Abstract
Background
Effective treatments in heart failure (HF) patients with ischemic etiology have not been fully established. Nicorandil, combination of nitrate component and sarcolemmal adenosine triphosphate-sensitive potassium channel opener, is a potent vasodilator of coronary and peripheral vessels and has been used as an antianginal agent. Therefore, we examined impacts of nicorandil on cardiac mortality in ischemic HF patients.
Methods
Consecutive 334 HF patients with ischemic etiology were retrospectively registered and divided into 2 groups based on oral administration of nicorandil: nicorandil group (n = 116) and non-nicorandil group (n = 218). We retrospectively examined cardiac mortality.
Results
In the Kaplan-Meier analysis (mean follow-up period 963 days), cardiac mortality was significantly lower in the nicorandil group than in the non-nicorandil group (11.2% vs. 19.7%, P = 0.032). In the Cox proportional hazard analysis, usage of nicorandil was a suppressor of cardiac mortality (hazard ratio 0.512, 95% confidence interval 0.275–0.953, P = 0.035), and this result was consistent in several subgroup analyses, such as left ventricular ejection fraction, percutaneous coronary intervention, coronary artery bypass graft, diabetes, β-blockers, and statins.
Conclusion
Nicorandil is potentially effective for reducing mortality in patients with ischemic heart failure.
Trial registration
This was a retrospective study.
Keywords: Ischemic heart failure, Nicorandil, Cardiac mortality, Prognosis
Background
Recent standard pharmacotherapy for heart failure (HF), such as beta-blockers and renin angiotensin system inhibitors, have much improved mortality in HF patients [1–3]. HF with ischemic etiology accounts for more than 50% of HF cases in Europe and North America, as well as 30–40% of HF cases in East Asia, and Latin America and the Caribbean [4]. Ischemic HF is associated with shorter survival than non-ischemic HF [5]. Percutaneous coronary intervention and mitral valve repair, except for coronary artery bypass graft (CABG), do not sufficiently improve the cardiac mortality rate in ischemic HF patients [6–9]. It has been recently reported that CABG added to pharmacotherapy decreases cardiovascular mortality as 10-year outcome [10]. A more comprehensive approach is necessary to refocus preventive and therapeutic strategies, and to decrease ischemic HF morbidity and mortality. Nicorandil, a combination of nitrate components and sarcolemmal adenosine triphosphate-sensitive potassium channel opener, is a potent vasodilator of coronary and peripheral vessels and has been used as an antianginal agent [11]. A recent meta-analysis revealed that nicorandil treatment in patients with ischemic heart disease did not reduce revascularization (relative risk, RR 0.95, 95% CI 0.70–1.29) or all-cause mortality (RR 0.81, 95% CI 0.64–1.02), but did reduce cardiovascular events (RR 0.77, 95% CI 0.69–0.86) [11]. Therefore, we examined the impacts of oral administration of nicorandil on cardiac mortality in ischemic HF patients.
Methods
Subjects and study protocol
This was a retrospective study. Consecutive 334 HF patients with ischemic etiology at Fukushima Medical University between 2009 and 2014 were divided into two groups based on oral administration of nicorandil at hospital discharge: a nicorandil group (guideline-based medical therapy + nicorandil 5 mg tid, n = 116) and non-nicorandil group (guideline-based medical therapy alone, n = 218). While the prescription of nicorandil was determined by the attending physician freely, patients with advanced coronary artery disease tended to be prescribed nicorandil in our hospital. Diagnosis of decompensated HF was defined based on the Framingham criteria [12]. Ischemic etiology was confirmed by either myocardial scintigraphy or coronary computed tomography angiography and/or coronary angiography. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a prior approval by the institution’s human research committee. We compared clinical features between the two groups. All patients were followed up for cardiac death until 2016. Cardiac death was adjudicated by independent experienced cardiologists and included death due to worsened HF in accordance with the Framingham criteria [12], ventricular fibrillation documented by electrocardiogram or other implantable devices, and acute coronary syndrome.
