Abstract
Purpose:
Ophthalmic complications in diabetes such as retinopathy, cataract, and infections have been extensively studied. Recently, attention has been drawn toward ocular surface changes in diabetes mellitus (DM). This study has been carried out to investigate the tear film and ocular surface abnormalities in type II DM patients.
Materials and Methods:
A total of 83 participants (130 eyes) were enrolled: 53 diabetics (80 eyes) and 30 healthy controls (50 eyes). Of the 53 diabetics, 24 patients (42 eyes) had some diabetic retinopathy. The tear film and ocular surface were evaluated using Schirmer test, tear film break-up time (TBUT), keratoepitheliopathy score (KES), Rose Bengal Staining (RBS) test, and conjunctival impression cytology.
Results:
When compared with the healthy controls, diabetics showed significantly reduced Schirmer, TBUT measurements and the higher grades of KES and RBS test (P < 0.001). Impression cytology analysis showed goblet cell loss and conjunctival squamous metaplasia in diabetics.
Conclusion:
Tear film abnormality is a significant feature of diabetic ocular surface diseases. These abnormalities are likely on account of poor quality and function of tears, combined with the subnormal ocular surface. Therefore, all diabetic patients especially those with evidence of retinopathy changes should undergo routine early examination and follow-up of tear function and ocular surface parameters.
Key words: Conjunctival metaplasia, keratoepitheliopathy score, Rose Bengal, Schirmer, tear break up time
Diabetes is one of the common causes of blindness in persons aged 20–70 years. Cataract and retinopathy are well known ocular complications of diabetes. However, recently, attention has been drawn to ocular surface problems, especially dry eye in diabetic patients.[1] Diabetic keratoepitheliopathy is sometimes hard to cure and can induce quantitative and qualitative abnormalities in tear secretion, contributing to decreased corneal sensitivity and poor adhesion of regenerating epithelial cells.[1,2] Research shows that most cases of dry eye associated with diabetes are caused by insufficient production of tears due to “autonomic neuropathy” affecting the nerves that control the lacrimal gland.[3]
Studies have shown an intimate relationship between dry eye and diabetes mellitus (DM) with changes of conjunctival surface, but the results remain controversial. In addition in India, there has been a lack of research relating changes of the conjunctival surface in diabetic patients to clinical parameters. Therefore, we aimed to study the changes of tear film and ocular surface in diabetics by assessing the keratoepitheliopathy score (KES), Schirmer test, tear film break-up time (TBUT), Rose Bengal Staining (RBS), goblet cell density (GCD), and conjunctival squamous metaplasia (CSM) grade using conjunctival impression cytology (CIC) and to compare the results with those of healthy controls.
Materials and Methods
After clearance from Institutional Ethical Committee in accordance with the guidelines of the Declaration of Helsinki and obtaining informed consent, 80 eyes of 53 type II diabetics (of which 42 eyes of 24 patients had evidence of diabetic retinopathy [DR]), and 50 eyes of 30 age- and gender-matched, nondiabetic healthy controls seeking medical attention for refractive errors were recruited from the Department of Ophthalmology. Patients with diabetes were further divided into diabetics with retinopathy (DR group: 24 patients) and without retinopathy (DM group: 29 patients). Retinopathy changes were evaluated as per Early Treatment of Diabetic Retinopathy Study criteria.[4] A diagnosis of diabetes was made if the fasting blood glucose ≥126 mg/dl on two separate occasions or random blood glucose (RBG) ≥200 mg/dl with symptoms or 2-h plasma glucose ≥200 mg/dl.[5] All participants were asked about subjective symptoms such as burning, itching, lacrimation, foreign body sensation, and photophobia. If ≥2 of these symptoms were present, the individual was classified as having subjective complaints. Individuals who had a history of chronic ocular drug abuse; contact lens wear; topical medication; ocular surgery in the previous 3 months; abnormalities in the cornea; conjunctiva or eyelid; secondary ocular; and systemic diseases with dry eyes as a manifestation, bilateral dense cataracts; bilateral central corneal opacities; or any opacification in the media were excluded from the study.
We carried out Schirmer test, TBUT, KES, RBS, and CIC analysis as described below.
