Abstract
We report herein a method for the recovery, purification, and application of OX063, a costly, commercially available non-toxic spin probe widely used for EPR imaging, as well as its corresponding quinone methide (QM) form. This precious probe can be successfully recovered after use in animal model experiments (25–47 % recovery from crude lyophilizate, with 98.5% purity), even from samples that are >2 years old. Significantly, the recovered trityl can be reused in further animal model EPR imaging experiments. The work also describes support for the observed formation of an air-sensitive radical derived from QM under reducing conditions.
Keywords: OX063 trityl, Quinone-methide, EPR imaging
TOC image
INTRODUCTION
Since Gomberg’s first report in 1900,1 trityl radicals have been the subject of numerous studies, and many novel derivatives possessing a variety of properties have been developed. The potential use of these persistent radicals as electron paramagnetic resonance (EPR) probes in biological applications was recognized early on, and many water-soluble triarylmethyl (TAM) radicals have been synthesized and studied.2,3 Of these, carboxylated TAMs have emerged as powerful tools for cancer biology and medicine, as they show good water-solubility, narrow line widths, and long relaxation times.4 These radicals were initially designed as polarizing agents for dynamic nuclear polarization (DNP) and contrast agents in Overhauser-enhanced magnetic resonance imaging (OMRI).5,6 Subsequently, TAMs have also been used for a variety of other biomedical applications, including the measurements of oxygen partial pressure (pO2), intracellular pH, glutathione redox state, and EPR imaging (EPRi).7–10 Among the many imaging modalities that have been used in the laboratory to measure oxygen, trityl-based EPRi offers high quality, three-dimensional distributions of pO2 with spatial resolution of 1 mm and pO2 resolution 1–3 mm Hg, which compares favorably with results obtained using OxyLite™ probe.11
Many symmetric, water-soluble trityls have been examined for diagnostic and therapeutic applications, but their toxicity has limited their use in animal studies. The so-called “Finland trityl,” for example, can be prepared by an efficient and general route,12 but it displays toxic properties and readily binds to serum albumin.13 On the other hand, the trityl OX063 1, which has high water solubility, good biological stability, and low toxicity in animal models (LD50, 8 mmol/kg), has proven of considerable use in biological studies and has been used successfully for in vivo imaging of mouse tumor and oxygen concentration mapping.6 Despite its desirable properties and demonstrated utility, its prohibitive cost (ca. $10,000/g from GE Healthcare) has limited its widespread use in biological investigations. Moreover, a single mouse imaging experiment requires ca. 50 mg of OX063.
MATERIALS AND METHODS
Preparation of lyophilized urine
Using an indwelling double lumen catheter, the subject mouse’s bladder was continually flushed with water at rate of 7 mL/h during imaging. Water was directed to the bladder through the inner lumen of the catheter. The resulting bladder effluent, a mixture of water and trityl loaded urine, was directed from the bladder to a collection vessel by the outer lumen of the catheter. At the end of imaging, bladder effluent was transferred to a common container for imaging effluent, and frozen. When accumulated frozen effluent reached a volume of a liter the effluent was thawed, filtered to remove any particulates and sediment, and then aliquoted into freeze dryer flasks. Flasks were frozen at −80 °C, and then mounted on the vacuum manifold of a Heto Lyolab 3000 freeze dryer. Flasks were kept on the manifold until the material inside was dry. The material was then scraped from the flasks and transferred to vials and stored in a fridge.
Reduction of quinone methide 2 to corresponding air-sensitive radical 3
Quinone methide 2 (1.37 mg, 1μmol) was added to a 2-dram vial containing 1 mL of methanol. The vial was swirled gently to dissolve the compound while flushing with a gentle stream of nitrogen for 15 minutes. After that time, 1 mL of saturated aqueous solution of Na2S2O4 was added to the purple mixture, forming an intensely green solution of phenolic radical 3, which can be stored under the inert atmosphere up to 5 hours. Similarly, treatment of a stirred methanolic solution of 2, maintained under a nitrogen atmosphere, with NaBH4 (10 mg, 2.63 mmol) resulted in vigorous hydrogen formation accompanied by formation of the intensely green QM radical. The radical was stored under inert atmosphere up to 5 hours without significant diminution of the color.
