Abstract
Two structural forms of a ternary alloy PtRuIr/C catalyst, one amorphous and one highly crystalline, were synthesized and compared to determine the effect of their respective structures on their activity and stability as anodic catalysts in methanol oxidation. Characterization techniques included TEM, XRD, and EDX. Electrochemical analysis using a glassy carbon disk electrode for cyclic voltammogram and chronoamperometry were tested in a solution of 0.5 mol L−1 CH3OH and 0.5 mol L−1 H2SO4. Amorphous PtRuIr/C catalyst was found to have a larger electrochemical surface area, while the crystalline PtRuIr/C catalyst had both a higher activity in methanol oxidation and increased CO poisoning rate. Crystallinity of the active alloy nanoparticles has a big impact on both methanol oxidation activity and in the CO poisoning rate.
Keywords: electrocatalysts, fuel cells, methanol oxidation, structure, crystallinity
1. Introduction
Increasing the electrocatalytic activity and stability of Pt-based catalysts has been the focus of much recent research [1,2,3] and remains a critical requirement for the future implementation of direct methanol fuel cells (DMFCs). Among the various Pt-based binary catalysts, the PtRu alloy has been reported as the most effective for methanol electro-oxidation [4,5,6], with further recent gains in activity and durability reported by incorporating a third metal, such as Co, Ni, Sn, Ir, etc. [7,8,9,10]. Among these ternary alloy catalysts, the PtRuIr/C system seems particularly promising [11,12,13]. Furthermore, the effect of composition for PtRuIr/C catalyst was systematically studied. However, the effect of its structure and morphology on methanol electro-oxidation is not focused on by other researchers.
Synthesis of nanostructured electrocatalysts is of great importance in developing the so-called “next-generation” catalysts [14]. The catalytic activity of such nanostructured electrocatalysts is highly dependent on the surface area, surface atomic structure, crystal size and shape. With control of nanostructure and morphology, large surface areas and abundant catalytic active sites can be realized, which enhance catalytic performance and utilization efficiency of the electrocatalyst [15]. In particular, amorphous structures in alloys can present unique compositions and catalytic surface structures as compared to conventional crystallized metal [16,17]. Some studies show that amorphous composition can have positive effects on the kinetics or stability of the methanol oxidation reaction due to amorphous alloys presenting unique compositions and surface structures for molecular reactions [18], while others show that intermetallic compounds with high-crystallinity have higher electrocatalytic activity for methanol oxidation reaction [19,20].
Inspired by the reports, the present work aimed to gain deeper insight into the effect of PtRuIr nanoparticle crystallinity on methanol electro-oxidation for carbon-supported PtRuIr catalysts. To this end, crystalline and amorphous carbon-supported PtRuIr structures were prepared, and then studied and compared using cyclic voltammetry and chronoamperometry.
2. Results and Discussion
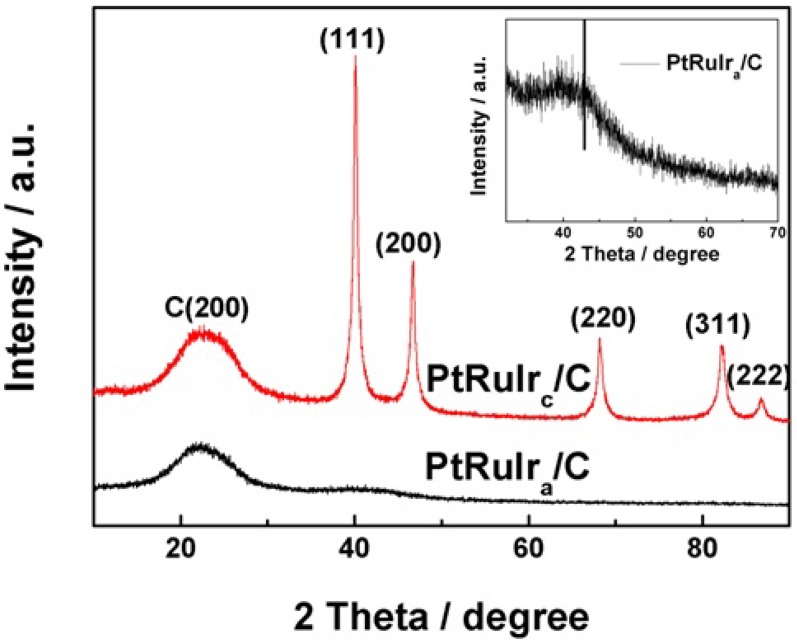
X-Ray Diffraction (XRD) analysis (Figure 1) produced clear differences in the peak distributions of the carbon-supported PtRuIrc/C (crystalline form) and PtRuIra/C (amorphous form) catalysts. In the diffractograms of the two catalysts, the first peak located at about 24.8° in all the XRD plots is associated with the Vulcan XC-72R support, and no peaks corresponding to the metals Ir and Ru were observed [11]. For clarity, the diffraction patterns of the PtRuIra/C catalyst between 32° and 70° have been enlarged in the inset of Figure 1. Here, the PtRuIra/C catalyst had only one wide, diffuse, broad peak at approximately 2θ = 45°, indicating that the sample’s internal structure was amorphous [18]. In contrast, the XRD pattern of the heat treated sample, PtRuIrc/C, have the five main characteristic peaks of the face-centered cubic (fcc) crystalline Pt alloy [13,21,22], corresponding to the planes (111), (200), (220), (311), and (222), at 2θ values of ca. 40°, 47°, 68°, 82°and 86°, respectively. On the other hand, a displacement of the peaks related to the polycrystalline Pt towards more positive values of 2θ is observed. This can be ascribed to the existence of alloys between the metals Pt, Ru and Ir. The formation of alloy results in a contraction of the crystalline lattice of Pt due to the substitution of some atoms of Pt with large size (rPt = 0.138 nm) for the atoms of Ir and/or Ru with small sizes (rRu = 0.134 nm) (rIr = 0.136 nm) [11,23]. These results indicate that the PtRuIrc alloy supported on carbon catalyst had fcc crystalline structure.
