Abstract
Hemophagocytic lymphohistiocytosis (HLH) is a hyperinflammatory syndrome characterized by unregulated macrophage and T-lymphocyte activation resulting in cytokine overproduction and subsequent histiocytic phagocytosis. Variant infections, particularly viruses have been postulated as the inciting factor for this potentially fatal disease. Herein, we will report a case of HLH associated with anaplasmosis.
Keywords: Anaplasmosis, hemophagocytic lymphohistiocytosis, hemophagocytic syndrome
INTRODUCTION
Human granulocytic anaplasmosis (HGA) is a tick-borne disease caused by Anaplasma phagocytophilum infection that is transmitted through Ixodes tick.[1,2,3] Initial symptoms typically present 1–2 weeks after being bitten by an infected tick. The clinical presentation is broad ranging from a mild flu-like illness characterized by fever/chills, headaches, myalgias, and malaise to serious and potentially fatal disease including disseminated intravascular coagulopathy if not detected and treated appropriately.[1,2,3] We propose that one of the more severe complications may be hemophagocytic lymphohistiocytosis (HLH). HLH is a hyperinflammatory syndrome characterized by unregulated macrophage and T-lymphocyte activation resulting in cytokine overproduction, hemophagocytosis, and tissue destruction.[4] HLH is associated with various malignancies, autoimmune diseases, and infections.[5,6] An association between macrophage activation and anaplasmosis has been reported by Dumier et al.[7] Herein, we will report a case of HLH associated with anaplasmosis.
CASE REPORT
A case of a 63-year-old male who presented to an area hospital with complaints of fever (104°F) and lethargy for 1 day. Three weeks before the presentation, he underwent a root canal surgery for a dental abscess. At that time, he received antibiotic prophylaxis. Patient also with a history of mechanical aortic valve replacement and aortic root graft in 1987 due to valvular insufficiency and ascending aortic aneurysm, respectively. On initial evaluation, he was found to have a normal white blood cell count with 39% bandemia and thrombocytopenia at 14 × 109/L. Blood and urine cultures were drawn before starting empiric antibiotics (vancomycin and ceftriaxone) for presumptive endocarditis. Within 24 h, the patient had become leukopenic (neutrophils count 2.73 × 109/L) and platelets had decreased further to 11 × 109/L. At this time, he was given filgrastim and platelet transfusion. Transthoracic echocardiogram was performed and noted an echodensity on the prosthetic aortic valve, and he was transferred to our institution for further diagnosis and treatment.
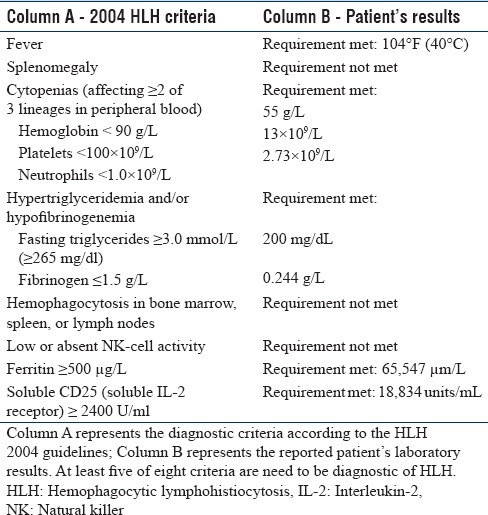
Upon arrival to our institution, the patient was noted to have persistent fever, a nondiagnostic physical examination with worsening oliguric renal failure, and thrombocytopenia. Other pertinent laboratory abnormalities were elevated ferritin (65,647 μg/L), triglycerides (200 mg/dL), lactate dehydrogenase (2067 unit/L), and soluble interleukin-2 receptor (18,834 U/mL) while the fibrinogen was decreased (0.244 g/L) [Table 1]. The hypothesis of HLH was considered, and our suspicion was further supported by the development of neurologic symptoms including dysarthria and clumsiness with fine motor skills. On further review, the patient reported tick attachment in August which was approximately 1 month before presentation. Doxycycline was, therefore, initiated pending serology for tick-borne illnesses.
Table 1.
Hemophagocytic Lymphohistiocytosis Diagnostic Criteria
Vancomycin and ceftriaxone were discontinued after negative blood cultures and transesophageal echocardiogram, whereas doxycycline was continued due to high suspicion of a tick-borne illness. Testing for Lyme disease, Rickettsiosis, and Babesiosis returned negative. However, immunoglobulin G and polymerase chain reaction for A. phagocytophilum were reactive, thus confirming the diagnosis of HGA. With doxycycline solely, the patient's symptomatology and before-mentioned laboratory abnormalities resolved within 1 week of therapy.
