Abstract
Peroxiredoxin (Prx) was previously known as a Cys-dependent thioredoxin. However, we unexpectedly observed that Prx1 from the green spotted puffer fish Tetraodon nigroviridis (TnPrx1) was able to reduce H2O2 in a manner independent of Cys peroxidation and reductants. This study aimed to validate a novel function for Prx1, delineate the biochemical features and explore its antioxidant role in cells. We have confirmed that Prx1 from the puffer fish and humans truly possesses a catalase (CAT)-like activity that is independent of Cys residues and reductants, but dependent on iron. We have identified that the GVL motif was essential to the CAT-like activity of Prx1, but not to the Cys-dependent thioredoxin peroxidase (POX) activity, and generated mutants lacking POX and/or CAT-like activities for individual functional validation. We discovered that the TnPrx1 POX and CAT-like activities possessed different kinetic features in the reduction of H2O2. The overexpression of wild-type TnPrx1 and mutants differentially regulated the intracellular levels of reactive oxygen species (ROS) and the phosphorylation of p38 in HEK-293T cells treated with H2O2. Prx1 is a dual-function enzyme by acting as POX and CAT with varied affinities towards ROS. This study extends our knowledge on Prx1 and provides new opportunities to further study the biological roles of this family of antioxidants.
Keywords: catalase-like activity, peroxiredoxin 1 (Prx1), puffer fish, ROS
Introduction
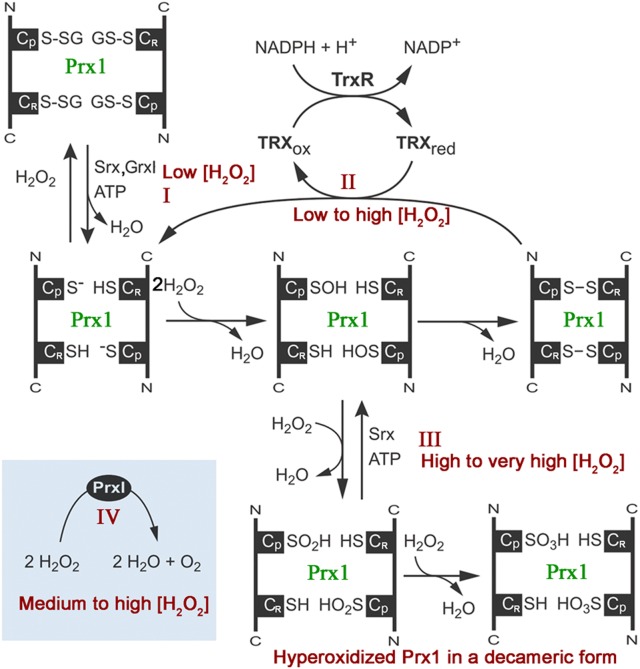
Peroxiredoxins (Prxs or Prdxs) are a family of ubiquitous antioxidant enzymes known to be involved in sensing and detoxifying hydrogen peroxide (H2O2) and other reactive oxygen species (ROS) in all kingdoms of life [1–3]. Mammalian Prxs also participate in the regulation of signal transduction by controlling the cytokine-induced peroxide levels [4–6]. Humans and other mammals possess six Prx isoforms, including four typical 2-cysteine (2-Cys Prxs) (Prx1–4), an atypical 2-Cys Prx5 and a 1-Cys Prx6 [7–9]. The thioredoxin peroxidase (POX) activity is the hallmark of Prx proteins. In the case of Prx1–4, the conserved N-terminal peroxidatic Cys residue (CP-SH), corresponding to the Cys51 in the mammalian Prx1, is oxidized by H2O2 to cysteine sulfenic acid (CP-SOH) and then resolved by a reaction with the C-terminal resolving Cys172 (CR-SH) in the adjacent monomer to form a disulfide-bond Cys51 and Cys172 (Figure 1, Pathway II). The disulfide linkage is reduced by NADPH-dependent thioredoxin (Trx)/thioredoxin reductase (TrxR) cycles to complete the Prx catalytic cycle in cells, or by a reducing agent such as dithiothreitol (DTT) commonly used in assaying POX activity [10–12]. Alternatively, the CP-SH and CR-SH residues in Homo sapiens Prx1 (HsPrx1) can be glutathionylated in the presence of a small amount of H2O2, and deglutathionylated by sulfiredoxin (Srx) or glutaredoxin I (Grx I) (Figure 1, Pathway I). CP-SH may also be hyperoxidized in the presence of excessive amount of H2O2 to form reversible sulfinic acid (CP-SO2H) that can be slowly recycled by Srx, or irreversible sulfonic acid (CP-SO3H), resulting in the loss of the POX activity and the formation of Prx1 decamers with protein chaperone function (Figure 1, Pathway III) [13–17]. Among these reactions, the rapid recycling of POX activity is responsible for the reduction of H2O2 and other ROS, while the other two appear to be involved in the regulation of Prx functions [18].
Figure 1. Illustration of pathways regulating vertebrate peroxiredoxin 1 (Prx1) to scavenge hydrogen peroxide.
Pathway I, the peroxidatic cysteine (CP-SH) and resolving cysteine (CR-SH) of Homo sapiens Prx1 (HsPrx1) can be glutathionylated in the presence of a small amount of H2O2, and deglutathianylated by sulfiredoxin (Srx) or glutaredoxin I (GrxI). Pathway II, the CP-SH of Prx1 is oxidized by H2O2 to cysteine-sulfenic acid (CP-SOH), which then reacts with CR-SH of the other subunit to produce an intermolecular disulfide, and the reducing equivalents for such typical peroxidase activity of Prx1 are ultimately derived from NADPH (reduced form of nicotinamideadenine dinucleotide phosphate) via thioredoxin reductase (TrxR) and Trx. Pathway III, the CP-SH is selectively oxidized by H2O2 to CP-SO2H or even CP-SO3H, which can be reversed by Srx and ATP (adenosine triphosphate). Pathway IV, the newly discovered catalase-like activity of Prx1, which directly converts H2O2 to O2.
Although Prxs could be oxidized in multiple ways, all these POX activities were known to rely on the Cys-dependent peroxidation cycles. However, in the present study, we unexpectedly observed that the Prx1 from the green spotted puffer fish Tetraodon nigroviridis (TnPrx1) was able to reduce H2O2 in a manner that was independent of Cys peroxidation and occurred in the absence of reducing agents. This Cys-independent activity observed in wild-type (WT) and site-mutated TnPrx1 proteins differs from the classic POX activity in Prxs, but resembles the catalase (CAT)-like activity (i.e. directly reducing H2O2 into H2O with the release of O2; or 2 H2O2 → 2 H2O + O2 for CAT-like activity vs. 2 H2O2 → 2 H2O for POX activity), making Prx1 a dual antioxidant protein. For clarity, we denoted Cys-dependent POX and Cys-independent CAT-like activities in TnPrx1 as TnPrx1-POX and TnPrx1-CAT, respectively. We have determined detailed kinetic features on the TnPrx1-CAT activity, and also identified that the 117GVL119 motif was essential to this activity. Using a HEK-293T cell transfection system, we showed that the TnPrx1-CAT participated in the regulation of H2O2 and H2O2-dependent phosphorylation of p38 protein in cells. Additionally, CAT activity was also confirmed in human Prx1 (HsPrx1), suggesting that the Cys-independent Prx1-CAT activity is conserved from fish to mammals.
Experimental
Cloning and expression of recombinant Prx1 proteins
The open reading frames (ORFs) of Prx1 genes from T. nigroviridis (TnPrx1, GenBank accession number: DQ003333) and Homo sapiens (HsPrx1, NM_001202431) were amplified by RT-PCR from mRNA isolated from pufferfish kidney and Hela cells, and cloned into pET28a or pMAL-c2E bacterial expression vector containing a His × 6-tag or maltose-binding protein (MBP) at the N-terminus, as described [19,20]. TnPrx1 mutants were generated by site-directed mutagenesis by replacing all three Cys residues (i.e. Cys52, Cys71 and Cys173) with Ser residues to eliminate POX activity (denoted by POX−CAT+), or the 117GVL119 motif with 117HLW119 to suppress CAT-like activity (POX+CAT−), or both (POX−CAT−) (see Table 1 for details on the genotypes of constructs). Site-directed mutants of TnPrx1 were constructed using the overlapping extension PCR strategy. Taking the construction of POX+CAT− as an example, two DNA fragments (named S1 and S2) were amplified by PCR using WT TnPrx1 ORF cDNA as the template and TnPrx1-EcoRI-F and TnPrx1-HLW-R or TnPrx1-HLW-F and TnPrx1-XhoI-R as the primer pair (see Table 2 for the description of primers). Then, the target POX+CAT− encoding sequence was amplified using templates containing both S1 and S2 and the primer pair TnPrx1-EcoRI-F and TnPrx1-XhoI-R. Typical PCR reactions were performed using Ex Taq™ Polymerase (TaKaRa) under the following conditions: 94°C for 5 min; 35 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 1 min; and a final extension at 72°C for 10 min. All constructs were subjected to Sanger sequencing to verify the correctness of the gene sequences and desired mutations.
Table 1. Kinetic parameters of wild-type (WT) TnPrx1 and various mutants deficient in thioredoxin peroxidase (POX) and/or catalase (CAT)-like activities on H2O2 compared with those reported for mammalian glutathione peroxidases (GPxs) and CATs.
NS, Not suitable. All data are presented as the mean values ± standard deviations (SDs of each group).
| Constructs | Genotype | DTT1 | Km or K′ (µM) | kcat (s−1) | kcat/Km/(×104 M−1 s−1) | n2 |
|---|---|---|---|---|---|---|
| TnPrx1 (POX+CAT+)3 | WT puffer fish Prx1 | − | 168 ± 8.8 | 1.8 ± 0.11 | ∼1.0 | 4.7 ± 0.33 |
| + | 214 ± 11.7 (Overall) | 2.5 ± 0.36 (Overall) | ∼1.1 (Overall) | 3.4 ± 0.34 (Overall) | ||
| 2.23 ± 0.03 (POX) | 0.21 ± 0.01 (POX) | ∼8 (POX) | ||||
| TnPrx1 (POX−CAT+) | All 3 Cys → Ser | − | 211 ± 7.1 | 2.3 ± 0.17 | ∼1.1 | 3.7 ± 0.42 |
| + | 227 ± 8.3 | 2.6 ± 0.26 | ∼1.1 | 3.3 ± 0.46 | ||
| TnPrx1 (POX+CAT−) | 117GVL → 117HLW | − | ∼250 | 0.013 ± 0.002 | ||
| + | 4.15 ± 0.6 | 0.23 ± 0.01 | ∼6 | |||
| TnPrx1 (POX−CAT−) | All 3 Cys → Ser and 117GVL → 117HLW | − | ∼250 | 0.016 ± 0.001 | ||
| + | ∼250 | 0.016 ± 0.002 | ||||
| HsPrx1 (WT) | WT human Prx1 | − | 347 ± 13.8 | 3.9 ± 0.16 | ∼1.1 | 10.1 ± 1.8 |
| + | 343 ± 11.2 | 4.0 ± 0.06 | ∼1.1 | 8.8 ± 1.0 | ||
| Mammalian GPx4 | NS | NS | 2 × 102–2 × 104 | 101 –102 | ∼104 | |
| Mammalian catalase4 | NS | NS | 104–105 | 104–105 | ∼102 |
Activity assayed with or without the reducing agent DTT (dithiothreitol, 100 µM). In the absence of DTT, only CAT-like activity plus a basal level of H2O2 consumption by oxidizing same molar amount of Cys residues in TnPrx1 and HsPrx1;
n = Hill coefficient;
Parameters in the presence of DTT were given for overall activity (i.e. POX + CAT) determined by allosteric sigmoidal model and for POX activity determined only by Michaelis-Menten model at the lower range of H2O2 concentrations (see curves in Figure 3a, inset);
Data acquired from http://www.brenda-enzymes.org/.