Figure 1.
X-Ray Diffraction (XRD) patterns of PtRuIrc/C and PtRuIra/C catalysts.
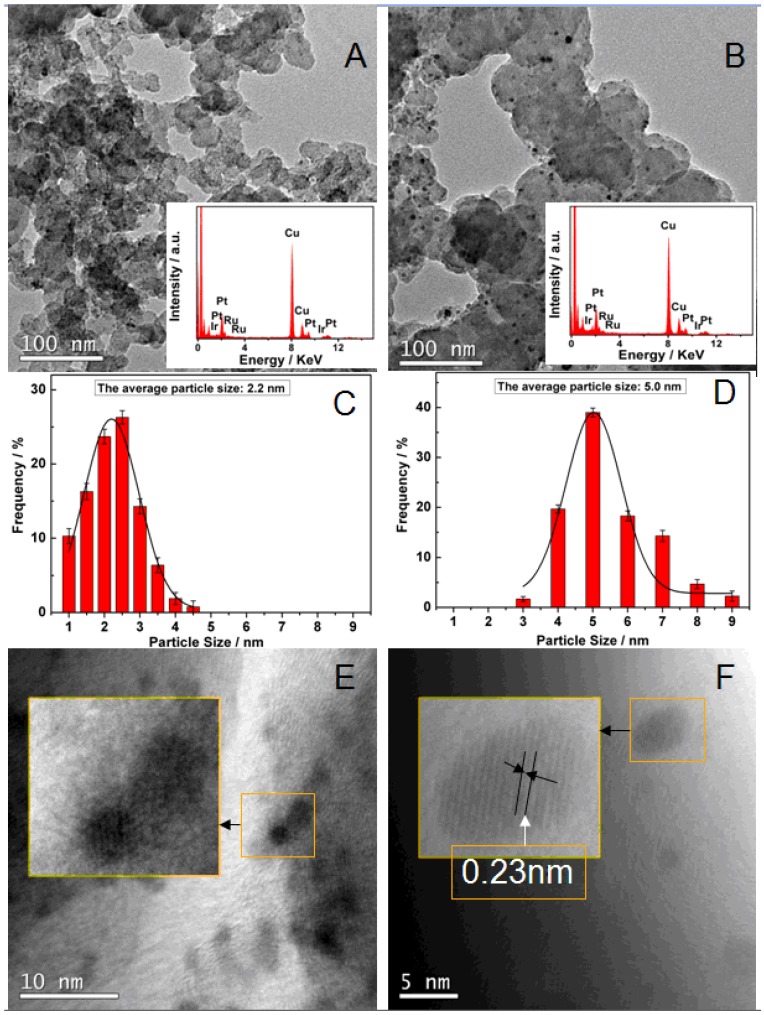
Figure 2 shows TEM images, corresponding particle sizes distribution histogram and EDX composition of PtRuIra/C and PtRuIrc/C. From the Figure 2A (PtRuIra/C) and 2B (PtRuIrc/C), it can be observed that both catalysts were highly dispersed on the carbon support. The particle size distribution histogram of PtRuIra/C (Figure 2C) and PtRuIrc/C (Figure 2D) catalyst based on examination of more than 300 particles show that the particle size varied from 1.0 to 4.5 nm for PtRuIra/C and 3 to 9 nm for PtRuIrc/C and a relatively narrow size distribution for both catalysts. The derived average particle size is about 2.2 ± 0.02 nm and 5.0 ± 0.02 nm for the PtRuIra/C and PtRuIrc/C catalysts (see Table 1), respectively. The HRTEM image of PtRuIra/C in Figure 2E shows an inexplicit lattice, indicating that the particles of PtRuIra/C are of mainly amorphous state [24]. In contrast, the HRTEM image (insets in Figure 2F) reveals that the PtRuIrc/C nanoparticles are crystalline, showing a lattice of ~0.23 nm identifiable as the d-spacing of the (111) plane of face-centered cubic Pt [25]. The EDS results of PtRuIrc/C and PtRuIra/C (insets in Figure 2A,B) indicate that the both catalysts consist of: C, Pt, Ru and Ir, and ca. 3.5:3:1 of atom ratio for Pt:Ru:Ir is obtained. The result is also confirmed by ICP analysis. The metal loading for the two catalysts is ca. 20%, close to the normal value.
