Abstract
Background
Vibrio campbellii is widely distributed in the marine environment and is an important pathogen of aquatic organisms such as shrimp, fish, and mollusks. An isolate of V. campbellii carrying the pirAB vp gene, causing acute hepatopancreatic necrosis disease (AHPND), has been reported. There are no previous reports about the complete genome of V. campbellii causing AHPND (VCAHPND). To extend our understanding of the pathogenesis of VCAHPND at the genomic level, the genome of V. campbellii 20130629003S01 isolated from a shrimp with AHPND was sequenced and analysed.
Results
The complete genome sequence of V. campbellii 20130629003S01 was generated using the PacBio RSII platform with single molecule, real-time sequencing. The 20130629003S01 strain consists of two circular chromosomes (3,621,712 bp in chromosome 1 and 2,245,751 bp in chromosome 2) and four plasmids of 70,066, 204,531, 143,140, and 86,121 bp. The genome contains a total of 5855 protein coding genes, 134 tRNA genes and 37 rRNA genes. The average nucleotide identity value of 20130629003S01 and other reference V. campbellii strains was 97.46%, suggesting that they are closely related.
Conclusions
The genome sequence of V. campbellii 20130629003S01 and its comparative analysis with other V. campbellii strains that we present here are important for a better understanding of the genomic characteristics of VCAHPND.
Electronic supplementary material
The online version of this article (doi:10.1186/s13099-017-0180-2) contains supplementary material, which is available to authorized users.
Background
Vibrio campbellii is widely distributed in the marine environment and is an important pathogen of wild and reared marine organisms such as shrimp, fish, and mollusks [1]. In recent years, an isolate of V. campbellii carrying the pirAB vp gene that causes acute hepatopancreatic necrosis disease (AHPND) has been reported [2]. Shrimp production in AHPND-affected regions (SE Asia and Mexico) has dropped sharply, which is causing heavy economic losses. Initially, V. parahaemolyticus, which becomes virulent by acquiring a unique extrachromosomal AHPND-associated plasmid carrying pirAB vp (VPAHPND), was the only pathogen known to cause AHPND. Later, non-V. parahaemolyticus AHPND-causing Vibrio started emerging, and V. harveyi-like, V. owensii and V. campbellii strains have been reported [2–4].
Recent studies have shown that VPAHPND possesses not only toxin genes but also a ~70 kb plasmid, which expresses Pirvp [5]. However, there are no reports about the complete genome of V. campbellii that causes AHPND (VCAHPND). In this paper, we obtained the complete genome sequence of one strain of V. campbellii, which was isolated in June 2013 from the hepatopancreas of diseased Litopenaeus vannamei in Guangxi, China. PCR amplifications were performed using VpPirA and VpPirB primers specific to the pirAB vp genes (pirA vp and pirB vp), and this strain was evaluated for its pathogenicity in L. vannamei [2]. The shrimp showed typical symptoms of AHPND, and cumulative mortalities reached 100% [2]. The genome sequencing of 20130629003S01 provides timely information for a better understanding of the genomic characteristics of the pathogen.
Methods
Genomic DNA isolation, sequencing and assembly
Strain 20130629003S01 is V. campbellii isolated in June 2013 from AHPND-affected L. vannamei in Guangxi, China. The genomic DNA of this strain was extracted using the Wizard Genomic DNA Purification Kit (Promega, Madison, WI, USA). The DNA was examined by 1% agarose gel electrophoresis and quantified using a NanoDrop 2000 spectrophotometer (Thermo Scientific, MA, USA). The genomic DNA was sequenced using the PacBio RSII platform by Majorbio Bio-Pharm Biotechnology Co., Ltd., Shanghai, China. A 10-kb DNA library was constructed according to the manufacturer’s protocols and sequenced using single-molecule real-time (SMRT) sequencing technology with P6-C4 chemistry. One SMART cell was used for sequencing, and the data were assembled de novo using the hierarchical genome assembly process (HGAP) [6]. The assembly was based on 1.02 Gb of PacBio data and polished with three successive passes through Quiver to reach a final consensus accuracy at 194× coverage. This assembly consisted of six contigs including two chromosomes and four plasmids. The repeat sequences at the end of the six contigs were removed to obtain the complete genome and plasmid sequences.
Genome annotation
Gene prediction was carried out using Glimmer [7], while rRNA and tRNA were analysed using RNAmmer [8] and tRNAscan-SE version 1.21 [9]. Gene annotation was carried out based on homology searches against the gene ontology (GO) database and clusters of orthologous groups (COG) protein database. Prophage regions were identified using the PHAge Search Tool (PHAST) [10]. Virulence genes were searched for using the virulence factor of pathogenic bacteria database (VFDB) [11] and BLAST.