Schirmer test was performed without topical anesthesia using standardized Whatman filter paper. The strips were placed in the lower fornix away from the cornea and left in place for 5 min. The distance wet was measured in millimeters, and a reading <5 mm was considered abnormal.[6,7]
KES was evaluated by staining the cornea with fluorescein and scoring the area and density of staining. The scores were multiplied, and the product was used as an index of corneal surface damage. The staining area was graded on a numerical scale of 0–3, with 0 representing no punctate staining, 1 representing less than one-third, 2 representing one-third to two-thirds, and 3 representing more than two-thirds staining. The staining density was also graded on a numerical scale of 0–3, with 0 representing no punctate staining, 1 representing sparse density, 2 representing moderate density, and 3 representing high density with overlapping lesions.[2,6]
TBUT test was performed by staining the tear film using a fluorescein strip without using topical anesthesia and asking the subjects to blink several times for few seconds to encourage its distribution. The tear film was observed using a blue cobalt filter without artificially holding the lids open. The interval between the last blink and the appearance of the first corneal dry spot in the stained tear film was measured. The procedure was repeated 3 times and the mean value recorded. A TBUT value <10 s was considered abnormal.[6,7]
Rose Bengal test was done using saline moistened Rose Bengal strips placed in the lower fornix. The staining of nasal and temporal conjunctiva was graded from 0 to 3 as described by van Bijsterveld.[8] Grade 0 referred to no staining, grade 1 to the staining of a few points, grade 2 to a scattered pattern, and grade 3 referred to staining of confluent areas of the ocular surface. If the sum of these staining scores were ≥3, it was considered abnormal.
Impression cytology was performed as follows we used Millipore cellulose acetate filter paper of order number 11106--47----ACN of 0.45 µ pore size of circular shape 1 cm diameter manufactured by Sartorius Stedim Biotech, Germany. After topically anesthetizing with 0.5% paracaine, the lids were retracted, and the lacrimal lake at medial canthus was dried with a swab. A strip of cellulose acetate filter paper, grasped with a forceps at one end, measuring 15 mm × 10 mm, with the straight side toward limbus was applied dull side down to the lower nasal bulbar conjunctiva adjacent to the corneal limbus, and the filter strip was pressed gently with blunt, smooth tipped forceps for 2–3 s. The strip was then gently removed in a peeling motion and was placed on a clean glass slide at room temperature in a manner such that surface with impression faced upward. The slide was then immediately placed horizontally in a Petridish for 20 min in a freshly prepared solution of glacial acetic acid, formalin, and ethyl alcohol in a volume ratio of (1:1:20). The slide was fixed in absolute alcohol, stained with hematoxylin and eosin and periodic acid-Schiff, and was then mounted.[9] Photographs were taken using a light microscope. The smear was scanned at low power (×4 and × 10), and then at high-power (×40), 4 high power fields (HPF) which contained maximum number of cells were observed. Goblet cells were counted, and the GCD in 1 HPF was represented as the number of cells per square millimeters. The degree of squamous metaplasia of the conjunctival epithelial surface was also reported using the modified Nelson's grading scheme: Grade 0: >500 goblet cells/mm2; Grade 1: 200–500 goblet cells/mm2; Grade 2: 100–199 goblet cells/mm2; and Grade 3: <100 goblet cells/mm2. GCD <200 cells/mm2 or CSM grade ≥2 were taken as abnormal.[10]
Statistical analysis
Independent t-test, analysis of variance (ANOVA) followed by post hoc Tukey HSD test, Kruskal–Wallis test were used. Statistical significance was considered P ≤ 0.05, 95% confidence interval was quoted.
Results
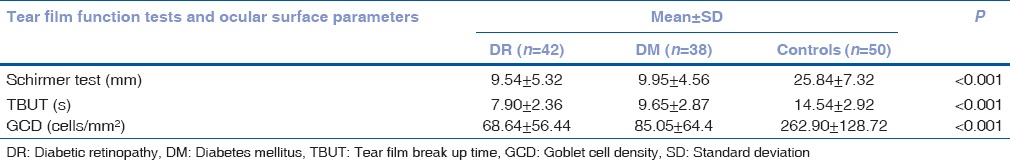
Mean age (years) in the three groups were DR group: 52.50 ± 4.76 versus DM group: 53.02 ± 5.55 versus controls: 51.66 ± 4.80 (P = 0.437) [Table 1]. Mean age of males and females were 52.96 ± 5.87 and 51.80 ± 4.14. Significantly poorer Schirmer, TBUT and GCD measurements were found in study groups (DR and DM groups) as compared to control group (P < 0.001) [Table 2].