Figure 2.
TEM, the corresponding particle size distributing histogram and HRTEM images of PtRuIra/C (A,C,E) and PtRuIrc/C (B,D,F) catalysts. Inset of (A) and (B): EDX spectrum of the PtRuIra/C (A) and PtRuIrc/C (B) catalysts.
Table 1.
Composition the average particle size, and the electrochemical performance of the PtRuIra/C and PtRuIrc/C catalysts.
| Catalyst | PtRuIra/C | PtRuIrc/C |
|---|---|---|
| Pt:Ru:Ir atom ratio | 3.5:3.0:1.0 | 3.5:3.0:1.0 |
| The average particle size/nm | 2.2 ± 0.02 | 5.0 ± 0.02 |
| ECSA/m2 g−1metal | 59.5 | 32.6 |
| The onset potential for CO oxidation/mV vs. RHE | 663 | 521 |
| The onset potential for methanol oxidation/mV vs. RHE | 370 | 338 |
| The mass activity for methanol oxidation/mA mg−1 | 147 | 298 |
| The specific activity for methanol oxidation/mA cm−2 | 0.25 | 0.91 |
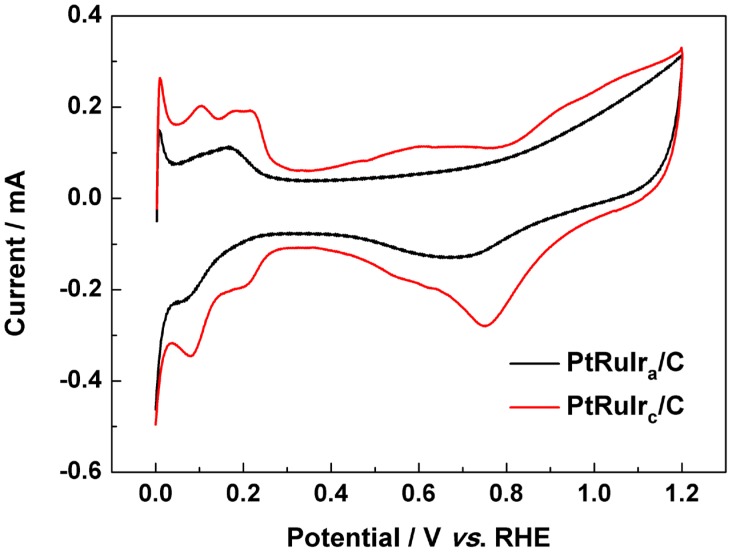
Typical cyclic voltammograms (CVs) of PtRuIrc/C and PtRuIra/C catalysts in 0.5 mol L−1 H2SO4 solution are shown in Figure 3. A well-defined CV feature of polycrystalline Pt is observable in the curve generated from PtRuIrc/C. Here, there are three pairs of redox peaks around 0.09, 0.173 and 0.214 V (vs. RHE), corresponding to the planes (110), (111), and (100), which can be ascribed to hydrogen adsorption/desorption on crystal surface sites of Pt [7,26]. In contrast, the CV curve of PtRuIra/C catalyst only has one large, broad peak and does not exhibit the typical peaks of pure polycrystalline Pt between 0 and 0.3 V (vs. RHE). This further suggests that the active components of PtRuIra/C catalyst had an amorphous structure. Furthermore, the oxide (OHads) stripping peak (0.75 V vs. RHE) of the PtRuIrc/C is 70 mV more positive than that of PtRuIra/C (0.68 V vs. RHE), suggesting faster hydroxyl desorption from the PtRuIrc/C surfaces [27].
Figure 3.
