Abstract
2-Deoxyglucose (2-DG) is a toxic glucose analog. To identify genes involved in 2-DG toxicity in Schizosaccharomyces pombe, we screened a wild-type overexpression library for genes which render cells 2-DG resistant. A gene we termed odr1, encoding an uncharacterized hydrolase, led to strong resistance and altered invertase expression when overexpressed. We speculate that Odr1 neutralizes the toxic form of 2-DG, similar to the Saccharomyces cerevisiae Dog1 and Dog2 phosphatases which dephosphorylate 2-DG-6-phosphate synthesized by hexokinase. In a complementary approach, we screened a haploid deletion library to identify 2-DG-resistant mutants. This screen identified the genes snf5, ypa1, pas1 and pho7. In liquid medium, deletions of these genes conferred 2-DG resistance preferentially under glucose-repressed conditions. The deletion mutants expressed invertase activity more constitutively than the control strain, indicating defects in the control of glucose repression. No S. cerevisiae orthologs of the pho7 gene is known, and no 2-DG resistance has been reported for any of the deletion mutants of the other genes identified here. Moreover, 2-DG leads to derepressed invertase activity in S. pombe, while in S. cerevisiae it becomes repressed. Taken together, these findings suggest that mechanisms involved in 2-DG resistance differ between budding and fission yeasts.
Keywords: fission yeast, glucose metabolism, 2-Deoxyglucose resistance, invertase activity, odr1 overexpression, deletion library
Genes that are required to provide resistance to the anticancer molecule 2-Deoxyglucose when overexpressed or deleted in fission yeast have been identified.
INTRODUCTION
There are two main reasons to study the mechanisms of action of the toxic glucose analog 2-Deoxyglucose (2-DG). First, in the budding yeast Saccharomyces cerevisiae 2-DG inhibits growth, affects cell wall synthesis, causes abnormal cell morphology and cell lysis, and interferes with glucose metabolism (Biely et al.1971; Krátký, Biely and Bauer 1975). A series of pioneering papers (Zimmermann and Scheel 1977; Entian and Zimmermann 1980; Neigeborn and Carlson 1987) have identified mutants resistant to 2-DG, and corresponding genes have been characterized leading to insights into the underlying mechanisms (for additional references, see McCartney et al.2014). Second, 2-DG shows anticancer activity in humans by inhibiting tumor growth with yeast providing a model system to understand its mode of action (Cairns, Harris and Mak 2011; Raez et al.2013). Aerobic glycolysis is a metabolic pathway that is of particular importance to cancer cells for generating energy, and 2-DG is thought to impede this pathway by inhibiting several of its enzymes (Pelicano et al.2006). Glycolysis is also the metabolic pathway used by rapidly proliferating yeast cells to ferment glucose to ethanol. After cellular uptake, hexokinase phosphorylates 2-DG to the highly toxic 2-DG-6-phosphate, which in turn cannot be further converted to fructose-6-phosphate (Jaspers and van Steveninck 1975; Lobo and Maitra 1977).
The fission yeast, Schizosaccharomyces pombe, is only remotely related to budding yeast and provides a valuable complementary model organism, being in several ways more closely related to humans than to budding yeast (Hoffman, Wood and Fantes 2015). Research by the Hoffman laboratory and others on glucose metabolism has advanced our understanding of glucose signaling in fission yeast. Glucose is sensed by the Git/Protein Kinase A (PKA) pathway (Hoffman 2005) and the glucose repression pathway involving glucose-6-phosphate (Roux et al.2009). Based on work in S. cerevisiae, 2-DG alters glucose sensing and induces a glucose starvation signal (O'Donnell et al.2015). During glucose starvation, S. pombe PKA forms a complex with its regulatory subunit, and it becomes phosphorylated and re-localized to the cytoplasm (Gupta et al.2011). Glucose starvation triggers the stress-activated protein kinase pathway in S. pombe (Madrid et al.2006, 2013), resulting in a gene expression response (Madrid et al.2004; Kato, Kira and Kawamukai 2013).
In S. pombe, 2-DG leads to deformed cells that lyse at the site of glucan synthesis (Megnet 1965; Johnson 1968). In addition, the 2-DG-resistant std1 mutant shows defects in glucose transport (Mehta et al.1998), and other 2-DG-resistant mutants exhibiting derepressed invertase activity and impaired glucose uptake map to four different genetic loci (Kig, Turkel and Temizkan 2005). Some glucose transport-deficient S. pombe mutants are also resistant to 2-DG, but 2-DG resistance and glucose transport deficiency have been attributed to different genetic loci (Milbradt and Höfer 1994). Here, we systematically identify genes that cause 2-DG resistance when overexpressed or when deleted to obtain functional information on 2-DG action in fission yeast.