Statistical analysis
The chi-square test was used for comparisons of categorical variables. Data of the two groups were compared using the independent Student’s t-test for normally distributed data, and the Mann-Whitney U test for non-normally distributed data. To assess the potential heterogeneity of nicorandil treatment effects on cardiac mortality, we conducted subgroup analyses. Interactions between nicorandil and the following clinically relevant variables, which are different between the two groups and/or generally known risk factors, were estimated by a Cox proportional hazards regression model: age, sex, left ventricular ejection fraction (LVEF), presence of left main trunk lesion, three-vessel disease, history of percutaneous coronary intervention or CABG, presence of diabetes, chronic kidney disease, dialysis, and use of β-blockers, statins, anti-platelet agents, and nitrate. A value of P < 0.05 was considered statistically significant for all comparisons. Analyses were performed using a statistical software package (SPSS ver. 21.0, IBM, Armonk, NY, USA).
Results
As shown in Table 1, the nicorandil group had higher prevalence of three-vessel disease, history of coronary artery bypass graft, usage of anti-platelet agents and statins, and tended to have higher prevalence of diabetes and usage of β-blockers and nitrates. In contrast, age, gender, New York Heart Association class, other co-morbidities, B-type natriuretic peptide, C-reactive protein, total protein, sodium, and LVEF did not differ between the two groups. During the follow-up period (mean 963 days), there were 56 cardiac deaths (13 in the nicorandil group and 43 in the non-nicorandil group). As shown in Fig. 1, the cardiac mortality was significantly lower in the nicorandil group than in the non-nicorandil group (P = 0.032). In the Cox proportional hazard analysis (Table 2), usage of nicorandil was a suppressor of cardiac mortality (HR 0.512, 95%CI 0.275–0.953, P = 0.035). Interactions between nicorandil use and clinically relevant variables were modeled using Cox regression and are shown in Table 2 for cardiac mortality. In the subgroup analysis, there was no interaction between nicorandil use and other important variables that affect cardiac mortality in any subgroups. Then, we focused on the history of CABG (Fig. 2), cardiac mortality was significantly lower in the nicorandil group than in the non-nicorandil group in patients with CABG (P = 0.019), and remained in a tendency in patients without CABG (P = 0.133).
Table 1.
Comparisons of clinical features (N = 334)
| Non-nicorandil group (n = 218) |
Nicorandil group (n = 116) |
P-value | |
|---|---|---|---|
| Age (years) | 71.7 ± 11.6 | 69.8 ± 10.5 | 0.146 |
| Male gender (n, %) | 169 (77.5) | 86 (74.1) | 0.488 |
| Body mass index (kg/cm2) | 23.7 ± 4.6 | 23.9 ± 4.2 | 0.708 |
| Systolic blood pressure (mmHg) | 132.3 ± 36.1 | 130.8 ± 35.9 | 0.716 |
| Diastolic blood pressure (mmHg) | 76.2 ± 23.3 | 72.4 ± 21.5 | 0.144 |
| Heart rate (bpm) | 82.2 ± 23.8 | 77.5 ± 21.2 | 0.071 |
| New York Heart Association class III or IV (n, %) | 5 (2.3) | 3 (2.6) | 0.868 |
| LVEF (%) | 43.3 ± 13.6 | 45.6 ± 14.5 | 0.211 |
| LMT lesion (n, %) | 9 (4.1) | 10 (8.6) | 0.134 |
| 3VD (n, %) | 45 (20.6) | 40 (34.5) | 0.008 |
| PCI (n, %) | 159 (72.9) | 86 (74.1) | 0.813 |
| CABG (n, %) | 27 (12.4) | 42 (36.2) | <0.001 |
| Co-morbidity | |||
| Hypertension (n, %) | 194 (89.0) | 105 (90.5) | 0.665 |
| Diabetes (n, %) | 128 (58.7) | 79 (68.1) | 0.092 |
| Dyslipidemia (n, %) | 193 (88.5) | 107 (92.2) | 0.286 |
| Atrial fibrillation (n, %) | 64 (29.4) | 27 (23.3) | 0.235 |