TLC analysis and purification of lyophilized mouse urine excretions
The lyophilized mouse urine powder was first examined by thin-layer chromatography (TLC) using Whatman silica gel 60 Å F254 plates (250 μm) and visualized using a UV lamp. The TLC was eluted in methanol:DCM:distilled water (10:10:1) and displayed 2 main spots, purple and green, characterized by Rf coefficient 0.25 and 0.20, respectively. Purification of the bulk lyophilizate was carried out by flash chromatography using SiliCycle SiliaFlash P60 silica gel (particle size 40–63 μm). About 120 mg of lyophilized powder was dissolved in 5 mL of methanol and 0.5 mL of distilled water. The prepared dark purple solution was loaded onto the silica gel column (dimensions: 23 cm × 3 cm), and the column was eluted using a solution of methanol:DCM:distilled water (10:10:1) as the eluent. The fractions were collected (10 mL each) and examined by TLC and each of two main fractions were combined and concentrated to afford pure 1 and 2.
HRMS and RP-HPLC measurements
High-resolution mass spectra were recorded on an Agilent 6224 TOF-MS (with positive Electrospray ionization mode [+ESI]). All HPLC measurements were carried out using Agilent 1290 LC System at 280 nm; C-18 RP column (Agilent Eclipse Plus C18, 3.5μm, 4.6 × 150 mm; PN 959963-902) was used and injection volume was 6μl; time of each measurement was 13 min. Conditions: 0–0.8 min (80% H2O (0.1% TFA); 20 % acetonitrile); 0.8–7 min (100 % acetonitrile); 7–13 min (80% H2O (0.1% TFA); 20 % acetonitrile). For details see Supporting Information.
UV-VIS and NMR spectroscopy
UV-VIS spectra were recorded on PerkinElmer Lambda 25 spectrometer. Electronic spectra were measured in the range 230 nm to 750 nm in methanolic solutions. 1H and 13C-NMR spectra were recorded on Bruker DRX-500 and DMX-500 (at 500 MHz and 125 MHz, respectively) and are reported relative to Me4Si (δ 0.0) or residual solvent signals, unless otherwise stated. All measurements were carried out in MeOD. For details see Supporting Information.
In vivo EPR imaging
EPR images of approximately 9 mm leg born FSa fibrosarcomas in C3H mice as described extensively previously14,15. The imaging was carried out using OX063 that was recovered and purified from the urinary effluent of mice, as described above. No toxicity from the recovered probe was noted through these experiments, similar to earlier experiments using available OX063.
EPR spectrum, T1, and T2 measurements
EPR spectra and pulse relaxation temporal profiles presented in this paper were obtained with the same spectrometer configurations described above,14,15 with the exceptional circumstance that magnetic field gradients were disabled and the temporal profiles and spectra were measured with 2 mL samples of 1 mM OX063 trityl. For details see Supporting Information.
RESULTS AND DISCUSSION
From the first in vivo experiments carried out in our lab using OX063, we had observed that the mouse’s urine was deeply green, suggesting that much of the persistent radical was being excreted unchanged. With these early observations and in the hope that a method could be developed for the isolation of pure 1 from the complex mixture of metabolites in urine, we began saving lyophilized excretions from all mouse experiments. We now report a simple protocol for purifying the preserved lyophilizate powder to reclaim high purity trityl radical 1, separating it from its main metabolite, quinone methide (QM) 2.16–18 We also report on the redox chemistry of QM and demonstrate that recovered 1 is of sufficiently high quality that it can be used in EPR studies. An extensive screen of adsorbents and solvent combinations was carried out and led to the identification of a simple protocol for the separation of pure 1 and 2. Silica gel flash chromatography of the mouse urine lyophilizate using methanol: DCM: distilled water (10:10:1) afforded two major colored fractions, purple and green (Rf 0.25 and 0.20 on TLC, respectively).