Table 2. List of primers and their applications.
| Primer | Sequences (5′ to 3′) | Application |
|---|---|---|
| TnPrx1-EcoRI-F | GAATTCATGGCTGCAGGCAAAGCTC | Cloning (pET28) |
| TnPrx1-XhoI-R | CTCGAGGTGCTTGGAGAAGAACTCTTTG | Cloning (pET28) |
| TnPrx1-KpnI-F | GGGTACCGATGGATTACAAGGATGAC | Cloning (pMal) |
| TnPrx1-HindIII-R | CAAGCTTGGCTTAGTGCTTGGAGAAG | Cloning (pMal) |
| TnPrx1-Ser52F | CTTCACCTTTGTGTCCCCCACTGAAG | Mutation |
| TnPrx1-Ser52R | CTTCAGTGGGGGACACAAAGGTGAAG | Mutation |
| TnPrx1-Ser71F | CGGAAAATTGGATCCGAGGTCATCG | Mutation |
| TnPrx1-Ser71R | CGATGACCT CGGATCCAATTTTCCG | Mutation |
| TnPrx1-Ser173F | GCATGGAGAAGTTTCCCCTGCCGGC | Mutation |
| TnPrx1-Ser173R | GCCGGCAGGGGAAACTTCTCCATGC | Mutation |
| TnPrx1-HLW-F | CAATCTCTACAGACTACCACTTATGGAAGGAAGACGAAGG | Mutation |
| TnPrx1-HLW-R | CCTTCGTCTTCCTTCCATAAGTGGTAGTCTGTAGAGATTG | Mutation |
| HsPrx1-F | GCTGATAGGAAGATGTCTTCAGGAA | Cloning |
| HsPrx1-R | GCCAACTCAGGCCATTCCTACC | Cloning |
| HsPrx1-EcoRI-F | CCGGAATTCATGTCTTCAGGAAATGCTAAAATTG | Expression |
| HsPrx1-XhoI-R | CCGCTCGAGCTTCTGCTTGGAGAAATATTC | Expression |
| siRNA-402 | GCACCAUUGCUCAGGAUUATT | Gene silencing |
| siRNA-525 | CCAGUUCACUGACAAACAUTT | Gene silencing |
| siRNA-625 | GCUCUGUGGAUGAGACUUUTT | Gene silencing |
| siRNA-Ctrl | UUCUCCGAACGUGUCACGUTT | Gene silencing control |
| TnPrx1-assay-F | TTTCCGGGAAATTGGA | qRT-PCR |
| TnPrx1-assay-R | AGATTGTGTGTCGTGT | qRT-PCR |
| HsPrx1-assay-F | CGCGTCTTGTTCTTGCCTGGTGTCG | qRT-PCR |
| HsPrx1-assay-R | CGCGTCTTGTTCTTGCCTGGTGTCG | qRT-PCR |
| Catalase-assay-F | TACCTGTGAACTGTCCCTACCGTGC | qRT-PCR |
| Catalase-assay-R | CATAGAATGCCCGCACCTGAGTAAC | qRT-PCR |
The expression of recombinant Prx1 proteins fused with His and MBP tags in Escherichia coli was performed at 37°C for 4 h after the addition of isopropyl β-D-1-thiogalactopyranoside (IPTG) (0.1 or 0.3 mM for His- or MBP-tagged proteins, respectively). Fusion proteins were purified from the soluble fractions by Ni-TNA agarose bead- or amylose resin-based affinity chromatography and eluted with elution buffer containing 250 mM imidazole (for His-tagged proteins) or 10 mM maltose (for MBP-tagged proteins) in accordance with the manufacturer's instructions (Novagen (pET28 system) and NEB (pMAL system)), or as specified [19,20]. Purified Prx1 proteins were subjected to SDS-PAGE analysis and stained with Coomassie Brilliant Blue. The protein purities were determined by densitometry using a 1D Image Analysis Software with Gel Logic 200 Imaging System (Eastman Kodak Co., USA).
A reversible monomer-to-dimer transition system was established to evaluate the Cys-dependent formation of dimers, in which the purified recombinant proteins in the form of monomers were first allowed to be oxidized to form dimers in air at 4°C, and then the resulting protein dimers were reduced to monomers by treating samples with 50 mM of DTT at room temperature for 10 min or as specified. The reduced and oxidized forms of Prx1 were detected by non-reducing SDS-PAGE. Prior to each activity assay, Prx1 proteins in the reduced form were subjected to extensive ultrafiltration with phosphate-buffered saline (PBS) to remove DTT.
Protein structure homology-modeling
TnPrx1 protein structure homology-modeling was performed using a rat Prx1 (PDB ID: 1QQ2; 80% amino acid identity to TnPrx1) as template. Global alignment of various structural models was performed by using PyMOL to produce various structural-model figures. The active site of TnPrx1 was predicted using an alpha-shape algorithm to determine potential active sites in the protein structures in MOE site finder. A pocket structure formed by conserved 117GVL119 amino acid residues was predicted to be an H2O2-binding site. To test whether the GVL pocket was essential for the Prx1-CAT activity, we replaced 117GVL119 with HLW residues containing large chains or aromatic rings to alter the pocket structure and abolish its ability to bind H2O2. The mutant was constructed by site-directed mutagenesis using overlapping extension PCR strategy as described above.
Enzyme activity assays
The reduction of H2O2 by TnPrx1 and HsPrx1 was determined by a modified sensitive Co(II catalysis luminol chemiluminescence assay as described [21]. Briefly, the luminol-buffer cocktail was composed by 100 µl of luminol (100 mg ml−1) in borate buffer (0.05 M, pH 10.0) and 1 ml of Co(II) (2 mg/ml)-EDTA (10 mg ml−1, pH 9.0). Reactions were started by mixing 50 µl of proteins (50 µg ml−1) with 50 µl of H2O2 at concentrations between 0–500 µM for 1 min at 25°C, following by the addition of 1.1 ml of the luminol-buffer cocktail to stop the reaction. The same amount of PBS was used to substitute proteins in the control and for generating standard curves.
The intensity of emission was measured with an FB12 luminometer (Berthold Detection Systems, Pforzheim) and the maximum values were recorded. The kinetic parameters of Prx1 proteins were determined using the Michaelis-Menten and/or the allosteric sigmoidal kinetic models. The production of oxygen was measured with an oxygen electrode (#341003038/ 9513468), Mettler Toledo. The reaction was performed in 4 ml of 600 µM H2O2 solutions, and the measurement was started by the addition of proteins (POX+CAT+ dimers, 0.32 µM or POX−CAT+ monomers, 0.64 µM) under soft stirring. Oxygen production rates were monitored at specified time points. Reactions with bovine catalase (8 nM) (Sigma-Aldrich) and BSA (6 µM) were used as positive and negative controls, respectively.
Determination of enzyme properties
The effect of pH on Prx1-CAT activity was evaluated by detecting the reduction of H2O2 in reactions carried out in 0.2 mM Na2HPO4/0.1 mM citrate buffer at pH 2.0–8.0 and 50 mM disodium pyrophosphate/NaOH buffer at pH 8.0–11.0, respectively. The effect of temperature was tested between 0–70°C at pH 7.0. The thermal and pH stabilities were similarly assayed, except that concentrated Prx1 proteins were first treated at various temperatures for 1 h, or 6 h under different pH conditions, and then their specific activities were determined under regular assay conditions (i.e. pH 7.0 at room temperature).
Specific activities were also assayed for iron-saturated proteins prepared by mixing proteins with FeCl3 (1:100 molar ratio), followed by ultrafiltration (MWCO = 10 kDa) to remove unbound iron. The role of iron in Prx1-CAT was further evaluated by iron chelation and rescue assays, in which TnPrx1 proteins were treated with 25 mM 4,5-dihydroxy-1,3-benzene disulfonic acid (tiron) and 50 mM 2,2′-dipyridyl (DP) at 4°C overnight, followed by ultrafiltration. Chelator-treated samples were then incubated with FeCl3 (200 µM) or as specified at 4°C overnight, followed by ultrafiltration to remove unbound iron. The residual TnPrx1-CAT activities of iron-free and iron-rescued proteins were determined in standard reactions as described above. To confirm that iron was truly bound to TnPrx1, iron-rescued samples were subjected to extensive ultrafiltration with PBS, and the iron content was detected by the inductively coupled plasma optical emission spectroscopy (ICP-OES), Optima 8000DV, Perkin-Elmer, as described [22]. To evaluate whether the 117GVL119 motif is involved in iron–binding, the pocket mutant (p.G117H/V118L/L119W) with the MBP tag was also subjected to iron content examination by ICP-OES.
The effects of seven other metals on the TnPrx1-CAT activity were also tested, in which WT TnPrx1 dimers were treated with tiron/DP mixture, and then reconstituted individually by incubating them with Mg2+, Ca2+, Cu2+, Mn2+, Co2+, Ni2+ or Zn2+ (protein:metal molar ratio = 1:5) at 4°C overnight. The effects of two classic CAT inhibitors (DTT and 3-amino-1,2,4-triazole [3-AT]) on TnPrx1 were assayed by pretreating proteins with 10 mM 3-AT at 4°C overnight or 1 mM DTT at 25°C for 30 min. Bovine catalase was used as positive control. Enzyme activities were assayed as described above.
Knockdown of HsPrx1 by siRNA in HEK-293T cells
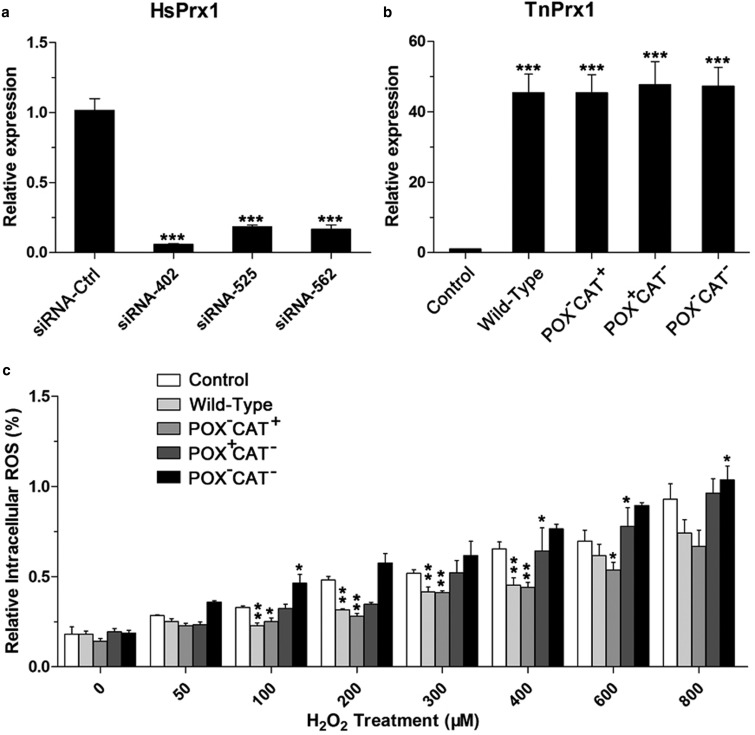
Three small interfering RNAs (siRNAs) targeting HsPrx1 (siRNA-402, -525, and -562) and one negative control siRNA (siRNA-Ctrl) were synthesized by GenePharma (Shanghai, China) (Table 1). HEK-293T cells were cultured at 37°C in Dulbecco's modified Eagle's medium (DMEM, Invitrogen) supplemented with 10% fetal bovine serum (Gibco) in the presence of 5% CO2. Transfection was performed using Polyethylenimine (PEI) in accordance with the manufacturer's recommended instructions (Sigma-Aldrich). Total RNA was isolated from HEK-293T cells transfected with siRNAs and/or TnPrx1 constructs using TRIzol reagent (Invitrogen). The levels of HsPrx1 transcripts after transfections were monitored by qRT-PCR using SYBR Premix Ex Taq™ II kit (TaKaRa) and primers described in Table 2, in which the samples were first denatured at 95°C for 1 min, and then subjected to 40 cycles at 95°C for 10 s, 60°C for 30 s, and 72°C for 20 s. Each experimental condition included three biological replicates, and each biological sample included three technical replicates. The relative RNA expression was calculated using a 2−ΔCT method.