| Chronic kidney disease (n, %) | 151 (69.3) | 78 (67.2) | 0.704 |
| Dialysis (n, %) | 28 (12.8) | 16 (13.8) | 0.865 |
| Anemia (n, %) | 141 (64.7) | 78 (67.2) | 0.639 |
| Smoking (n, %) | 155 (71.1) | 74 (63.8) | 0.171 |
| Medications | |||
| Angiotensin converting enzyme inhibitors (n, %) | 123 (56.4) | 71 (61.2) | 0.417 |
| Angiotensin receptor blockers (n, %) | 67 (30.7) | 36 (31.0) | 1.000 |
| Aldosterone antagonists (n, %) | 91 (41.7) | 42 (36.2) | 0.325 |
| β-blockers (n, %) | 176 (80.7) | 102 (87.9) | 0.094 |
| Calcium channel blockers (n, %) | 94 (43.1) | 47 (40.5) | 0.727 |
| Diuretics (n, %) | 153 (70.2) | 79 (68.1) | 0.694 |
| Inotropic agents (n, %) | 27 (12.4) | 11 (9.5) | 0.426 |
| Anti-platelet agents (n, %) | 186 (85.3) | 113 (97.4) | <0.001 |
| Anti-coagulations (n, %) | 97 (44.5) | 50 (43.1) | 0.807 |
| Anti-diabetic agents (n, %) | 92 (42.2) | 59 (50.9) | 0.135 |
| Statins (n, %) | 132 (60.6) | 88 (75.9) | 0.005 |
| Nitrates (n, %) | 44 (20.2) | 34 (29.3) | 0.077 |
| Laboratory data | |||
| BNP (pg/ml)a | 306.5 (865.1) | 377.5 (619.8) | 0.374 |
| C-reactive protein (mg/dl)a | 0.32 (1.19) | 0.21 (0.78) | 0.132 |
| Total protein (g/dl) | 7.0 ± 0.8 | 7.0 ± 0.7 | 0.816 |
| Sodium (mEq/l) | 138.2 ± 4.2 | 138.6 ± 3.5 | 0.492 |
LVEF left ventricular ejection fraction, LMT left main trunk, 3VD three-vessel disease, PCI percutaneous coronary intervention, CABG coronary artery bypass graft, BNP B-type natriuretic peptide
aData are presented as median (interquartile range)
Fig. 1.
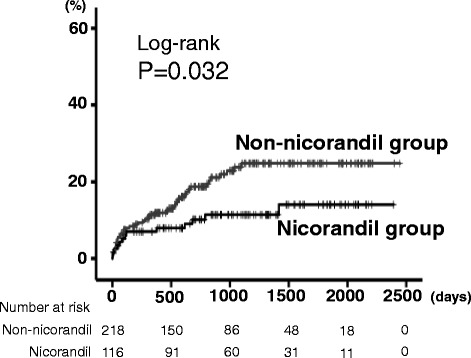
Comparison of cardiac mortality between the nicorandil (n = 116) and non-nicorandil groups (n = 218)
Table 2.
Subgroup analysis for cardiac mortality: Nicorandil use
| Factor | Subgroup | n | HR | 95% Cl | P value | Interaction P value |
|---|---|---|---|---|---|---|
| Total | 334 | 0.512 | 0.275–0.953 | 0.035 | - | |
| Age | ≥75 | 143 | 0.807 | 0.344–1.890 | 0.621 | 0.252 |
| <75 | 191 | 0.380 | 0.153–0.942 | 0.037 | ||
| Sex | Male | 255 | 0.449 | 0.216–0.932 | 0.032 | 0.403 |
| Female | 79 | 0.737 | 0.213–2.547 | 0.629 | ||
| LVEF | Reduced | 244 | 0.623 | 0.325–1.192 | 0.153 | 0.405 |
| Preserved | 90 | 0.245 | 0.029–2.102 | 0.200 | ||
| LMT | Present | 19 | 1.240 | 0.000–3.420 | 0.581 | 0.968 |
| Absent | 315 | 0.492 | 0.259–0.934 | 0.030 | ||
| 3VD | Present | 85 | 0.672 | 0.244–1.849 | 0.441 | 0.482 |
| Absent | 249 | 0.425 | 0.188–0.962 | 0.040 | ||
| PCI | Present | 245 | 0.556 | 0.272–1.138 | 0.108 | 0.646 |
| Absent | 89 | 0.422 | 0.120–1.483 | 0.179 | ||
| CABG | Present | 69 | 0.181 | 0.036–0.897 | 0.036 | 0.128 |
| Absent | 265 | 0.718 | 0.366–1.409 | 0.336 | ||
| Diabetes | Present | 207 | 0.412 | 0.177–0.957 | 0.039 | 0.361 |
| Absent | 127 | 0.742 | 0.296–1.858 | 0.523 | ||
| CKD | Present | 229 | 0.434 | 0.217–0.871 | 0.019 | 0.206 |
| Absent | 105 | 1.252 | 0.280–5.596 | 0.769 | ||
| Dialysis | Present | 44 | 0.338 | 0.073–1.568 | 0.166 | 0.595 |
| Absent | 290 | 0.557 | 0.282–1.100 | 0.092 | ||
| β-blockers | Present | 278 | 0.483 | 0.229–1.022 | 0.057 | 0.469 |
| Absent | 56 | 0.830 | 0.273–2.523 | 0.743 | ||
| Statins | Present | 220 | 0.720 | 0.324–1.604 | 0.422 | 0.425 |
| Absent | 114 | 0.400 | 0.139–1.153 | 0.090 | ||
| Anti-platelet agents | Present | 299 | 0.600 | 0.316–1.140 | 0.119 | 0.907 |
| Absent | 35 | 0.041 | 0.000–215.058 | 0.464 | ||
| Nitrates | Present | 78 | 0.551 | 0.188–1.616 | 0.277 | 0.814 |
| Absent | 256 | 0.474 | 0.219–1.027 | 0.058 |
LVEF left ventricular ejection fraction, LMT left main trunk, 3VD three-vessel disease, PCI percutaneous coronary intervention, CABG coronary artery bypass graft, CKD chronic kidney disease
Fig. 2.