The second fraction was determined to be the desired trityl OX063 1, and the first, to be quinone methide 2, the oxidation product of 1. Analysis of recovered 1 by reverse phase-HPLC revealed two characteristic peaks with retention times of 1.49 min and 1.90 min in a 1:1 integrated ratio. The chromatogram of OX063 from commercial source (Nycomed, 91% assay) closely mirrored the output obtained from recovered OX063. The presence of two peaks in the chromatogram is intriguing and may reflect the presence of conformational diastereomers. In Finland trityl, the parent congener of OX063, the three aryl units have a propensity to adopt a C3 symmetric arrangement, with the aryl units canted parallel to one another, analogous to that found in a three-bladed propeller.19–21 The greater steric congestion combined with possible hydrogen bonding interactions may allow the three aryl units of OX063 to exist in either fully parallel arrangement or with two of the aryl units parallel and one anti-parallel.
The two compounds were further characterized by mass, NMR, and UV-VIS spectroscopy. Compound 1 was obtained as a green film, and a molecular formula of C52H63O18S12 was supported by HRMS (calculated m/z=1359.0677, observed m/z=1359.0663, error 1.04 ppm) and confirmed by LC-MS (ESI+, m/z= 1360). Compound 2 was obtained as a purple film; a molecular formula of C51H60Na2O17S12 was supported by HRMS (calculated m/z=1374.0274, observed m/z=1374.0404, error 9.42 ppm) and confirmed by LC-MS (ESI+, m/z=1374). Attempted NMR spectroscopy of 1 gave very broad signals, as expected for a radical species. The 1H and 13C-NMR spectra of 2 were consistent with its assigned structure (Fig 1). Characteristic 13C-NMR resonances for the carboxylic acids (172 ppm), quinone (162 ppm) and dithioacetal carbons (3 peaks, ~67 ppm) were noted. All signals in the NMR spectra were assigned with the assistance of C-H correlations. The UV-VIS spectrum of recovered OX063 displayed two absorption peaks, at 388 nm and 467 nm, whereas that of QM 2 presented a unique λmax of 550 nm. DFT calculations performed using SPARTAN 14 indicate that the two flanking aryl rings in 2 are non-coplanar with the quinone methide unit, presumably to avoid steric interactions.
Figure 1.
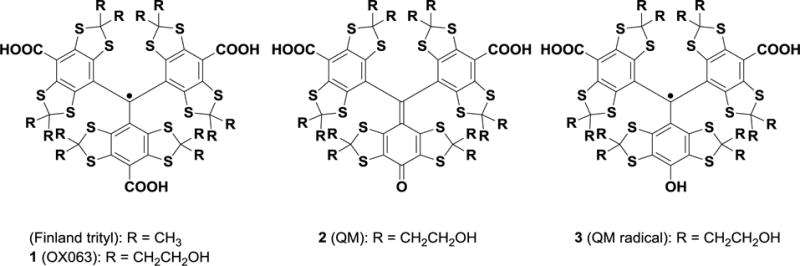
Chemical structures of trityl radicals (Finland and OX063), quinone methide 2, and QM-derived air-sensitive radical 3.
Interestingly, when we treated QM 2 with a saturated aqueous solution of sodium dithionate, a reducing agent known to convert quinones to their corresponding phenols, the characteristic purple solution of quinone 2 was transformed to an intense green solution, which we tentatively assigned to the air-sensitive phenolic radical 3. In the absence of an inert atmosphere, this species rapidly oxidized back to the quinone form. CW-EPR spectroscopy measurements of 3 (see Supporting Information) reveal the characteristic splitting associated with a para-substituted hydrogen atom. When the reduction was conducted in D2O, the unique splitting was not observed, supporting the structural assignment. The UV-VIS spectrum of 3 showed absorbance maxima at 360 and 600 nm. Furthermore, the characteristic green color associated with radical formation was also observed upon subjecting an aqueous solution of QM 2 to other common reducing agents (H2 and Pd/C, or NaBH4). As before, exposure to air allowed the green radical to oxidize back to QM. An evaluation of several samples (9 samples of lyophilized mouse urine) indicated that older batches generally contained a lower concentration of trityl probe. We have also noted less oxidation byproducts of OX063 when the processing and lyophilization of mouse urine was carried out immediately after the in vivo experiment.
