ABSTRACT
Protein synthesis, the translation of mRNA into a polypeptide facilitated by the ribosome, is assisted by a variety of protein factors, some of which are GTPases. In addition to four highly conserved and well-understood GTPases with known function, there are also a number of noncanonical GTPases that are implicated in translation but whose functions are not fully understood. LepA/EF4 is one of these noncanonical GTPases. It is highly conserved and present in bacteria, mitochondria, and chloroplasts, but its functional role in the cell remains unknown. LepA's sequence and domain arrangement are very similar to those of other translational GTPases, but it contains a unique C-terminal domain (CTD) that is likely essential to its specific function in the cell. Three main hypotheses about the function of LepA have been brought forward to date: (i) LepA is a back-translocase, (ii) LepA relieves ribosome stalling or facilitates sequestration, and (iii) LepA is involved in ribosome biogenesis. This review examines the structural and biochemical information available on bacterial LepA and discusses it on the background of the available in vivo information from higher organisms in order to broaden the view regarding LepA's functional role in the cell and how the structure of its unique CTD might be involved in facilitating this role.
KEYWORDS: LepA/EF4, back-translocation, ribosome stalling, ribosomes, translational GTPase, ribosome biogenesis
INTRODUCTION
Protein synthesis is fundamental to all living organisms. The process of protein synthesis (translation) is carried out on the ribosome with the help of a number of protein factors, many of which are GTPases. These translational GTPases (trGTPases) act as molecular switches and exist in either an “on” (GTP-bound) or “off” (GDP-bound) conformation (1), regulating ribosome-dependent protein synthesis. The process of translation occurs in four stages that are universally conserved in both bacteria and eukaryotes: initiation, elongation, termination, and ribosome recycling. These steps are facilitated by four trGTPases that are conserved in all bacteria (2): initiation factor 2 (IF2), elongation factor Tu (EF-Tu), elongation factor G (EF-G), and release factor 3 (RF3). During bacterial initiation, IF2's role is to recruit initiator tRNA to the P site of the 30S ribosomal subunit, forming the 30S initiation complex (3, 4). EF-Tu and EF-G then participate during the elongation cycle: EF-Tu delivers aminoacyl-tRNA to the A site of the ribosome, while EF-G catalyzes the translocation of tRNAs from the PRE state (pretranslocation, tRNAs in A and P sites) to the POST state (posttranslocation, tRNAs in P and E sites). Elongation continues to extend the polypeptide chain until termination occurs, facilitated by RF3. Ribosome recycling then occurs with the help of EF-G.
In addition to these four canonical trGTPases, there are a number of noncanonical GTPases that are implicated during translation yet are poorly understood with regard to their cellular role and functional mechanism. Conflicting evidence and poor biochemical characterization often make it difficult to properly assign function to these factors. One such factor is the highly conserved GTPase LepA/elongation factor 4 (EF4). LepA was identified as the first gene in the bicistronic leader peptidase operon, which also encodes signal peptidase I in Escherichia coli (5), giving it the name LepA. Due to its localization to the membrane (6), LepA was initially believed to be a membrane-associated protein. This, along with the fact that signal peptidase I functions in protein export, led to the hypothesis that LepA may be involved in protein export as well. However, lepA knockout strains showed no observable effect on protein export (7), suggesting a non-membrane transport-related function and sparking the debate regarding LepA's cellular role.
LepA is extremely well conserved; based on representative bacteria from different phyla, it is reported as the third most highly conserved protein in bacteria, falling behind EF-Tu and EF-G, with an amino acid identity of 55 to 68% between bacterial orthologs (8). In addition to being present in all bacteria, with the exceptions of Streptococcus pyogenes and Carsonella ruddii (2), LepA is found in the mitochondria and chloroplasts of eukaryotes, but it is absent from archaea and the cytosol of eukaryotes. LepA is not essential under optimal growing conditions (7), but it is reported to be required for growth under stress conditions, such as low pH, high magnesium, and high or low temperature (9–13).
LepA's sequence and domain arrangement are very similar to those of other trGTPases (6). However, it contains a C-terminal domain (CTD) with a unique sequence not seen in other GTPases. LepA's CTD is likely essential to the function of LepA in the cell and should therefore be a focus in studies that aim to elucidate the function of LepA from a structural and/or mechanistic angle. The specific roles of LepA and its CTD have not been identified, although a number of hypotheses exist. These include back-translocation, ribosome sequestering or rescuing, or ribosome biogenesis (8, 14–16). Here, we examine the available information on LepA and try to understand its role in the cell in the context of its unique CTD by integrating the available structural, biochemical, and in vivo observations in bacteria as well as its eukaryotic homologs.
STRUCTURE OF LepA: INSIGHTS FROM CRYO-EM AND X-RAY CRYSTALLOGRAPHY
The structure of LepA.
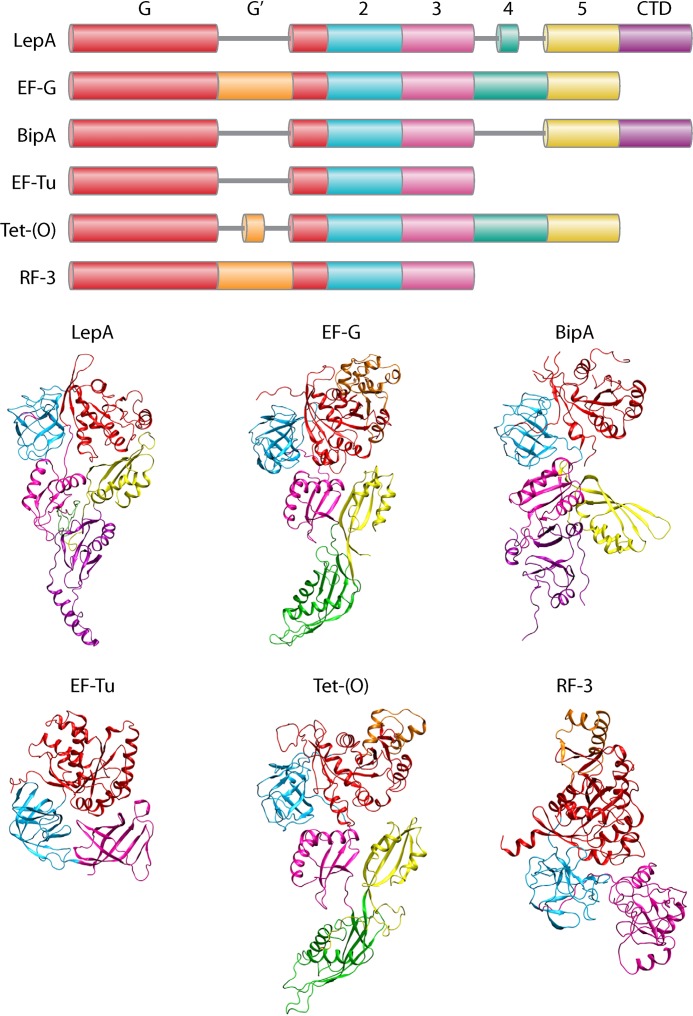
Evans et al. provided the first high-resolution structural data on LepA with the crystal structure of the E. coli protein in its apo (nucleotide-free) form at a resolution of 2.8 Å (17). The structure revealed that LepA consists of six domains, including one shortened linker domain. Four of LepA's domains are shared with EF-G (Fig. 1). Domains I and II are common to all trGTPases. Domain I, the G domain, is responsible for nucleotide binding and interacts with ribosomal proteins L7/L12 and L11 at the GTPase-activating center (GAC) in the 50S ribosomal A site (18, 19). Missing in LepA is the G′ subdomain that is present in EF-G as part of domain I. The G′ subdomain has been shown to interact with L7/L12 proteins and to be important in ribosome-stimulated GTP hydrolysis (20); this subdomain is present in the trGTPases Tet-(O) and RF3 (Fig. 1) but also absent in EF-Tu and IF2. Domain II is characterized by an OB fold, a β-barrel structure that functions in oligonucleotide/oligosaccharide binding (17). LepA shares domains III and V with EF-G, both of which contain the RNA recognition motif that is seen in many ribosomal proteins (21). Interestingly, LepA is missing EF-G's domain IV but contains a smaller corresponding domain linking domains III and V (17), which here is referred to as domain IV. The most interesting aspect of LepA's structure is the presence of a distinctive C-terminal domain (CTD) with a fold unique to LepA, containing a large number of positively charged residues (17). However, the crystal structure of unbound LepA does not include the fully resolved CTD; the last 44 amino acids are missing, which is thought to be due to their flexibility. It has been recently reported that the last 44 amino acids of the CTD constitute a subdomain that is important for LepA's nucleotide-dependent function on the ribosome (22). Through molecular dynamics analysis, it was shown that a helix-turn-helix (HTH) motif of the CTD is flexible, free both in solution and when bound to the ribosome (23).
