Abstract
Objective
To assess whether multiplex polymerase chain reaction (mPCR) vs non-mPCR testing impacts the use of antibiotics, chest radiographs, and isolation precautions.
Study design
We retrospectively compared use of antibiotics, chest radiographs, and isolation precautions for patients <18 years old (excluding neonates) hospitalized at a tertiary referral center tested for respiratory pathogens in the emergency department or during the first 2 hospital days, during 2 periods: June 2010-June 2012 (non-mPCR group) vs October 2012-May 2014 (mPCR group).
Results
Subjects (n = 2430) in the mPCR group were older, had more complex chronic conditions, and were admitted to the pediatric intensive care unit more often compared with the non-mPCR (n = 2349) group. Subjects in the mPCR group had more positive tests (42.4% vs 14.4%, P < .01), received fewer days of antibiotics (4 vs 5 median antibiotic days, P < .01), fewer chest radiographs performed, (59% vs 78%, P < .01), and were placed in isolation longer (20 vs 0 median isolation-hours, P < .01) compared with the non-mPCR group. In multivariable regression, patients tested with mPCR were less likely to receive antibiotics for ≥2 days (OR 0.5, 95% CI 0.5-0.6), chest radiographs at admission (OR 0.4, 95% CI 0.3-0.4), and more likely to be in isolation for ≥2 days (OR 2.4, 95% CI 2.1-2.8) compared with the non-mPCR group.
Conclusions
Use of mPCR testing for respiratory viruses among hospitalized patients was significantly associated with decreased healthcare resource utilization, including decreased use of antibiotics and chest radiographs, and increased use of isolation precautions.
Keywords: Respiratory pathogen testing, Health resources, Respiratory viral panel
Abbreviations: CUMC, Columbia University Medical Center; EMR, Electronic medical record; ICD-9, International Classification of Diseases, Ninth Revision; mPCR, Multiplex polymerase chain reaction; PCR, Polymerase chain reaction; PHIS, Pediatric Health Information System; PICU, Pediatric intensive care unit; RSV, Respiratory syncytial virus
Multiplex polymerase chain reaction (mPCR) for diagnosis of respiratory pathogens is increasingly used in pediatric inpatient facilities.1, 2 Food and Drug Administration-approved mPCR assays now enable detection of a broader array of viruses with higher specificity, sensitivity, and faster turnaround time than previous testing using immunoassays or cultures.3, 4 Although rapid identification of a viral etiology for clinical illness could affect healthcare resource utilization, this has not been established conclusively in hospitalized children.5 Previous work assessing the impact of mPCR testing on clinical outcomes such as duration of antibiotic therapy or length of stay in pediatric clinical settings has shown inconsistent results.2, 6 These studies assessed mPCR use in the emergency department7 or in an ambulatory care setting,2 limited subjects to those tested with mPCR6 or those with specific diagnoses,8 and did not adjust for seasonal trends.4
From July to September 2012, New York-Presbyterian Morgan Stanley Children's Hospital transitioned from the use of non-mPCR testing methods to the use of mPCR testing to identify respiratory pathogens. The objectives of this study were to compare the impact of using mPCR vs non-mPCR testing for respiratory pathogens at hospital admission on the utilization of healthcare resources for pediatric inpatients as measured by duration of inpatient antibiotic therapy, chest radiograph use on admission, and duration of isolation precautions. We hypothesized that using mPCR testing at admission would decrease use of antibiotics and chest radiographs, and increase the use of isolation precautions compared with these outcomes when using non-mPCR testing.
Methods
We conducted a retrospective cohort study of children hospitalized at New York-Presbyterian Morgan Stanley Children's Hospital, a 200-bed tertiary referral children's hospital located in New York City, who underwent testing for a respiratory pathogen either in the emergency department prior to admission or within the first 2 days of hospitalization. The Columbia University Medical Center (CUMC) Institutional Review Board approved this study with a waiver of consent.
Study subjects eligible for inclusion were hospitalized infants, children, and adolescents under 18 years of age, who were tested for respiratory pathogens in the emergency department prior to admission, in inpatient units, or in the pediatric intensive care unit (PICU) within the first 2 calendar days of hospitalization. Children tested after the first 2 calendar days of hospitalization were not included to restrict the sample to patients with community-acquired illnesses. Newborns admitted to the well-baby nursery and neonatal intensive care unit patients were excluded. All other neonates and infants (hospitalized in units other than well-baby nursery and neonatal intensive care unit), were included in the study population, regardless of age.