In order to verify the effectiveness of the above recovery methodology for in vivo imaging, the reclaimed OX063 was administered to a C3H/He mouse bearing an FSa F10 tumor on its right calf. During the imaging experiment, the mouse was anesthetized with a breathing atmosphere of isoflurane and its core temperature was maintained within 1 °C of 37 °C by radiant heating. The mouse, weighing 19.1 g, was injected with an initial i.v. bolus of 38mg of recycled OX063 (2g/kg), followed by continuous infusion at 0.27 ml/hr (1.4 g/kg/hr) for 40 minutes. Three pO2 images were acquired during the forty minute interval. Throughout the administration of the recycled 1, and for an additional hour after imaging, the mouse’s bladder was cannulated with a double lumen catheter.22 Fresh water was introduced into the bladder at a rate of 7.5mL/h and the green-tinged effluent, the color of the OX063 radical that flowed from the bladder through the interluminal space was collected in a beaker. The results indicate that OX063 is eliminated from the animal bloodstream primarily by kidney filtration without oxidation of the radical. During the infusion of OX063, the bladder catheter line remained distinctly greenish. Following cessation of infusion, at the end of the third image, the color of the effluent progressively lightened until it was barely green and the mouse’s skin color, including paws and ears, returned to normal. A total of ~13 ml of deeply colored green effluent was collected. Breathing was monitored every five minutes during imaging, and isoflurane was adjusted to maintain respiratory rate of 100 bpm. Following cessation of isoflurane administration after imaging, the mouse recovered normal ambulation within a few minutes. Robust and continuous clearance of trityl, together with rapid recovery following anesthesia support the suitability of recycled trityl for animal experimentation. Amplitude (relative OX063 concentration) and pO2 images obtained with recycled OX063 are as typically observed (Fig 2). Central tumor location is roughly indicated by the region outlined in black. The color bars represent values of oxygen partial pressure in the solid tumor model, and the histogram shows distributions of pO2 for all voxels in the cursor area (tumor tissue). OX063 is preferentially retained in the tumor, and its highest concentrations, indicated by red/orange, are in the rough tumor center. Reflecting chaotic, poorly organized vasculature, the rough tumor center is poorly oxygenated with a median pO2 of 8.4 torr and a standard deviation of 9.8 torr.
Figure 2.
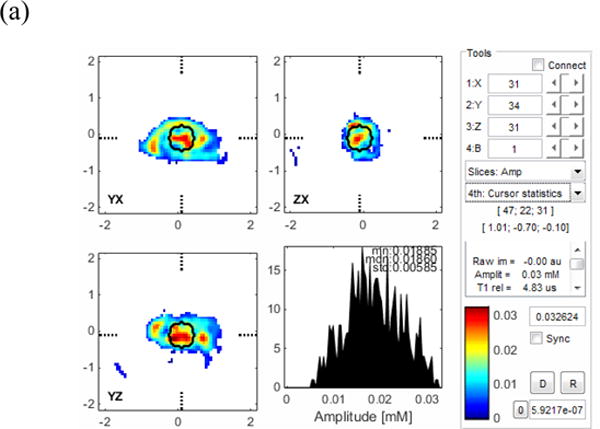

Amplitude (a) and pO2 (b) EPR oxygen images of C3H/He mouse leg bearing FSa F10 tumor (marked with black line).
CONCLUSIONS
In conclusion, we have developed a simple and robust method for recovering the valuable spin probe OX063, and have successfully used the recycled probe for in vivo imaging. Additionally, we have also described simple conditions for the reduction of QM, the oxidation product of 1, to an oxygen-sensitive radical 3, which was characterized by EPR spectroscopy.
Supplementary Material
Acknowledgments
This work was supported by NIH grants P41 EB002034 and R01 CA098575.