FIG 1.
Domain arrangement and crystal structures of trGTPases LepA, EF-G, BipA, EF-Tu, Tet-(O), and RF3 (PDB IDs 4W2E, 4V9P, 5A9W, 1EFT, 4V6V, and 4V85, respectively). Homologous domains are shown as similar colors. CTDs (purple) are unique to each protein.
LepA on the ribosome.
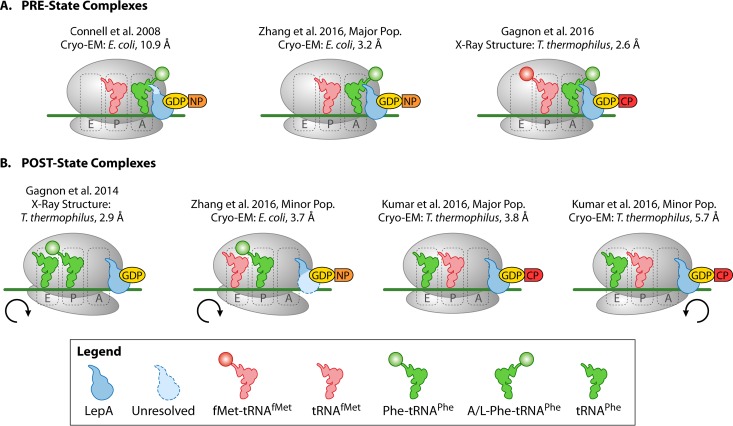
Seven structures of LepA bound to the ribosome have been reported to date (Fig. 2 and Table 1). These structures demonstrate that LepA's binding site is similar to that of trGTPases EF-G and EF-Tu, both of which bind to the GTPase-activating center (GAC) and the sarcin-ricin loop (SRL) in the A site of the ribosome. LepA makes contacts with both the 50S and 30S subunits, similarly to EF-G (24). When structures of ribosome-bound LepA and EF-G are superimposed, their domains I, III, and V occupy similar locations on the ribosome. However, their unique domains occupy markedly different positions. While EF-G's fourth domain penetrates into the decoding center of the 30S A site, LepA's shortened fourth domain prevents this from occurring (25, 26). Additionally, LepA's CTD extends into the peptidyl transferase center (PTC) to interact with the P-site tRNA (P-tRNA) and 23S rRNA, while also interacting with the A site tRNA (A-tRNA) when present in the structure (15, 23, 26, 27).
FIG 2.
Cartoon representations of all available structures of LepA in complex with 70S ribosomes, mRNA, and tRNAs. The 30S rotation state is indicated below the respective complexes.
TABLE 1.
Characteristics of the available LepA structures in complex with 70S ribosomes
| Structure information | PRE complexes |
POST complexes |
|||||
|---|---|---|---|---|---|---|---|
| LepA-GDPNP-PRE | LepA-GDPNP-PRE (major group) | LepA-GDPCP-PRE | LepA-GDP-POST | LepA-GDPNP-POST (minor group) | LepA-GDPCP-POST (major group) | LepA-GDPCP-POST (minor group) | |
| Reference | 25 | 23 | 15 | 27 | 23 | 35 | 35 |
| Method | Cryo-EM | Cryo-EM | X-ray crystallography | X-ray crystallography | Cryo-EM | Cryo-EM | Cryo-EM |
| Resolution (Å) | 10.9 | 3.2 | 2.6 | 2.9 | 3.7 | 3.8 | 5.7 |
| Organism | Escherichia coli | Escherichia coli | Thermus thermophilus | Thermus thermophilus | Escherichia coli | Thermus thermophilus | Thermus thermophilus |
| mRNA (nt) | MF-mRNAa (46), AUG-UUC | MF-mRNA (81), SDb (GGAGG) AUG-UUC | MF-mRNA (24), SD (GGAGG) AUG-UUC | mRNA (18), SD (GGAGG) UUC | MF-mRNA (81), SD (GGAGG) AUG-UUC | MF-mRNA (25), SD (GGAGG) AUG-UUC | MF-mRNA (25), SD (GGAGG) AUG-UUC |
| A-tRNA (state) | Ac[14C]Phe-tRNAPhe (A/L) | Ac[14C]Phe-tRNAPhe (A/4) | Phe-tRNAPhe (A/L) | None | None | None | None |
| P-tRNA | [32P]deacyl-tRNAfMet | Deacyl-tRNAfMet | fMet-tRNAfMet | Phe-tRNAPhe | Ac[14C]Phe-tRNAPhe | tRNAfMet | tRNAfMet |
| E-tRNA | None | None | None | tRNAPhe (modeled) | Deacyl-tRNAfMet | tRNAPhe | tRNAPhe |
| 30S rotation (°) | Unrotated | Unrotated | Unrotated | Clockwise (5) | Clockwise (4) | Unrotated | Counterclockwise (5.6) |
| PDB ID | 3DEG | 3JCE | 5J8B | 4W2E | 3JCD | 51MQ | 51MR |
| Prepn | LepA and GDPNP incubated with POST state 70S | LepA and GDPNP incubated with POST state 70S | LepA and GDPCP incubated with PRE state 70S | LepA and GDPCP incubated with 70S and P-site Phe-tRNAPhe | PRE state 70S incubated with EF-G to yield POST state; LepA and GDPNP incubated with POST state 70S | LepA and GDPCP incubated with PRE state 70S | LepA and GDPCP incubated with PRE state 70S |
| Comments | Missing last 55 aa of CTD | None | Engineered L9 fusion; highest-resolution structure so far | Engineered L9 fusion | Only modeled 26 residues of CTD | Engineered L9 fusion | Engineered L9 fusion |
MF-mRNA, mRNa containing the codons for methionine (M) and phenylalanine (F).
SD, Shine-Dalgarno element. The sequences of the SD (in parentheses) and the codons are indicated.