MATERIALS AND METHODS
Media, reagents and strains
Standard methods and media were used for growth of Schizosaccharomyces pombe strains. The minimal media used were Edinburgh minimal medium 2 (EMM2) (Moreno, Klar and Nurse 1991) and MM as described (Schweingruber and Edenharter 1990), containing either 2% or 0.5% glucose. In contrast to EMM, MM allows to study growth as well as mating and sporulation. It was therefore chosen to characterize the 2-DG-resistant strains. The two media differ in their ammonium content. All media contained leucine, adenine and uracil supplements as required by the strains at 50 μg/ml. 2-deoxy D glucose ≥99% pure was purchased from Sigma Aldrich, USA. The GOD-POD assay kit (Autospan liquid gold glucose kit) for measuring invertase activity was obtained from SPAN diagnostics (India). The primers were obtained from Chromous Biotech, Bangalore, India. The ura4-D18 h− strain and the haploid deletion mutant library, along with the parent control strain ade6-M210 ura4-D18 leu1-32 h+, were from the collection of the Bähler laboratory. It corresponds to the latest haploid disruption library version from Bioneer (v5.0), covering 98% of all non-essential genes. The generation of this mutant library has been described, and correct genotypes of the deletion mutants have been tested by PCR as described (Kim et al.2010; Rallis et al.2014). Table 1 lists the strains used in this study.
Table 1.
List of strains used in this study.
| Strain name | Genotype | Plasmid |
|---|---|---|
| wt | 972 h− | |
| ura− | ura4 D18 h− | |
| pREP4X | ura4D18 h− | pREP4X |
| pODR1 | ura4 D18 h− | pODR1 |
| pYSP2 | ura4D18 h− | pYSP2 |
| Parent | ade6 M210 ura4 D18 leu1 h+ | |
| odr1Δ | ade6 M210 ura4 D18 leu1 SPBC215.10::KanMX h+ | |
| a clr5Δ | ade6 M210 ura4 D18 leu1 clr5::KanMX h+ | |
| snf5Δ | ade6 M210 ura4 D18 leu1snf5::KanMX h+ | |
| fyv7Δ | ade6 M210 ura4 D18 leu1fyv7::KanMX h+ | |
| pho7Δ | ade6 M210 ura4 D18 leu1pho7::KanMX h+ | |
| ypa1Δ | ade6 M210 ura4 D18 leu1ypa1::KanMX h+ |
This deletion could not be verified by PCR.
Growth experiments
Resistance to 2-DG of strains on solid MM media was tested by growing cells in liquid MM to mid-log phase at 30°C, the cell density was normalized, and in a volume of 5 μl ∼1.6 × 104 and 0.4 × 104 cells were applied on the plates shown in Figs 1 and 3.
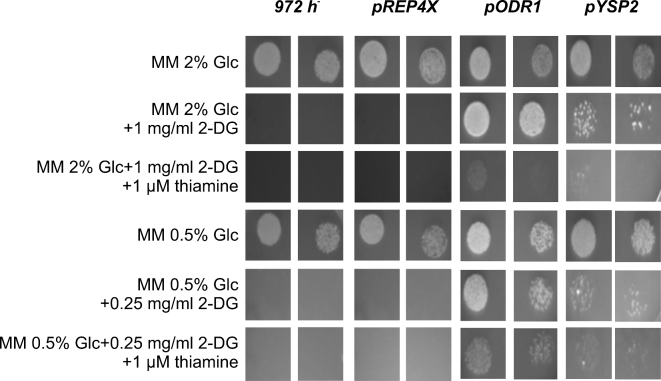
Figure 1.
Growth of odr1 and ysp2 overexpressor strains on solid media in presence and absence of thiamine. Cells containing the plasmids pODR1 and pYSP2 under the control of the nmt1 promoter, along with the control cells containing the plasmids pREP4X and wild-type cells 972, were spotted at two different cell densities on solid MM containing 2% and 0.5% glucose (Glc). Plates contain thiamine and 2-DG as indicated (Materials and Methods). Growth resulting after plating ∼1.6 × 104 and 0.4 × 104 cells as 5 μl drops was assayed. When cells were diluted more, they were not growing at all or only very poorly on 2-DG plates, indicating that 2-DG resistance is highly dependent on initial cell density.