Funding Sources
NIH grants P41 EB002034 and R01 CA098575
ABBREVIATIONS
- DNP
dynamic nuclear polarization
- EPRi
electron paramagnetic resonance imaging
- OMRI
Overhauser-enhanced magnetic resonance imaging
- pO2
partial oxygen pressure
- QM
quinone methide
- RP-HPLC
reversed phase high pressure liquid chromatography
- TAM
triarylmethyl radical
Footnotes
ASSOCIATED CONTENT
The Supporting Information is available free of charge on ACS publication website at: http://pubs.acs.org.
HPLC profiles of trityl derivatives, HRMS, UV-VIS and EPR spectra.
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
References
- 1.Gomberg M. AN INSTANCE OF TRIVALENT CARBON: TRIPHENYLMETHYL. J Am Chem Soc. 1900;22:757–771. [Google Scholar]
- 2.Bobko AA, Dhimitruka I, Eubank TD, Marsh CB, Zweier JL, Khramtsov VV. Trityl-based EPR probe with enhanced sensitivity to oxygen. Free Radic Biol Med. 2009;47:654–658. doi: 10.1016/j.freeradbiomed.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frank J, Elewa M, Said M, El Shihawy HA, El-Sadek M, Müller D, Meister A, Hause G, Drescher S, Metz H, Imming P, Mäder K. Synthesis, Characterization, and Nanoencapsulation of Tetrathiatriarylmethyl and Tetrachlorotriarylmethyl (Trityl) Radical Derivatives-A Study To Advance Their Applicability as in Vivo EPR Oxygen Sensors. J Org Chem. 2015;80:6754–66. doi: 10.1021/acs.joc.5b00918. [DOI] [PubMed] [Google Scholar]
- 4.Elas M, Magwood JM, Butler B, Li C, Wardak R, DeVries R, Barth ED, Epel B, Rubinstein S, Pelizzari CA, Weichselbaum RR, Halpern HJ. EPR Oxygen Images Predict Tumor Control by a 50% Tumor Control Radiation Dose. Cancer Res. 2013;73:5328–5335. doi: 10.1158/0008-5472.CAN-13-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michaelis VK, Smith AA, Corzilius B, Haze O, Swager TM, Griffin RG. High-Field 13 C Dynamic Nuclear Polarization with a Radical Mixture. J Am Chem Soc. 2013;135:2935–2938. doi: 10.1021/ja312265x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krishna MC, English S, Yamada K, Yoo J, Murugesan R, Devasahayam N, Cook JA, Golman K, Ardenkjaer-Larsen JH, Subramanian S, Mitchell JB. Overhauser enhanced magnetic resonance imaging for tumor oximetry: Coregistration of tumor anatomy and tissue oxygen concentration. Proc Natl Acad Sci. 2002;99:2216–2221. doi: 10.1073/pnas.042671399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bobko AA, Dhimitruka I, Zweier JL, Khramtsov VV. Trityl Radicals as Persistent Dual Function pH and Oxygen Probes for in Vivo Electron Paramagnetic Resonance Spectroscopy and Imaging: Concept and Experiment. J Am Chem Soc. 2007;129:7240–7241. doi: 10.1021/ja071515u. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y, Villamena FA, Song Y, Sun J, Rockenbauer A, Zweier JL. Synthesis of 14 N- and 15 N-labeled Trityl-nitroxide Biradicals with Strong Spin−Spin Interaction and Improved Sensitivity to Redox Status and Oxygen. J Org Chem. 2010;75:7796–7802. doi: 10.1021/jo1016844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Y, Song Y, Rockenbauer A, Sun J, Hemann C, Villamena FA, Zweier JL. Synthesis of Trityl Radical-Conjugated Disulfide Biradicals for Measurement of Thiol Concentration. J Org Chem. 2011;76:3853–3860. doi: 10.1021/jo200265u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bobko AA, Dhimitruka I, Komarov DA, Khramtsov VV. Dual-Function pH and Oxygen Phosphonated Trityl Probe. Anal Chem. 2012;84:6054–6060. doi: 10.1021/ac3008994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elas M, Ahn KH, Parasca A, Barth ED, Lee D, Haney C, Halpern HJ. Electron paramagnetic resonance oxygen images correlate spatially and quantitatively with Oxylite oxygen measurements. Clin Cancer Res. 2006;12:4209–17. doi: 10.1158/1078-0432.CCR-05-0446. [DOI] [PubMed] [Google Scholar]