Effects of WT TnPrx1 and mutants on intracellular ROS level and the phosphorylation of P38 MAPK
The ORFs of WT and mutated TnPrx1 genes were subcloned into pCMV-Tag2B vector. For transient transfection, WT or HsPrx1-silenced HEK-293T cells were plated in 100 mm culture plates (1.4 × 106 cells/plate), grown overnight, and transfected with 17 µg of WT Prx1 or mutant plasmids using Fugene reagent (Promega). Blank pCMV-Tag2B vector was used as negative control. After 48 h of post-transfection, cells were washed with PBS, and incubated with 2′7′-dichlorodihydrofluorescein diacetate (DCFH-DA, 200 µM, Sigma) in serum-free medium at 37°C for 30 min to allow uptake by cells and intracellular cleavage of the diacetate groups by thioesterase. Cells were washed with PBS to remove free DCFH-DA from the medium, and counted by trypan blue (0.4% exclusion method). Viable cells were then plated into collagen-coated 96-well plates (2 × 104 cells/well), and treated with H2O2 for 60 min at final concentrations of 0–850 µM or 0–800 µM for WT or HsPrx1-silenced HEK-293T cells, respectively. Intracellular fluorescence signals of oxidized DCFH at 0 and 1 h time points (T0 and T1) followed by the H2O2 treatment were measured with a Synergy H1 Hybrid Reader (BioTek, USA) (λex/λem = 485/525 nm). The relative fluorescence signal for each sample (RFsample) was calculated using the following equations:
| 1 |
| 2 |
In order to evaluate the effect of TnPrx1 constructs on the phosphorylation of intracellular p38 MAPK, transfected cells treated with H2O2 (0–1200 µM or 0–800 µM for WT or HsPrx1-silenced cells) were collected and lysed. This was followed by Western blot analysis using antibodies against p38 (1:1000 dilution; Catalog No. 8690, Cell Signaling Technology, Danvers) and phosphorylated p38 protein (p-p38) (1:1000 dilution; Catalog No. 4511, Cell Signaling Technology, Danvers), respectively. The immuno-reactive bands were visualized using an enhanced chemiluminescence (ECL) system (Pierce, Rockford, IL).
Statistical analysis
All experiments were performed independently at least three times. Data were presented as the mean ± standard deviation of the mean (SD). Two-tailed Student's t-test was used to assess statistical significance between experimental and control groups.
Results
Cys-independent CAT-like activity in TnPrx1
Thioredoxin peroxidase (POX) was previously the only known enzyme activity in Prxs that relied on NADPH-dependent oxidoreduction between Trx and TrxR to maintain the continuation of their POX activity (Figure 1, Pathway II). In the absence of Trx/TrxR/NADPH cycle or a reducing agent (e.g. DTT), the reactions stop after the formation of a CP-CR disulfide bound, in which one pair of Prx monomers may only reduce two H2O2 molecules. Four to six H2O2 molecules may be reduced when hyperoxidized without the formation of disulfide bonds (Figure 1, Pathway III).
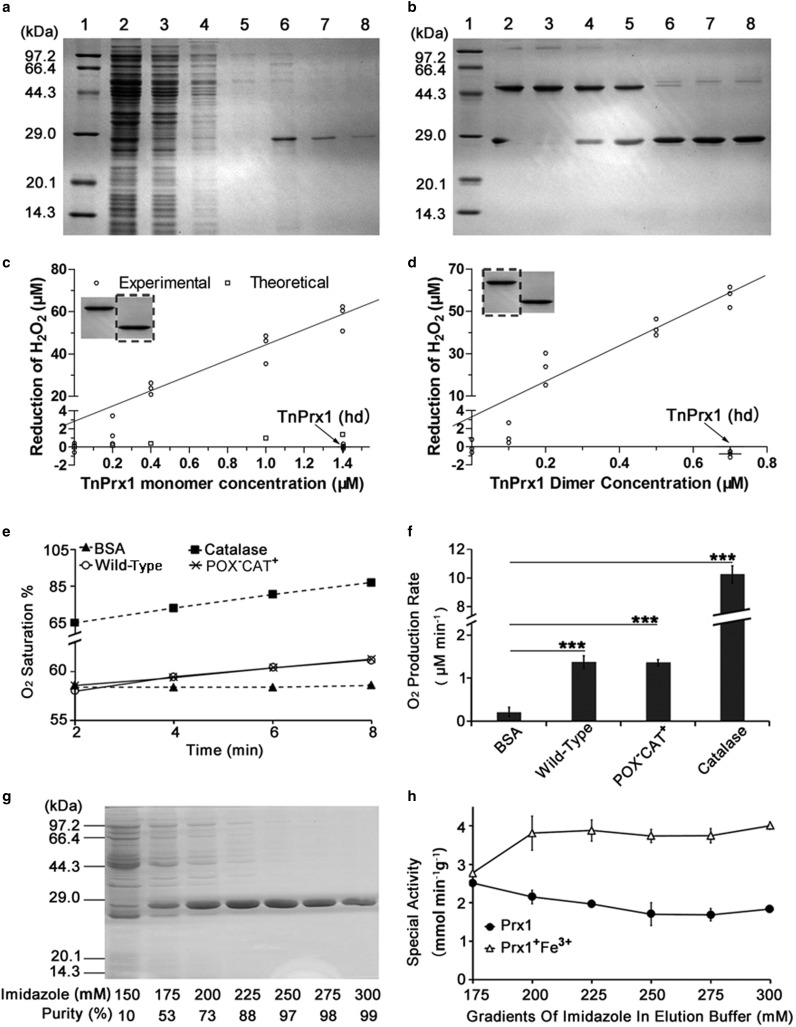
Surprisingly, however, in the absence of a reducing agent, we observed that the recombinant WT TnPrx1 monomers (>99% purity) in reduced status were able to continuously reduce H2O2 molecules (Figure 2a–c), implying the presence of non-POX oxidoreduction activity in TnPrx1. Similar activity was observed when TnPrx1 was fully oxidized to form dimers (Figure 2b,d), confirming that the observed non-POX activity was independent of the status of Cys residues. The observed activity was not attributed to nonspecific background reactions as it was not observed in reactions containing no, or denatured, TnPrx1 (Figure 2c,d; first and last columns). Additionally, we also detected the O2 production (Figure 2e,f), and the calculated ratio between the reduced H2O2 and the produced O2 was 2.29:1, indicating that this activity was derived from a CAT-like activity (i.e. 2 H2O2 → 2 H2O + O2), rather than the POX activity that only produces H2O.
Figure 2. Verification of the catalase (CAT)-like activity of TnPrx1 and mutants.
(a) Expression and purification of soluble TnPrx1 protein. Lanes: 1, protein markers; 2, crude cell lysate; 3, flow-through; 4–5, 40 mM imidazole wash; 6–8, eluted recombinant protein (250 mM imidazole); (b) Reductive dissociation of TnPrx1 dimer induced by DTT. Lanes: 1, protein markers; 2–8, proteins treated with different concentrations of DTT (dithiothreitol) (0, 0.1, 0.5, 1, 5, 10 and 50 mM); (c,d) Activities of TnPrx1 in monomers (c) and dimers (d) in the absence of a reducing agent by detecting the reduction of H2O2 using a luminol chemiluminescence assay after incubation with 300 µM H2O2 for 10 min at 25°C. The bands in the dashed box denote the TnPrx1 monomers and dimers used in the corresponding assays. Theoretical values represented the maximal reduction of H2O2 possibly achieved by the oxidation of three TnPrx1 Cys residues in a given amount of TnPrx1 protein in the absence of reductants or redox recycling. hd = heated denatured TnPrx1 protein (at 100°C for 10 min); (e,f) Detection of O2 production in reactions containing H2O2 and various protein constructs (i.e. 0.32 µM wild-type dimers, 0.64 µM POX−CAT+ monomers, 8 nM catalase and 6 µM BSA) with oxygen electrode technique; POX = Cys-dependent thioredoxin peroxidase; (g,h) Gradient elution of TnPrx1 protein with varied concentrations of imidazole in elution buffer (g), and their corresponding activity by measuring the reduction of H2O2 with or without the addition of extra iron (200 µM) by luminol chemiluminescence (h). TnPrx1 concentration was determined by thin-layer gel optical scanning. Activity was normalized to mmol of H2O2 reduced per min per gram of TnPrx1 protein. Data are representative of at least three independent experiments. The error bars represent standard deviations (SDs), and statistical significances between experimental and control groups were determined by Student's t-test. ***P < 0.001.
To fully rule out the possibility that a trace amount of contaminating CAT from E. coli was present in the TnPrx1 preparations (despite >99% purity) and contributed to the activity, we prepared TnPrx1 proteins under different elution stringency (i.e. imidazole at 150–300 mM) to allow various impurities (i.e. containing various amounts of contaminants). We confirmed again that the activity was derived from TnPrx1, as it was correlated with the amount of TnPrx1, rather than with the level of impurity (Figure 2g,h).
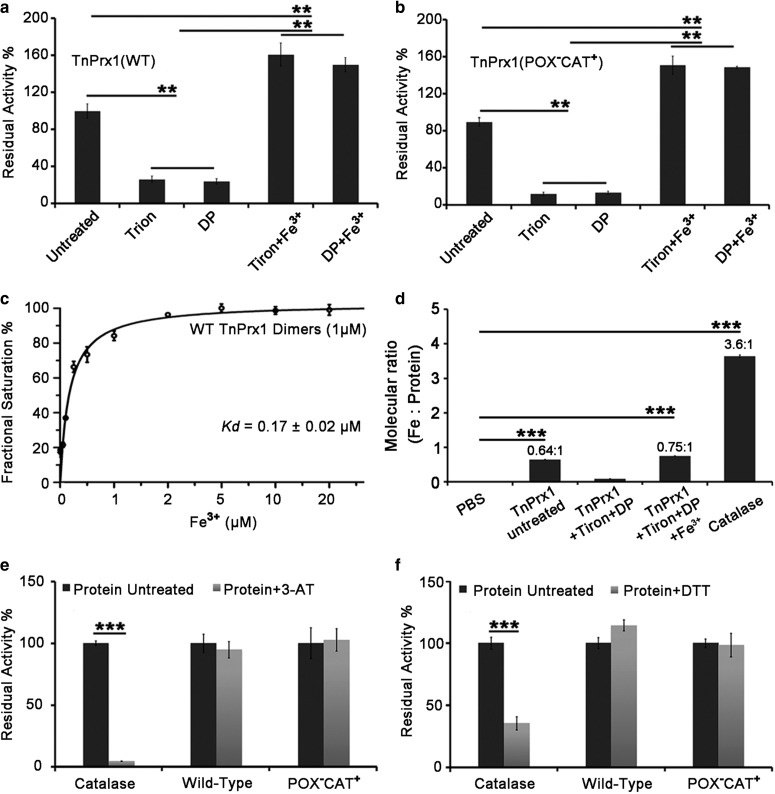
The activity was iron-dependent, as it could be inhibited by ferrous/ferric chelators tiron and DP, and the addition of Fe3+ not only increased the activity of untreated TnPrx1, but also reversed the inhibition by chelators (Figures 2h and 3a). Fe3+ displayed low nanomolar-level binding affinity with TnPrx1 (apparent Kd = 0.17 µM), and a ∼1:1 (metal:Prx1) stoichiometry (Figure 3c). To confirm the iron-TnPrx1 binding, we directly evaluated the iron content of recombinant TnPrx1 proteins under various conditions. Proteins were subjected to extensive ultrafiltration to remove unbound iron. The molecular ratio between iron and untreated TnPrx1 protein was 0.64 (±0.002):1 (Figure 3d). Treatment by chelators reduced the ratio to 0.08 (±0.003):1, whereas the addition of FeCl3 (200 µM) restored the ratio to 0.75 (±0.006):1 (Figure 3d). These observations indicated that each TnPrx1 binds to one iron, and up to 75% of the recombinant TnPrx1 proteins were in their active form.
Figure 3. Iron-dependency and inhibition of TnPrx1 and mutants determined by measuring the reduction of H2O2.