LepA's conformation upon binding to the ribosome remains relatively unchanged from its apo state, but some changes do occur. Two quasi-rigid bodies are formed by domains I and III and domains IV and V, while the CTD appears to move independently (25). Consistent with conformational changes upon GTP binding by other trGTPases, the superimposition of apo-LepA onto the structure of LepA bound to a nonhydrolyzable GTP analog (LepA-GDPCP) reveals changes in the switch I (SWI) and switch II (SWII) regions (26). Compared to LepA alone, the LepA-GDPCP-70S complex shows a reorientation of SWII toward domain III. SWI and SWII are flexible regions that are known to adopt different conformations upon binding to either GTP or GDP in order to facilitate the function of trGTPases on the ribosome (28). It has been proposed that GTP hydrolysis in trGTPases triggers conformational changes in SWI and SWII that lead to further conformational changes in domains III and IV (24). The position of LepA's catalytic residue, His81 (14, 22), changes upon ribosome binding. In its apo form, LepA's His81 is oriented toward domain III, but upon ribosome binding it is reoriented closer to the GDPCP nucleotide (26), consistent with the GTPase activation upon binding to the ribosome.
Comparison of LepA bound to the ribosome in the GDP- versus GTP-bound form reveals that GTP hydrolysis causes structural rearrangements. In the X-ray structure of LepA-GDPCP (15), the SWI region is stabilized and LepA is in a more compact conformation than in the crystal structure of LepA-GDP (27). It appears that the changes that take place during GTP hydrolysis affect the positioning of LepA's SWII and domain III regions, which are similar in the apo and GDP-bound forms but differ when LepA is bound to GDPCP (26). The most recent cryo-electron microscopy (cryo-EM) structure of LepA-GDPCP bound to POST state ribosomes reveals a well-resolved nucleotide binding pocket, which shows that the sarcin-ricin loop (SRL) interacts with LepA's catalytic histidine residue and places it into an activated position (26).
The recent surge of structures of LepA in complex with different functional states of the ribosome have shown that LepA is able to interact with a variety of translational intermediates. Complexes of LepA bound to PRE state ribosomes (tRNAs in the A and P sites) have yielded structures of ribosomes only in their unrotated states, but POST state complexes (tRNAs in the P and E sites) exhibit a variety of ribosome conformations (Fig. 2). In X-ray structures of LepA-GDP bound to the POST state ribosome, the 30S subunit is rotated by 5° relative to the 50S subunit (27). This rotation is also seen in the cryo-EM structure of LepA-GDPNP (another nonhydrolyzable GTP analog) bound to the POST state ribosome, although with a smaller angle of 4° (23). Both of these clockwise-rotated POST state complexes include aminoacylated P-tRNAs which may suggest that the rotated POST state of the ribosome is dependent on the presence of an amino acid in the P site. The two recent cryo-EM structures of LepA bound to POST state ribosomes reveal a major population of structures showing the ribosome in an unrotated state, while in a minor population of the ribosomal complexes, the 30S subunit is rotated counterclockwise (26). Both of these structures are inconsistent with the previously established POST state complexes. However, they contain only deacylated tRNAs, and Kumar et al. (23) suggest that nucleotide contamination could have affected the conformation of the minor group structure from Zhang et al. (23), where LepA-GDPNP is bound to POST state ribosomes and exhibits clockwise subunit rotation, therefore displaying a GDP-bound state of LepA on the ribosome. If this is the case, the available structural information would suggest that LepA-GDP-POST exhibits clockwise rotation (23, 27), LepA-GTP-PRE is unrotated (23, 25), and LepA-GTP-POST exhibits either a counterclockwise rotated or an unrotated conformation (26). Missing from this list is LepA-GDP-PRE. However, it may not be possible to obtain this structure if clockwise rotation of the ribosome is required for LepA-GDP binding.
Interactions of LepA's CTD.
Interestingly, in contrast to the structural changes to the ribosome, the position of LepA's CTD remains relatively unaltered in each of the different structures of LepA on the ribosome, although more details have become clear with increased resolution. In the initial low-resolution cryo-EM structure of LepA-70S, LepA's CTD was shown to interact solely with an intermediate-state tRNA in the A site, termed the A/L-tRNA (25). However, this structure did not contain the entire CTD and was lacking the C-terminal 44 amino acids. Interactions have been revealed in the recent higher-resolution structures of LepA bound to PRE state ribosomes, which show an extended CTD conformation with a helix-turn-helix (HTH) module interacting with the CCA end of the A/L-tRNA (15, 23). The CTD engages with the A/L-tRNA at the acceptor and D-stem regions, while LepA's other unique domain, its reduced domain IV, interacts with the anticodon-stem region. These two LepA unique domains make contacts with the A/L-tRNA mostly via its sugar-phosphate backbone, and they encompass both helical domains of the tRNA; in doing so, LepA's CTD has been proposed to probe the L shape of the A/L-tRNA in order to remodel it into its intermediate conformation (15).
In each structure containing LepA's complete CTD, the CTD has been shown to extend into the peptidyl transferase center (PTC) of the 50S subunit (15, 23, 26, 27). The positively charged CTD can interact with either the P-tRNA or the 23S rRNA. In the structure of LepA-GDPNP bound to the POST state ribosome (23), the CCA end of the P-tRNA is not in its canonical position required for base-pairing to the P-loop nucleotides G2251 and G2252 of the 23S rRNA, inconsistent with the crystal structure of LepA-GDP bound to the POST state ribosome (27). The disrupted base-pairing between the P loop and the P-tRNA is proposed to be due to three conserved LepA residues, Arg560, Lys563, and Asp557, all in the CTD (23) and ultimately remodeling the 23S rRNA and the PTC. These observations, along with biochemical analysis (22), suggest that the CTD is crucial to LepA's function in the cell. This is consistent with the in vivo observations that a LepA variant lacking the CTD (LepA-ΔCTD) fails to complement the reported synthetic phenotypes (14).
LepA and BipA: two paralogs of EF-G with unique C-terminal domains.
LepA's domain layout follows that of most trGTPases, but it most strikingly resembles that of BipA, another noncanonical trGTPase that is also a paralog of EF-G (Fig. 1). Like LepA, BipA is extremely well conserved (2) and is not essential under optimal growth conditions, yet it associates with the ribosome and becomes important for cell growth under suboptimal conditions such as low temperature and low pH (29–31). BipA contains domains I, II, III, and V but is missing the equivalent of domain IV and the G′ subdomain. Most interestingly, BipA contains its own unique C-terminal domain (CTD) which is likely crucial for BipA's interaction with the ribosome. A recent cryo-EM structure of BipA-GDPCP bound to the E. coli 70S ribosome reveals that BipA's CTD does not occupy a position comparable to that of LepA's CTD when bound to the ribosome but rather is positioned similarly to EF-G's domain IV (26). Interestingly, BipA has been suggested to be involved in 50S subunit biogenesis at low temperatures (32). The shared structures of BipA and LepA are consistent with a general design strategy in which the first four domains are responsible for the interaction with the ribosome, while the CTD is modified to carry out a particular task specific to the respective protein (Fig. 1).
PROPOSED FUNCTIONS: THE DEBATE ON LepA'S CELLULAR ROLE
LepA is a translational GTPase.