Comparison of cardiac mortality between the nicorandil and non-nicorandil groups in patients with or without coronary artery bypass graft (CABG): a. Without CABG (n = 265) and b. With CABG (n = 69)
Discussion
In the present study, we firstly demonstrated that oral administration of nicorandil was associated with lower cardiac mortality in ischemic HF patients, and this result was consistent in several subgroup analyses, such as LVEF, percutaneous coronary intervention, coronary artery bypass graft, diabetes, β-blockers, and statins.
Intravenous nicorandil for decompensated HF patients, regardless of ischemic etiology, improves cardiac pump function, New York Heart Association class, left ventricular function, myocardial microvascular circulation, pulmonary capillary wedge pressure, pulmonary arterial pressure, and peripheral resistance [13], and oral administration of nicorandil decreases the composite end point of mortality and hospitalization for cardiac causes (HR 0.35, 95% CI 0.16–0.54) [13]. Oral administration of nicorandil suppresses sympathetic nervous activity, prevents left ventricular remodeling in HF patients (LVEF <45%, ischemic etiology 43.5%), and may reduce cardiac events (cardiac mortality, HR 0.502, 95% CI 0.268–0.940; major adverse cardiac effect, HR 0.436, 95% CI 0.266–0.715) [14]. These previous reports [13, 14] are partially concordant with our results.
Several favorable effects of nicorandil on cardiovascular system have been reported, such as reduction in preload and afterload, improvement of myocardial perfusion, protection of cardiomyocytes from ischemic damage, prevention of Ca2+ overload by opening adenosine triphosphate-sensitive potassium channels, anti-inflammatory and anti-proliferative effects, anti-apoptosis, anti-arrhythmic effects, protection of endothelial, mitochondrial, and energy-modulating functions, and preservation of kidney function [11, 13, 14].
Study limitations
There are several limitations in the present study. First, it is a nonrandomized and retrospective study of a single institution, so the number of subjects was relatively small and there are potential biases and confounders that may be responsible for our findings. Second, we have conducted this study using only variables on hospitalization, without consideration for changes in medical parameters and post-discharge treatment. Third, our results has not established a cause-effect relationship between the usage of nicorandil and improvement of cardiac mortality. Thus, the results of the present study should be viewed as preliminary, and further studies with larger populations and randomization are needed.
Conclusions
In conclusion, nicorandil potentially reduces cardiovascular mortality in patients with ischemic HF.
Acknowledgements
The authors acknowledge Ms. Kumiko Watanabe, Ms. Tomiko Miura and Ms. Hitomi Kobayashi for their outstanding technical assistance.
Funding
Not applicable.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
AY and YT, making article, drafting the article and conception of this study; SW and TY, performing statistical analysis; TS, SS, MO, and AK obtaining general data; YT revising the article critically for important intellectual content. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a prior approval by the institution’s human research committee (Fukushima Medical University).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- CABG
Coronary artery bypass graft
- CI
Confidence interval
- HF
Heart failure
- HR
Hazard ratio
- LVEF
Left ventricular ejection fraction
- RR
Relative risk
Contributor Information
Akiomi Yoshihisa, Phone: +81 24 547 1190, Email: yoshihis@fmu.ac.jp.