(a,b) Effects of iron chelators (25 mM 4,5-dihydroxy-1,3-benzene disulfonic acid [tiron] and 50 mM 2,2′-dipyridyl [DP]) on the CAT activity of wild-type (WT) TnPrx1 and mutants, and restoration of the activity by the addition of Fe3+ (200 µM). Residual activities were expressed as the percentage activity (vs. untreated WT TnPrx1); (c) Dose-dependent WT TnPrx1 activity on Fe3+. Residual activities were expressed as the percentage activity (vs. WT TnPrx1 treated with 200 µM Fe3+); (d) The molar ratio between TnPrx1 protein and bound iron determined by ICP-OES (inductively coupled plasma optical emission spectroscopy). TnPrx1 was treated as specified, followed by extensive washes with water by ultrafiltration prior to ICP. Bovine catalase and PBS were used as controls; (e,f) Effects of catalase inhibitors 3-Amino-1,2,4-triazole (3-AT) and dithiothreitol (DTT) on the CAT activity of WT TnPrx1 and mutants. CAT was used as positive control. Protein samples (50 µl, 50 µg ml−1, pretreated with 3-AT or DTT) were reacted with 50 µl of H2O2 solutions (300 µM) for 1 min. The residual activities were measured by luminol chemiluminescence assay and expressed as the percent activity (vs. untreated WT TnPrx1). Data are representative of at least three independent experiments. The error bars represent standard deviations (SDs), and statistical significances between experimental and control groups were determined by Student's t-test. **P < 0.01, ***P < 0.001. CAT = Catalase-like activity of peroxiredoxin 1.
Additionally, the effect of other metals, including Mg2+, Ca2+, Cu2+, Mn2+, Co2+, Ni2+ and Zn2+ on the CAT-like activity was tested, but no enhancement activity was observed (data not shown). The dependence on iron, but not on reducing agents and Cys residues were characteristic to CATs, further confirming that the observed activity was not derived from the POX activity of Prxs. Instead, it resembled a CAT that was previously unknown to Prxs. However, TnPrx1 was insensitive to the inhibitors of typical CATs, such as DTT and the irreversible inhibitor 3-AT (Figure 3e,f), suggesting that Prx1 might represent a new class of CAT-like enzyme. Indeed, unlike typical CATs, TnPrx1 lacked the Soret absorbance peak unique to heme-containing moieties (data not shown), indicating that it is a heme-less metalloprotein, rather than a heme-containing protein.
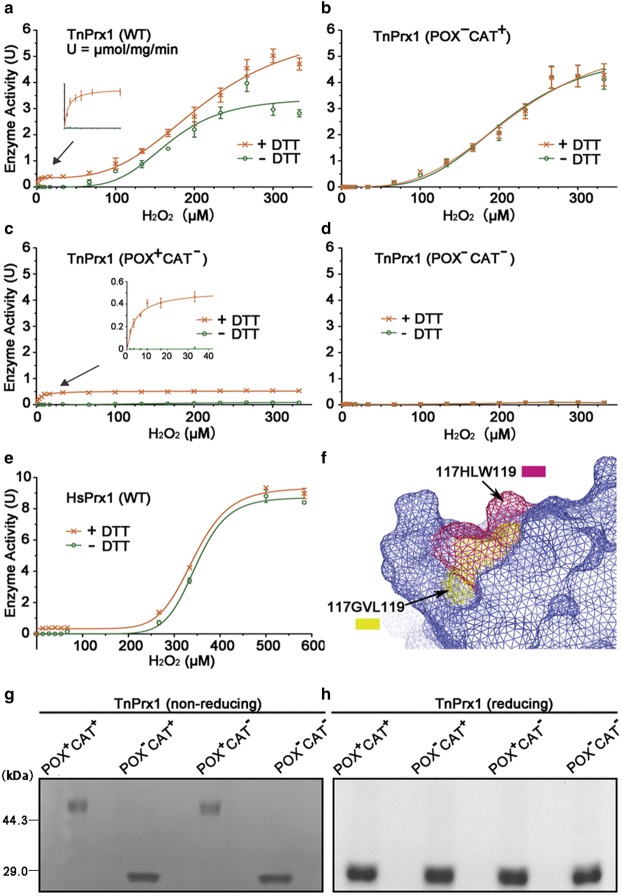
In the presence of DTT, WT TnPrx1 displayed Michaelis-Menten kinetics on low concentrations of H2O2 (i.e. <100 µM) (Figure 4a). The Km value was 2.2 µM, which was comparable to the Km values previously reported for Prx1-POX activities that were typically much lower than 20 µM [7]. At higher H2O2 concentrations (>50 µM), TnPrx1 exhibited allosteric kinetics, suggesting a positive cooperativity, i.e. K′app = 214 µM and Hill coefficient (n) = 3.4. This result implies that reducing Prx1 has a relatively low affinity for O2. However, binding of one H2O2 to Prx1 might change the structure of the binding site, which allows the second H2O2 to bind more easily or the bounded H2O2 to be catalyzed by iron more rapidly, thereby increasing O2 affinity. In the absence of DTT, however, TnPrx1 showed little or no activity until [H2O2] reached >50 µM (K′app = 168 µM, n = 4.7) (Figure 4a; Table 1). The data were in agreement with the notion that TnPrx1 possessed both POX and CAT activities, since the activities with DTT (POX + CAT) were higher than those without DTT (CAT only) by a relatively constant rate (i.e. 2.5 s−1), determined by a ‘Michaelis-Menten + allosteric sigmoidal' model.
Figure 4. Kinetic features of Prx1 proteins.
(a–e) Enzyme kinetics curves for wild-type (WT) and mutated pufferfish Prx1 (TnPrx1) and WT human Prx1 (HsPrx1) with or without DTT (dithiothreitol). (f) Structural comparison of the potential cavity of WT Prx1 protein (yellow) and its mutant (warm pink) in mesh form. The image is a merged model of the two Prx1 proteins; (g,h) The dimeric versus monomeric status of TnPrx1 proteins in non-reducing or reducing SDS-PAGE (Sodium dodecyl sulfate-Polyacrylamide Gel Electrophoresis), respectively. All TnPrx1 proteins were treated with monomer-to-dimer transition protocol prior to the assays.
Since CAT activity was described for the first time in a Prx1 of fish origin, we wanted to know whether it was also present in mammalian Prx1. We expressed recombinant human Prx1 (HsPrx1) and performed a similar assay with and without a reducing agent. Our data supported the contention that HsPrx1 was also bifunctional, since it possessed POX and CAT activities with kinetic parameters comparable to those of TnPrx1 (i.e. K′app(−DTT) = 347 µM, n(−DTT) = 10.1, K′app(+DTT) = 342 µM, and n(+DTT) = 8.8, respectively) (Figure 4e; Table 1). Although Prxs from more species need to be examined to make a firm conclusion, the data here suggest that the CAT-like activity is probably conserved among vertebrate Prx1 from fish to mammals.
To further validate TnPrx1-CAT activity, we performed a site-directed mutagenesis and constructed a mutant by replacing all three Cys residues with Ser residues to completely eliminate its Cys-dependent POX activity. The resulting mutant (POX−CAT+) was unable to form dimers as expected (Figure 4g,h), but was still capable of converting H2O2 to O2 (Figure 2e,f). The POX−CAT+ mutant displayed virtually identical sigmoidal curves when assayed with and without DTT, which resembled those of WT TnPrx1 without DTT and those of similar kinetic parameters (i.e. K′app(−DTT) = 211 µM, n(−DTT) = 3.7, K′app(+DTT) = 227 µM, and n(+DTT) = 3.3), respectively (Figure 4b; Table 1). These observations confirmed that the observed TnPrx1-CAT activity was truly independent of the Cys residues and the reducing agent.
Potential active site for the CAT-like activity in TnPrx1
The discovery of a previously unknown Prx1-CAT activity prompted us to search for the functional motif. By examining a previously reported structure of rat Prx1 (PDB ID: 1QQ2), and homology-based modeling of TnPrx1, we observed a flexible loop consisting of six residues, Gly117, Val118, Leu119, Phe127 (rat Prx1) or Tyr127 (TnPrx1), Ile142 and Ile144 at the dimer interface, in which a H2O2 molecule could well fit into a pocket formed by the highly conserved 117GVL119 residues (Figure 5). To test whether this pocket might contribute to the TnPrx1-CAT activity, we generated a TnPrx1 construct by replacing 117GVL119 with 117HLW119 (denoted by POX+CAT−) to alter the pocket structure (Figure 4f). Indeed, the mutant POX+CAT− lost CAT-like activity (i.e. no activity without DTT in the reactions), but retained only DTT-dependent POX activity, which followed Michaelis-Menten kinetics that were characteristic to Prx1-POX activity (Km = 4.15 µM) (Figure 4c; Table 1).
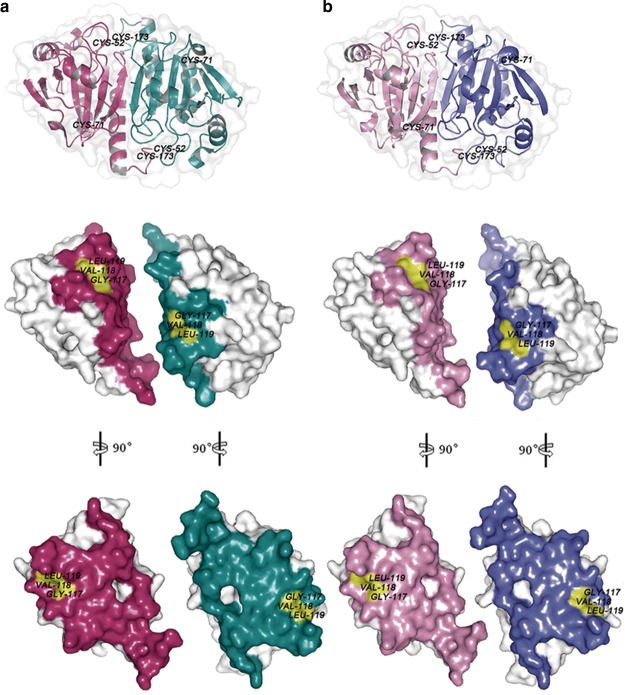
Figure 5. Structural comparison between rat Prx1 (PDB ID:1QQ2) (a) and TnPrx1 determined by homology-modelling (b).
Structural models were represented in surface forms prepared using PyMOL software (www.pymol.org). The amino acids located at the dimer interface are shown in colors. The pockets containing the 117GVL119 motif in rat Prx1 and TnPrx1 are highlighted in yellow.
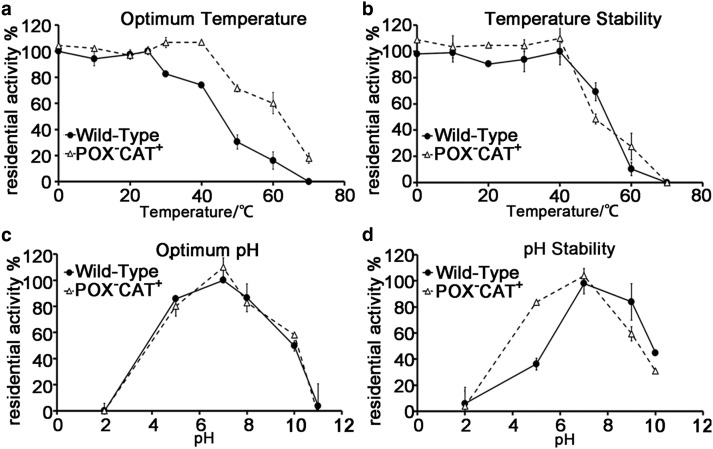
To further dissect individual TnPrx1-POX and Prx1-CAT activities, we generated a double-mutation (POX−CAT−), in which all Cys residues and 117GVL119 were replaced by Ser and 117HLW119, respectively. As expected, this double-negative mutant lost both POX and CAT activities and was unable to reduce H2O2 regardless of whether DTT was present or not (Figure 4d). Among all the mutants tested, POX−CAT+ also displayed expected iron-dependency, in which iron chelators inhibited its activity that could be restored by adding iron (Figure 3b), whereas the two CAT− mutants (i.e. POX+CAT− and POX−CAT−) only retained low activity (6% vs. WT) that were unaffected by iron chelators and iron (data not shown). Moreover, the molecular ratio between iron and TnPrx1 protein of the two CAT− mutants remained approximately 1:1 (i.e. iron:POX+CAT− = 0.93 (±0.005):1; iron:POX−CAT− = 0.80 (±0.002):1), which was not significantly different from that between iron and WT TnPrx1 (P = 0.09 and P = 0.14, respectively). This result suggests that the replacement of 117GVL119 by 117HLW119 merely destroyed the substrate binding site but not the iron binding site (Supplementary Figure S1). Additionally, the TnPrx1-CAT activity tolerated low temperature more than pH, as it was able to retain virtually constant peak activity between 0–40°C, but only retained peak activity at ∼pH 7.0 (Figure 6).