The gene coding for LepA was discovered in E. coli as the first open reading frame (ORF) of the lep operon, followed by an ORF encoding signal peptidase I that cleaves the N-terminal signal sequence of exported proteins (5). Because it was found to be localized to the cell membrane (along with signal peptidase I), LepA was suggested to work together with signal peptidase I to help the passage of secreted proteins across the membrane. This notion was rejected later when it was discovered that LepA is not essential and is not required for protein transport (7).
Based on its primary sequence homology to other trGTPases (IF2, EF-Tu, and EF-G), LepA was proposed to be a membrane-bound GTPase that is involved in translation (6). LepA was found to be a ribosome-binding protein through cross-linking experiments in Staphylococcus aureus and human ribosomes using oxazolidinone antibiotics (33). The antibiotic was shown to cross-link in vivo to A-2602 of the 23S rRNA, placing its site of action in the peptidyl transferase center (PTC), as well as to ribosome-bound LepA, indicating that LepA binds to the ribosome and therefore may be involved in some step of translation. Molecular modeling of the specific binding site of oxazolidinone antibiotics placed it within the A site (34), which is in accordance with the experimentally determined binding site of LepA (15, 23, 25, 27, 35).
LepA as a back-translocase.
The first experimental support for LepA's role in translation elongation came from in vitro assays that suggested that LepA can catalyze the backwards movement of tRNAs from the POST state to the PRE state—the opposite of the translocation reaction catalyzed by EF-G (8). In these experiments, three different functional states of ribosome were used: a “Pi” state resembling the 70S initiation complex (Phe-tRNAPhe in the P site), the PRE state (A-site Phe-tRNAPhe and P-site deacyl-tRNAfMet), and the POST state (PRE state ribosomes incubated with EF-G to yield ribosomes with P- and E-site tRNAs). These complexes can be compared to the available structures of LepA-70S complexes (Fig. 2). In the presence of LepA, ribosomes in the Pi state reacted with puromycin and aminoacyl-tRNA (aa-tRNA) as usual, but POST state ribosomes were no longer able to carry out the puromycin reaction (puromycin is a 50S A site binding analog of the 3′ end of aa-tRNA) and were also unable to catalyze dipeptide formation (8). These results suggest that in the presence of LepA, the movement of the POST state tRNAs back into the PRE state is catalyzed, precluding the binding of puromycin or aa-tRNA to the A site. Further in vitro studies were carried out to investigate this back-translocation activity of LepA. Footprinting, Pb2+ cleavage, and toeprinting assays each produced evidence that when LepA is added to POST state ribosomes, the resulting tRNA footprint/Pb2+ cleavage/mRNA toeprint signals are converted to those specific to the PRE state (8). The fact that LepA is missing domain IV helps to promote this model of back-translocation. In EF-G, domain IV acts as a doorstop that occupies the A site to prevent the backwards movement of the tRNA-mRNA complex during elongation (36). Along the same lines, initial studies from Kurt Fredrick's lab reported that overexpression of LepA reduces tmRNA tagging in bacteria (a process that removes stalled ribosomes from aberrant mRNAs). They noticed that, when overexpressed, LepA inhibits A site mRNA cleavage in this process, which they hypothesized to be due to reverse translocation activity of LepA resulting in a tRNA in the A site protecting the codon at this site (37). Kinetic analysis of LepA's back-translocase activity by Liu et al. demonstrates that LepA's back-translocation activity occurs through three intermediates and enhances the rate of back-translocation 2-fold (38).
A physiological role of the back-translocation activity has been suggested based on the observation that LepA increases the yield of active protein being synthesized, as shown in experiments where increasing amounts of LepA was added to a GFP-based reporter system (8). If LepA's cellular role is to facilitate back-translocation, it may serve the purpose of lowering the rate of translation when the cell is under stress, improving cotranslational folding and thereby increasing the active fraction of protein. Alternatively, it has been suggested that LepA can recognize ribosomes that have been improperly translocated (and are in a dysfunctional POST state), bind to them, and catalyze the movement of tRNAs back into the PRE state in order to allow EF-G another attempt at proper translocation (8). If this is the case, a number of questions remain. What is the exact nature of these mistranslocated ribosomes, and how is LepA able to recognize them? Further, can LepA specifically recognize ribosomes translating certain mRNAs?
Additional support for LepA's back-translocation activity comes from the first cryo-EM structure of LepA bound to the E. coli ribosome (25): this complex was obtained by incubating LepA-GDPNP with ribosomes in the POST state, revealing LepA bound to a PRE state ribosome with an intermediate A/L-tRNA in the A site. This suggests a model in which LepA binds to the ribosome in the POST state and induces a clockwise rotation to open up the decoding center, allowing for the backwards movement of the E- and P-site tRNAs into the P and A sites (27). The tRNA moved from the P site would then occupy the A site in an intermediate conformation (A/L state), and the positions of the new P- and A/L-tRNAs could then be stabilized by the positively charged CTD of LepA, which has been shown to reach into the PTC (25, 27). It might therefore be interesting to study the specific interactions made by the CTD as the ribosome shifts from the POST to the PRE state in order to elucidate a possible role of the CTD in sensing the state of the ribosome. Consistent with this, it has been shown that LepA's CTD plays a role in the nucleotide-dependent regulation of ribosome binding (22). The questions of how and when GTP hydrolysis occurs during LepA's functional cycle and how the CTD is involved are important for future studies of LepA's mechanism of action.
A mechanism for the proposed back-translocation activity of LepA has recently been suggested by Zhang and colleagues. They used CTD variants in toeprinting and chemical footprinting experiments to show that when LepA is bound to PRE state ribosomes, the CTD makes extensive contacts with the A/L-state (A/4-state) tRNA that allow LepA to “capture” the back-translocated tRNA and stabilize it in the PRE state (23). Additionally, they reported that when LepA initially binds to the POST state ribosome, it disengages the CCA end of the P-tRNA from the P loop of the PTC on the 50S subunit, shifting the equilibrium to favor movement of the tRNA to the A site (23).
When considering the overall mechanism of back-translocation, it will be useful to keep in mind the thermodynamics of EF-G-mediated translocation and the various affinities of specific tRNAs for different binding sites on the ribosome. It has been shown that in the absence of EF-G, translocation is reversible provided that the ribosome contains both P- and E-site tRNAs (39). That study found that for spontaneous reverse translocation to occur, the E-tRNA must be cognate to the mRNA codon in the E site. However, this occupancy may be transient, as in the case of fMet-tRNAfMet, which has a weak affinity for the E site. The authors proposed that LepA may act to shift the equilibrium from the POST to the PRE state, as EF-G does for the opposite reaction (39).
LepA as a ribosome-sequestering factor.