Cyclic voltammograms of PtRuIra/C and PtRuIrc/C catalysts in 0.5 mol L−1 H2SO4 solution under N2 atmosphere; scan rate = 50 mV s−1.
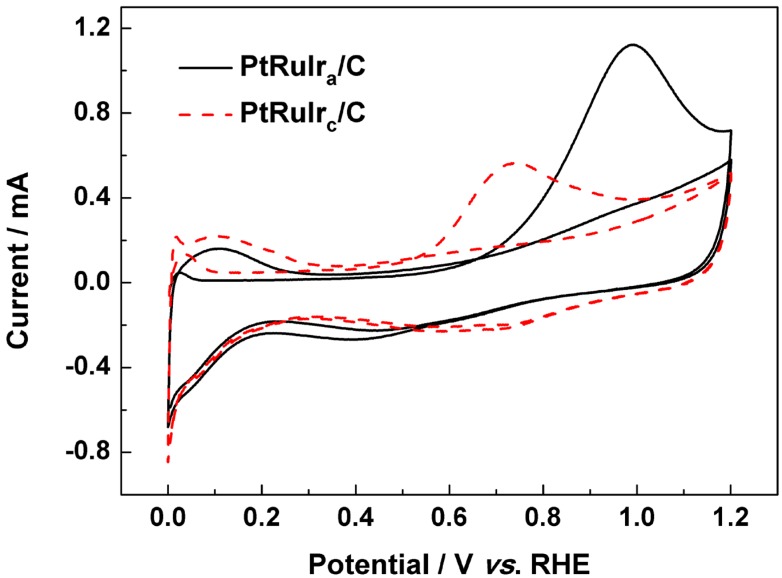
The CVs for CO electro-oxidation on PtRuIrc/C and PtRuIra/C catalysts are shown in Figure 4. Here, the hydrogen desorption peaks were completely suppressed in the first scan in the lower potential region (0 to 0.3 vs. RHE), due to the saturated coverage of COads species on the surface of PtRuIr alloy active sites [28]. However, hydrogen desorption peaks recovered in the second cycle after the CO was removed by oxidation.
Figure 4.
CO stripping voltammograms of PtRuIrc/C and PtRuIra/C catalysts in a solution of 0.5 mol L−1 H2SO4 at room temperature.
It can be seen from Table 1 that the onset potential of CO electro-oxidation with PtRuIrc/C (0.54 V vs. RHE) is lower than that of PtRuIra/C (0.67 V vs. RHE), which demonstrates that crystallinity of PtRuIr alloy influences the CO oxidation ability (the onset oxidation potential). The peak potential on the PtRuIrc/C catalyst (0.73 V vs. RHE) show a negative shift of around 0.45 V (vs. RHE) compared to the PtRuIra/C catalyst (0.98 V vs. RHE). The lower peak potential and onset potential of the COads oxidation on PtRuIra/C indicate that PtRuIrc/C catalyst was kinetically more active for COads oxidation [17]. The electrochemical surface area (ECSA) of the catalyst was calculated using the Equation (1) [29]:
| (1) |
where QCO is the charge for CO desorption electro-oxidation in microcoulomb (μC), 484 is the charge required to oxidize a monolayer of CO on the catalyst in μC cm−2 and ω is the precious metal loading, respectively. The ECSACO for PtRuIrc/C and PtRuIra/C were 32.6 m2 g−1metal and 59.5 m2 g−1 metal respectively. PtRuIrc/C had a lower ECSACO than the PtRuIra/C.The real electrochemical surface area is determined by the active sites on the surface of the metal particle. The number of the active sites is related to the composition of the surface, the size of the particle and the structure of the surface [30]. After heat treatment, the PtRuIrc/C nanoparticles agglomerated resulting in increased particle size, which has been proved by TEM. The large particle size results in the small ECSA [31,32,33]. Therefore, we believe that the different ECSACO mainly originated from the effect of particle size and structure.