Figure 6. The effect of pH and temperature on catalase (CAT)-like activity and the stability of TnPrx1 proteins.
(a) The effect of temperature on residual Prx1-CAT activity. The activity assay was performed at pH 7.0 and at various temperatures. (b) The effect of temperature on Prx1-CAT stability. All the proteins were incubated at pH 7.0 and at various temperatures for 1 h and then the residual activity was estimated. (c) The effect of pH on residual Prx1-CAT activity. The activity assay was performed at room temperature and at various pH values. (d) The effect of pH on Prx1-CAT stability. The proteins were incubated with various pH at 4°C for 6 h and then the residual activity was measured.
Collectively, these observations confirm that TnPrx1 possesses both POX and CAT activities, and the residues 117GVL119 are critical to Prx1-CAT activity. TnPrx1-POX acted on H2O2 with much higher affinity (Km = 4.15 µM), but had a relatively low maximal activity (kcat = 0.23 s−1) with a wider range of H2O2 levels (Table 1; Figure 4c); whereas Prx1-CAT acted on H2O2 with lower affinity (K′(−DTT) = 210.7 µM), but had a much higher activity (kcat = 2.3 s−1) (Table 1).
Implication of TnPrx1-CAT in regulating ROS level and signaling
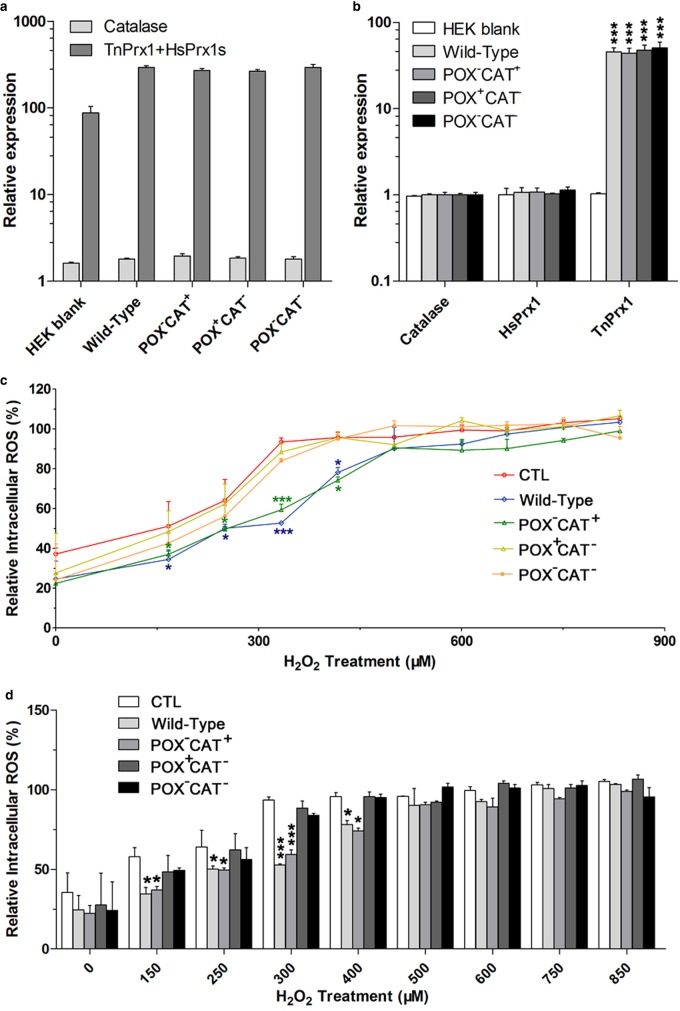
The physiological roles of TnPrx1-CAT activity were investigated using a mammalian cell transfection system. First, we transfected HEK-293T cells to overexpress various TnPrx1 constructs, and examined the effects in regulating intracellular ROS (iROS) in response to H2O2 treatment. The expression of TnPrx1 constructs was confirmed by qRT-PCR (Figure 7a,b). We observed a general trend that cells overexpressing CAT+ proteins (i.e. WT and POX−CAT+) had lower iROS levels than those expressing CAT− proteins (i.e. blank vector, POX+CAT− and POX−CAT−) in response to the treatment of 150–600 µM exogenous H2O2 (Figure 7c). The iROS levels were significantly downregulated in cells overexpressing CAT+ proteins compared with the control cells (transfected with blank vector) at exogenous H2O2 concentrations between 150 and 400 µM (Figure 7c; P < 0.05 or P < 0.001). However, no significant differences were observed in those expressing CAT− proteins in response to 150–400 µM exogenous H2O2 treatment (Figure 7c,d).
Figure 7. Involvement of TnPrx1 constructs in regulating intracellular ROS (iROS) in HEK-293T cells.
(a,b) The expression of various TnPrx1 constructs in transfected cells were confirmed by qRT-PCR (real-time quantitative polymerase chain reaction) compared with those of endogenous HsPrx1 and catalase (CAT) genes. The relative levels of Prx1 transcripts (HsPrx1) only in blank control, or HsPrx1 + TnPrx1 in transfected cells were determined using a pair of primers derived from regions conserved between fish and mammalian Prx1 genes (Table 2). (a) Fold changes of Prx1 and catalase transcripts were expressed relative to the catalase transcripts in the blank control. (b) Fold changes of HsPrx1 and TnPrx1 transcripts were expressed relative to the transcripts of their own genes in the blank control. (c,d) Effects of TnPrx1 constructs on intracellular ROS in transfected cells treated with exogenous H2O2 as determined by DCFH (dichlorodihydrofluorescein diacetate) fluorescence assay. Cells transfected with blank vector were used as the negative control. The error bars represent standard deviations (SDs), and statistical significances between experimental and control groups were determined by Student's t-test. *P < 0.05, ***P < 0.001.
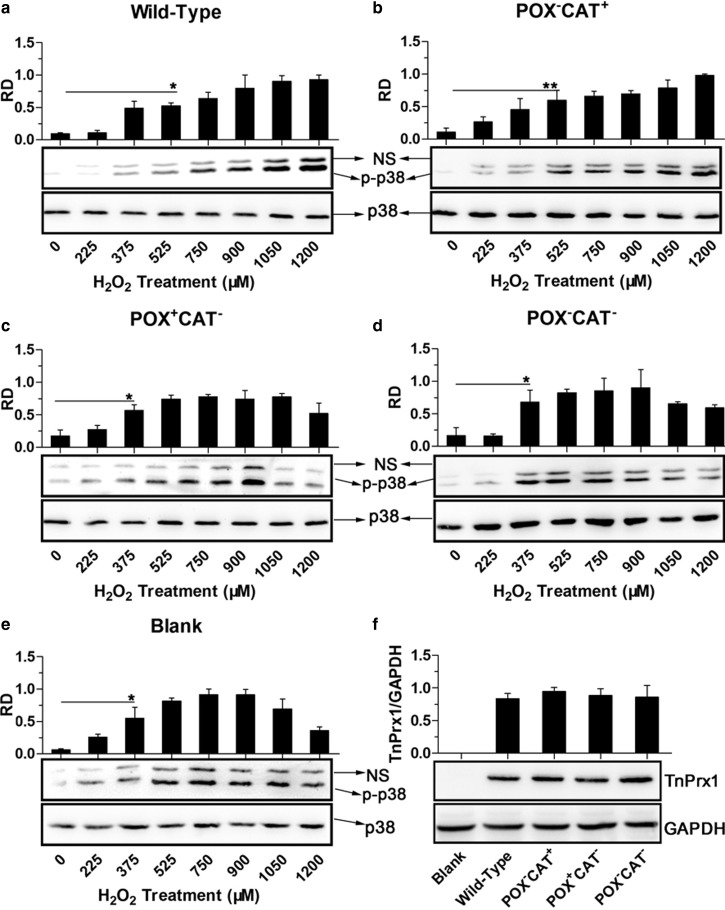
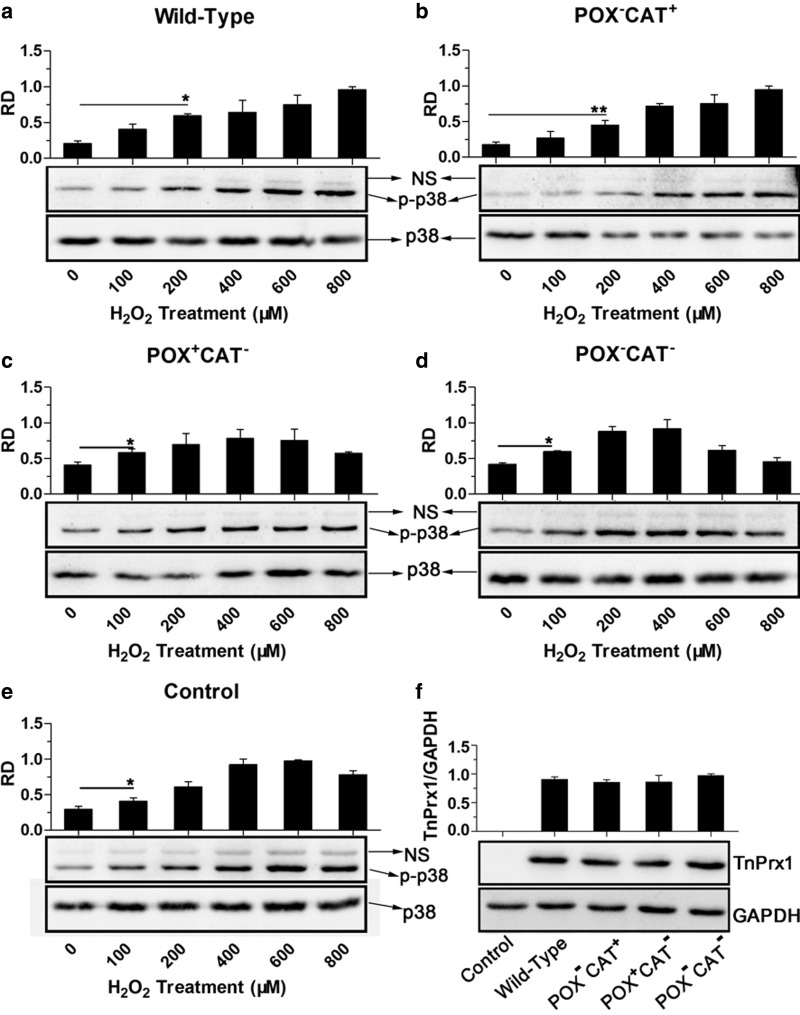
Second, since H2O2 was known to also function as a signaling molecule, particularly in regulating kinase-driven pathways [23], we tested whether Prx1-CAT-associated regulation of intracellular H2O2 affected the phosphorylation of p38 protein that played a central role in the p38 mitogen-activated protein kinase (MAPK) signaling pathway. In HEK-293T cells transfected with blank or double-negative (POX−CAT−) plasmids, there were low background levels of phosphorylated p38 (p-p38) in the absence of H2O2 stimulation (0 µM) (Figure 8). The levels of p-p38 in cells treated with 225–1200 µM H2O2 displayed a bell-curve that peaked in the 525–900 µM H2O2 groups, which was comparable to previously reported data [24]. When CAT+ constructs (i.e. WT and POX−CAT+) were overexpressed, a considerable delay of phosphorylation of p38 was observed, as p-p38 was significantly (P < 0.05) up-regulated in cells challenged with H2O2, starting at 525 µM, peaked in the 900–1200 µM.