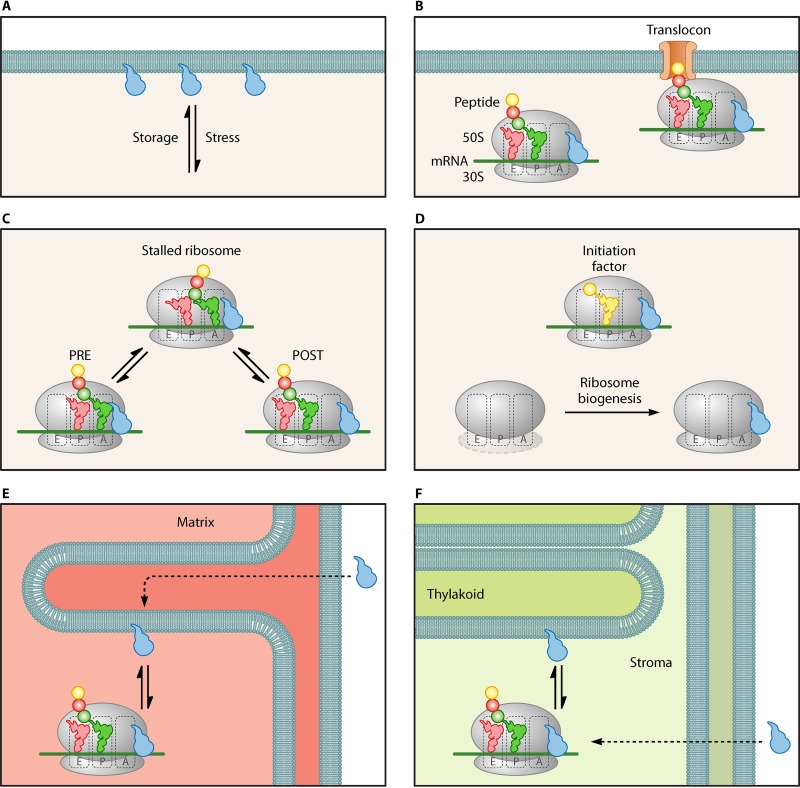
In contrast to the proposed function of LepA as a back-translocase that preferentially binds to POST state ribosomes, it has been suggested that LepA may be able to sequester a translating ribosome by competing with EF-G for binding to the PRE state ribosome. Under normal conditions, the cytosolic concentration of EF-G is 50-fold higher than that of LepA (11). Liu et al. demonstrated that at high enough LepA concentrations (5- to 10-fold higher than the EF-G concentration), LepA is able to compete with EF-G for binding to the PRE state ribosome, as shown by the conversion of the PRE complex to an X3 intermediate (16). This intermediate state exhibits properties between those of the PRE and POST states with regard to puromycin reactivity and fluorescence of complexes programed with pyrene-mRNA-09. The X3 intermediate was shown to be favored over the I3 state, which was previously shown by this group to form during the conversion from POST to PRE (38). This suggests a preference for LepA to bind to the PRE state ribosomes under conditions where the cytosolic LepA concentration is increased. The resulting increased concentration of LepA in the cytosol may lead to an increase of LepA-bound PRE state ribosomes, slowing down translation and improving cotranslational folding. This would be consistent with the increased protein activity observed in the presence of LepA in vitro (8). Pech et al. showed that LepA moves to the cytosol at high Mg2+ concentrations and low pH, as well as at low temperatures (11), leading to a model in which LepA is released under stress conditions to perform its function in the cytosol (Fig. 3A). An argument against this model is that only a 20-fold increase has been shown to occur, which may not be substantial enough to compete with EF-G (11). However, it can be speculated that LepA may bind to a different functional ribosomal complex than EF-G. An alternative hypothesis that could explain LepA's membrane-bound nature is that LepA may function as part of the translocon complex (Fig. 3B).
FIG 3.
Schematic representation of proposed models regarding LepA's function. (A) LepA associating with the membrane. (B) LepA associating with ribosomes involved in the translocon complex. (C) LepA as an elongation factor that binds to one of two ribosomal states and results in stalling. (D) LepA as an initiation factor or a ribosome biogenesis factor (E) LepA (GUF1) function in the mitochondria. LepA associates with the inner membrane and dissociates under stress conditions to aid in the translation of mitochondrial membrane proteins. (F) LepA (cpLepA) function in the chloroplast. LepA associates with the thylakoid membrane and dissociates under stress conditions to aid in the translation of chloroplast proteins.
Regardless of the ribosomal state to which LepA binds these observations suggest that LepA may catalyze the formation of an intermediate that would result in the stalling of translation, sequestering the ribosome either temporarily or to guide it to a subsequent process (Fig. 3C). The recent X-ray structure of LepA bound to the 70S ribosome obtained by Gagnon et al. may support LepA's function as a ribosome-sequestering factor. This structure shows that LepA's CTD reaches into the P site to interact with the P-tRNA likely protecting the attached amino acid from hydrolysis. For the CTD to reach into the PTC, the classical A-tRNA would have to be displaced. This may occur as a consequence of the clockwise rotating movement of the ribosome upon LepA binding (27).
To study this particular model, it will be important to further characterize LepA's response to stress. For example, it may be beneficial to look at the change in stoichiometry of ribosomes bound to LepA under different stress conditions. If ribosome sequestering is in fact the main function of LepA in the cell, the following questions remain. What is the signal for LepA binding, and does it select for specific mRNAs to bind to and sequester? Does LepA's CTD have a role in sensing the peptide being synthesized? If this is the case, which genes are targeted by LepA? Additionally, what is the role of GTP hydrolysis in LepA-induced ribosome sequestration?
Alternative hypotheses regarding LepA's cellular role.
The recent crystal structure of LepA-GDPCP bound to the PRE state ribosome has shown that LepA's CTD displaces the CCA end of the A/L-tRNA away from the PTC (15). This observation led to a number of suggestions. It is possible that the displacement of the CCA end destabilizes the A/L-tRNA and causes it to dissociate from the ribosome, a function that would be useful under conditions when deacylated tRNAs bind to the A site to trigger a stress response. Alternatively, LepA may facilitate tRNA movement to resolve a stalled ribosome by “pulling” on the acceptor stem of the A/L-tRNA to pull the peptide chain backward along the peptide exit tunnel; this suggestion is in contrast to the previously postulated idea that LepA functions to sequester ribosomes (16). The “pulling” of the acceptor stem may lead to the unfolding of a misfolded protein outside the exit tunnel in order to give it a second chance to fold, which would be in agreement with the observation that LepA increases the active fraction of protein synthesized (8).
The phenomenon of ribosome tunnel perturbation in stalling translation is not uncommon. An example includes the stimulatory effect of erythromycin on the expression of ErmB. In this case, the presence of erythromycin during translation of the leader peptide ErmBL causes stalling of the ribosome and subsequent conformational changes to the ermB gene that induce expression. It was shown through cryo-EM and molecular dynamics experiments that ErmBL and erythromycin perturb the PTC at the A- and P-tRNA positions in order to inhibit formation of peptide bonds and induce translational arrest (40).
LepA's proposed function in ribosome biogenesis.
Although a role in translocation has been the main focus of mechanistic investigation in the past, evidence that LepA may play a role in translation initiation rather than elongation has been reported (14). Balakrishnan et al. screened for synthetic phenotypes in LepA knockout strains using the Keio collection. They found that deletion of genes involved in gene regulation, ribosome assembly, transport, or respiration caused growth phenotypes. Supplementing LepA in the form of a plasmid rescued, at least partially, the effects caused by these deletions. More interestingly, LepA lacking its CTD or catalytic histidine (H81) could not rescue these phenotypes, suggesting that both the CTD and LepA's GTPase activity are essential for the in vivo function of LepA (14), a hypothesis supported by recent in vitro observations focusing on these elements (22). Furthermore, LepA generally increases average ribosome density (14), thereby enhancing translation efficiency of a large number of genes. Together with the evidence that LepA has only subtle effects on elongation, the authors suggested that LepA's primary role has to do with initiation either directly as an initiation factor or indirectly by having a role in ribosome biogenesis (Fig. 3D). New evidence from the Fredrick lab supports the latter hypothesis. They found that LepA deletion strains exhibit the accumulation of immature 30S particles and suggest that LepA is involved in biogenesis of the 30S subunit (41).