Testing for respiratory pathogens for the study period was ordered by the treating clinicians as part of routine care. Though there were no official guidelines, testing was recommended year round for all patients with respiratory symptoms and for febrile infants less than 2 months of age.
Subjects hospitalized between June 2010 and June 2012 were tested with non-mPCR methods. Non-mPCR testing included enzyme immunoassay for influenza and respiratory syncytial virus (RSV), direct fluorescent antigen for parainfluenza and adenovirus, and polymerase chain reaction (PCR) for influenza and RSV, and/or viral cultures, with a turnaround time of 2-5 days. These assays often were performed sequentially (eg, PCR testing was performed first followed by direct fluorescent antigen and/or culture if PCR results were negative). Mycoplasma (serology and PCR sent to a commercial laboratory) and Bordetella pertussis (culture and PCR sent to a commercial laboratory) testing were offered in the non-mPCR period but were not included in this analysis because results usually were not available within 48 hours and, hence, would less likely influence clinical decisions made during this period. Subjects hospitalized between October 2012 and May 2014 were tested with mPCR methods. Testing used the Food and Drug Administration-approved mPCR Film Array Respiratory panel (BioFire Diagnostics, Inc, Salt Lake City, Utah),9 which identifies adenovirus, coronavirus (strains HKU1, NL63, 229E, OC43); human metapneumovirus, rhinovirus/enterovirus; influenza (strains A, A/H1, A/H3, A/H1-2009, B); parainfluenza virus (strains 1, 2, 3, 4); and RSV as well as the bacterial respiratory pathogens Mycoplasma, B pertussis, and Chlamydophilia. This panel has high sensitivity (85%-100%), specificity (95%-100%), and at our institution a turnaround time of approximately 3 hours from order entry to results being viewed by providers.9, 10 Hospitalizations from July to September 2012 were excluded to allow providers to become familiar with mPCR testing.
Clinical, laboratory, and demographic data for study subjects were obtained from the CUMC clinical data warehouse and linked with CUMC data from the Pediatric Health Information System (PHIS) database.11 The CUMC clinical data warehouse includes patient data from the electronic medical record (EMR) and PHIS is a validated administrative database that contains inpatient data from hospitals affiliated with Children's Hospital Association (Overland Park, Kansas). PHIS contains demographic data, International Classification of Diseases, Ninth Revision (ICD-9) diagnostic codes, charge data for medications, and laboratory and radiology utilization.11 Data quality and reliability are assured through a joint effort between the Children's Hospital Association and participating hospitals. Demographic data included age, sex, and insurance status. Race/ethnicity data were not included in the final analysis because these data were unreliable and incomplete. Hospitalization data included dates of admission and discharge, dates/times of transfer to and discharge from the inpatient unit and/or PICU, length of hospital stay, and principal ICD-9 diagnosis. Principal ICD-9 diagnoses were categorized as either respiratory or nonrespiratory by study investigators (Table I; available at www.jpeds.com). Patients with complex chronic conditions were also identified using ICD-9 diagnoses.12 The date and results of respiratory pathogen testing were also collected.
Outcome Variables: Antibiotic and Chest Radiograph Utilization, Duration of Isolation Precautions
To measure the duration of antibiotic therapy, contiguous days of antibiotics commonly used to treat community-acquired respiratory illnesses ordered in the emergency department or within the first 2 calendar days of hospitalization were extracted (Table II; available at www.jpeds.com). Contiguous days of antibiotics could include different agents to account for narrowing or broadening therapy during a single antibiotic course. To determine utilization of imaging, chest radiographs ordered in the emergency department or within the first 2 calendar days of hospitalization were included to reflect those obtained to manage community-acquired illness. To measure the duration of isolation, the hours of contact isolation, droplet isolation, and/or contact/droplet isolation were calculated using the date/time for initiation and for discontinuation of isolation orders.
