Abstract
Aeromonas salmonicida is associated with superficial skin infections in fish. Its virulence factors allow colonization of water including surface water such as salt water, beaches, and fresh water wells. Moreover, it is possible for immunocompromised patients to develop invasive disease after chronic exposure to Aeromonas spp. through contaminated water. While there are reports of Aeromonas spp. bacteremia following water ingestion, there have been no reports of A. salmonicida bacteremia from water consumption. We report the first case of A. salmonicida bacteremia in a patient with diabetes due to chronic consumption of well water.
Keywords: Aeromonas salmonicida, bacteremia, water
INTRODUCTION
We present the first reported case of Aeromonas salmonicida bacteremia in humans following chronic well water consumption. Aeromonas spp. bacteremia is more common in patients with immunocompromised conditions defined as cancer, liver cirrhosis, diabetes mellitus, end-stage renal disease, and receiving immunosuppressive therapy. We describe how A. salmonicida causes bacteremia and why physicians should ask about water sources when interviewing immunocompromised patients.
CASE REPORT
A 62-year-old African-American male with diabetes (hemoglobin A1c 10.1% in 07/2016, +2.3% from 10/2015) with sarcoidosis and stage 3b chronic kidney disease (baseline creatinine 1.8–2.0 mg/dL) was admitted with right lower lobe (RLL) pneumonia and bacteremia. He drank wine socially but never used tobacco products or illicit drugs and lived on a rural property with private well water.
The week before admission, he was working on this property and drank lots of well water. He developed congestion and muscle soreness, followed by a productive cough with blood-streaked sputum, wheezing, and shortness of breath. He went to the closest emergency department. His temperature was 37.8°C, heart rate 104/min, respirations 20/min, blood pressure 167/78 mmHg, and oxygen saturation 93% on room air. Peripheral white blood cell (WBC) count was 2.9 K/μL with 80% polymorphonuclear leukocytes (PMNs). Basic metabolic profile revealed sodium 133 mmol/L, blood urea nitrogen (BUN) 39 mg/dL, creatinine 1.8 mg/dL, estimated glomerular filtration rate (eGFR) 38 mL/min/SA, and glucose 319 mg/dL. Chest X-ray [Figure 1] read airspace disease with right lung base consolidation suggestive of acute lobar pneumonia. The patient left against medical advice and received a 10-day course of oral cefdinir 300 mg twice a day and methylprednisolone 4 mg 6-day taper. The following week, blood cultures taken from his recent ED visit grew nonlactose fermenting Gram-negative rods in the aerobic bottle; VITEK® MS identified A. salmonicida sensitive to ampicillin, ampicillin/sulbactam, cefepime, ceftazidime, ceftriaxone, gentamicin, levofloxacin, meropenem, piperacillin/tazobactam, tobramycin, and trimethoprim-sulfamethoxazole and intermediate to cefazolin. He was admitted to our service for treatment of bacteremia and pneumonia.
Figure 1.
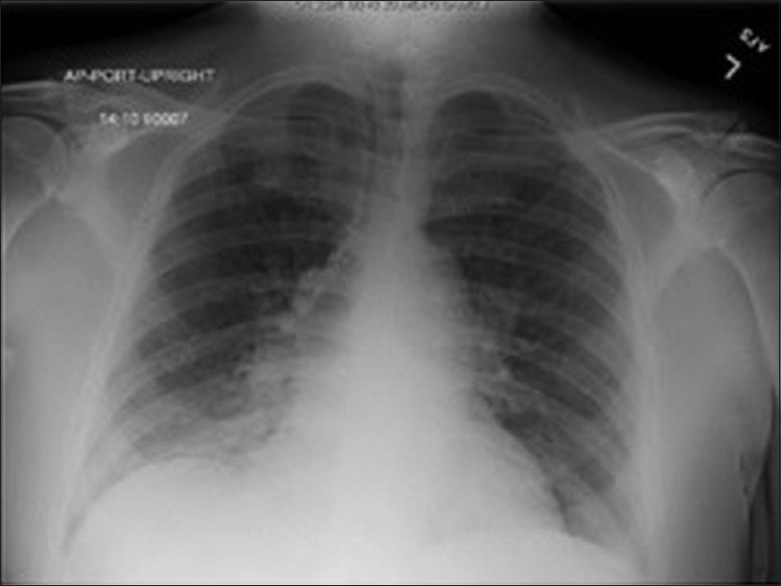
Chest X-ray, presentation
On admission, his temperature was 36.2°C, heart rate 72/min, respirations 18/min, blood pressure 158/56 mmHg, and oxygen saturation 95% on room air. He had rales in the RLL lobe with egophony and expiratory wheezing throughout lower lung lobes bilaterally, remainder of examination unremarkable. Peripheral WBC count was 12.8 K/μL with 89% PMNs. Complete metabolic profile remarkable for sodium 133 mmol/L, BUN 54 mg/dL, creatinine 2.28 mg/dL, eGFR 34 mL/min/SA, and glucose 648 mg/dL.