Comparative genome analysis
The complete reference genome sequences of V. campbellii strains were downloaded from NCBI and used for comparative genome analysis. The accession numbers of the reference V. campbellii strains were DS40M4 (AGIE01000001–AGIE01000121), CAIM_519 (AMDG01000001–AMDG01000213), BAA-1116 (CP006605–CP006607), UMTGB204 (JSFE01000001–JSFE01000060), HY01 (DS179406–DS179608), LMB29 (CP019293–CP019298) and 051011E (BBKU01000001–BBKU01000219). The JSpecies program was used for calculating the average nucleotide identity (ANI) value [12] among the 8 strains, which were cut into fragments of 1020 bp for calculating the ANI values by using the BLAST algorithm [13]. Next, a distance dendrogram was constructed using the R program.
Quality assurance
The genomic DNA used for sequencing was isolated from a pure culture of V. campbellii strain 20130629003S01. The 16S rRNA gene was amplified and sequenced, and BLAST was performed against the NCBI database.
Initial findings
Genome properties
The complete genome of V. campbellii strain 20130629003S01 includes two circular DNA chromosomes of 3,621,712 and 2,245,751 bp with GC content of 45.26–45.56%, and four plasmids of 70,066, 204,531, 143,140, and 86,121 bp with GC content of 39.56–45.90%. chromosome 1 consists of 3216 protein coding genes, 34 rRNA genes and 117 tRNA genes. Chromosome 2 consists of 2057 protein coding genes, 3 rRNA genes and 15 tRNA genes. Plasmid 1 consists of 86 protein coding genes. Plasmid 2 consists of 229 protein coding genes. Plasmid 3 consists of 145 protein coding genes and 2 tRNA genes. Plasmid 4 consists of 122 protein coding genes. Information about the features of the complete genomic sequence of V. campbellii strain 20130629003S01 is provided in Fig. 1. The genome of this strain contains four incomplete phage sequences on chromosome 1, one incomplete phage sequence on chromosome 2, and one intact phage sequence on chromosome 2.
Fig. 1.
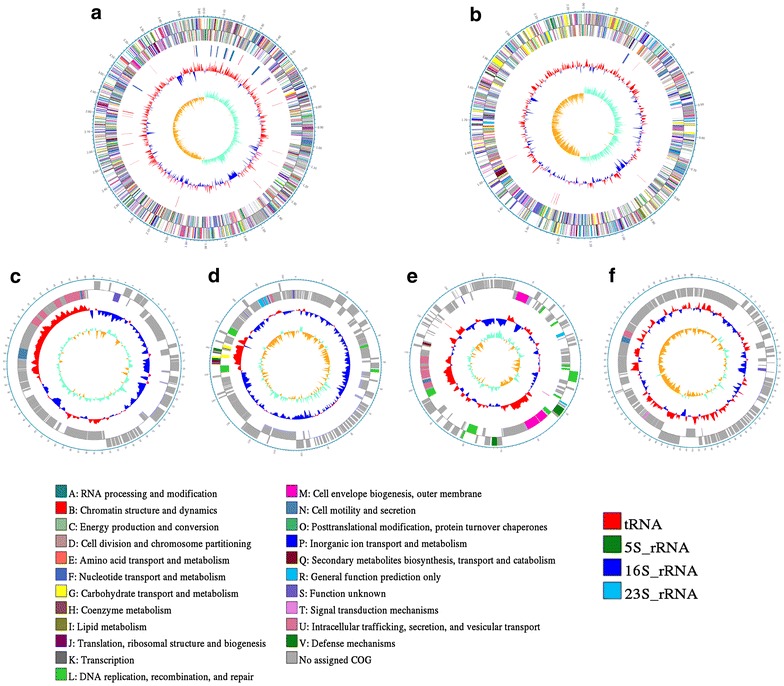
The circular genome maps of the strain 20130629003S01. a Chromosome 1. b Chromosome 2. c Plasmid 1. d Plasmid 2. e Plasmid 3. f Plasmid 4. The tracks from outside to inside represent the identity of the genome size, CDS (+), CDS (−), rRNA and tRNAs, GC contents, GC skews. Between these circles, all annotated ORFs were colored differently according to the COG assignments
Functional categorization
The results of COG categorization of the predicted open reading frames (ORFs) are shown in Additional file 1: Figure S1. The ORFs could be categorized into 22 classes, which include S (424 ORFs, function unknown), E (339, amino acid transport and metabolism), K (293 ORFs, transcription), R (273 ORFs, general function prediction only), T (262 ORFs, signal transduction mechanisms), G (256 ORFs, carbohydrate transport and metabolism), P (253 ORFs, inorganic ion transport and metabolism), C (236 ORFs, energy production and conversion), M (229 ORFs, cell wall/membrane/envelope biogenesis), J (190 ORFs, translation, ribosomal structure and biogenesis), O (171 ORFs, post-translational modification, protein turnover, chaperones), L (163 ORFs, replication, recombination and repair), H (131 ORFs, coenzyme transport and metabolism), U (128 ORFs, intracellular trafficking, secretion, and vesicular transport), N (122 ORFs, cell motility), I (101 ORFs, lipid transport and metabolism), F (90 ORFs, nucleotide transport and metabolism), Q (73 ORFs, secondary metabolites biosynthesis, transport and catabolism), V (68 ORFs, defence mechanisms), D (37 ORFs, cell cycle control, cell division, chromosome partitioning), A (1 ORF, RNA processing and modification), and B (1 ORF, chromatin structure and dynamics).