Yu Sato, Email: yu-sato@fmu.ac.jp.
Shunsuke Watanabe, Email: sw232304@fmu.ac.jp.
Tetsuro Yokokawa, Email: yokotetu@fmu.ac.jp.
Takamasa Sato, Email: takamasa@fmu.ac.jp.
Satoshi Suzuki, Email: ssatoshi@fmu.ac.jp.
Masayoshi Oikawa, Email: moikawa@fmu.ac.jp.
Atsushi Kobayashi, Email: koba-a@fmu.ac.jp.
Yasuchika Takeishi, Email: takeishi@fmu.ac.jp.
References
- 1.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 2.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2013;62(16):e147–239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 3.Fonarow GC, Albert NM, Curtis AB, Gheorghiade M, Liu Y, Mehra MR, et al. Incremental reduction in risk of death associated with use of guideline-recommended therapies in patients with heart failure: a nested case-control analysis of IMPROVE HF. J Am Heart Assoc. 2012;1(1):16–26. doi: 10.1161/JAHA.111.000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khatibzadeh S, Farzadfar F, Oliver J, Ezzati M, Moran A. Worldwide risk factors for heart failure: a systematic review and pooled analysis. Int J Cardiol. 2013;168(2):1186–1194. doi: 10.1016/j.ijcard.2012.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Felker GM, Shaw LK, O'Connor CM. A standardized definition of ischemic cardiomyopathy for use in clinical research. J Am Coll Cardiol. 2002;39(2):210–218. doi: 10.1016/S0735-1097(01)01738-7. [DOI] [PubMed] [Google Scholar]
- 6.Smith PK, Puskas JD, Ascheim DD, Voisine P, Gelijns AC, Moskowitz AJ, et al. Surgical treatment of moderate ischemic mitral regurgitation. N Engl J Med. 2014;371(23):2178–88. doi: 10.1056/NEJMoa1410490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mohr FW, Morice MC, Kappetein AP, Feldman TE, Stahle E, Colombo A, et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet. 2013;381(9867):629–38. doi: 10.1016/S0140-6736(13)60141-5. [DOI] [PubMed] [Google Scholar]
- 8.Deja MA, Grayburn PA, Sun B, Rao V, She L, Krejca M, et al. Influence of mitral regurgitation repair on survival in the surgical treatment for ischemic heart failure trial. Circulation. 2012;125(21):2639–48. doi: 10.1161/CIRCULATIONAHA.111.072256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Velazquez EJ, Lee KL, Deja MA, Jain A, Sopko G, Marchenko A, et al. Coronary-artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med. 2011;364(17):1607–16. doi: 10.1056/NEJMoa1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petrie MC, Jhund PS, She L, Adlbrecht C, Doenst T, Panza JA, et al. Ten-year outcomes after coronary artery bypass grafting according to age in patients with heart failure and left ventricular systolic dysfunction: an analysis of the extended follow-up of the STICH trial (Surgical Treatment for Ischemic Heart Failure) Circulation. 2016;134(18):1314–24. doi: 10.1161/CIRCULATIONAHA.116.024800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo B, Wu P, Bu T, Zeng Z, Lu D. All-cause mortality and cardiovascular events with nicorandil in patients with IHD: systematic review and meta-analysis of the literature. Int J Cardiol. 2014;176(3):661–669. doi: 10.1016/j.ijcard.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 12.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285(26):1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 13.Zhao F, Chaugai S, Chen P, Wang Y, Wang DW. Effect of nicorandil in patients with heart failure: a systematic review and meta-analysis. Cardiovasc Ther. 2014;32(6):283–296. doi: 10.1111/1755-5922.12097. [DOI] [PubMed] [Google Scholar]
- 14.Kasama S, Toyama T, Iwasaki T, Sumino H, Kumakura H, Minami K, et al. Effects of oral nicorandil therapy on sympathetic nerve activity and cardiac events in patients with chronic heart failure: subanalysis of our previous report using propensity score matching. Eur J Nucl Med Mol Imaging. 2014;41(1):144–54. doi: 10.1007/s00259-013-2538-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.