Table 1.
Demographics of study population
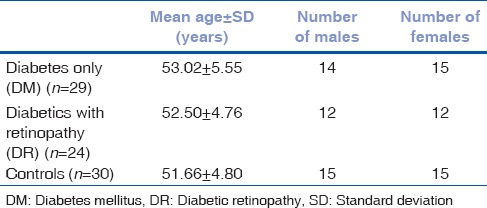
Table 2.
Comparison of Schirmer test, tear film break up time and goblet cell density among cases and controls
KES and RBS grading were significantly poorer in DR group than DM group; median values were 1 versus 0 (P < 0.001). None of the controls showed any evidence of staining. CSM was found significantly worse in DR and DM group than in control group, median CIC grade 3 (DR and DM group) versus 2 (control) (P < 0.001) [Table 3].
Table 3.
Comparison of keratoepitheliopathy score, Rose Bengal Staining and conjunctival squamous metaplasia among cases and controls

Discussion
Our study shows that the tear film parameters such as Schirmer test and TBUT were significantly reduced, KES was abnormal, and RBS was worse in the diabetic patients as compared to controls. The CIC comprising GCD and CSM results also supported these changes. These results indicate that dry eye is a significant feature of the diabetic ocular surface disease.
Schirmer is still the simplest, fastest and least expensive of the relatively few diagnostic tests available for assessing the tear production rate. Similar to our study, numerous other authors have shown that tear secretion is significantly decreased in diabetics.[6,11,12,13] Unlike us, Li et al. on 111 noninsulin-dependent DM (NIDDM) patients and 100 age- and gender-matched control, found comparable Schirmer values: (10.61 ± 6.86 mm) versus (10.92 ± 7.05 mm); P < 0.05.[14] Even worsening retinopathy returned nonsignificant differences in Schirmer tests: No-DR (10.95 ± 6.89 mm) versus BDR (11.71 ± 7.30 mm) versus PDR (7.63 ± 5.20 mm); (P > 0.05). Evidence from our study is essentially in accordance with the majority of that in literature: that diabetics with retinopathy fare significantly worse on Schirmer test than those without, and both do worse than healthy controls. This could be due to decrease corneal and conjunctival sensitivity in diabetics, and due also to damage to microvasculature of lacrimal gland leading to its impaired functioning.[6,11,12,13,14]
While some authors have reported results similar to ours,[6,12,13,14] one study has found no significant difference in TBUT between diabetics and normal controls.[11]
Yoon evaluated KES on 94 NIDDM-eyes and 60 control eyes and reported significantly higher scores in diabetic group (1.14 ± 0.89) than in the control group (0.34 ± 0.48) (P < 0.001).[13] This study went on to report that significantly worsening KES were associated with poor metabolic control and the presence of diabetic neuropathy, and not with age, gender, and duration of diabetes.
Ozdemir demonstrated that significantly (P < 0.001) greater proportion of diabetic cases had abnormal RBS as compared to healthy controls: 13 of 41 (31%) versus 2 of 10 (20%).[12] We did not come across any study where RBS was studied with respect to worsening retinopathy.