- 12.Reddy TJ, Iwama T, Halpern HJ, Rawal VH. General Synthesis of Persistent Trityl Radicals for EPR Imaging of Biological Systems. J Org Chem. 2002;67:4635–4639. doi: 10.1021/jo011068f. [DOI] [PubMed] [Google Scholar]
- 13.Song Y, Liu Y, Liu W, Villamena FA, Zweier JL. Characterization of the Binding of the Finland Trityl Radical with Bovine Serum Albumin. RSC Adv. 2014;4:47649–47656. doi: 10.1039/C4RA04616A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Epel B, Sundramoorthy SV, Barth ED, Mailer C, Halpern HJ. Comparison of 250 MHz electron spin echo and continuous wave oxygen EPR imaging methods for in vivo applications. Med Phys. 2011;38:2045. doi: 10.1118/1.3555297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sundramoorthy SV, Epel B, Halpern HJ. Orthogonal resonators for pulse in vivo electron paramagnetic imaging at 250MHz. J Magn Reson. 2014;240:45–51. doi: 10.1016/j.jmr.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Decroos C, Li Y, Bertho G, Frapart Y, Mansuy D, Boucher JL. Oxidative and Reductive Metabolism of Tris(p -carboxyltetrathiaaryl)methyl Radicals by Liver Microsomes. Chem Res Toxicol. 2009;22:1342–1350. doi: 10.1021/tx9001379. [DOI] [PubMed] [Google Scholar]
- 17.Decroos C, Balland V, Boucher JL, Bertho G, Xu-Li Y, Mansuy D. Toward Stable Electron Paramagnetic Resonance Oximetry Probes: Synthesis, Characterization, and Metabolic Evaluation of New Ester Derivatives of a Tris-(para -carboxyltetrathiaaryl)methyl (TAM) Radical. Chem Res Toxicol. 2013;26:1561–1569. doi: 10.1021/tx400250a. [DOI] [PubMed] [Google Scholar]
- 18.Decroos C, Boucher JL, Mansuy D, Xu-Li Y. Reactions of amino acids, peptides, and proteins with oxidized metabolites of tris(p-carboxyltetrathiaaryl)methyl radical EPR probes. Chem Res Toxicol. 2014;27:627–636. doi: 10.1021/tx400467p. [DOI] [PubMed] [Google Scholar]
- 19.Ściebura J, Skowronek P, Gawronski J. Trityl Ethers: Molecular Bevel Gears Reporting Chirality through Circular Dichroism Spectra. Angew Chemie. 2009;121:7203–7206. doi: 10.1002/anie.200902167. [DOI] [PubMed] [Google Scholar]
- 20.Tormyshev VM, Genaev AM, Sal’nikov GE, Rogozhnikova OY, Troitskaya TI, Trukhin DV, Mamatyuk VI, Fadeev DS, Halpern HJ. Triarylmethanols with Bulky Aryl Groups and the NOESY/EXSY Experimental Observation of a Two-Ring-Flip Mechanism for the Helicity Reversal of Molecular Propellers. European J Org Chem. 2012;2012:623–629. doi: 10.1002/ejoc.201101243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Driesschaert B, Robiette R, Lucaccioni F, Gallez B, Marchand-Brynaert J. Chiral properties of tetrathiatriarylmethyl spin probes. Chem Commun. 2011;47:4793. doi: 10.1039/c1cc10988j. [DOI] [PubMed] [Google Scholar]
- 22.Haney CR, Parasca AD, Ichikawa K, Williams BB, Elas M, Pelizzari CA, Halpern HJ. Reduction of image artifacts in mice by bladder flushing with a novel double-lumen urethral catheter. Mol Imaging. 2006;5:175–9. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