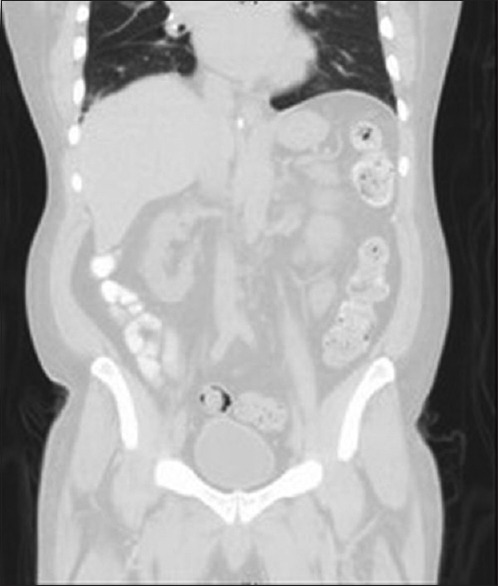
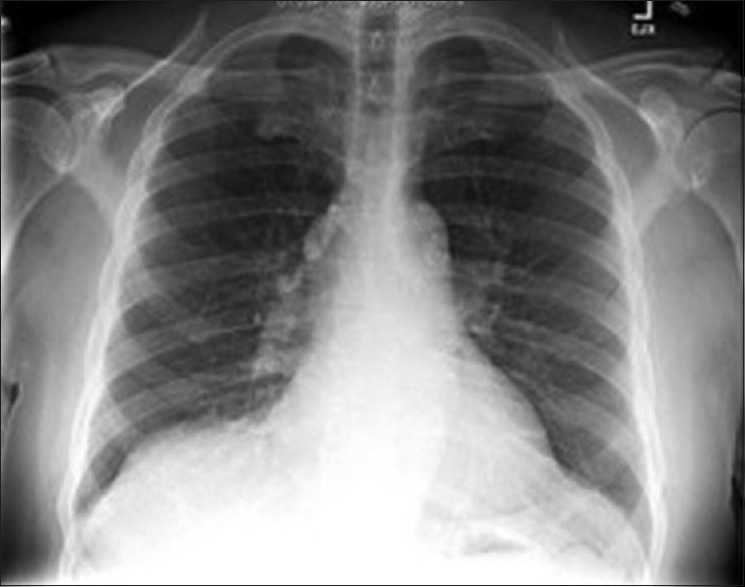
Given the unusual bacteria, we asked the patient about exposure to fish or other bodies of water: he had not been swimming, fishing, or boating for several decades; he ate store-bought fish 2 days after he went to the emergency department; and he drank well water at his property. We asked the laboratory at the referring hospital to repeat the patient's blood culture manually; using matrix-assisted laser desorption ionization time-of-flight technology, it grew the same A. salmonicida pathogen, reporting >98% accuracy. Sputum cultures grew normal flora; bronchoscopy did not reveal other organisms or malignancy. Workup for an intra-abdominal source was negative: Patient's last colonoscopy in 2010 did not reveal masses, hepatitis panel was nonreactive, computed tomography of the chest/abdomen/pelvis [Figure 2] was unremarkable, and barium swallow did not reveal aspiration. We began intravenous cefepime 2.0 g every 12 h. Two days after initiation of therapy, the patient's coughing improved without additional episodes of hemoptysis. Repeat blood cultures were negative, chest X-ray [Figure 3] demonstrated resolution of RLL pneumonia. His acute-on-chronic kidney disease and hyperglycemia returned to baseline following fluid resuscitation and insulin. After 5 days of antibiotic therapy, his respiratory symptoms resolved completely, and he was discharged to home on a 10-day course of intravenous ceftriaxone 2.0 g/day.
Figure 2.
Chest X-ray, posttreatment
Figure 3.