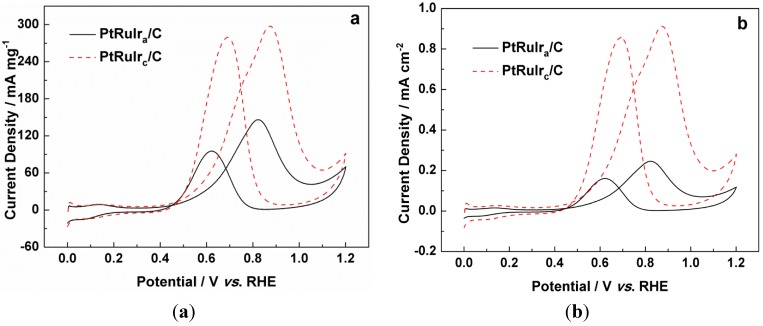
The electrocatalytic activity of PtRuIrc/C and PtRuIra/C catalysts in methanol oxidation is shown in Figure 5. The onset potential and the activity for methanol oxidation on both catalysts are shown in Table 1. In the forward scan in Figure 5a, the current density (mass activity) of PtRuIrc/C (298 mA mg−1) is 50% higher than that of PtRuIra/C (147 mA mg−1). In Figure 5b, the current density (specific activity) of the PtRuIrc/C is 3.6 times as large as that of PtRuIra/C. Although the particle size of PtRuIrc is obviously larger than that of PtRuIra, the PtRuIrc/C showed superior catalytic activity to PtRuIra/C, i.e., lower onset potential, and higher oxidation current density due to the effect of the structure. Moreover, the mass and specific activities of PtRuIrc/C are distinctly higher than those of the PtRuIra/C catalyst (see Table 1).
Figure 5.
Cyclic voltammograms of PtRuIrc/C and PtRuIra/C catalysts normalized to the metal loading on the electrodes (a) and ECSACO (b), in 0.5 mol L−1 CH3OH + 0.5 mol L−1 H2SO4 solution under N2 atmosphere; scan rate = 50 mV s−1.
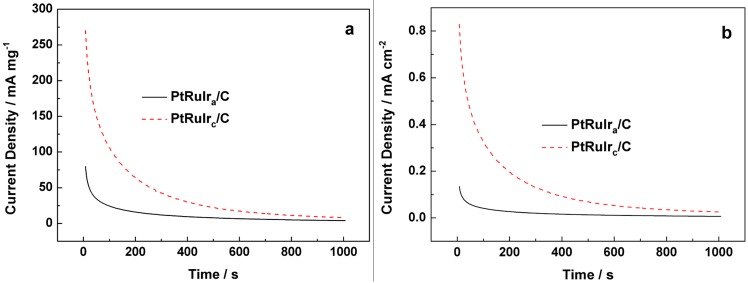
Figure 6 shows the chronoamperometry curves for the PtRuIrc/C and PtRuIra/C catalysts in 0.5 mol L−1 H2SO4 and 0.5 mol L−1 CH3OH at a constant potential of 0.8 V (vs. RHE), the current density is normalized to the metal loading on the electrodes (a) and ECSACO (b), respectively. Figure 6, shows that the potentiostatic current for all the catalysts initially decreased rapidly owing to the formation of COads and other intermediate species during the methanol oxidation reaction. With time, the current density decayed more gradually and a pseudo-steady state was achieved. The decay can be attributed to the adsorbed anion SO42− on the surface of the catalyst, thus restricting the methanol oxidation reaction. We calculated the long-term poisoning rate (δ) by measuring the linear decay of the current for a period of more than 500 s from Figure 6 by the following Equation (2) [33]:
| (2) |
where is the slope of the linear portion of the current decay and i0 is the current at the start of polarization back extrapolated from the linear current decay. The current densities of the PtRuIrc/C at 1000 s are 8.30 mA mg−1 and 0.025 mA cm−2, while those of PtRuIra/C catalysts at 1000 s are 4.0 mA mg−1 and 0.0067 mA cm−2, respectively. The calculated δ values show that the poisoning rate 0.10 of the PtRuIra/C catalysts is slow compared to 0.13 of the PtRuIrc/C catalyst. This indicates that the PtRuIra/C catalyst had a relatively lower poisoning rate than the PtRuIrc/C catalyst. Thus, although the PtRuIrc/C catalyst had a larger ECSACO, the poisoning rate was faster than that of the PtRuIra/C catalyst. This is probably because the faster and higher activities for the methanol oxidation reaction on the PtRuIrc/C electrode generated a larger amount of reactive intermediates and the ultimate poisoning species, rapidly producing the larger δ value.
Figure 6.
Chronoamper ometry curves of PtRuIra/C and PtRuIrc/C catalysts for methanol oxidation, polarized at a constant potential of 0.6 V vs. Ag/AgCl at room temperature.
3. Experimental Section
3.1. Preparation of PtRuIr/C Catalysts with Different Crystallinity
Amorphous PtRuIr/C catalyst (PtRuIra/C) was prepared by a modified organic colloid method in ethylene glycol (EG) solution. In a typical process, a PtRuIra/C catalyst with a nominal weight Pt:Ru:Ir ratio of 3:3:1 was prepared as follows: 4.85 mL 20 mg mL−1 H2PtCl6·aqueous solutions, 1.99 mL 20 mg mL−1 RuCl3, 2.56 mL 10 mg mL−1 H2IrCl6 and sodium citrate (220 mg) were dissolved in 30 mL ethylene glycol (EG) and stirred for 0.5 h. Pretreated carbon black Vulcan® XC72R (100 mg) was added to the mixture under stirring conditions. The pH of the system was adjusted to ~9 by drop-wise addition of a 5 wt % KOH/EG solution with vigorous stirring. The mixture was transferred to a flask and heated at 160 °C for 6 h and the resultant product was collected by filtration, washed with ultrapure water to remove all residual chloride ion, and then dried in air at 60 °C for 12 h. The metal loading is 20%. Crystalline PtRuIr/C catalyst (PtRuIrc/C) was prepared by heating the above-prepared PtRuIra/C powder in a tube furnace under H2/N2 atmosphere at 700 °C for 2 h.