Statistical Analyses
Demographic and clinical characteristics of the subjects in the non-mPCR vs the mPCR group were compared using parametric (Student t test) and nonparametric (Wilcoxon rank sum test) tests for continuous variables, and the χ2 test for categorical variables. As PCR was used to detect RSV and influenza in both the non-mPCR and mPCR groups, rates of positivity for these 2 pathogens were compared to assess for testing patterns over time. Bivariate analysis determined the association between the use of mPCR testing and the 3 outcome variables: duration of antibiotic therapy, performance of chest radiograph, and duration of isolation precautions. We compared overall antibiotic and isolation utilization rates for mPCR and non-mPCR groups (calculated as summed number of antibiotic or isolation days divided by summed patient days) using the Fisher exact test. In addition, to assess if secular trends in antibiotic use could confound any observed association between antibiotic use and respiratory pathogen testing, we used data from PHIS to determine days of antibiotic use for patients with primary ICD-9 diagnoses of acute gastroenteritis, which are community-acquired diagnoses not diagnosed by the respiratory viral panel.
Multivariable analysis used logistic regression models to test the association of the exposure variables (use of the mPCR test vs a non-mPCR test) with 3 binary outcome variables: (1) duration of antibiotic therapy (≥2 days vs <2 antibiotic days); (2) use of chest radiograph (within 2 days vs none within 2 days of hospitalization); and (3) duration of isolation precautions (≥2 vs <2 days). These outcome variables were dichotomized as described because, although results from respiratory testing performed within the first 2 days of admission would influence the immediate use of antibiotics, chest radiograph, and isolation precautions, overall use of these resources throughout the hospital stay, particularly if prolonged, could be impacted by other factors (eg, management of complex chronic conditions or hospital-acquired conditions).
The following covariates were included in the 3 primary models: age in years (as a categorical variable with each year as a category); having public insurance, a complex chronic condition, primary respiratory diagnosis, and/or PICU admission. To adjust for seasonal variation, we included an indicator variable for one-quarter of the year (with the first one-quarter of the year as a reference group). Likelihood ratio tests were used to determine the addition of variables into the model. ORs were calculated for all variables of interest and an alpha error of 0.05 was prespecified. We repeated the regression analysis with varying cut points for antibiotic use (no antibiotic vs any antibiotics, ≥3 days vs <3 days of antibiotic therapy) and isolation implementation (any isolation vs no isolation) to ensure robustness of any regression findings seen in the above analysis.
To determine the impact of test positivity on resource utilization, we compared clinical outcomes between those patients who had positive and negative tests within each group. We used t tests for continuous variables and χ2 tests for categorical variables to compare antibiotic use, chest radiograph use, and duration of isolation between patients with positive and negative tests within the non-mPCR and within the mPCR groups separately.
Results
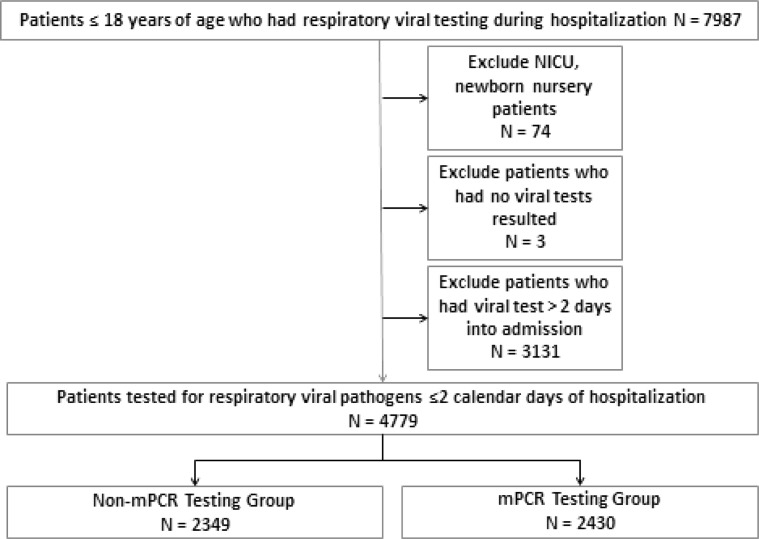
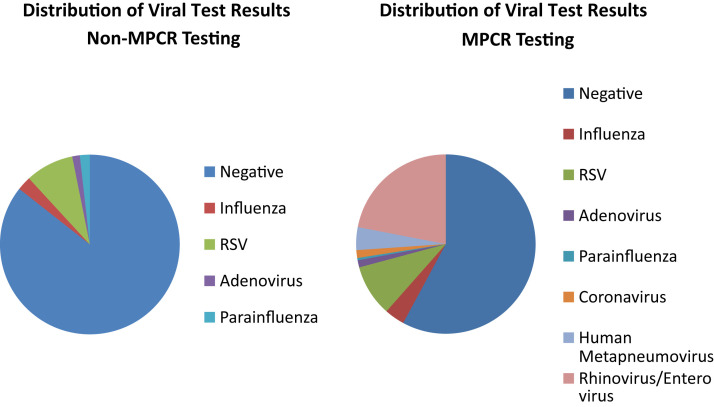
During the study period, 4779 hospitalized patients were included; 2349 patients tested with non-mPCR assays and 2430 tested with mPCR (Figure 1; available at www.jpeds.com). Subjects tested with mPCR were older, more likely to have a complex chronic condition, or be admitted to the PICU during their hospitalization (Table III ). Test positivity was higher in the mPCR group compared with the non-mPCR group (42.4% vs 14.4%, P < .01) (Figure 2; available at www.jpeds.com). Rates of RSV (8.7% vs 9.3%, P = .49) and influenza (2.6% vs 3.6%, P = .03) were similar in the 2 groups.