Figure 8. Effects of various TnPrx1 constructs on ROS-mediated phosphorylation of p38 MAPK in HEK-293T cells.
(a–e) Western blot analysis was conducted on total protein extracts from cells transfected with various TnPrx1 constructs and treated with various concentrations of exogenous H2O2 using antibodies specific to p38 and phosphorylated p38 (p-p38), respectively. Blank control cells received no transfection. Representative data from one of the three or more independent experiments were shown. Note: Antibody to p38 recognized a single band at 43 kDa, while antibody to p-p38 also produced a nonspecific band (NS) above the major band at 43 kDa for each sample. The NS bands were much weaker than—but proportional to—the major bands, a finding that was also observed by other investigators (e.g. [55–57]). Bar charts showed relative density (RD) of p-p38 major bands (vs. p38). The error bars represented standard deviations (SDs) from three replicated blots. An asterisk in each bar chart indicates the lowest concentration of exogenous H2O2 starting to give statistical significance between H2O2-treated and untreated controls (*P < 0.05 by Student's t-test); (f) Confirmation on the protein expression from TnPrx1 constructs by Western blot analysis using antibodies specific to His-tag and to human GAPDH control, respectively. RD = relative density; POX = Cys-dependent thioredoxin peroxidase; CAT = Catalase-like activity of peroxiredoxin 1.
On the other hand, in cells overexpressing POX+CAT− TnPrx1, no significant delay of p38 phosphorylation was observed, as p-p38 was only significantly up-regulated in cells challenged with H2O2; this started at 375 µM, peaked at approximately 750 µM and declined at 1200 µM (Figure 8), with a pattern was similar to that of the blank or double-negative group. Although further studies are needed to fully dissect the physiological roles of individual Prx1-POX and Prx1-CAT activities in cells and in vivo, these observations provide primary evidence on the involvement of the TnPrx1-CAT activity in regulating ROS-mediated p38 signaling pathway when cells were incubated with high micromolar to low millimolar level of H2O2.
We also employed an siRNA-silencing technology to minimize the effect of endogenous HsPrx1 in assaying the activity of TnPrx1 constructs on iROS and the phosphorylation of p38. Among the three siRNA constructs, siRNA-402 showed the highest efficacy in silencing HsPrx1 (i.e. ∼94% with siRNA-402 vs. 81–83% with siRNA-525 and siRNA-562) (Figure 9a). Thus, siRNA-402 was used for subsequent functional investigations. Various TnPrx1 constructs were co-transfected with siRNA-402 into HEK-293T cells. iROS and p-p38 levels were measured in WT and HsPrx1-silenced cells after H2O2 treatment (Figure 9b). Results showed that the iROS levels in cells transfected with CAT+ constructs (i.e. WT and POX−CAT+) were significantly lower than those transfected with CAT− constructs (i.e. blank vector, POX+CAT−, and POX−CAT−) in response to the treatment of H2O2 at 100–600 µM (Figure 9c; P < 0.05, or P < 0.01). Similarly, a considerable delay of p38 phosphorylation upon H2O2 stimulation was observed in cells expressing CAT+ TnPrx1 proteins compared with those expressing CAT− proteins; the peak point for significant upregulation of p-p38 was shifted from 600 µM in cells expressing CAT− TnPrx1 proteins to 800 µM in cells expressing CAT+ TnPrx1 proteins (Figure 10; (P < 0.05 or P < 0.01). These observations were consistent with the results using WT HEK-293T cells (Figures 7 and 8), further supporting the functional role of TnPrx1-CAT activity in regulating iROS level and signaling.
Figure 9. Involvement of TnPrx1 constructs in regulating intracellular ROS (iROS) in wild-type and HsPrx1-silienced HEK-293T cells.
(a) Interference of HsPrx1 expression in HEK-293T cells by three siRNA species targeting HsPrx1. Negative control used siRNA-Ctrl. The interference efficiency was shown as relative levels of HsPrx1 transcripts determined by qRT-PCR (real-time quantitative polymerase chain reaction). (b) Expression of various TnPrx1 constructs in cells co-transfected with siRNA-402. (c) Effects of TnPrx1 constructs on iROS (intracellular reactive oxygen species) levels in siRNA-402-transfected cells treated with exogenous H2O2 as determined by DCFH Dichlorodihydrofluorescein Diacetate fluorescence assay. Cells transfected with siRNA-402 only were used as control. Error bars represent standard deviations (SDs). Asterisks show statistical significances between the experimental and control groups by Student's t-test (*P < 0.05, **P < 0.01 and ***P < 0.001). POX = Cys-dependent thioredoxin peroxidase; CAT = Catalase-like activity of peroxiredoxin 1.
Figure 10. Effects of various TnPrx1 constructs on the ROS-mediated phosphorylation of p38 MAPK in HsPrx1-silienced HEK-293T cells.
(a–e) Western blot analysis of total protein extracts from cells co-transfected with HsPrx1-siRNA-402 and various TnPrx1 constructs after the treatment of various concentrations of exogenous H2O2 using antibodies specific to p38 and phosphorylated p38 (p-p38) proteins. Control cells were transfected with siRNA-402 only. Representative data from one of the three independent experiments are shown. (f) Protein expression of TnPrx1 constructs by Western blot analysis using antibodies specific to His-tag and to human GAPDH (glyceraldehyde-3-phosphate dehydrogenase) (control). An asterisk in each bar chart indicates the lowest concentration of exogenous H2O2 yielding statistical significance between H2O2-treated cells and untreated controls (*P < 0.05, **P < 0.01 by Student's t-test). RD = relative density.
Discussion
Eukaryotic cells contain a complex system to detoxify and regulate H2O2 and other reactive oxygen species. These include small molecules, such as ascorbic acid, β-carotene, glutathione, and α-tocopherol, and various enzymes, such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx) and peroxiredoxin (Prx) [25]. Some of these enzymes or isoforms are mainly cytosolic (e.g. Prx1, Prx2, Prx5, Prx6, SOD1 and GPx1), while others may be compartmentalized (e.g. catalase in peroxisome, SOD2 and Prx3 in mitochondria, SOD3, GPx3 and Prx4 in extracellular region), which constitutes a precise antioxidant network for the defense against various oxidative stresses in the diverse cellular activities [4,26,27].
Cells are known to rely heavily on Prxs in scavenging H2O2 and other ROS molecules. In fact, they are the third most abundant proteins in erythrocytes, or represent 0.1–1% of total soluble proteins in other cells [4,7,26,28,29], and Prx1/2-knockout in mice might lead to the development of severe blood cell diseases (e.g. hemolytic anemia and hematopoietic cancer) [30,31]. Prxs are widely distributed and have been found in animals, plants, fungi, protists, bacteria and cyanobacteria, suggesting that they are a family of ancient proteins essential to a variety of critical cellular activities [32,33].
Prxs were previously recognized only as a family of thioredoxin POX, for which the biochemical features and biological functions were subjected to extensive investigations [23]. In the present study, we discovered that TnPrx1 and HsPrx1 are bifunctional and possess both Cys-dependent POX and Cys-independent CAT-like activities, which further extends our understanding of this important family of antioxidant proteins. The CAT-like activity in TnPrx1 was validated by the identification of the active site containing the GVL motif, which also enabled us to generate mutants lacking CAT and/or POX activity for dissecting their individual activities. Our data suggested that previously observed antioxidant activity in WT Prx1 (at least in some animals) were in fact a combined activity of Prx1-POX and Prx1-CAT. The alternation of GVL motif abolished TnPrx1-CAT activity, but not TnPrx1-POX activity (Figure 4c, Table 1). This suggests that Prx1 contained two independent H2O2 binding sites, which is in agreement with previous reports that the H2O2-binding site for the Cys-dependent POX activity was near the Cys51 and Cys172 residues but distant from the GVL site [34–36]. In fact, Prx1 was identified as early as the 1990s. However, the CAT activity of this protein has never been reported. This shortfall might be due to the limitations of the sensitive H2O2-detection method previously available and the universal usage of NADPH-dependent examination on Prx1 activity in early investigations. The latter of which was suitable for the determination of Prx1-POX activity (but not CAT activity), which was reflected indirectly by the consumption of NADPH. In addition, the concentration of H2O2 used in previous Prx1-POX studies was rather low because of the high affinity of Prx1-POX to H2O2 but the limited hydrolysis capacity for H2O2 (<20 µM). The underlying substrate (H2O2) concentration is extremely low for detection considering the CAT activity.
CATs are heme-containing enzymes [37]. Mammalian Prx1 was previously identified as a heme-binding protein 23 kDa (HBP23) [10,38], and a bacterial 2-Cys peroxiredoxin alkyl hydroperoxide reductase C (AhpC) was also reported to be able to bind heme [39], although heme-binding is non-essential to their functions. Our data indicated that TnPrx1-CAT activity was not heme-related, but rather was dependent on mononuclear iron. However, the exact iron-binding site remains to be determined. Sequence analysis indicates that Prx1 proteins from T. nigroviridis and mammals contain a 2-His-1-carboxylate facial triad-like motif (e.g. motif 81HX2HX36E121 in TnPrx1) that is conserved in mononuclear non-heme iron enzymes [40]. Additionally, a Trp87 residue is also present at the motif. Aromatic residues, particularly Trp and Tyr, are known to be enriched at the Fe-sites of iron-proteins [41]. The involvement of aromatic residues in redox catalysis and/or electron transfer is not yet fully understood, but their capability to mediate electron transfer reactions makes them most suitable for tunneling electrons to/from redox sites [41]. On the other hand, the putative facial triad is not in the immediate proximity of the GVL motif. Therefore, its involvement in iron-binding and the mechanism of iron-mediated electron transfer for the Prx1-CAT activity need to be verified by further structure-based analysis.
Previous studies showed the existence of non-heme catalases in lactic acid bacteria, such as Pedicoccus cerevisiae and Lactobacillus plantarum, which are dependent on non-heme manganese [42,43]. A recent study has reported that a non-heme iron(III) CAT mimic, the complexes of 14-membered tetraaza macrocycles, can catalytically decompose H2O2 to H2O and O2, similarly to the native CAT enzyme [44]. Moreover, other non-heme iron binding proteins, such as phenylalanine hydroxylase, isopenicillin N synthase and anthocyanidin synthase, were identified from Rattus norvegicus, Aspergillus nidulans, and Streptomyces clavuligerus, respectively [40]. However, a native non-heme iron catalase has not been reported yet in any vertebrates and other organisms. To the best of our knowledge, the present study is the first to report a native non-heme iron catalase-like enzyme in vertebrate species, adding a new member to the CAT superfamily.
The sequence identity and similarity of vertebrate Prx1 are over 77% and 88% (Table 3) respectively, and the active site of CAT activity is completely conserved among Prx1 proteins, suggesting that CAT activity may be a ubiquitous function of Prx1 family members. The confirmation of reductant-independent HsPrx1-CAT activity indicates that this new function is probably conserved, at least in some vertebrates. Furthermore, 117GVL119 are conserved in Prx1–3, while 117GVY119 are conserved in Prx4. Although the Prx5 and Prx6 share low similarity with Prx1–4, they have similar 3D structures [45], suggesting that CAT activity might be present in other Prxs, at least Prx1–3. It might also explain why some parasites and cyanobacteria do not contain CAT and GPx, but have diverse Prx homologies [32,46].