3.2. Measurements
The catalysts were characterized by recording their XRD patterns on a Shimadzu XD–3A (Japan), using filtered Cu-Kα radiation (λ = 0.15418 nm), generated at 40 kV and 30 mA. Scans for 2θ values were recorded at 4°/min between 10° and 90°. All X–ray diffraction patterns were analyzed using Jade 7.5 of Material Data, Inc. (MDI): peak profiles of individual reflections were obtained by a nonlinear least-square fit of the Cu-Kα corrected data. TEM measurements were carried out on a Tecnai G220 S–TWIN (FEI Company); the acceleration voltage was 200 kV. The average chemical compositions of the two catalysts were determined by the energy-dispersive X-ray spectroscopy (EDS) analysis and IRIS advantage inductively coupled plasma atomic emission spectroscopy (ICP-AES) system (Thermo Electron Corporation, America).
A common three-electrode cell was used for the electrochemical measurements, using a CHI 650D electrochemical work station. The counter and reference electrode were a platinum wire and an Ag/AgCl (3 M KCl) electrode, respectively, and the working electrode was a glassy carbon disk (5 mm in diameter). The thin-film electrode was prepared as follows: 5 mg of catalyst was dispersed ultrasonically in 1 mL Nafion/ethanol (0.25% Nafion) for 15 min. 8 μL of the dispersion was transferred onto the glassy carbon disk using a pipette, and then dried in the air. The metal loading on the film is 40.8 μg cm−2.
4. Conclusions
Carbon-supported PtRuIr alloy catalysts of amorphous and crystalline structure were successfully synthesized and characterized. Electrochemical characterization found that although PtRuIra/C had a larger electrochemical surface area mainly due to the small size of the particles, the PtRuIrc/C had the better electrochemical performance in the methanol oxidation reaction. However, the poisoning rate of the PtRuIrc/C catalyst was faster than that on the PtRuIra/C catalyst. The difference in activity originates from the effect of the structure. Therefore, these results show that control of crystallinity of the active alloy nanoparticles can play an important role in both methanol oxidation activity and in the CO poisoning rate.
Acknowledgments
Thank you to the National Natural Science Foundation of China (21163018), the National Science Foundation for Post-Doctoral Scientists of China (20110490847, 2012T50587) and the research fund of the Key Laboratory of Fuel Cell Technology of Guangdong Province for financially supporting this work.
References
- 1.Peng Z., Yang H. Designer platinum nanoparticles: Control of shape, composition in alloy, nanostructure and electrocatalytic property. Nano Today. 2009;4:143–164. doi: 10.1016/j.nantod.2008.10.010. [DOI] [Google Scholar]
- 2.Zhang H., Jin M., Xia Y. Noble-Metal Nanocrystals with Concave Surfaces: Synthesis and Applications. Angew. Chem. Int. Ed. 2012;51:7656–7673. doi: 10.1002/anie.201201557. [DOI] [PubMed] [Google Scholar]
- 3.Rabis A., Rodriguez P., Schmidt T.J. Electrocatalysis for Polymer Electrolyte Fuel Cells: Recent Achievements and Future Challenges. ACS Catal. 2012;2:864–890. doi: 10.1021/cs3000864. [DOI] [Google Scholar]
- 4.An X.-S., Fan Y.-J., Chen D.-J., Wang Q., Zhou Z.-Y., Sun S.-G. Enhanced activity of rare earth doped PtRu/C catalysts for methanol electro-oxidation. Electrochim. Acta. 2011;56:8912–8918. doi: 10.1016/j.electacta.2011.07.106. [DOI] [Google Scholar]
- 5.Daimon H., Kurobe Y. Size reduction of PtRu catalyst particle deposited on carbon support by addition of non-metallic elements. Catal. Today. 2006;111:182–187. doi: 10.1016/j.cattod.2005.10.023. [DOI] [Google Scholar]