Figure 1.
Study population. NICU, neonatal intensive care unit.
Table III.
Characteristics of the subjects in the non-mPCR and mPCR testing groups
| Characteristics of subjects | Non-mPCR testing June 2010-May 2012 |
mPCR testing October 2012-May 2014 |
P value |
|---|---|---|---|
| N = 2349 | N = 2430 | ||
| Age in y, median (IQR) | 1 (0-5) | 2 (0-7) | <.01 |
| Sex, female, n (%) | 1064 (45) | 1102 (45) | .97 |
| Public insurance, n (%) | 1603 (68) | 1616 (67) | .2 |
| LOS in d, median | 3 | 4 | .51 |
| PICU admission, n (%) | 512 (21) | 745 (31) | <.01 |
| Primary respiratory ICD-9 diagnosis, n (%) | 705 (30) | 670 (28) | .06 |
| Complex chronic condition, n (%) | 1033 (44) | 1150 (47) | .02 |
LOS, length of stay.
Bold values indicate P < .05.
Figure 2.
Distribution of respiratory pathogen testing results comparing non-mPCR and mPCR testing groups.
Impact of mPCR Testing on Use of Antibiotics, Chest Radiograph, and Isolation Precautions
Overall, subjects in the mPCR group received fewer days of antibiotics than subjects in the non-mPCR group (4 vs 5 median antibiotic days, P < .01), although the number of antibiotic days per patient days was similar (75 vs 86 antibiotic days per 100 patient days, P = .4; Table IV ). Fewer subjects in the mPCR group had chest radiograph performed during the first 2 days of admission compared with those in the non-mPCR group (P < .01). Overall, subjects in the mPCR group spent more days in isolation (34 vs 15 isolation days per 100 patient days, P < .01) with a longer median duration of isolation (P < .01; Table IV). More subjects in the mPCR group were placed on isolation precautions within the first 2 hospitalization days (60.3% vs 35.3%, P = .01) and had isolation precautions extended ≥2 days compared with those in the non-mPCR group (34.8% vs 18.0%, P < .01).
Table IV.
Healthcare resource utilization in the non-mPCR vs mPCR testing groups
| Healthcare resources | Non-mPCR Testing June 2010-May 2012 |
mPCR Testing October 2012-May 2014 |
P value |
|---|---|---|---|
| N = 2349 | N = 2430 | ||
| Antibiotic d, median (IQR) | 5 (2-9) | 4 (1-8) | <.01 |
| Antibiotic d per 100 patient d, overall mean | 86 | 75 | .4 |
| Chest radiograph performance, n (%) | 1820 (78) | 1426 (59) | <.01 |
| Isolation precaution duration in h, median (IQR) | 0 (0-36) | 20.2 (0-70) | <.01 |
| Isolation precaution duration in d per 100 patient d, overall mean | 15 | 34 | <.01 |
Bold values indicate P < .05.