CIC analysis showed significantly higher grade of squamous metaplasia and lower GCD in both DR group and DM group than in control group (P < 0.0001) [Table 3]. GCD illustrates the condition of the ocular surface. The loss of goblet cells is a sign of squamous metaplasia. Epithelial cells were larger and polygonal, and the nucleocytoplasmic ratio was increased. In the majority of severe cases, pyknotic, or even absent nuclei were found. The mechanisms producing these morphological changes in the ocular surface in due course of the diabetic disease are still not clear. Although we observed abnormal CIC grading on healthy control eyes also, we felt that it may possibly be due to age-related changes and external harsh environmental conditions at the place of study. Dogru et al. and Yoon et al. described an increase of squamous metaplasia and a reduction in GCD in the conjunctiva of diabetic patients.[6,13]
Conclusion
Our study indicates that tear film and conjunctival surface changes in patients with DM include decreased tear secretion and stability, evidence of keratoepitheliopathy, abnormal RBS, squamous metaplasia, and poor GCD. On comparison of the DR and DM groups with control group, the results were significant and in greater proportion. Interestingly, diabetics with retinopathy fared significantly worse on tear function tests and ocular surface parameters tests than those without, and both did worse than healthy controls. It is likely that on account of poor quality and function of tears, combined with subnormal ocular surface, diabetic patients are often symptomatic and have ocular surface disease as compared to healthy controls. Since most of the data on dry eye disease in diabetics is based on western and European studies and since there is increasing trend in the incidence of diabetics in India, it is, therefore, important to know the pattern in the Indian population. Hence, more such studies should be carried out keeping Indian population in mind, and thus routine and early examination of tear function and ocular surface parameters should form a part of the workup of all diabetics, especially those with retinopathy changes.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Saini JS, Khandalavla B. Corneal epithelial fragility in diabetes mellitus. Can J Ophthalmol. 1995;30:142–6. [PubMed] [Google Scholar]
- 2.Inoue K, Kato S, Ohara C, Numaga J, Amano S, Oshika T. Ocular and systemic factors relevant to diabetic keratoepitheliopathy. Cornea. 2001;20:798–801. doi: 10.1097/00003226-200111000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Chous P. Dry Eyes and Diabetes Often Go Hand in Hand: dLife Blog. Connecticut: LifeMed Media, Inc; 2013. [Last cited on 2015 Oct 02]. Available from: http://www.dlife.com/diabetes/complications/eye-care/chous_sept2006 . [Google Scholar]
- 4.Grading diabetic retinopathy from stereoscopic color fundus photographs – An extension of the modified Airlie House classification. ETDRS Report Number 10. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98(5 Suppl):786–806. [PubMed] [Google Scholar]
- 5.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33:S62–9. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dogru M, Katakami C, Inoue M. Tear function and ocular surface changes in noninsulin-dependent diabetes mellitus. Ophthalmology. 2001;108:586–92. doi: 10.1016/s0161-6420(00)00599-6. [DOI] [PubMed] [Google Scholar]
- 7.Jones LT. The lacrimal secretory system and its treatment. Am J Ophthalmol. 1966;62:47–60. doi: 10.1016/0002-9394(66)91676-x. [DOI] [PubMed] [Google Scholar]
- 8.van Bijsterveld OP. Diagnostic tests in the sicca syndrome. Arch Ophthalmol. 1969;82:10–4. doi: 10.1001/archopht.1969.00990020012003. [DOI] [PubMed] [Google Scholar]
- 9.Anshu, Munshi MM, Sathe V, Ganar A. Conjunctival impression cytology in contact lens wearers. Cytopathology. 2001;12:314–20. doi: 10.1046/j.1365-2303.2001.00349.x. [DOI] [PubMed] [Google Scholar]
- 10.Nelson JD. Impression cytology. Cornea. 1988;7:71–81. [PubMed] [Google Scholar]
- 11.Goebbels M. Tear secretion and tear film function in insulin dependent diabetics. Br J Ophthalmol. 2000;84:19–21. doi: 10.1136/bjo.84.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ozdemir M, Buyukbese MA, Cetinkaya A, Ozdemir G. Risk factors for ocular surface disorders in patients with diabetes mellitus. Diabetes Res Clin Pract. 2003;59:195–9. doi: 10.1016/s0168-8227(02)00244-9. [DOI] [PubMed] [Google Scholar]
- 13.Yoon KC, Im SK, Seo MS. Changes of tear film and ocular surface in diabetes mellitus. Korean J Ophthalmol. 2004;18:168–74. doi: 10.3341/kjo.2004.18.2.168. [DOI] [PubMed] [Google Scholar]
- 14.Li HY, Pang GX, Xu ZZ. Tear film function of patients with type 2 diabetes. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2004;26:682–6. [PubMed] [Google Scholar]