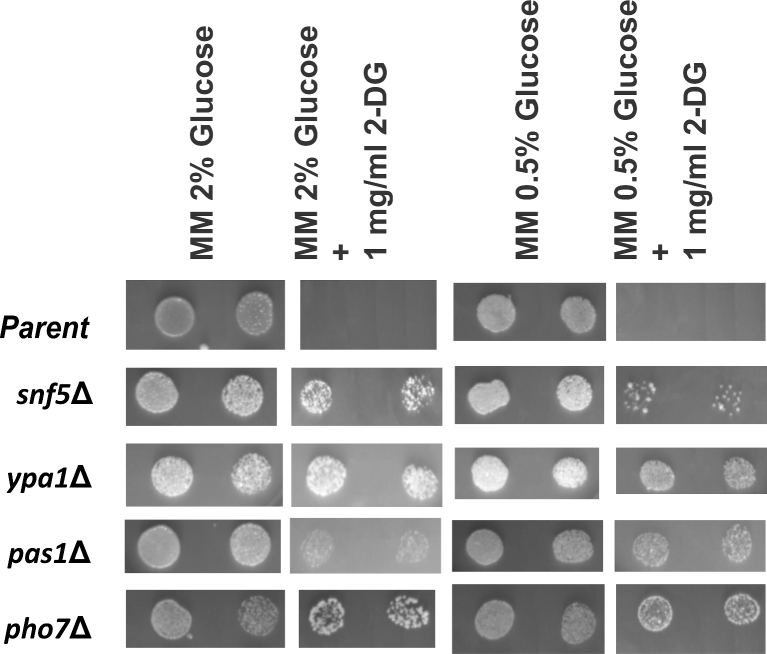
Figure 3.
Growth of deletion mutants in absence and presence of 2-DG on solid media. The mutants as indicated, along with the parent strain (ade6-M210 ura4-D18 leu1-32 h+), were spotted at two different cell densities on MM and MM containing 2-DG plates, both under glucose-repressing and derepressing conditions as given for Fig. 1.
Growth in liquid media was tested by inoculating cells pre-cultured to early mid-log phase in the liquid media given in Figs 2 and 4, and growing cells at 30°C in a rotary shaker at 180–200 rpm. To estimate growth, the optical density was measured at 600 nm and percentage growth inhibition was determined by calculating the percentage decrease between growth in the absence and presence of 2-DG.
Figure 2.
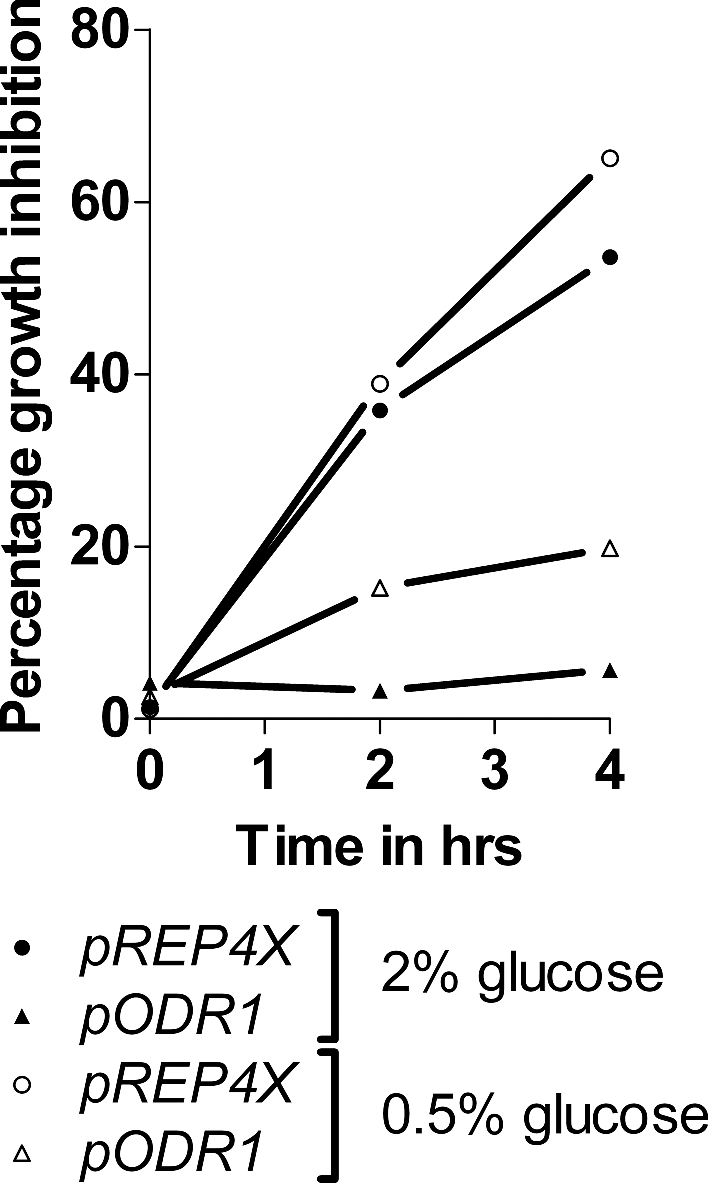
Growth inhibition by 2-DG of the odr1 overexpressor strain in liquid medium. Cells containing the plasmids pODR1 and control cells containing the plasmid pREP4X were grown at 30°C in MM containing 2% or 0.5% glucose as indicated, the presence and absence of 2-DG (0.25 mg/ml). Growth was tested at 2 and 4 h, and percentages of growth inhibition by 2-DG were plotted. The given values represent the mean of two independent experiments.