Factors associated with healthcare resource utilization assessed by multivariable logistic regression are shown in Table V . Patients who had mPCR testing or a primary respiratory diagnosis were less likely to have antibiotics continued for ≥2 days. Patients who had mPCR testing were significantly less likely to have a chest radiograph performed. In contrast, being admitted to the PICU, having a primary respiratory diagnosis, or a complex chronic condition was predictive of increased chest radiograph use. Those who had mPCR testing, a primary respiratory diagnosis, or PICU admission were more likely to have had isolation precautions extended for ≥2 days. Repeating the analysis of healthcare resource utilization with varying durations of antibiotic use and isolation did not alter the findings of the primary regression models (data not shown). To assess for secular trends in antibiotic treatment, we compared antibiotic use for acute gastroenteritis and found that the use was similar in the non-mPCR testing period vs the mPCR testing period (19.8 antibiotic days vs 21.1 antibiotic days/100 patient days, respectively, P = .9).
Table V.
Factors associated with resource utilization, including non-mPCR vs mPCR testing, assessed by logistic regression∗
| Healthcare resources | OR (95% CI) |
|---|---|
| Antibiotic utilization (≥2 antibiotic-d) | |
| mPCR vs non-mPCR | 0.5 (0.5-0.6) |
| PICU admission (yes vs no) | 1.1 (0.9-1.2) |
| Primary ICD-9 respiratory diagnosis (yes vs no) | 0.6 (0.5-0.7) |
| Complex chronic condition (yes vs no) | 0.9 (0.8-1.1) |
| Insurance status (public vs not public) | 1.1 (0.9-1.2) |
| Chest radiograph use (within 2 d) | |
| mPCR vs non-mPCR | 0.4 (0.3-0.4) |
| PICU admission (yes vs no) | 3.9 (3.2-4.7) |
| Primary respiratory diagnosis (yes vs no) | 3.6 (2.9-4.2) |
| Complex chronic condition (yes vs no) | 2.3 (2.0-2.7) |
| Insurance status (public vs not public) | 0.8 (0.7-0.9) |
| Isolation for ≥2 d | |
| mPCR vs non-mPCR | 2.4 (2.1-2.8) |
| PICU admission (yes vs no) | 2.1 (1.8-2.4) |
| Primary respiratory diagnosis (yes vs no) | 1.8 (1.5-2.1) |
| Complex chronic condition (yes vs no) | 1.2 (1.0-1.4) |
| Insurance status (public vs not public) | 0.9 (0.8-1.0) |
Bold values indicate P < .05.
All models adjusted for age and one-quarter of year.
Impact of Positive vs Negative Results on Use of Antibiotics, Chest Radiograph, and Isolation Precautions
The association of positive tests vs negative test results on utilization of healthcare resources is shown (Table VI ). Subjects with positive tests in both the mPCR and non-mPCR groups received less antibiotics, but more chest radiographs. In the mPCR group, a higher proportion of subjects with positive test results were placed on isolation for ≥2 days, and in the non-mPCR group there was no difference in this variable between those with positive vs negative test results.
Table VI.
Resource utilization among subjects with positive vs negative test results within the non-mPCR and mPCR groups
| Healthcare resources | Non-mPCR test results |
mPCR test results |
||
|---|---|---|---|---|
| Positive |
Negative |
Positive |
Negative |
|
| n = 338 | n = 2011 | n = 1030 | n = 1400 | |
| Antibiotic utilization (≥2 d) | 219 (64.8%)∗ | 1488 (74%) | 559 (54.3%)∗ | 876 (62.6%) |
| Chest radiograph utilization (≤2 d of hospitalization) | 277 (81.9%)† | 1543 (76.7%) | 678 (65.8%)† | 748 (53.4%) |
| Isolation precautions (≥2 d) | 60 (17.8%) | 363 (18.1%) | 617 (59.9%)∗ | 230 (16.4%) |
P < .01.
P < .05.
Discussion
In this study of a large cohort of hospitalized children, mPCR testing on admission was associated with less use of antibiotics and chest radiographs, and increased duration of isolation compared with testing with non-mPCR based methods. These results expand on previous work assessing the impact of mPCR based testing in pediatric settings. These studies have shown a decrease in antibiotic use after introduction of mPCR testing4; and shorter duration of antibiotic therapy for those with positive tests and common respiratory diagnoses,8 or those cared for by specific admitting services.6 The impact of mPCR testing in other clinical settings has been inconsistent; use of rapid viral testing has shown little impact on antibiotic use in ambulatory and emergency department settings.2, 7 This heterogeneity in findings could indicate that provider decisions for antibiotic use in the emergency department or ambulatory setting may be more impacted by clinical factors (eg, physical examination or past medical history) and less impacted by mPCR test results. In addition, in these settings, decisions are made within shorter time frames, and despite the relatively rapid turnaround time for mPCR testing, results may still not be timely enough to impact decision making. Furthermore, inpatient providers may benefit from a longer period of observation and serial clinical examinations to verify and validate results from diagnostic evaluations, possibly allowing for “bolder” decisions to stop antibiotics.