Table 3. Percentage amino acid identity and similarity of vertebrate Prx1*.
| Rat | Mouse | Bovine | Platypus | Chicken | Zebrafinch | Lizard | Frog | Zebrafish | Catfish | Tetraodon | Fugu | Rainbow trout | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Human | 97/98 | 95/98 | 96/98 | 92/98 | 88/94 | 87/94 | 86/94 | 84/94 | 81/92 | 84/93 | 79/89 | 80/90 | 82/92 |
| Rat | 96/100 | 96/97 | 91/97 | 88/93 | 87/93 | 86/93 | 85/92 | 81/91 | 84/92 | 79/88 | 79/89 | 82/91 | |
| Mouse | 95/97 | 90/97 | 87/93 | 87/93 | 85/94 | 82/93 | 79/92 | 82/92 | 77/88 | 77/89 | 79/91 | ||
| Bovine | 90/97 | 87/93 | 88/93 | 85/93 | 84/93 | 80/91 | 83/92 | 79/89 | 79/90 | 82/92 | |||
| Platypus | 87/95 | 87/95 | 89/95 | 83/94 | 80/92 | 82/94 | 80/90 | 80/91 | 81/93 | ||||
| Chicken | 97/99 | 90/97 | 83/93 | 83/92 | 89/96 | 85/92 | 85/93 | 85/93 | |||||
| Zebrafinch | 89/97 | 83/92 | 82/93 | 88/96 | 84/92 | 84/93 | 84/93 | ||||||
| Lizard | 82/92 | 81/93 | 82/95 | 83/91 | 83/92 | 80/92 | |||||||
| Frog | 77/91 | 80/93 | 77/90 | 77/90 | 77/90 | ||||||||
| Zebrafish | 90/95 | 83/91 | 84/92 | 87/92 | |||||||||
| Catfish | 87/93 | 88/94 | 89/94 | ||||||||||
| Tetraodon | 98/99 | 88/94 | |||||||||||
| Fugu | 88/95 |
Data shown as identity/similarity (%).
The intracellular concentrations of H2O2 and other ROS molecules in vivo are not precisely known, but may range from sub- to lower micromolar levels in various prokaryotic and eukaryotic cells. However, intracellular H2O2 levels may rise to the order of 100 µM in phagocytes, and the transient H2O2 levels may reach >200 µM in brain cells [47,48]. Moreover, appropriately stimulated polymorphonuclear leukocytes (PMN) and monocytes can produce up to 1.5 nmol of H2O2 in 104 cells per hour (which is roughly equivalent to >350–450 mM of H2O2) if it is not removed and accumulated per hour, given their cell sizes at ∼330 and 420 fL [49,50]. In the present study, we have shown that Prx1 acts mainly (if not only) as POX under low-level H2O2 environment with high affinity and relatively low capacity (Km and kcat at ∼2.23–4.15 µM and ∼0.23 s−1), but as both POX and CAT when H2O2 level reaches ∼50 µM or higher, where the latter behaves as an allosteric enzyme with activity 10-times higher than the former (Km and kcat at ∼210 µM and 2.3 s−1).
In vitro transfection experiments also confirmed the functional difference between these two activities, since HEK-293T cells overexpressing WT TnPrx1 and mutant retaining CAT activity were capable of scavenging more iROS than those overexpressing mutants lacking CAT or both POX and CAT activities at low to middle micromolar H2O2 levels (Figures 7c and 9c). The levels of exogenous H2O2 necessary to produce significant effects on cellular activities, such as on the phosphorylation of p38 in cells transfected with various TnPrx1 mutants, were ∼375–1050 µM or higher (Figure 8), which corresponded to ∼50–150 µM intracellular H2O2 based on the model that predicted that intracellular H2O2 concentrations were ∼seven-fold or even 10–100-folds lower than that applied exogenously [48,51,52]. The corresponding intracellular levels of H2O2 fell within the levels for physiologically relevant signaling (i.e. 15–150 µM) [52].
Collectively, the existence of a two-fold difference in Km between Prx1-POX and Prx1-CAT activities enables Prx1 to function on a wider range of ROS concentrations than many other proteins in the cytosol, in which Prx1-POX acts on sub- to lower micromolar iROS normally present in cells, whereas Prx1-CAT (probably along with GPx) and classic CAT enzymes acts on moderate to higher micromolar iROS concentrations that are present in certain types of cells (e.g. some brain and immune cells and/or required for H2O2 signaling) (Table 1). The increased oxidative stress in cells with moderate to higher micromolar iROS levels might cause Prx1 to undergo a functional switch from Prx1-POX to Prx1-CAT by hyperoxidation and/or phosphorylation [13–17]. The exact functional connection between Prx1-POX and Prx1-CAT activities and the molecular mechanism underlying the conversion of the two Km values remains to be clarified. The development of a more sensitive and accurate method for local iROS detection is crucial to this clarification.
However, it is noticeable that although TnPrx1 and HsPrx display CAT-like activity, their catalytic efficiencies are ∼100-fold smaller than those of regular CATs (i.e. kcat/KmPrx1-CAT at ∼104 M−1 s−1 vs. kcat/KmCAT at ∼106 M−1 s−1), which raises the question of whether Prx1-CAT function is critical to organisms, since a higher level of iROS may be quickly scavenged by regular CAT. Prx1 is a cytosol protein, whereas native CATs are typically present in peroxisomes. Data-mining the Multi-Omics Profiling Expression Database (MOPED) (https://www.proteinspire.org/) also reveals that human Prx1 is much more abundant than CAT in most cells/tissues (Supplementary Figure S2a). Therefore, we speculate that the CAT-like activity in Prx1 and possibly in other Prxs may act as one of the first line of scavengers for cytosolic ROS. Prx-CAT may also play more critical role in scavenging and/or regulating ROS in certain cells and tissues that are deficient, or contain extremely low levels of CAT. For example, in human bone, oral epithelium and retina, the CAT protein levels are 132-, 45- and 36-fold less than Prx1 (i.e. 13 vs. 1730, 55 vs. 2490, and 110 vs. 4020 ppm, respectively). Some cancer cells might also take advantage of the Prx1-CAT activity, since the expressions of CAT were deficient or highly down-regulated in many cancer cells [53], whereas those of Prx1 were up-regulated in cancer cells including breast, lung and urinary cancers and hepatocellular carcinoma [54]. The down- and up-regulation of CAT and Prx1 was also clearly supported by comparing the MOPED protein expression profiles between cancer and non-cancer cells (Supplementary Figure S2b). Additionally, we also confirmed by qRT-PCR that the mRNA level of CAT in HEK-293T cells was ∼50–200-fold less than that of Prx1 (Figure 7a).
The Prx-CAT function might also explain how some invertebrates lacking CAT and GPx regulate high levels of intracellular ROS. For example, some parasitic helminths (e.g. Fasciola hepatica and Schistosoma mansoni) and roundworms (e.g. filarial parasites), as well as some protozoa (e.g. Plasmodium sp.) are deficient in CAT and GPx, but possess highly expressed Prx genes [32,46].
In summary, we observed a CAT-like activity in the pufferfish and human Prx1 proteins that were independent of Cys residue and reductants, but dependent on non-heme mononuclear iron. TnPrx1-CAT activity was capable of regulating intracellular ROS and the ROS-dependent phosphorylation of p38 in transfected HEK-293T cells. These newly discovered features extended our knowledge of Prx1 and provided a new opportunity to further dissect its biological roles.
Abbreviations
- 3-AT
3-amino-1,2,4-triazole
- CAT
Catalase
- DCFH-DA
2′7′-dichlorodihydrofluorescein diacetate
- DMEM
Dulbecco's modified Eagle's medium
- DP
2,2′-dipyridyl
- DTT
Dithiothreitol
- ECL
Enhanced chemiluminescence
- GPx
Glutathione peroxidase
- Grx
Glutaredoxin
- HBP23
Heme-binding protein 23 kDa
- HsPrx
Homo sapiens (human) peroxiredoxin
- ICP-OES
Inductively coupled plasma optical emission spectroscopy
- iROS
Intracellular reactive oxygen species
- MAPK
mitogen-activated protein kinase
- MOPED
Multi-Omics Profiling Expression Database
- ORF
Open reading frame
- p38
p38 mitogen-activated protein kinase (MAPK)
- PMN
Polymorphonuclear leukocytes
- POX
Thioredoxin peroxidase
- p-p38
Phosphorylated p38 MAPK
- Prx
Peroxiredoxin
- Prx1-CAT
Catalase-like activity of peroxiredoxin 1
- Prx1-POX
Thioredoxin peroxidase activity of peroxiredoxin 1
- qRT-PCR
Quantitative reverse transcription-polymerase chain reaction
- ROS
Reactive oxygen species
- SD
Standard deviation of the mean
- SOD
Superoxide dismutase
- Srx
Sulfiredoxin
- Tiron
4,5-dihydroxy-1,3-benzene disulfonic acid
- TnPrx
Tetraodon nigroviridis (green spotted puffer fish) peroxiredoxin
- Trx
Thioredoxin
- TrxR
Thioredoxin reductase
- WT
Wild-type
Author contribution
J.Z.S., G.Z., C.C.S., W.R.D. contributed to the experimental design. C.C.S., W.R.D., S.T. and L.J.Y. performed most of the experiments and data analysis. J.Z. constructed a cysteine mutant TnPrx1 (POX−CAT+) and performed protein expression. L.N. cloned the Prx1 gene of Tetraodon nigroviridis and Homo sapiens. C.C.S., W.R.D., S.T., G.Z., J.Z.S. participated in manuscript preparation. L.X.X., G.Z., J.Z.S. reviewed and edited the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China [31630083, 31472298, 31372554, 31572641, 31272691]; Stem Cell and Translational Research, the National Key Research and Development Program of China [2016YFA0101001]; National Basic Research Program of China (973) [2012CB114404, 2012CB114402], Hi-Tech Research and Development Program of China (863) [2012AA092202]; Zhejiang Major Special Program of Breeding [2016C02055-4]; Recruitment Program of Global Experts, Zhejiang Province (2013).
Competing Interests
The Authors declare that there are no competing interests associated with the manuscript.