- 6.Gómez de la Fuente J.L., Martínez-Huerta M.V., Rojas S., Hernández-Fernández P., Terreros P., Fierro J.L.G., Peña M.A. Tailoring and structure of PtRu nanoparticles supported on functionalized carbon for DMFC applications: New evidence of the hydrous ruthenium oxide phase. Appl. Catal. B. 2009;88:505–514. doi: 10.1016/j.apcatb.2008.10.016. [DOI] [Google Scholar]
- 7.Huang T., Wang X.Y., Zhuang J.H., Cai W.B., Yu A.S. Preparation of Porous PtRuCo Catalyst by One-Step Codeposition and Its Electrocatalytic Performance for Methanol Oxidation. Electrochem. Solid State Lett. 2009;12:B112–B115. doi: 10.1149/1.3125286. [DOI] [Google Scholar]
- 8.Zhu J., Cheng F., Tao Z., Chen J. Electrocatalytic Methanol Oxidation of Pt0.5Ru0.5−xSnx/C (x = 0−0.5) J. Phys. Chem. C. 2008;112:6337–6345. doi: 10.1021/jp8000543. [DOI] [Google Scholar]
- 9.Liao S., Holmes K.-A., Tsaprailis H., Birss V.I. High Performance PtRuIr Catalysts Supported on Carbon Nanotubes for the Anodic Oxidation of Methanol. J. Am. Chem. Soc. 2006;128:3504–3505. doi: 10.1021/ja0578653. [DOI] [PubMed] [Google Scholar]
- 10.Khorasani-Motlagh M., Noroozifar M., Ekrami-Kakhki M.-S. Investigation of the nanometals (Ni and Sn) in platinum binary and ternary electrocatalysts for methanol electrooxidation. Int. J. Hydrog. Energy. 2011;36:11554–11563. doi: 10.1016/j.ijhydene.2011.06.071. [DOI] [Google Scholar]
- 11.Eguiluz K.I. B., Salazar-Banda G.R., Miwa D., Machado S.A. S., Avaca L.A. Effect of the catalyst composition in the Ptx(Ru–Ir)1−x/C system on the electro-oxidation of methanol in acid media. J. Power Sources. 2008;179:42–49. doi: 10.1016/j.jpowsour.2007.12.070. [DOI] [Google Scholar]
- 12.Geng D.S., Matsuki D., Wang J.J., Kawaguchi T., Sugimoto W., Takasu Y. Activity and Durability of Ternary PtRuIr/C for Methanol Electro-oxidation. J. Electrochem. Soc. 2009;156:B397–B402. doi: 10.1149/1.3060111. [DOI] [Google Scholar]
- 13.Liang Y., Zhang H., Zhong H., Zhu X., Tian Z., Xu D., Yi B. Preparation and characterization of carbon-supported PtRuIr catalyst with excellent CO-tolerant performance for proton-exchange membrane fuel cells. J. Catal. 2006;238:468–476. doi: 10.1016/j.jcat.2006.01.005. [DOI] [Google Scholar]
- 14.Arico A.S., Bruce P., Scrosati B., Tarascon J.-M., van Schalkwijk W. Nanostructured materials for advanced energy conversion and storage devices. Nat. Mater. 2005;4:366–377. doi: 10.1038/nmat1368. [DOI] [PubMed] [Google Scholar]
- 15.Qiao Y., Li C.M. Nanostructured catalysts in fuel cells. J. Mater. Chem. 2011;21:4027–4036. doi: 10.1039/c0jm02871a. [DOI] [Google Scholar]
- 16.Loukrakpam R., Wanjala B.N., Yin J., Fang B., Luo J., Shao M., Protsailo L., Kawamura T., Chen Y., Petkov V., Zhong C.-J. Structural and Electrocatalytic Properties of PtIrCo/C Catalysts for Oxygen Reduction Reaction. ACS Catal. 2011;1:562–572. doi: 10.1021/cs200022s. [DOI] [Google Scholar]
- 17.Cui Z., Kulesza P.J., Li C.M., Xing W., Jiang S.P. Pd nanoparticles supported on HPMo-PDDA-MWCNT and their activity for formic acid oxidation reaction of fuel cells. Int. J. Hydrog. Energy. 2011;36:8508–8517. doi: 10.1016/j.ijhydene.2011.04.072. [DOI] [Google Scholar]
- 18.Wang H., Zhang X., Wang R., Ji S., Wang W., Wang Q., Lei Z. Amorphous CoSn alloys decorated by Pt as high efficiency electrocatalysts for ethanol oxidation. J. Power Sources. 2011;196:8000–8003. doi: 10.1016/j.jpowsour.2011.05.062. [DOI] [Google Scholar]