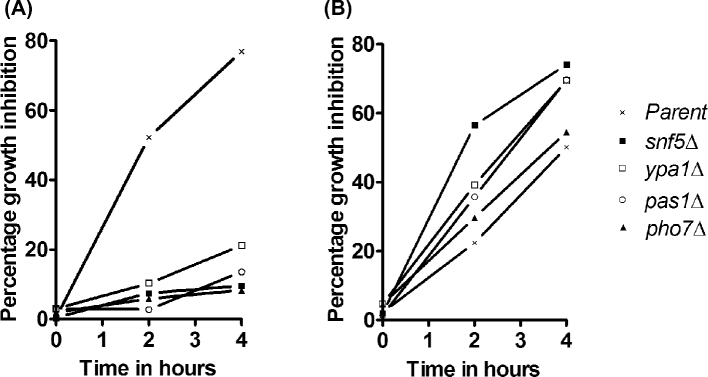
Figure 4.
Growth inhibition of deletion mutants by 2-DG in liquid medium. The mutants as indicated, along with the parent strain (ade6-M210 ura4-D18 leu1-32 h+), were grown at 30°C in MM medium containing 2% glucose (A) or 0.5% glucose (B) in the presence and absence of 2-DG (0.25 mg/ml). Growth was tested after 2 and 4 h and percentages of growth inhibition by 2-DG were plotted. The data represent the mean of two independent experiments.
Plasmids
To overexpress wild-type genes, we used a partial Sau3A genomic library ligated into the shuttle vector pURL18 as described previously (Barbet, Muriel and Carr 1992) (a gift from Viesturs Simanis). To check for accurate structure of the plasmid, we sequenced it (Chromous Biotech, India) (data not shown). Plasmid pREP4X, containing the thiamine repressible nmt1 promoter, was obtained from Susan Forsburg (Forsburg 1993).
Transformations
Strains were transformed by the alkali cation method as described (Moreno, Klar and Nurse 1991) or by the more rapid transformation method (Kanter-Smoler, Dahlkvist and Sunnerhagen 1994).
Screening of the disruption strain library
Screening was performed as described (Rallis et al.2014), but with each mutant represented in quadruplicate. The initial screening was performed on EMM containing 1 mg 2-DG/ml. We re-tested the 2-DG-resistant strains on solid MM containing 1 mg 2-DG/ml. The strains examined in more detail have been tested for co-segregation of the 2-DG and kanamycin resistance marker. In addition, the deletions were validated by colony PCR as previously described (Rallis et al.2014) (Supplementary Table 2, Supporting Information; Supplementary Fig. 2, Supporting Information)
Construction of plasmids containing odr1 and ysp2 under control of nmt1 promoter
Using standard PCR-based methods (Moreno, Klar and Nurse 1991), the genes starting with the translation start codon were fused to the nmt1 promoter. The primers used were for odr1 TAAGCACTCGAGATGCCGTCTAAAGAA (forward primer) and TA-AGCAGGATCCTTATTATTAATTAAAATCAGGAGGGATATTAT (reverse primer) and for ysp1 TAAGCACTCGAGATGAAGGGT-TTAGGTCT and TAAGCAGGATCCTTATTACTAATACTTGCGAGCG. The REP4X plasmid was cut using a Xho1 and BamH1 double digest. The correct sequences of the constructs were verified by sequencing. We call the plasmids containing the odr1 and ysp2 genes under the control of the nmt1 promoter pODR1 and pYSP2, respectively.
Invertase assay
Cells were harvested and washed twice with ice-cold 10 mM sodium azide. Cells were pelleted and resuspended in sterile water to an optical density of 1 at 600 nm, corresponding to ∼107 cells/ml. A total of 100 μl cell suspensions were aliquoted into fresh tubes, centrifuged, cell pellets were resuspended in 50 μl of 50 mM sodium acetate pH 5.1 and invertase activity was determined as described (Harkness and Arnason 2014), using the GOD-POD assay kit. Activities are given as units (micromolar glucose produced per minute) per 106 cells. Statistical analysis was performed using GraphPad Prism 5® (GraphPad Software Inc., La Jolla, CA, USA). Results are expressed as mean ± SEM (n = 3).
RESULTS
odr1 and ysp2 cause increased 2-DG resistance when overexpressed
To identify genes involved in 2-DG toxicity, we first screened for strains that are resistant to 2-DG when transformed with the wild-type gene library pURL18. Out of 77 resistant transformants, we could only isolate two intact nuclear genes. The remaining cells either contained plasmids that harbored truncated nuclear or mitochondrial genes, or the plasmids could not be recovered.
