMed] [Google Scholar]
- 19.Erden G, Barazi AO, Tezcan G, Yildirimkaya MM. Biological variation and reference change values of CA 19–9, CEA, AFP in serum of healthy individuals. Scand J Clin Lab Invest. 2008;68:212–218. doi: 10.1080/00365510701601699. [DOI] [PubMed] [Google Scholar]
- 20.Kim CW, Yoon YS, Park IJ, Lim SB, Yu CS, Kim JC. Elevation of preoperative s-CEA concentration in stage IIA colorectal cancer can also be a high risk factor for stage II patients. Ann Surg Oncol. 2013;20:2914–2920. doi: 10.1245/s10434-013-2919-4. [DOI] [PubMed] [Google Scholar]
- 21.Sadahiro S, Suzuki T, Ishikawa K, Nakamura T, Tanaka Y, Masuda T, Mukoyama S, Yasuda S, Tajima T, Makuuchi H, Murayama C. Recurrence patterns after curative resection of colorectal cancer in patients followed for a minimum of ten years. Hepatogastroenterology. 2003;50:1362–1366. [PubMed] [Google Scholar]
- 22.Meyerhardt JA, Mangu PB, Flynn PJ, Korde L, Loprinzi CL, Minsky BD, Petrelli NJ, Ryan K, Schrag DH, Wong SL, Benson AB., 3rd Follow-up care, surveillance protocol, and secondary prevention measures for survivors of colorectal cancer: American Society of Clinical Oncology clinical practice guideline endorsement. J Clin Oncol. 2013;31:4465–4470. doi: 10.1200/JCO.2013.50.7442. [DOI] [PubMed] [Google Scholar]
- 23.Labianca R, Nordlinger B, Beretta GD, Mosconi S, Mandala M, Cervantes A, Arnold D. Early colon cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(suppl 6):vi64–vi72. doi: 10.1093/annonc/mdt354. [DOI] [PubMed] [Google Scholar]
- 24.Moertel CG, Fleming TR, Macdonald JS, Haller DG, Laurie JA, Tangen C. An evaluation of the carcinoembryonic antigen (CEA) test for monitoring patients with resected colon cancer. JAMA. 1993;270:943–947. [PubMed] [Google Scholar]
- 25.Bhatnagar J, Tewari HB, Bhatnagar M, Austin GE. Comparison of carcinoembryonic antigen in tissue and serum with grade and stage of colon cancer. Anticancer Res. 1999;19:2181–2187. [PubMed] [Google Scholar]
- 26.Khoo SK, Warner NL, Lie JT, Mackay IR. Carcinoembryonic antigenic activity of tissue extracts: a quantitative study of malignant and benign neoplasms, cirrhotic liver, normal adult and fetal organs. Int J Cancer. 1973;11:681–687. doi: 10.1002/ijc.2910110319. [DOI] [PubMed] [Google Scholar]