Considerations for future LepA studies.
With evidence to support such different functions of LepA, further work is required in order to narrow down LepA's specific cellular function. However, the available information clearly indicates that LepA's CTD is paramount to its role in the cell. LepA's CTD contains a 44-amino-acid subdomain that is important for its GTP-dependent function on the ribosome (22) and that has also been shown to be highly flexible (23, 27). The flexibility of the subdomain likely plays a key role in the CTD's interaction with the ribosome. It may be able to sense the functional state of the ribosome, which would be significant either for the back-translocation reaction or during ribosome sequestration. Alternatively, the CTD is able to sense the peptide being translated, helping LepA to specifically bind to ribosomes that translate certain mRNAs.
It will be important to reconcile the available in vitro observations with in vivo data and to carry out further in vivo studies to home in on the role of LepA in the cell. Important considerations will include the mechanism by which cytoplasmic concentrations of LepA are regulated and whether the type of stress experienced by the cell specifies which mRNAs/ribosomes LepA is likely to act on. Integrating the information regarding eukaryotic homologs of LepA will also be necessary for our understanding due to the high conservation between bacterial and eukaryotic LepAs.
EUKARYOTIC HOMOLOGS OF LepA
Homologs of LepA are present in all known bacterial and eukaryotic genomes (8) and have been studied in yeast, plants, nematodes, and mice. In eukaryotes, LepA homologs are present exclusively in the mitochondria and chloroplasts. Mitochondrial LepA is often referred to as GUF1 (for GTPase of unknown function) or as mtLepA (for mitochondrial LepA), while chloroplast LepA is referred to as cpLepA (for chloroplast LepA). Conservation between homologs in each of these species is quite high: GUF1 in Saccharomyces cerevisiae shares 65% similarity (43% identity) with E. coli LepA and 64% similarity (42% identity) with Caenorhabditis elegans (42), cpLepA in Arabidopsis thaliana shares 64% to 87% identity with its bacterial and eukaryotic homologs (43), and mouse mtLepA shares 50% identity with E. coli LepA (44). This high conservation between such divergent groups is indicative of some function that has remained crucial over billions of years of evolution. Encoded by the nuclear genome, GUF1/cpLepA contains an N-terminal signaling sequence that targets the protein for transport into the mitochondria or chloroplast (8, 9, 43) (Fig. 3E and F).
Localization of LepA homologs parallels that seen in the bacterial protein, which localizes to the cell membrane under normal conditions (5). Similarly, GUF1 is found in the mitochondrial matrix and localizes to the inner membrane (9, 12), and cpLepA localizes to the thylakoid membrane (43). Also similar to the case for bacterial LepA, the eukaryotic homologs are nonessential under normal conditions but are required for successful growth and protein synthesis under stress conditions such as suboptimal temperature or nutrient limitation (9, 12, 43). Additionally, GUF1 was shown to bind mitochondrial ribosomes in a GTP-dependent manner (9).
Most interestingly, LepA homologs seem to be required for the synthesis of proteins that are essential to the function of mitochondria and chloroplasts, indicating a strong evolutionary pressure for the conservation of the protein. In yeast, the deletion of GUF1 results in the defective assembly of cytochrome oxidase complexes (9), suggesting that GUF1 is essential for the efficient translation of these proteins (Fig. 3E). Studies on GUF1 in C. elegans appear to yield similar results, as deletion of the protein results in reduced mitochondrial translation and the disruption of respiratory chain supercomplexes at low temperature (12). Similarly, it has been suggested that cpLepA in plants is essential for the synthesis of photosystem II proteins (Fig. 3F), as cpLepA mutants showed decreased photosynthesis activity and impaired chloroplast development in A. thaliana (43). It is unknown how LepA and its homologs might increase the translation efficiency of specific proteins under suboptimal temperatures, but it may be reasonable to speculate that the mechanism of action of GUF1/cpLepA is similar to that of bacterial LepA. This would be remarkable, considering that the structures of the bacterial and mitochondrial ribosomes differ significantly (45).
Recently, LepA knockouts in mice have revealed that LepA is essential for spermatogenesis, a process that depends largely on mitochondrial translation (44). When LepA was knocked out, mice exhibited abnormal mitochondria in their spermatogonia, spermatocytes, and sperm; in some cases, these cells lacked an inner membrane system. Additionally, knockout mice had reduced levels of the complexes involved in oxidative phosphorylation and complex IV, which is encoded only by mitochondrial DNA (mtDNA), was especially reduced (44). This is consistent with the observation that mtLepA is crucial for quality control of protein synthesis in the mitochondria (9, 12).
The fact that LepA is found in bacteria, mitochondria, and chloroplasts but is missing from the eukaryotic cytosol and from archaea raises the question of how it evolved to function only in these areas. Mitochondrial and chloroplast ribosomes are more similar to those found in bacteria than to those in the cytosol. It is possible that LepA functions only on bacterial (or bacterium-like) ribosomes because eukaryotic ribosomes may have evolved a protein with functionality resembling that of LepA or because eukaryotic translation does not proceed through a stage that requires the action of LepA.
More work is required to define how closely the functions of bacterial and eukaryotic homologs of LepA are linked. LepA appears to aid in protein synthesis under stress conditions in both bacteria and eukaryotes, but we do not have enough evidence to determine which stage of translation LepA is most prominently implicated in. LepA has recently been suggested to function during initiation/ribosome biogenesis in bacteria (14, 41); no direct evidence for this has been shown in mitochondria. However, study of cpLepA in A. thaliana suggests the possibility that cpLepA gene knockout strains contain a lower-than-normal level of chloroplast-encoded mRNAs associated with ribosomes due to defective translation initiation (43).
CONCLUSION
Although much structural and biochemical information regarding LepA/EF4 has become available over the last 10 years, its cellular function still remains unclear. No doubt exists that the function of LepA in the cell is related to protein synthesis; however, the precise role remains a matter of debate.
This is particularly interesting because LepA is highly conserved on the sequence level, suggesting a role that is very sensitive to sequence variations. This can be the result of a restrained sequence space caused by the necessity to maintain the ability to interact with several molecular partners during the functional cycle, creating high evolutionary pressure. Examples for this are EF-Tu and EF-Ts (46), where EF-Ts is drastically less conserved than EF-Tu although they interact during their individual functional cycles. Such a strong evolutionary constraint on LepA's sequence, comparable that for to EF-Tu and EF-G, is surprising given that LepA is not equally essential for bacterial growth under optimal conditions. However, a central role for the translation machinery is consistent with the fact the LepA gene is one of the core genes retained in the reduced genomes of mollicutes (47). With this background in mind, it will be interesting to see what is different about the translation machinery in organisms that either do not have or have lost LepA (e.g., archaea and the bacteria Streptococcus pyogenes and Carsonella ruddii). The absence of LepA in archaea and eukaryotes might make LepA a bacterial invention that was retained throughout evolution even in mitochondria and chloroplasts. The latter is particularly remarkable and might hold interesting hints at the cellular function of LepA. The mitochondrial translation machinery has substantially diverged from the bacterial translation machinery (45), specializing in the translation of only 14 mostly hydrophobic proteins and their cotranslational delivery to the membrane. These proteins are all critical for the function of the respiratory chain complexes located in the mitochondrial membrane. The observed defects in the respiratory chain complexes of LepA knockout mouse testis mitochondria support a role in membrane protein synthesis, such as folding or membrane insertion (44).