The identified genes were put under the control of the expression vector pREP4X containing the thiamine-repressible promoter nmt1; 2-DG resistance was then examined by growing strains in the presence and absence of thiamine. Knowing from work in Saccharomyces cerevisiae, growth conditions such as carbon sources can drastically alter 2-DG resistance (McCartney et al.2014). We therefore tested the strains both under glucose repressing (2%) and derepressing (0.5%) conditions, and identified two genes leading to 2-DG resistance when overexpressed. For gene SPBC215.10, called here odr1 (for overexpression causing deoxyglucose resistance), the effect of thiamine was strong under glucose-repressing conditions, while under glucose-derepressing conditions, the overexpressing strain still weakly grew in the presence of thiamine (Fig. 1). This effect is not surprising given that full repression of the nmt1 promoter occurs only at 15 μmolar thiamine (Forsburg 1993), a concentration that causes weak 2-DG resistance for unknown reasons (unpublished results). The other gene, ysp2, showed only weak 2-DG resistance when overexpressed (Fig. 1). The few resistant colonies growing on 2-DG-containing plates probably represent cells containing high plasmid copy numbers. The resistant colonies were unlikely to reflect spontaneous 2-DG-resistant mutants, given that no resistant cells were observed for the wild-type cells, parent control cells and the deletion cells (Fig. 3). Given its weak resistance, we did not further study ysp2.
To quantify 2-DG resistance of the odr1 overexpressor strain, we tested its growth in liquid medium. Neither the wild-type nor parent strain did grow with 1 mg/ml 2-DG, so growth was examined at 0.25 mg/ml 2-DG. Cells were inoculated in the presence and absence of 2-DG and growth was measured after 2 and 4 h incubation. Figure 2 shows the growth inhibition by 2-DG. The data confirm that odr1 overexpression leads to 2-DG resistance, although the effect was weaker in liquid low-glucose medium than in high-glucose medium.
Knowing that overexpression of odr1 causes resistance to 2-DG, we tested whether the corresponding deletion mutant (odr1::kanMX ade6-M210 ura4-D18 leu1-32) was more sensitive than its parent strain. However, quantitative measurements in liquid medium showed no increased sensitivity to 2-DG for the odr1 mutant (data not shown).
Identification of four genes causing 2-DG resistance when deleted
In a complementary approach to identify genes involved in 2-DG toxicity, we pre-screened a haploid deletion library for mutants resistant to 2-DG on EMM plates containing 1.0 mg/ml 2-DG. This first screen identified 14 mutants exhibiting 2-DG resistance phenotypes in both of the two independent repeats carried out (Supplementary Fig. 1, Supporting Information; Supplementary Table 1, Supporting Information). We also performed a second screen for mutants resistant to 2-DG on EMM plates containing the lower dose of 0.5 mg/ml 2-DG. This screen identified 59 mutants exhibiting 2-DG resistance phenotypes in both of the two independent repeats carried out. The hits of the second screen included all 14 mutants of the first screen (Supplementary Table 1, Supporting Information).
To characterize growth of the 14 resistant mutants from the first screen, we tested them under the same physiological conditions as the odr1 overexpressor strain in MM containing 2-DG (1.0 mg/ml). For seven mutant strains, deleted for the genes plc1, iec1, adn3, vps38, rxt3, trk2 and moe1, resistance to 2-DG was weak (data not shown), while one mutant, deleted for the gene snz1, did not grow after re-streaking on either MM or EMM for unknown reasons (data not shown). These differences in resistance and growth under the two conditions tested highlight the effect that physiological conditions can have for experiments involving glucose metabolism. For one mutant, deleted for the gene fyv7, the kanamycin resistance did not co-segregate with 2-DG resistance, revealing that the resistance was not caused by the deletion.
Four of these 14 strains identified in the screen exhibited moderate to strong resistance under glucose-repressing conditions on MM plates containing 1.0 mg/ml 2-DG. A fifth strain, clr5Δ, also caused 2-DG resistance, but we could not confirm the deletion by PCR genotyping (Supplementary Fig. 2, Supporting Information). The four PCR-verified strains were deleted for the following genes: snf5, ypa1, pas1 and pho7. For all of these mutants, the kanamycin marker co-segregated with the 2-DG resistance phenotype (data not shown) shows that the resistance is caused by the gene deletion. Examination of cell morphologies of the four resistant mutants did not reveal any evident differences to wild-type cells (data not shown). Except for the mutant snf5Δ, which has previously been reported to be 2-DG resistant (Monahan et al.2008), the other four mutants also grew well under derepressing conditions on solid medium (Fig. 3). Growth tests of the four mutants in liquid medium exhibited strong 2-DG resistance under glucose-repressing conditions (Fig. 4A). On the other hand, these mutants did not show any 2-DG resistance under derepressing conditions, with most showing even higher sensitivity than the parental strain (Fig. 4B). We assume that osmotic stabilization effects acting in colonies but not in liquid medium may be responsible for the different growth behavior.