Computed tomography chest/abdomen/pelvis
DISCUSSION
A. salmonicida, a Gram-negative nonlactose fermenting rod, is the bacterial pathogen responsible for furunculosis in salmonid Atlantic salmon and superficial ulcerative diseases in nonsalmonid carp (Cyprinus carpio), goldfish (Carassius auratus), and flounder (Platichthys flesus), and more lethal infections such as hemorrhagic septicemia in black-tip reef shark (Carcharhinus melanopterus).[1,2] Virulence factors – outer protein coat and several pili – are responsible for host colonization and subsequent bacteremia.[3] A. salmonicida and other Aeromonas spp. have been isolated in surface water including salt water lagoons and beaches and fresh water wells.[4,5]
The incidence of A. salmonicida in humans is very rare. It was cultured from stool of patients with diarrheal diseases following ingestion of contaminated food.[6] A patient with diabetes undergoing continuous ambulatory peritoneal dialysis developed A. salmonicida peritonitis after eating fish.[7] To date, A. salmonicida bacteremia has not been reported. The most common species of Aeromonas associated with bacteremia are A. hydrophila, followed by A. sobria, A. caviae, and A. veronii.[6] Tang et al. noticed 75% of Aeromonas spp. bacteremia involved patients with immunocompromised conditions defined as cancer, liver cirrhosis, diabetes, end-stage renal disease, or receiving immunosuppressive therapy.[5] There are several reports of bacteremia due to Aeromonas spp. after water ingestion including one case of A. hydrophila bacteremia following years of well water consumption.[8,9] Chronic exposure of immunocompromised persons to Aeromonas through contaminated water can lead to invasive disease including bacteremia.[10] Our patient had no underlying malignancies or liver cirrhosis and was not taking immunosuppressants; however, his diabetes had progressively worsened in the midst of chronic kidney disease and sarcoidosis. All of these factors, combined with his long-time exposure to A. salmonicida through well water, resulted in septicemia.
More importantly, complications from Aeromonas spp. bacteremia are significant: mortality rate is 33%, and relapse of septicemia is possible.[6] It is critical to treat patients early with extended-spectrum cephalosporins, avoiding narrow-spectrum β-lactams as Aeromonas has inducible β-lactamase activity, and adding additional coverage with fluoroquinolones. Given that our patient had a quinolone allergy, we extended his course for 2 weeks total to provide additional coverage and avoid relapse.
This case suggests that A. salmonicida can cause bacteremia in immunocompromised individuals who chronically consume contaminated well water, and clinicians should consider water exposures in patients who present with Aeromonas spp. bacteremia then promptly treat to avoid increased medical expenditures related to managing serious illnesses.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to thank the Department of Internal Medicine at East Tennessee State University, James H. Quillen College of Medicine, for supporting our endeavors to publish this case report.
REFERENCES
- 1.Wiklund T, Dalsgaard I. Occurrence and significance of atypical Aeromonas salmonicida in non-salmonid and salmonid fish species: A review. Dis Aquat Organ. 1998;32:49–69. doi: 10.3354/dao032049. [DOI] [PubMed] [Google Scholar]
- 2.Briones V, Fernández A, Blanco M, Ramiro F, de Vicente ML, García J, et al. Haemorrhagic septicaemia by Aeromonas salmonicida subsp. salmonicida in a black-tip reef shark (Carcharhinus melanopterus) Zentralbl Veterinarmed B. 1998;45:443–5. doi: 10.1111/j.1439-0450.1998.tb00814.x. [DOI] [PubMed] [Google Scholar]
- 3.Dacanay A, Boyd JM, Fast MD, Knickle LC, Reith ME. Aeromonas salmonicida type I pilus system contributes to host colonization but not invasion. Dis Aquat Organ. 2010;88:199–206. doi: 10.3354/dao02157. [DOI] [PubMed] [Google Scholar]
- 4.Sechi LA, Deriu A, Falchi MP, Fadda G, Zanetti S. Distribution of virulence genes in Aeromonas spp. isolated from Sardinian waters and from patients with diarrhoea. Appl Microbiol. 2002;92:221–7. doi: 10.1046/j.1365-2672.2002.01522.x. [DOI] [PubMed] [Google Scholar]
- 5.Tang HJ, Lai CC, Lin HL, Chao CM. Clinical manifestations of bacteremia caused by Aeromonas species in Southern Taiwan. PLoS One. 2014;9:e91642. doi: 10.1371/journal.pone.0091642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Janda JM, Abbott SL. The genus Aeromonas: Taxonomy, pathogenicity, and infection. Clin Microbiol Rev. 2010;23:35–73. doi: 10.1128/CMR.00039-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang X, Yang QQ, Guo QY, Yi CY, Mao HP, Lin JX, et al. Aeromonas salmonicida peritonitis after eating fish in a patient undergoing CAPD. Perit Dial Int. 2008;28:316–7. [PubMed] [Google Scholar]
- 8.Blair JE, Woo-Ming MA, McGuire PK. Aeromonas hydrophila bacteremia acquired from an infected swimming pool. Clin Infect Dis. 1999;28:1336–7. doi: 10.1086/517793. [DOI] [PubMed] [Google Scholar]
- 9.Katz MJ, Parrish NM, Belani A, Shah M. Recurrent Aeromonas bacteremia due to contaminated well water. Open Forum Infect Dis. 2015;2:ofv142. doi: 10.1093/ofid/ofv142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leclerc H, Schwartzbrod L, Dei-Cas E. Microbial agents associated with waterborne diseases. Crit Rev Microbiol. 2002;28:371–409. doi: 10.1080/1040-840291046768. [DOI] [PubMed] [Google Scholar]