Integrating the information summarized here, it will be critical to step back from the perceived function of LepA as a back-translocase and allow for a wider systems view, which is necessary to instruct future investigation into the cellular function and molecular mechanism of LepA.
ACKNOWLEDGMENTS
This work was supported by funding from the Canadian Institutes of Health Research operating grants program (H.J.W.) and Alberta Innovates Technology Futures (H.-J.W., Strategic Chairs Program).
We thank Emily Wilton for helpful comments on the manuscript, Dylan Girodat for assistance with Fig. 1, and Harland Brandon for excellent discussion.
REFERENCES
- 1.Bourne HR, Sanders DA, McCormick F. 1990. The GTPase superfamily: a conserved switch for diverse cell functions. Nature 348:125–132. doi: 10.1038/348125a0. [DOI] [PubMed] [Google Scholar]
- 2.Margus T, Remm M, Tenson T. 2007. Phylogenetic distribution of translational GTPases in bacteria. BMC Genomics 8:15. doi: 10.1186/1471-2164-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Canonaco MA, Calogero RA, Gualerzi CO. 1986. Mechanism of translational initiation in prokaryotes. Evidence for a direct effect of IF2 on the activity of the 30 S ribosomal subunit. FEBS Lett 207:198–204. [DOI] [PubMed] [Google Scholar]
- 4.Milon P, Carotti M, Konevega AL, Wintermeyer W, Rodnina MV, Gualerzi CO. 2010. The ribosome-bound initiation factor 2 recruits initiator tRNA to the 30S initiation complex. EMBO Rep 11:312–316. doi: 10.1038/embor.2010.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.March PE, Inouye M. 1985. Characterization of the lep operon of Escherichia coli. Identification of the promoter and the gene upstream of the signal peptidase I gene. J Biol Chem 260:7206–7213. [PubMed] [Google Scholar]
- 6.March PE, Inouye M. 1985. GTP-binding membrane protein of Escherichia coli with sequence homology to initiation factor 2 and elongation factors Tu and G. Proc Natl Acad Sci U S A 82:7500–7504. doi: 10.1073/pnas.82.22.7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dibb NJ, Wolfe PB. 1986. lep operon proximal gene is not required for growth or secretion by Escherichia coli. J Bacteriol 166:83–87. doi: 10.1128/jb.166.1.83-87.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qin Y, Polacek N, Vesper O, Staub E, Einfeldt E, Wilson DN, Nierhaus KH. 2006. The highly conserved LepA is a ribosomal elongation factor that back-translocates the ribosome. Cell 127:721–733. doi: 10.1016/j.cell.2006.09.037. [DOI] [PubMed] [Google Scholar]
- 9.Bauerschmitt H, Funes S, Herrmann JM. 2008. The membrane-bound GTPase Guf1 promotes mitochondrial protein synthesis under suboptimal conditions. J Biol Chem 283:17139–17146. doi: 10.1074/jbc.M710037200. [DOI] [PubMed] [Google Scholar]
- 10.Bijlsma JJ, Lie ALM, Nootenboom IC, Vandenbroucke-Grauls CM, Kusters JG. 2000. Identification of loci essential for the growth of Helicobacter pylori under acidic conditions. J Infect Dis 182:1566–1569. doi: 10.1086/315855. [DOI] [PubMed] [Google Scholar]
- 11.Pech M, Karim Z, Yamamoto H, Kitakawa M, Qin Y, Nierhaus KH. 2011. Elongation factor 4 (EF4/LepA) accelerates protein synthesis at increased Mg2+ concentrations. Proc Natl Acad Sci U S A 108:3199–3203. doi: 10.1073/pnas.1012994108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang F, Gao Y, Li Z, Chen L, Xia Z, Xu T, Qin Y. 2014. Mitochondrial EF4 links respiratory dysfunction and cytoplasmic translation in Caenorhabditis elegans. Biochim Biophys Acta 1837:1674–1683. doi: 10.1016/j.bbabio.2014.05.353. [DOI] [PubMed] [Google Scholar]
- 13.Yang F, Li Z, Hao J, Qin Y. 2014. EF4 knockout E. coli cells exhibit lower levels of cellular biosynthesis under acidic stress. Protein Cell 5:563–567. doi: 10.1007/s13238-014-0050-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balakrishnan R, Oman K, Shoji S, Bundschuh R, Fredrick K. 2014. The conserved GTPase LepA contributes mainly to translation initiation in Escherichia coli. Nucleic Acids Res 42:13370–13383. doi: 10.1093/nar/gku1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gagnon MG, Lin J, Steitz TA. 2016. Elongation factor 4 remodels the A-site tRNA on the ribosome. Proc Natl Acad Sci U S A 113:4994–4999. doi: 10.1073/pnas.1522932113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu H, Chen C, Zhang H, Kaur J, Goldman YE, Cooperman BS. 2011. The conserved protein EF4 (LepA) modulates the elongation cycle of protein synthesis. Proc Natl Acad Sci U S A 108:16223–16228. doi: 10.1073/pnas.1103820108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evans RN, Blaha G, Bailey S, Steitz TA. 2008. The structure of LepA, the ribosomal back translocase. Proc Natl Acad Sci U S A 105:4673–4678. doi: 10.1073/pnas.0801308105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diaconu M, Kothe U, Schlunzen F, Fischer N, Harms JM, Tonevitsky AG, Stark H, Rodnina MV, Wahl MC. 2005. Structural basis for the function of the ribosomal L7/12 stalk in factor binding and GTPase activation. Cell 121:991–1004. doi: 10.1016/j.cell.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 19.Savelsbergh A, Mohr D, Wilden B, Wintermeyer W, Rodnina MV. 2000. Stimulation of the GTPase activity of translation elongation factor G by ribosomal protein L7/12. J Biol Chem 275:890–894. doi: 10.1074/jbc.275.2.890. [DOI] [PubMed] [Google Scholar]
- 20.Nechifor R, Murataliev M, Wilson KS. 2007. Functional interactions between the G′ subdomain of bacterial translation factor EF-G and ribosomal protein L7/L12. J Biol Chem 282:36998–37005. doi: 10.1074/jbc.M707179200. [DOI] [PubMed] [Google Scholar]
- 21.Laurberg M, Kristensen O, Martemyanov K, Gudkov AT, Nagaev I, Hughes D, Liljas A. 2000. Structure of a mutant EF-G reveals domain III and possibly the fusidic acid binding site. J Mol Biol 303:593–603. doi: 10.1006/jmbi.2000.4168. [DOI] [PubMed] [Google Scholar]
- 22.De Laurentiis EI, Wieden HJ. 2015. Identification of two structural elements important for ribosome-dependent GTPase activity of elongation factor 4 (EF4/LepA). Sci Rep 5:8573. doi: 10.1038/srep08573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang D, Yan K, Liu G, Song G, Luo J, Shi Y, Cheng E, Wu S, Jiang T, Lou J, Gao N, Qin Y. 2016. EF4 disengages the peptidyl-tRNA CCA end and facilitates back-translocation on the 70S ribosome. Nat Struct Mol Biol 23:125–131. doi: 10.1038/nsmb.3160. [DOI] [PubMed] [Google Scholar]