2-DG-resistant mutants exhibit altered invertase expression
The invertase Inv1 of Schizosaccharomyces pombe is a glucose-repressible cell wall glycoprotein (Moreno et al.1985). Inv1 expression is partially diagnostic for the control of glucose metabolism and particularly for the state of glucose repression. We tested the Inv1 activity in the 2-DG-resistant mutants grown under repressed and derepressed conditions. The mutant strains varied in their activity levels, both in the repressed and derepressed state, when compared to the parent strain, with the greatest upregulation observed in pas1Δ. The deletion mutants snf5Δ and pho7Δ were more affected in glucose derepression, whereas ypa1Δ was more affected under glucose repression (Fig. 5). In summary, comparing the ratios of the activities of cells grown under high- and low-glucose conditions, the mutants and the odr1-overexpressing strain featured a more constitutive invertase phenotype than the control strains (Table 2). Examination of the corresponding odr1Δ mutant revealed a hyperderepression (Table 2). These results indicate that the odr1 gene, in its wild-type configuration, plays a role in glucose repression.
Figure 5.
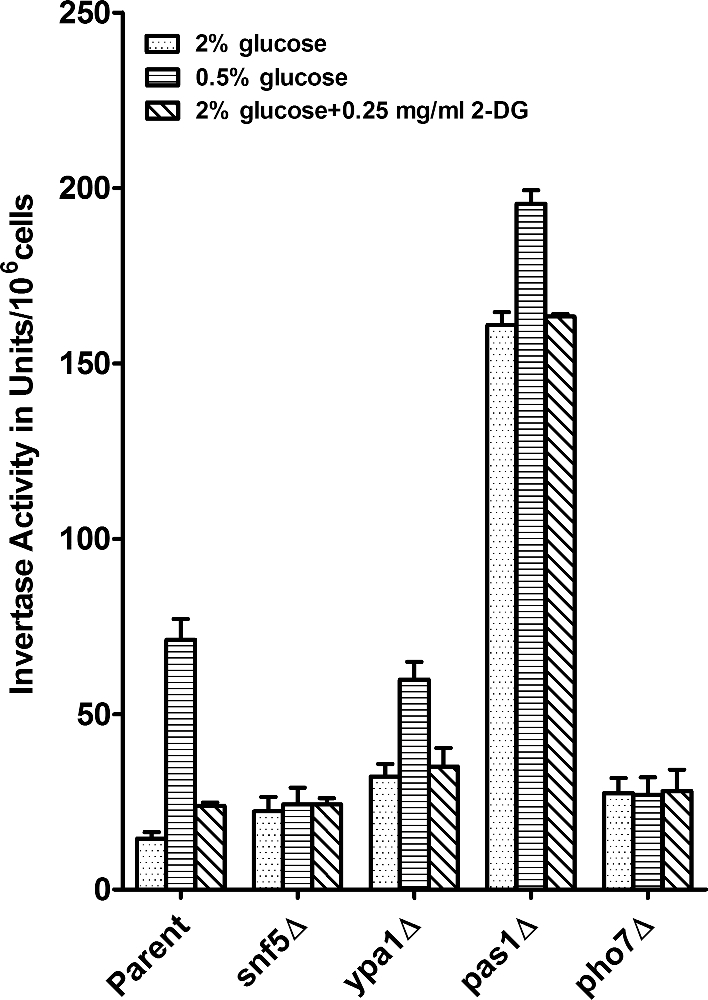
Invertase activity of the 2-DG-resistant mutants. Cells from the 2-DG-resistant deletion mutants and the corresponding parent strains ade6-M210 ura4-D18 leu1-32 h+ were grown in MM medium containing 2% or 0.5% glucose and in 2% glucose medium containing 2-DG as indicated. Invertase activity was determined and is given as activity per cells (Materials and Methods). The values given represent the mean of three measurements, with samples from each measured in five replicates, originating from three independent experiments. Error bars represent SEM (n = 3).
Table 2.
Ratios of invertase activity of 2-DG-resistant mutants under glucose-repressed and derepressed conditions and in the presence of 2-DG.
| Strain | Fold increase of invertase activity from 2% to 0.5% glucose | Fold increase of invertase activity in 2% glucose containing 2-DG |
|---|---|---|
| pREP4X | 3.9 ± 0.26 | 2.2 ± 0.19 |
| pODR1 | 1.6 ± 0.06 | 1.3 ± 0.07 |
| Parent | 5.0 ± 0.44 | 1.7 ± 0.6 |
| odr1Δ | 6.9 ± 0.83 | 3.6 ± 0.61 |
| snf5Δ | 1.1 ± 0.01 | 1.2 ± 0.19 |
| ypa1Δ | 1.9 ± 0.22 | 1.1 ± 0.05 |
| pas1Δ | 1.2 ± 0.004 | 1.0 ± 0.03 |
| pho7Δ | 1.0 ± 0.08 | 1.0 ± 0.06 |
pREP4X: control strain for overexpressor strain pODR1.
Parent: parent strain of deletion strains (ade6-M210 ura4-D18 leu1-32 h+).