References
- 1.Rhee S. G. (2016) Overview on peroxiredoxin. Mol. Cells 39, 1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Netto L. E. and Antunes F. (2016) The roles of peroxiredoxin and thioredoxin in hydrogen peroxide sensing and in signal transduction. Mol. Cells 39, 65–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perkins A., Nelson K. J., Parsonage D., Poole L. B. and Karplus P. A. (2015) Peroxiredoxins: guardians against oxidative stress and modulators of peroxide signaling. Trends Biochem. Sci. 40, 435–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rhee S. G., Kang S. W., Jeong W., Chang T.-S., Yang K.-S. and Woo H. A. (2005) Intracellular messenger function of hydrogen peroxide and its regulation by peroxiredoxins. Curr. Opin. Cell Biol. 17, 183–189 [DOI] [PubMed] [Google Scholar]
- 5.Rhee S. G., Woo H. A., Kil I. S. and Bae S. H. (2012) Peroxiredoxin functions as a peroxidase and a regulator and sensor of local peroxides . J. Biol. Chem. 287, 4403–4410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown J. D., Day A. M., Taylor S. R., Tomalin L. E., Morgan B. A. and Veal E. A. (2013) A peroxiredoxin promotes H2O2 signaling and oxidative stress resistance by oxidizing a thioredoxin family protein. Cell Rep. 5, 1425–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chae H. Z., Kim H. J., Kang S. W. and Rhee S. G. (1999) Characterization of three isoforms of mammalian peroxiredoxin that reduce peroxides in the presence of thioredoxin. Diabetes Res. Clin. Pract. 45, 101–112 [DOI] [PubMed] [Google Scholar]
- 8.Seo M. S., Kang S. W., Kim K., Baines I. C., Lee T. H. and Rhee S. G. (2000) Identification of a new type of mammalian peroxiredoxin that forms an intramolecular disulfide as a reaction intermediate . J. Biol. Chem. 275, 20346–20354 [DOI] [PubMed] [Google Scholar]
- 9.Wang X., Phelan S. A., Petros C., Taylor E. F., Ledinski G., Jürgens G. et al. (2004) Peroxiredoxin 6 deficiency and atherosclerosis susceptibility in mice: significance of genetic background for assessing atherosclerosis. Atherosclerosis 177, 61–70 [DOI] [PubMed] [Google Scholar]
- 10.Wood Z. A., Schröder E., Robin Harris J. and Poole L. B. (2003) Structure, mechanism and regulation of peroxiredoxins . Trends Biochem. Sci. 28, 32–40 [DOI] [PubMed] [Google Scholar]
- 11.Chae H. Z., Chung S. J. and Rhee S. G. (1994) Thioredoxin-dependent peroxide reductase from yeast. J. Biol. Chem. 269, 27670–27678 [PubMed] [Google Scholar]
- 12.Chae H. Z., Uhm T. B. and Rhee S. G. (1994) Dimerization of thiol-specific antioxidant and the essential role of cysteine 47. Proc. Natl. Acad. Sci. U.S.A. 91, 7022–7026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park J. W., Mieyal J. J., Rhee S. G. and Chock P. B. (2009) Deglutathionylation of 2-Cys peroxiredoxin is specifically catalyzed by sulfiredoxin . J. Biol. Chem. 284, 23364–23374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chae H. Z., Oubrahim H., Park J. W., Rhee S. G. and Chock P. B. (2012) Protein glutathionylation in the regulation of peroxiredoxins: a family of thiol-specific peroxidases that function as antioxidants, molecular chaperones, and signal modulators. Antioxid. Redox Signal. 16, 506–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jang H. H., Lee K. O., Chi Y. H., Jung B. G., Park S. K., Park J. H. et al. (2004) Two enzymes in one: two yeast peroxiredoxins display oxidative stress-dependent switching from a peroxidase to a molecular chaperone function. Cell 117, 625–635 [DOI] [PubMed] [Google Scholar]
- 16.Biteau B., Labarre J. and Toledano M. B. (2003) ATP-dependent reduction of cysteine-sulphinic acid by S. cerevisiae sulphiredoxin. Nature 425, 980–984 [DOI] [PubMed] [Google Scholar]
- 17.Chang T.-S., Jeong W., Woo H. A., Lee S. M., Park S. and Rhee S. G. (2004) Characterization of mammalian sulfiredoxin and its reactivation of hyperoxidized peroxiredoxin through reduction of cysteine sulfinic acid in the active site to cysteine . J. Biol. Chem. 279, 50994–51001 [DOI] [PubMed] [Google Scholar]
- 18.Neumann C. A., Cao J. and Manevich Y. (2009) Peroxiredoxin 1 and its role in cell signaling. Cell Cycle 8, 4072–4078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong W.-R., Sun C.-C., Zhu G., Hu S.-H., Xiang L.-X. and Shao J.-Z. (2014) New function for Escherichia coli xanthosine phophorylase (xapA): genetic and biochemical evidences on its participation in NAD+ salvage from nicotinamide. BMC Microbiol. 14, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo F. and Zhu G. (2012) Presence and removal of a contaminating NADH oxidation activity in recombinant maltose-binding protein fusion proteins expressed in Escherichia coli. BioTechniques 52, 247–253 [DOI] [PubMed] [Google Scholar]
- 21.Parejo I., Petrakis C. and Kefalas P. (2000) A transition metal enhanced luminol chemiluminescence in the presence of a chelator. J. Pharmacol. Toxicol. Methods 43, 183–190 [DOI] [PubMed] [Google Scholar]
- 22.Kniemeyer O. and Heider J. (2001) Ethylbenzene dehydrogenase, a novel hydrocarbon-oxidizing molybdenum/iron-sulfur/heme enzyme. J. Biol. Chem. 276, 21381–21386 [DOI] [PubMed] [Google Scholar]
- 23.Park J., Lee S., Lee S. and Kang S.W. (2014) 2-cys peroxiredoxins: emerging hubs determining redox dependency of Mammalian signaling networks. Int. J. Cell Biol. 2014, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aggeli I.-K.S., Gaitanaki C. and Beis I. (2006) Involvement of JNKs and p38-MAPK/MSK1 pathways in H2O2-induced upregulation of heme oxygenase-1 mRNA in H9c2 cells. Cell. Signal. 18, 1801–1812 [DOI] [PubMed] [Google Scholar]
- 25.Mittler R., Vanderauwera S., Gollery M. and Van Breusegem F. (2004) Reactive oxygen gene network of plants. Trends Plant Sci. 9, 490–498 [DOI] [PubMed] [Google Scholar]
- 26.Panfili E., Sandri G. and Ernster L. (1991) Distribution of glutathione peroxidases and glutathione reductase in rat brain mitochondria. FEBS Lett. 290, 35–37 [DOI] [PubMed] [Google Scholar]
- 27.Bendayan M. and Reddy J. K. (1982) Immunocytochemical localization of catalase and heat-labile enoyl-CoA hydratase in the livers of normal and peroxisome proliferator-treated rats. Lab Invest. 47, 364–369 [PubMed] [Google Scholar]
- 28.Benfeitas R., Selvaggio G., Antunes F., Coelho P.M. and Salvador A. (2014) Hydrogen peroxide metabolism and sensing in human erythrocytes: A validated kinetic model and reappraisal of the role of peroxiredoxin II. Free Radic. Biol. Med. 74, 35–49 [DOI] [PubMed] [Google Scholar]
- 29.Matte A., Low P.S., Turrini F., Bertoldi M., Campanella M.E., Spano D. et al. (2010) Peroxiredoxin-2 expression is increased in β-thalassemic mouse red cells but is displaced from the membrane as a marker of oxidative stress. Free Radic. Biol. Med. 49, 457–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee T.-H., Kim S.-U., Yu S.-L., Kim S. H., Park D. S., Moon H.-B. et al. (2003) Peroxiredoxin II is essential for sustaining life span of erythrocytes in mice. Blood 101, 5033–5038 [DOI] [PubMed] [Google Scholar]
- 31.Han Y.-H., Kwon T.-H., Kim S.-U., Ha H.-L., Lee T.-H., Kim J.-M. et al. (2012) Peroxiredoxin I deficiency attenuates phagocytic capacity of macrophage in clearance of the red blood cells damaged by oxidative stress. BMB Rep. 45, 560–564 [DOI] [PubMed] [Google Scholar]
- 32.Henkle-Dührsen K. and Kampkötter A. (2001) Antioxidant enzyme families in parasitic nematodes . Mol. Biochem. Parasitol. 114, 129–142 [DOI] [PubMed] [Google Scholar]
- 33.Knoops B., Loumaye E. and Van Der Eecken V. (2007) Evolution of the peroxiredoxins. Subcell. Biochem. 44, 27–40 [DOI] [PubMed] [Google Scholar]
- 34.Choi H.-J., Kang S. W., Yang C.-H., Rhee S. G. and Ryu S.-E. (1998) Crystal structure of a novel human peroxidase enzyme at 2.0 Å resolution. Nat. Struct. Biol. 5, 400–406 [DOI] [PubMed] [Google Scholar]
- 35.Hall A., Nelson K., Poole L. B. and Karplus P. A. (2011) Structure-based insights into the catalytic power and conformational dexterity of peroxiredoxins. Antioxid. Redox Signal. 15, 795–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baek J. Y., Han S. H., Sung S. H., Lee H. E., Kim Y.-M., Noh Y. H. et al. (2012) Sulfiredoxin protein is critical for redox balance and survival of cells exposed to low steady-state levels of H2O2. J. Biol. Chem. 287, 81–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alfonso-Prieto M., Vidossich P. and Rovira C. (2012) The reaction mechanisms of heme catalases: an atomistic view by ab initio molecular dynamics. Arch. Biochem. Biophys. 525, 121–130 [DOI] [PubMed] [Google Scholar]
- 38.Hirotsu S., Abe Y., Okada K., Nagahara N., Hori H., Nishino T. et al. (1999) Crystal structure of a multifunctional 2-Cys peroxiredoxin heme-binding protein 23 kDa/proliferation-associated gene product. Proc. Natl. Acad. Sci. U.S.A. 96, 12333–12338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lechardeur D., Fernandez A., Robert B., Gaudu P., Trieu-Cuot P., Lamberet G. et al. (2010) The 2-Cys peroxiredoxin alkyl hydroperoxide reductase c binds heme and participates in its intracellular availability in Streptococcus agalactiae . J. Biol. Chem. 285, 16032–16041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koehntop K. D., Emerson J. P. and Que L. Jr (2005) The 2-His-1-carboxylate facial triad: a versatile platform for dioxygen activation by mononuclear non-heme iron(II) enzymes. J. Biol. Inorg. Chem. 10, 87–93 [DOI] [PubMed] [Google Scholar]
- 41.Andreini C., Bertini I., Cavallaro G., Najmanovich R. J. and Thornton J. M. (2009) Structural analysis of metal sites in proteins: non-heme iron sites as a case study. J. Mol. Biol. 388, 356–380 [DOI] [PubMed] [Google Scholar]
- 42.Delwiche E. A. (1961) Catalase of pedicoccus cerevisiae . J. Bacteriol. 81, 416–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnston M. A. and Delwiche E. A. (1965) Isolation and characterization of the cyanide-resistant and azide-resistant catalase of lactobacillus plantarum . J. Bacteriol. 90, 352–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Whittaker J. W. (2012) Non-heme manganese catalase – the ‘other’ catalase. Arch. Biochem. Biophys. 525, 111–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karplus P. A. and Hall A. (2007) Structural survey of the peroxiredoxins. Subcell. Biochem. 44, 41–60 [DOI] [PubMed] [Google Scholar]
- 46.Robinson M. W., Hutchinson A. T., Dalton J. P. and Donnelly S. (2010) Peroxiredoxin: a central player in immune modulation. Parasite Immunol. 32, 305–313 [DOI] [PubMed] [Google Scholar]
- 47.Qutub A. A. and Popel A. S. (2008) Reactive oxygen species regulate hypoxia-inducible factor 1α differentially in cancer and ischemia. Mol. Cell. Biol. 28, 5106–5119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Avshalumov M. V., Bao L., Patel J. C. and Rice M. E. (2007) H2O2 signaling in the nigrostriatal dopamine pathway via ATP-sensitive potassium channels: issues and answers. Antioxid. Redox Signal. 9, 219–231 [DOI] [PubMed] [Google Scholar]
- 49.Szatrowski T. P. and Nathan C. F. (1991) Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 51, 794–798 [PubMed] [Google Scholar]
- 50.Nibbering P. H., Zomerdijk T. P., Corsèl-Van Tilburg A. J. and Van Furth R. (1990) Mean cell volume of human blood leucocytes and resident and activated murine macrophages. J. Immunol. Methods. 129, 143–145 [DOI] [PubMed] [Google Scholar]
- 51.Antunes F. and Cadenas E. (2000) Estimation of H2O2 gradients across biomembranes. FEBS Lett. 475, 121–126 [DOI] [PubMed] [Google Scholar]
- 52.Rice M. E. (2011) H2O2: a dynamic neuromodulator. Neuroscientist 17, 389–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang D., Li F., Chi Y. and Xiang J. (2012) Potential relationship among three antioxidant enzymes in eliminating hydrogen peroxide in penaeid shrimp. Cell Stress Chaperones 17, 423–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cai C.-Y., Zhai L.-L., Wu Y. and Tang Z.-G. (2015) Expression and clinical value of peroxiredoxin-1 in patients with pancreatic cancer. Eur. J. Surg. Oncol. 41, 228–235 [DOI] [PubMed] [Google Scholar]
- 55.Matsumoto M., Sudo T., Saito T., Osada H. and Tsujimoto M. (2000) Involvement of p38 mitogen-activated protein kinase signaling pathway in osteoclastogenesis mediated by receptor activator of NF-κB ligand (RANKL). J. Biol. Chem. 275, 31155–31161 [DOI] [PubMed] [Google Scholar]
- 56.Reyes-Moreno C., Girouard J., Lapointe R., Darveau A. and Mourad W. (2004) CD40/CD40 homodimers are required for CD40-induced phosphatidylinositol 3-kinase-dependent expression of B7.2 by human B lymphocytes. J. Biol. Chem. 279, 7799–7806 [DOI] [PubMed] [Google Scholar]
- 57.Madrid L. V., Mayo M. W., Reuther J. Y. and Baldwin A. S. Jr (2001) Akt stimulates the transactivation potential of the relA/p65 subunit of NF-κB through utilization of the IκB kinase and activation of the mitogen-activated protein kinase p38. J. Biol. Chem. 276, 18934–18940 [DOI] [PubMed] [Google Scholar]