We found an association between mPCR testing and decreased use of chest radiographs. We theorize that more rapid and more sensitive identification of viral pathogens could have influenced providers' decisions not to obtain chest radiographs, particularly in patients without complex chronic conditions or clinical features suggestive of bacterial pneumonia. Supporting this, patients with more severe illness requiring a PICU stay or those with complex chronic conditions were more likely to have had a chest radiograph performed.
Use of mPCR testing increased the duration of isolation precautions. Current isolation strategies to prevent healthcare-associated transmission of respiratory viruses are largely based on older data,13 and the efficacy of these strategies for several of the less common pathogens identified by mPCR has not been tested rigorously.14, 15 Use of these precautions also requires costly hospital resources,15 has been associated with adverse events such as falls, and fewer physician visits in adult patient populations14 and may interfere with family-centered care.16 Future studies should assess the optimal use of isolation precautions for preventing healthcare transmission of the viral pathogens identified by mPCR testing.
In both mPCR and non-mPCR groups, test results impacted antibiotic and chest radiograph utilization. In both groups, patients with positive tests received less antibiotics compared with those with negative tests, although the proportions of patients with positive tests who were treated with 2 or more antibiotic days remained high. These results indicate the need for continued antibiotic stewardship activities to avoid unnecessary antibiotic therapy for viral infections. Similarly, patients with positive tests in both groups had higher rates of utilization of chest radiograph compared with patients with negative tests. We speculate this might have been due to more prominent respiratory symptoms in those testing positive. Finally, a higher proportion of patients with positive test results in the mPCR group were isolated for 2 or more days compared with those with negative test results. This observation is not unexpected given the detection of more viral pathogens by mPCR and active surveillance of test results by the Department of Infection Prevention and Control staff to ensure appropriate isolation rather than only relying on front-line clinicians to implement isolation precautions.
This study has limitations. It was performed at a referral children's hospital and findings may not be generalizable to other populations or settings. To assess the overall impact of mPCR testing, we included all patients who underwent respiratory testing within the first 2 days of hospitalization and did not restrict the study population to a specific diagnosis (eg, community-acquired pneumonia). We used a combination of EMR and administrative data collected retrospectively, which may have increased the imprecision of our estimates of antibiotic utilization, chest radiograph use, and isolation precautions. However, during the study period, there were no substantial changes to the EMR, or to our hospital's contributions to the PHIS database that should minimize bias. Although we adjusted for likely confounding variables, including seasonal variation, it is possible that other secular trends could have contributed to the differences in antibiotic and chest radiograph use observed over the years of the study. The publication of the pediatric community-acquired pneumonia guidelines and revised guidelines for bronchiolitis during the study period may have impacted individual clinicians' testing and treating practices among patients hospitalized with respiratory symptoms. There has also been increased interest nationally in minimizing radiation exposure in children, which may have influenced provider behavior and, thus, limited use of chest radiographs. However, during the study period, the New York-Presbyterian Morgan Stanley Children's Hospital did not implement substantial changes in antimicrobial stewardship, or other hospital-wide initiatives specifically to reduce the use of antibiotics or radiographs. In addition, the mPCR group had more patients with complex chronic conditions and more patients admitted to the PICU, which would more likely have been associated with an increase in use of antibiotics and imaging. The database does not permit assessment of the provider's interpretation of positive test results relative to causality of the patient's clinical syndrome or appropriateness of antibiotic therapy. Further work should focus on ways to improve decision support to aid in more effective use of mPCR testing by providers. In addition, the effect of mPCR testing on preventing healthcare transmission of viral pathogens should be assessed.
Acknowledgments
We acknowledge Barbara Ross, RN, MS, CIC, and Rohit Chaudhry, MS (NewYork-Presbyterian Hospital) for their help in obtaining the data for this study, as well as Lisa Kesting, MPA (New York University Langone Medical Center), Samantha Cox (Harvard Business School), and Allison Baxterbeck, MD (NewYork-Presbyterian Hospital, Columbia University Medical Center), for help in data cleaning and verification.
Footnotes
The authors declare no conflicts of interest.