- 24.Gao YG, Selmer M, Dunham CM, Weixlbaumer A, Kelley AC, Ramakrishnan V. 2009. The structure of the ribosome with elongation factor G trapped in the posttranslocational state. Science 326:694–699. doi: 10.1126/science.1179709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Connell SR, Topf M, Qin Y, Wilson DN, Mielke T, Fucini P, Nierhaus KH, Spahn CM. 2008. A new tRNA intermediate revealed on the ribosome during EF4-mediated back-translocation. Nat Struct Mol Biol 15:910–915. doi: 10.1038/nsmb.1469. [DOI] [PubMed] [Google Scholar]
- 26.Kumar V, Chen Y, Ero R, Ahmed T, Tan J, Li Z, Wong AS, Bhushan S, Gao YG. 2015. Structure of BipA in GTP form bound to the ratcheted ribosome. Proc Natl Acad Sci U S A 112:10944–10949. doi: 10.1073/pnas.1513216112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gagnon MG, Lin J, Bulkley D, Steitz TA. 2014. Crystal structure of elongation factor 4 bound to a clockwise ratcheted ribosome. Science 345:684–687. doi: 10.1126/science.1253525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maracci C, Rodnina MV. 2016. Review: translational GTPases. Biopolymers 105:463–475. doi: 10.1002/bip.22832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kiss E, Huguet T, Poinsot V, Batut J. 2004. The typA gene is required for stress adaptation as well as for symbiosis of Sinorhizobium meliloti 1021 with certain Medicago truncatula lines. Mol Plant Microbe Interact 17:235–244. doi: 10.1094/MPMI.2004.17.3.235. [DOI] [PubMed] [Google Scholar]
- 30.Pfennig PL, Flower AM. 2001. BipA is required for growth of Escherichia coli K12 at low temperature. Mol Genet Genomics 266:313–317. doi: 10.1007/s004380100559. [DOI] [PubMed] [Google Scholar]
- 31.Starosta AL, Lassak J, Jung K, Wilson DN. 2014. The bacterial translation stress response. FEMS Microbiol Rev 38:1172–1201. doi: 10.1111/1574-6976.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choudhury P, Flower AM. 2015. Efficient assembly of ribosomes is inhibited by deletion of bipA in Escherichia coli. J Bacteriol 197:1819–1827. doi: 10.1128/JB.00023-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colca JR, McDonald WG, Waldon DJ, Thomasco LM, Gadwood RC, Lund ET, Cavey GS, Mathews WR, Adams LD, Cecil ET, Pearson JD, Bock JH, Mott JE, Shinabarger DL, Xiong L, Mankin AS. 2003. Cross-linking in the living cell locates the site of action of oxazolidinone antibiotics. J Biol Chem 278:21972–21979. doi: 10.1074/jbc.M302109200. [DOI] [PubMed] [Google Scholar]
- 34.Leach KL, Swaney SM, Colca JR, McDonald WG, Blinn JR, Thomasco LM, Gadwood RC, Shinabarger D, Xiong L, Mankin AS. 2007. The site of action of oxazolidinone antibiotics in living bacteria and in human mitochondria. Mol Cell 26:393–402. doi: 10.1016/j.molcel.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 35.Kumar V, Ero R, Ahmed T, Goh KJ, Zhan Y, Bhushan S, Gao YG. 2016. Structure of the GTP form of elongation factor 4 (EF4) bound to the ribosome. J Biol Chem 291:12943–12950. doi: 10.1074/jbc.M116.725945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Connell SR, Takemoto C, Wilson DN, Wang H, Murayama K, Terada T, Shirouzu M, Rost M, Schuler M, Giesebrecht J, Dabrowski M, Mielke T, Fucini P, Yokoyama S, Spahn CM. 2007. Structural basis for interaction of the ribosome with the switch regions of GTP-bound elongation factors. Mol Cell 25:751–764. doi: 10.1016/j.molcel.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 37.Shoji S, Janssen BD, Hayes CS, Fredrick K. 2010. Translation factor LepA contributes to tellurite resistance in Escherichia coli but plays no apparent role in the fidelity of protein synthesis. Biochimie 92:157–163. doi: 10.1016/j.biochi.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu H, Pan D, Pech M, Cooperman BS. 2010. Interrupted catalysis: the EF4 (LepA) effect on back-translocation. J Mol Biol 396:1043–1052. doi: 10.1016/j.jmb.2009.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Konevega AL, Fischer N, Semenkov YP, Stark H, Wintermeyer W, Rodnina MV. 2007. Spontaneous reverse movement of mRNA-bound tRNA through the ribosome. Nat Struct Mol Biol 14:318–324. doi: 10.1038/nsmb1221. [DOI] [PubMed] [Google Scholar]
- 40.Arenz S, Bock LV, Graf M, Innis CA, Beckmann R, Grubmuller H, Vaiana AC, Wilson DN. 2016. A combined cryo-EM and molecular dynamics approach reveals the mechanism of ErmBL-mediated translation arrest. Nat Commun 7:12026. doi: 10.1038/ncomms12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gibbs MR, Moon K-M, Chen M, Balakrishnan R, Foster LJ, Fredrick K. 2017. Conserved GTPase LepA (elongation factor 4) functions in biogenesis of the 30S subunit of the 70S ribosome. Proc Natl Acad Sci U S A 114:980–985. doi: 10.1073/pnas.1613665114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kiser GL, Weinert TA. 1995. GUF1, a gene encoding a novel evolutionarily conserved GTPase in budding yeast. Yeast 11:1311–1316. doi: 10.1002/yea.320111312. [DOI] [PubMed] [Google Scholar]
- 43.Ji DL, Lin H, Chi W, Zhang LX. 2012. CpLEPA is critical for chloroplast protein synthesis under suboptimal conditions in Arabidopsis thaliana. PLoS One 7:e49746. doi: 10.1371/journal.pone.0049746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gao Y, Bai X, Zhang D, Han C, Yuan J, Liu W, Cao X, Chen Z, Shangguan F, Zhu Z, Gao F, Qin Y. 2016. Mammalian elongation factor 4 regulates mitochondrial translation essential for spermatogenesis. Nat Struct Mol Biol 23:441–449. doi: 10.1038/nsmb.3206. [DOI] [PubMed] [Google Scholar]
- 45.Greber BJ, Ban N. 2016. Structure and function of the mitochondrial ribosome. Annu Rev Biochem 85:103–132. doi: 10.1146/annurev-biochem-060815-014343. [DOI] [PubMed] [Google Scholar]
- 46.De Laurentiis EI, Mercier E, Wieden HJ. 2016. The C-terminal helix of Pseudomonas aeruginosa elongation factor Ts tunes EF-Tu dynamics to modulate nucleotide exchange. J Biol Chem 291:23136–23148. doi: 10.1074/jbc.M116.740381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grosjean H, Breton M, Sirand-Pugnet P, Tardy F, Thiaucourt F, Citti C, Barre A, Yoshizawa S, Fourmy D, de Crecy-Lagard V, Blanchard A. 2014. Predicting the minimal translation apparatus: lessons from the reductive evolution of mollicutes. PLoS Genet 10:e1004363. doi: 10.1371/journal.pgen.1004363. [DOI] [PMC free article] [PubMed] [Google Scholar]