- 19.Zhang L., Xia D. Electrocatalytic activity of ordered intermetallic PtSb for methanol electro-oxidation. Appl. Surf. Sci. 2006;252:2191–2195. doi: 10.1016/j.apsusc.2005.03.221. [DOI] [Google Scholar]
- 20.Youn D.H., Han S., Bae G., Lee J.S. Carbon-supported PtPb intermetallic compounds for electrooxidation of methyl formate. Electrochem. Commun. 2011;13:806–809. doi: 10.1016/j.elecom.2011.05.008. [DOI] [Google Scholar]
- 21.Wang H., Wang R., Li H., Wang Q., Kang J., Lei Z. Facile synthesis of carbon-supported pseudo-core@shell PdCu@Pt nanoparticles for direct methanol fuel cells. Int. J. Hydrog. Energy. 2011;36:839–848. doi: 10.1016/j.ijhydene.2010.09.033. [DOI] [Google Scholar]
- 22.Wang H., Linkov V., Ji S., Zhang W., Lei Z., Wang R. Highly Active, Carbon-supported, PdSn Nano-core, Partially Covered with Pt, as Catalysts for Methanol Oxidation. South Afr. J. Chem. 2012;65:69–74. [Google Scholar]
- 23.Shrestha S., Liu Y., Mustain W.E. Electrocatalytic Activity and Stability of Pt clusters on State-of-the-Art Supports: A Review. Catal. Rev. 2011;53:256–336. doi: 10.1080/01614940.2011.596430. [DOI] [Google Scholar]
- 24.Park K.-W., Choi J.-H., Kwon B.-K., Lee S.-A., Sung Y.-E., Ha H.-Y., Hong S.-A., Kim H., Wieckowski A. Chemical and Electronic Effects of Ni in Pt/Ni and Pt/Ru/Ni Alloy Nanoparticles in Methanol Electrooxidation. J. Phys. Chem. B. 2002;106:1869–1877. doi: 10.1021/jp013168v. [DOI] [Google Scholar]
- 25.Wang R., Jia J., Li H., Li X., Wang H., Chang Y., Kang J., Lei Z. Nitrogen-doped carbon coated palygorskite as an efficient electrocatalyst support for oxygen reduction reaction. Electrochim. Acta. 2011;56:4526–4531. doi: 10.1016/j.electacta.2011.02.066. [DOI] [Google Scholar]
- 26.Gasteiger H.A., Markovic N., Ross P.N., Cairns E.J. Methanol electrooxidation on well-characterized platinum-ruthenium bulk alloys. J. Phys. Chem. 1993;97:12020–12029. doi: 10.1021/j100148a030. [DOI] [Google Scholar]
- 27.Peng Z., Yang H. Synthesis and Oxygen Reduction Electrocatalytic Property of Pt-on-Pd Bimetallic Heteronanostructures. J. Am. Chem. Soc. 2009;131:7542–7543. doi: 10.1021/ja902256a. [DOI] [PubMed] [Google Scholar]
- 28.Prabhuram J., Zhao T.S., Tang Z.K., Chen R., Liang Z.X. Multiwalled Carbon Nanotube Supported PtRu for the Anode of Direct Methanol Fuel Cells. J. Phys. Chem. B. 2006;110:5245–5252. doi: 10.1021/jp0567063. [DOI] [PubMed] [Google Scholar]
- 29.Zhou C., Wang H., Peng F., Liang J., Yu H., Yang J. MnO2/CNT Supported Pt and PtRu Nanocatalysts for Direct Methanol Fuel Cells. Langmuir. 2009;25:7711–7717. doi: 10.1021/la900250w. [DOI] [PubMed] [Google Scholar]
- 30.Mohamed R., Gouws S., Ferg E. Characterization of Pt Catalysts for PEM Fuel Cells. Mol. Cryst. Liquid Cryst. 2012;555:149–157. doi: 10.1080/15421406.2012.635509. [DOI] [Google Scholar]
- 31.Schulenburg H., Durst J., Müller E., Wokaun A., Scherer G.G. Real surface area measurements of Pt3Co/C catalysts. J. Electroanal. Chem. 2010;642:52–60. doi: 10.1016/j.jelechem.2010.02.005. [DOI] [Google Scholar]
- 32.Jiang J., Kucernak A. Electrooxidation of small organic molecules on mesoporous precious metal catalysts. I: CO and methanol on platinum. J. Electroanal. Chem. 2002;533:153–165. doi: 10.1016/S0022-0728(02)01083-5. [DOI] [Google Scholar]
- 33.Jiang J., Kucernak A. Electrooxidation of small organic molecules on mesoporous precious metal catalysts. II: CO and methanol on platinum–ruthenium alloy. J. Electroanal. Chem. 2003;543:187–199. doi: 10.1016/S0022-0728(03)00046-9. [DOI] [Google Scholar]