Pathogenesis and virulence factors
Key virulence factors in AHPND-causing V. campbellii are pirAB vp (pirA vp and pirB vp) [2, 5]. The strain 20130629003S01 harbours four plasmids, pVCGX1, pVCGX2, pVCGX3, and pVCGX4. Interestingly, pVCGX1 has a pirA vp gene and pirB vp gene related to AHPND, and both pirA vp and pirB vp of pVCGX1 share 100% sequence identities with their orthologues in the plasmids pVA1 and pVPA3-1 of Vibrio parahaemolyticus [5, 14]. Therefore, pVCGX1 may contribute to pathogenesis.
Comparative genome analysis
Seven complete reference genome sequences of V. campbellii and their annotations were collected from the GenBank database. The ANI values were calculated using 8 strains, and all values between every two strains were greater than 95%. Furthermore, the 20130629003S01 strain was found to cluster with the LMB29 strain (Fig. 2). The LMB29 strain (GenBank accession number: CP019293.1) was isolated from cage-cultured red drum with skin ulcers in China. Comparative data are shown as a dendrogram in Fig. 2 and tabulated in Additional file 1: Table S1.
Fig. 2.
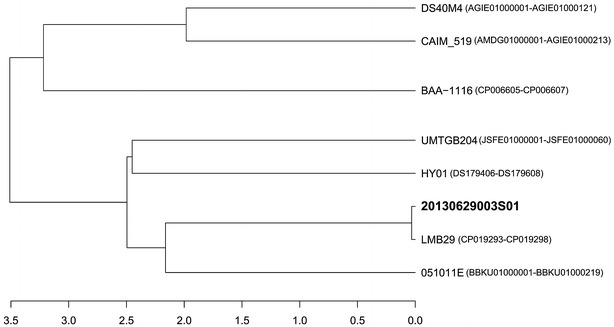
Distance dendrogram among Vibrio campbellii strains based on ANI values. The complete genome sequences of V. campbellii (DS40M4, CAIM_519, BAA_1116, UMTGB204, HY01, 20130629003S01, LMB29 and 051011E) revealed that the LMB29 strain has the closest evolutionary relationship (ANI value 98.53) with an isolate LMB29 from cage-cultured red drum with skin ulcer in China
Future directions
In conclusion, we report the 5.3 Mbp complete genome sequence of V. campbellii strain 20130629003S01. Additional comparative studies of the genomes of AHPND-causing Vibrio with the genome sequence of strain 20130629003S01 should provide genomic insights into the pathogenicity and virulence mechanisms of VCAHPND.
Author’s contributions
The experiments, data analysis and manuscript writing were performed by XD and JH. HLW isolated and preserved the bacterial strain 20130629003S01. PZZ and JYC performed experiments. ZL performed assembly and annotation sequencing data. XPW discussed the results and revised the manuscript. JH provided the sample for isolation of the strain 20130629003S01, vital guidance, technical support, and proofreading for the work. All authors read and approved the final manuscript.
Ackonwledgements
The authors thanks Mr. Daxiang Xie in Guangxi Aquaculture Research Institute and Mr. Debin Pang in Zhengyu Biotechnology Ltd. for their assistance during sampling of the strain 20130629003S01.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The genome sequence of Vibrio campbellii 20130629003S01 has been deposited in NCBI Genbank server under the accession number CP020076–CP020081 for chromosome 1, chromosome 2 and plasmids.
Funding
This work was supported by projects under Central Public-interest Scientific Institution Basal Research Fund, YSFRI, CAFS (20603022016012), the Project of the Aoshan Sci. & Tech. Innovation Program of Qingdao National Laboratory for Marine Science and Technology (2015ASKJ02), the China Agriculture Research System (CARS-47), and the Construction Programme for “Taishan Scholarship” of Shandong Province of China.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- L. vannamei
Litopenaeus vannamei
- ANI
average nucleotide identity
- rRNA
ribosomal RNA
- tRNA
transport RNA
- COG
cluster of orthologous groups
- GO
gene ontology
- BLAST
basic local alignment search tool
- PHAST
PHAge Search Tool
- VFDB
virulence factor of pathogenic bacteria database
- ORFs
open reading frames
Additional file
Additional file 1: Figure S1. Functional categorization of 20130629003S01 based on the COG database. Table S1. ANI values among Vibrio campbellii strains.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1186/s13099-017-0180-2) contains supplementary material, which is available to authorized users.