Different ratios of activities in pREP4X and parent strains are due to plasmid effects. Activity was measured in MM containing 2%, 0.5% or 2% glucose plus 0.25 mg/ml 2-DG (see Materials and Methods) and the ratios ±SEM (n = 3) are indicated here.
We also tested the effect of 2-DG on the regulation of invertase activity under glucose-repressed conditions. In the parent strains, 2-DG enhanced Inv1 activity, in contrast to the situation in S. cerevisiae where 2-DG represses its activity (Randez-Gil, Prieto and Sanz 1995). Similar results were obtained for all tested strains, including control strains 972, ura4-D18 and the pREP4X-containing strain (data not shown). These results suggest that 2-DG is mimicking glucose starvation. For the 2-DG-resistant mutants, the effects of 2-DG on Inv1 expression are qualitatively similar as for low glucose (Table 2).
DISCUSSION
The genetics of 2-DG resistance is complicated as 2-DG and its metabolic products are involved in a variety of processes. This makes it difficult to understand mechanism of 2-DG actions or its resistance. We identified the Schizosaccharomyces pombe odr1 gene causing strong 2-DG resistance when overexpressed. Despite examining many 2-DG resistant transformants, we could detect only one gene exhibiting this strong overexpression phenotype. In Saccharomyces cerevisiae, a similar screening yielded two genes, DOG1 and DOG2 (Randez-Gil et al.1995). These findings suggest that the number of genes whose overexpression confers 2-DG resistance is limited. On the other hand, we uncovered several deletion mutants that render cells resistant to 2-DG. Among 14 initially selected mutants, four mutants conferred moderate to good resistance to 2-DG. By screening at a 2-fold lower 2-DG concentration, we isolated 59 resistant mutants (Supplementary Fig. 1, Supporting Information; Supplementary Table 1, Supporting Information). These findings suggest that control of 2-DG resistance is a complex process involving many genes, considering also that we have not tested any genes that are essential for growth. In a similar screen performed in S. cerevisiae, 19 deletion mutants that are resistant to 2-DG have been reported (Ralser et al.2008), although it has later been suggested that 16 of these mutants are not actually causing 2-DG resistance (McCartney et al.2014). Out of the three remaining genes, deletion of HXK2 and REG1 provides strong resistance on glucose-rich media, whereas deletion of LSM6 is only weakly resistant (Neigeborn and Carlson 1987; Ralser et al.2008; McCartney et al.2014).
The transcription of the invertase gene is repressed by the presence of glucose (Tanaka et al.1998). The deletion mutants are affected to different degrees in the control of invertase activity (Fig. 5). According to the invertase activity ratios in the repressed versus derepressed states, the mutants exhibit constitutive invertase activity (Table 2). The corresponding proteins could be directly or indirectly involved in this control. The invertase activity data also indicate that 2-DG induces a glucose starvation signal and thus derepresses the invertase activity, while the deletion mutants resistant to 2-DG show more constitutive invertase activities across different conditions (Fig. 5).
Decreased growth on glucose has been reported for mutants deleted for clr5, ypa1 and pho7, consistent with these genes being defective in the control of glucose metabolism (Vachon et al.2013; Doi et al.2015). The genes snf5, pas1 and pho7 are involved in transcription (Table 3), raising the possibility that mutants of these genes prevent some changes in gene expression induced by 2-DG that could promote resistance to 2-DG. The snf5, ypa1 and pas1 genes show orthologs in S. cerevisiae, but the corresponding mutants have not been reported to be 2-DG resistant. The S. cerevisiae protein Snf1 is involved in controlling 2-DG sensitivity (O'Donnell et al.2015), but its S. pombe ortholog, Ssp2, has neither been reported to be involved in resistance to 2-DG, nor did it come up in our screen.
Table 3.
List of genes and their annotations identified in this study.
| Gene standard name | Gene product | Molecular function | References |
|---|---|---|---|
| snf5 | Chromatin remodeling (SWItch/Sucrose Non-Fermentable; SWI/SNF) complex subunit | Regulation of transcription and glucose import | Monahan et al. (2008) |
| ypa1 | Protein phosphatase type 2A regulator | Regulation of protein phosphatase activity | Goyal and Simanis (2012) |
| pho7 | Transcription factor | Regulation of RNA polymerase II-mediated transcription | Estill, Kerwin-Iosue and Wykoff (2015) |
| pas1 | Cyclin | Regulation of G1/S transition of cell cycle | van Slegtenhorst, Mustafa and Henske (2005) |
Only the most important annotations of the genes are given. For more details refer to S. pombe genome data and annotations database (Wood et al.2012).