Appendix.
Table I.
Primary respiratory ICD-9 diagnosis codes
| ICD-9 codes | Primary diagnosis names |
|---|---|
| 464.4 | Croup |
| 465.9 | Acute URI NOS |
| 466 | Acute bronchitis |
| 466.11 | Acute bronchiolitis due to RSV |
| 466.19 | Acute bronchiolitis due to organism NEC |
| 480 | Adenoviral pneumonia |
| 480.1 | RSV pneumonia |
| 480.02 | Parainfluenza viral pneumonia |
| 480.8 | Viral pneumonia NEC |
| 480.9 | Viral pneumonia NOS |
| 481 | Pneumococcal pneumonia |
| 482 | Klebsiella pneumoniae pneumonia |
| 482.1 | Pseudomonal pneumonia |
| 482.3 | Strep pneumonia NOS |
| 482.31 | Group A Step pneumonia |
| 482.39 | Strep pneumonia NEC |
| 482.41 | Staph aureus pneumonia |
| 482.42 | MRSA pneumonia |
| 482.49 | Staph pneumonia NEC |
| 482.82 | E coli pneumonia |
| 482.83 | Gram-negative pneumonia NEC |
| 482.9 | Bacterial pneumonia NOS |
| 483 | Mycoplasma pneumoniae pneumonia |
| 483.1 | Chlamydial pneumonia |
| 485 | Bronchopneumonia org NOS |
| 486 | Pneumonia organism NOS |
| 487 | Influenza w pneumonia |
| 487.1 | Flu with respiratory manifestations NEC |
| 487.8 | Flu with manifestations NEC |
| 488.11 | Flu 2009 H1N1 with pneumonia |
| 488.12 | Flu 2009 H1N1 with respiratory NEC |
| 488.19 | Flu 2009 H1N1 with manifestations NEC |
| 488.81 | Flu nov influenza A with pneumonia |
| 488.82 | Flu nov influenza A-respiratory NEC |
| 488.89 | Flu nov influenza A-manifestations NEC |
| 493.01 | Extrinsic asthma with status asthmaticus |
| 493.02 | Extrinsic asthma with exacerbation |
| 493.12 | Intrinsic asthma with exacerbation |
| 493.21 | Chronic obstructive asthma with status |
| 493.22 | Chronic obstructive asthma with exacerbation |
| 493.9 | Asthma NOS |
| 493.91 | Asthma with status asthmaticus |
| 493.92 | Asthma NOS with exacerbation |
| 511.81 | Mal pleural effusion |
| 511.89 | Other pleural effusion not TB |
| 512.89 | Pneumothorax NEC |
| 518.52 | PI NEC following trauma/surgery |
| 518.81 | Acute respiratory failure |
| 518.82 | Other pulmonary insufficiency |
| 518.84 | Acute and chronic respiratory failure |
| 518.89 | Other lung disease NEC |
| 519.01 | Tracheostomy infection |
| 519.09 | Tracheostomy complication NEC |
| 519.11 | Acute bronchospasm |
| 519.19 | Trachea/bronchus disease NEC |
| 786.03 | Apnea |
| 786.06 | Tachypnea |
| 786.09 | Respiratory abnormality NEC |
URI, upper respiratory infection; NOS, not otherwise specified; RSV, respiratory syncytial virus; NEC, not elsewhere classifiable; MRSA, methicillin-resistant staphylococcus aureus; TB, Tuberculosis; PI, Pulmonary insufficiency.
Table II.