Contributor Information
Xuan Dong, Email: dongxuan311@163.com.
Hailiang Wang, Email: whl846130@126.com.
Peizhuo Zou, Email: zoupeizhuo@qq.com.
Jiayuan Chen, Email: 2532585117@qq.com.
Zhen Liu, Email: zhen.liu@majorbio.com.
Xuepeng Wang, Email: xpwang@sdau.edu.cn.
Jie Huang, Phone: +86-0532-85823062, Email: huangjie@ysfri.ac.cn.
References
- 1.Thompson FL, Iida T, Swings J. Biodiversity of vibrios. Microbiol Mol Biol Rev. 2004;68:403–431. doi: 10.1128/MMBR.68.3.403-431.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dong X, Wang H, Xie G, Zou P, Guo C, Liang Y, et al. An isolate of Vibrio campbellii carrying the pirVP gene causes acute hepatopancreatic necrosis disease. Emerg Microbes Infect. 2017;6:e2. doi: 10.1038/emi.2016.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kondo H, Van PT, Dang LT, Hirono I. Draft genome sequence of non-Vibrio parahaemolyticus acute hepatopancreatic necrosis disease strain KC13.17.5, isolated from diseased shrimp in Vietnam. Genome Announc. 2015;3:e00978-15. doi: 10.1128/genomeA.00978-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu L, Xiao J, Xia X, Pan Y, Yan S, Wang Y. Draft genome sequence of Vibrio owensii strain SH-14, which causes shrimp acute hepatopancreatic necrosis disease. Genome Announc. 2015;3:e01395-15. doi: 10.1128/genomeA.01395-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee CT, Chen IT, Yang YT, Ko TP, Huang YT, Huang JY, et al. The opportunistic marine pathogen Vibrio parahaemolyticus becomes virulent by acquiring a plasmid that expresses a deadly toxin. Proc Natl Acad Sci USA. 2015;112:10798–10803. doi: 10.1073/pnas.1503129112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chin CS, Alexander DH, Marks P, Klammer AA, Drake J, Heiner C, et al. Nonhybrid, finished microbial genome assemblies from long-read SMRT sequencing data. Nat Methods. 2013;10:563–569. doi: 10.1038/nmeth.2474. [DOI] [PubMed] [Google Scholar]
- 7.Delcher AL, Bratke KA, Powers EC, Salzberg SL. Identifying bacterial genes and endosymbiont DNA with Glimmer. Bioinformatics. 2007;23:673–679. doi: 10.1093/bioinformatics/btm009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lagesen K, Hallin P, Rodland EA, Staerfeldt HH, Rognes T, Ussery DW. RNAmmer: consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res. 2007;35:3100–3108. doi: 10.1093/nar/gkm160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lowe TM, Eddy SR. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997;25:955–964. doi: 10.1093/nar/25.5.0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou Y, Liang Y, Lynch KH, Dennis JJ, Wishart DS. PHAST: a fast phage search tool. Nucleic Acids Res. 2011;39:W347–W352. doi: 10.1093/nar/gkr485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen L, Xiong Z, Sun L, Yang J, Jin Q. VFDB 2012 update: toward the genetic diversity and molecular evolution of bacterial virulence factors. Nucleic Acids Res. 2012;40:D641–D645. doi: 10.1093/nar/gkr989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richter M, Rossello-Mora R. Shifting the genomic gold standard for the prokaryotic species definition. Proc Natl Acad Sci USA. 2009;106:19126–19131. doi: 10.1073/pnas.0906412106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goris J, Konstantinidis KT, Klappenbach JA, Coenye T, Vandamme P, Tiedje JM. DNA-DNA hybridization values and their relationship to whole-genome sequence similarities. Int J Syst Evol Microbiol. 2007;57:81–91. doi: 10.1099/ijs.0.64483-0. [DOI] [PubMed] [Google Scholar]
- 14.Han JE, Tang KF, Tran LH, Lightner DV. Photorhabdus insect-related (Pir) toxin-like genes in a plasmid of Vibrio parahaemolyticus, the causative agent of acute hepatopancreatic necrosis disease (AHPND) of shrimp. Dis Aquat Organ. 2015;113:33–40. doi: 10.3354/dao02830. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The genome sequence of Vibrio campbellii 20130629003S01 has been deposited in NCBI Genbank server under the accession number CP020076–CP020081 for chromosome 1, chromosome 2 and plasmids.