DISCUSSION
HGA is a tick-borne illness transmitted by Ixodes species infected with A. phagocytophilum, a small, obligate intracellular coccobacillus. Typically presenting as a nonspecific viral illness, its prodrome is associated with fever, malaise, myalgias, headache, and sometimes arthralgia.[1] Laboratory data supportive of anaplasmosis include thrombocytopenia, leukopenia, mild anemia, elevated transaminases, especially aspartate transaminase, and less commonly increasing serum creatinine.[1]
High suspicion of HGA is probable upon visualization of morulae on peripheral smear.[1] Morulae are clusters of A. phagocytophilum growing within cytoplasmic vacuoles of neutrophils. However, this is not the standard of care for the diagnosis, as the percentage of granulocytes containing morulae is often low.[2] Moreover, to make peripheral smear more sensitive for the diagnosis most efficacious, it is recommended that at least 200 granulocytes are examined.[2] Definitive diagnosis is based on polymerase chain reaction for A. phagocytophilum as serology testing is often negative during the 1st week of illness.[2,3] In our patient, no morulae were identified, and this is likely related to his severe neutropenia. Diagnosis was confirmed by positive polymerase chain reaction for A. phagocytophilum.
The associated clinical finding and laboratory abnormalities are usually self-limited and resolve with 7–14 days of doxycycline.[3] However, our patient experienced a more severe disease course as exemplified by the development of HLH, a potentially life-threatening syndrome triggered by increased cytokine release resulting in the unregulated activation of monocytes and macrophages.[4,5] This results in hemophagocytosis in the bone marrow and lymphoid tissues. Various terms have been used to describe HLH including primary HLH (also called familial HLH) which is seen in patients with gene mutation; secondary (or sporadic, acquired) HLH seen in patients without familial mutation and with a known trigger; and macrophage activation syndrome which is seen in patients with rheumatologic disorders. Recognized triggers of HLH consist of malignancy, autoimmune disease, and variant infections.[5,6] Of the infectious triggers, viruses in particular Epstein–Barr virus have been most commonly associated with HLH. Other infectious causes include viruses (such as H1N1 influenza virus, measles virus, HIV, human herpesviruses-8, herpes, and varicella zoster virus), parasites (especially leishmaniasis and malaria), bacteria (including Ehrlichia, tuberculosis, and brucella), and fungi.[6]
HLH is diagnosed based on fulfillment of five of the eight criteria.[4,5] These criteria include fever; splenomegaly; cytopenias affecting at least 2 lineages; elevated triglycerides and/or low fibrinogen; hemophagocytosis evident on examination of bone marrow, spleen, or lymph nodes; reduced to absent natural killer cell activity; elevated ferritin >500 μg/L; and a high soluble CD25 (interleukin-2 receptor).[4,5] The criterion fulfilled in our patient included fever, bicytopenia with leukopenia and thrombocytopenia, hypertriglyceridemia, elevated ferritin, and high soluble interleukin-2 receptor.
A. phagocytophilum as a cause of HLH was first reported by Dumier et al., who reported on 29 patients with corroborated HGA ranging mild to fatal disease.[7] Severely affected patients had fever, cytopenia, elevated Interferon gamma levels, hepatic injury, elevated ferritin level, and elevated triglycerides. Patients with fatal disease were found on autopsy to hemophagocytic macrophages in their spleen and other organs, consistent with current diagnostic criteria for macrophage activation and hemophagocytic syndromes.[7] The data presented by Dumier et al. provide evidence that infection with A. phagocytophilum may represent manifestations of macrophage activation and that the clinical severity could be dependent on the degree of macrophage activation.[7] Macrophage activation syndrome and HLH should be considered as an advanced complication of anaplasmosis, especially when diagnosis and treatment are delayed. Although treatment for HLH typically entails chemotherapy and immunosuppressive agents, our patient's symptomatology reverted with doxycycline only, thus additionally supporting our belief that HLH was incited by anaplasmosis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Dumler JS, Choi KS, Garcia-Garcia JC, Barat NS, Scorpio DG, Garyu JW, et al. Human granulocytic anaplasmosis and Anaplasma phagocytophilum. Emerg Infect Dis. 2005;11:1828–34. doi: 10.3201/eid1112.050898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rand JV, Tarasen AJ, Kumar J, Homan SM, Tobin E. Intracytoplasmic granulocytic morulae counts on confirmed cases of ehrlichiosis/anaplasmosis in the northeast. Am J Clin Pathol. 2014;141:683–6. doi: 10.1309/AJCP6Q2BOKYALDYZ. [DOI] [PubMed] [Google Scholar]
- 3.Bakken JS, Dumler JS. Human granulocytic anaplasmosis. Infect Dis Clin North Am. 2015;29:341–55. doi: 10.1016/j.idc.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henter JI, Horne A, Aricó M, Egeler RM, Filipovich AH, Imashuku S, et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48:124–31. doi: 10.1002/pbc.21039. [DOI] [PubMed] [Google Scholar]
- 5.Mehta RS, Smith RE. Hemophagocytic lymphohistiocytosis (HLH): A review of literature. Med Oncol. 2013;30:740. doi: 10.1007/s12032-013-0740-3. [DOI] [PubMed] [Google Scholar]
- 6.Rouphael NG, Talati NJ, Vaughan C, Cunningham K, Moreira R, Gould C. Infections associated with haemophagocytic syndrome. Lancet Infect Dis. 2007;7:814–22. doi: 10.1016/S1473-3099(07)70290-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dumler JS, Barat NC, Barat CE, Bakken JS. Human granulocytic anaplasmosis and macrophage activation. Clin Infect Dis. 2007;45:199–204. doi: 10.1086/518834. [DOI] [PubMed] [Google Scholar]