Antibiotics commonly used to treat respiratory illnesses
| Amoxicillin |
| Amoxicillin-clavulanate |
| Ampicillin |
| Ampicillin-sulbactam |
| Azithromycin |
| Cefepime |
| Cefixime |
| Cefotaxime |
| Ceftazidime |
| Ceftriaxone |
| Cefuroxime |
| Cephalexin |
| Clindamycin |
| Erythromycin |
| Levofloxacin |
| Linezolid |
| Oxacillin |
| Penicillin G benzathine |
| Penicillin G sodium |
| Penicillin V potassium |
| Piperacillin-tazobactam |
| Sulfamethoxazole-trimethoprim |
| Vancomycin |
References
- 1.Wishaupt J.O., Versteegh F.G.A., Hartwig N.G. PCR testing for paediatric acute respiratory tract infections. Paediatr Respir Rev. 2015;16:43–48. doi: 10.1016/j.prrv.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wishaupt J.O., Russcher A., Smeets L.C., Versteegh F.G.A., Hartwig N.G. Clinical impact of RT-PCR for pediatric acute respiratory infections: a controlled clinical trial. Pediatrics. 2011;128:e1113–e1120. doi: 10.1542/peds.2010-2779. [DOI] [PubMed] [Google Scholar]
- 3.Wang J., Simons D.B., Adams J.L., Jerris R.C., Rogers B.B. Multiplex viral polymerase chain reaction testing using the FilmArray device compared with direct fluorescent antibody testing. Lab Med. 2014;45:62–64. doi: 10.1309/lmomixq6n4japdx1. [DOI] [PubMed] [Google Scholar]
- 4.Rogers B.B., Shankar P., Jerris R.C., Kotzbauer D., Anderson E.J., Watson J.R. Impact of a rapid respiratory panel test on patient outcomes. Arch Pathol Lab Med. 2015;139:636–641. doi: 10.5858/arpa.2014-0257-OA. [DOI] [PubMed] [Google Scholar]
- 5.Robinson C.C. The value of RVP in children's hospitals. J Clin Virol. 2007;40(Suppl 1):S51–S52. doi: 10.1016/S1386-6532(07)70011-2. [DOI] [PubMed] [Google Scholar]
- 6.Schulert G.S., Lu Z., Wingo T., Tang Y.-W., Saville B.R., Hain P.D. Role of a respiratory viral panel in the clinical management of pediatric inpatients. Pediatr Infect Dis J. 2013;32:467–472. doi: 10.1097/INF.0b013e318284b146. [DOI] [PubMed] [Google Scholar]
- 7.Doan Q., Enarson P., Kissoon N., Klassen T.P., Johnson D.W. Rapid viral diagnosis for acute febrile respiratory illness in children in the Emergency Department. Cochrane Database Syst Rev. 2014;9:CD006452. doi: 10.1002/14651858.CD006452.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schulert G.S., Hain P.D., Williams D.J. Utilization of viral molecular diagnostics among children hospitalized with community acquired pneumonia. Hosp Pediatr. 2014;4:372–376. doi: 10.1542/hpeds.2014-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li H., McCormac M.A., Estes R.W., Sefers S.E., Dare R.K., Chappell J.D. Simultaneous detection and high-throughput identification of a panel of RNA viruses causing respiratory tract infections. J Clin Microbiol. 2007;45:2105–2109. doi: 10.1128/JCM.00210-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poritz M.A., Blaschke A.J., Byington C.L., Meyers L., Nilsson K., Jones D.E. FilmArray, an automated nested multiplex PCR system for multi-pathogen detection: development and application to respiratory tract infection. PLoS One. 2011;6:e26047. doi: 10.1371/journal.pone.0026047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerber J.S., Newland J.G., Coffin S.E., Hall M., Thurm C., Prasad P.A. Variability in antibiotic use at children's hospitals. Pediatrics. 2010;126:1067–1073. doi: 10.1542/peds.2010-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simon T.D., Berry J., Feudtner C., Stone B.L., Sheng X., Bratton S.L. Children with complex chronic conditions in inpatient hospital settings in the United States. Pediatrics. 2010;126:647–655. doi: 10.1542/peds.2009-3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siegel J.D., Rhinehart E., Jackson M., Chiarello L., Health Care Infection Control Practices Advisory Committee 2007 guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control. 2007;35(10 Suppl 2):S65–S164. doi: 10.1016/j.ajic.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stelfox H.T., Bates D.W., Redelmeier D.A. Safety of patients isolated for infection control. JAMA. 2003;290:1899–1905. doi: 10.1001/jama.290.14.1899. [DOI] [PubMed] [Google Scholar]
- 15.Verlee K., Berriel-Cass D., Buck K., Nguyen C. Cost of isolation: daily cost of isolation determined and cost avoidance demonstrated from the overuse of personal protective equipment in an acute care facility. Am J Infect Control. 2014;42:448–449. doi: 10.1016/j.ajic.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 16.Cohen E., Austin J., Weinstein M., Matlow A., Redelmeier D.A. Care of children isolated for infection control: a prospective observational cohort study. Pediatrics. 2008;122:e411–e415. doi: 10.1542/peds.2008-0181. [DOI] [PubMed] [Google Scholar]