Even though some molecular functions of the corresponding proteins have been described (Table 3), we do not have a straightforward explanation for how the genes identified affect 2-DG resistance and glucose repression when deleted. The protein Snf5 is a subunit of the SWI/SNF chromatin remodeling complex involved in transcription and chromatin-related processes. Interestingly, the human Snf5 ortholog is involved in cancer development (Roberts and Orkin 2004). Deletion of snf5 leads to increased mRNAs encoding hexose transports (Monahan et al.2008), which could explain the increased 2-DG resistance in the high-glucose condition only, where increased influx of glucose may titrate out 2-DG (Figs 3 and 4). This result is of interest in light of findings in S. cerevisiae that 2-DG acts by reducing the levels of hexose transporters by α-arrestin-mediated endocytosis and degradation in vacuoles (O'Donnell et al.2015). Deletion of snf5 in S. pombe may thus counteract this effect of 2-DG. Except for the snf5 deletion, the other four deletion mutants showed resistance to 2-DG in both high- and low-glucose conditions, suggesting that these gene deletions affect resistance by a different mechanism.
We speculate that the S. pombe Odr1 hydrolase might function in detoxifying toxic forms of 2-DG, similar to the S. cerevisiae Dog1/Dog2 proteins that encode haloacid dehalogenase-like (HAD-like) hydrolase enzymes exhibiting specific 2-DG-6-phosphatase activities and thus render cells 2-DG resistant when overexpressed. The physiological function of these enzymes is not known (Sanz, Randez-Gil and Prieto 1994; Randez-Gil, Prieto and Sanz 1995; Randez-Gil et al.1995). An HAD-like hydrolase domain is also present in Odr1, besides a Cof-subfamily domain believed to be required for enzymes acting on phosphorylated sugars (Marchler-Bauer et al.2015). It is therefore plausible that Odr1 detoxifies the toxic form of 2-DG. Notably, these proteins have opposite effects on invertase expression: overexpression of Odr1 causes derepression of S. pombe invertase, whereas overexpression of Dog1 causes repression of S. cerevisiae invertase (Randez-Gil, Prieto and Sanz 1995).
A BLAST search (Altschul et al.1990) for Dog1/Dog2 orthologs in S. pombe yielded protein SPCC1020.07 (annotated as a pseudouridine 5΄ phosphatase), and a search for Odr1 paralogs in S. pombe revealed a protein (SPAC25B8.12c) with 45% identity. None of these proteins, however, did come up in our screen for genes causing 2-DG resistance during overexpression, but the deletion mutant of SPCC1020.07 renders the cells weakly 2-DG resistant (Supplementary data, screening at 0.5 mg/ml 2-DG, Supporting Information).
The S. pombe orthologs of genes conferring resistance when overexpressed or deleted in S. cerevisiae (DOG1/DOG2, REG1 and HXK2) did not come up in our screen. Moreover, the odr1 gene, rendering S. pombe cells 2-DG resistant, shows the opposite effect on invertase activity to the DOG1 gene, rendering S. cerevisiae 2-DG resistant. Notably, the finding that 2-DG derepresses the invertase activity in S. pombe, unlike in S. cerevisiae where it is repressed (Randez-Gil, Prieto and Sanz 1995), suggests that control of 2-DG resistance in the two yeasts is achieved by at least partially different mechanisms. The mechanisms by which the identified genes affect 2-DG-induced gene expression, sensitivity and glucose repression in S. pombe require further studies.
Supplementary Material
Supplementary data are available at FEMSYR online.
Acknowledgments
We thank Anne-Marie Schweingruber, Viesturs Simanis and Susan Forsburg for their invaluable help that made the establishment of a pombe lab and in particular this project possible. They helped to establish methods for cloning and PCR, provided reagents, strains and made useful comments on the manuscript.
SUPPLEMENTARY DATA
FUNDING
This work was supported by the Dr Erwin Braun Foundation, Switzerland, by a grant to MES at UOM for work on the effects of water-filtered infrared A (wIRA) on yeast. We also thank Vision Group on Science and Technology (VGST)/K-FIST, Government of Karnataka (GOK) [VGST/K-FIST (2010-11)/GRD-36/2013-14] grant towards Molecular biology lab of DOS in Biochemistry, UOM. Work in the Bähler laboratory was funded by a Wellcome Trust Senior Investigator Award (grant no. 095598/Z/11/Z to JB).
Conflict of interest. None declared.
REFERENCES
- Altschul SF, Gish W, Miller W et al. Basic local alignment search tool. J Mol Biol 1990;215:403–10 [DOI] [PubMed] [Google Scholar]
- Barbet N, Muriel WJ, Carr AM. Versatile shuttle vectors and genomic libraries for use with Schizosaccharomyces pombe. Gene 1992;114:59–66 [DOI] [PubMed] [Google Scholar]
- Biely P, Z Krátký, Kovařík J et al. Effect of 2-Deoxyglucose on cell wall formation in Saccharomyces cerevisiae and its relation to cell growth inhibition. J Bacteriol 1971;107:121–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer 2011;11:85–95 [DOI] [PubMed] [Google Scholar]
- Doi A, Fujimoto A, Sato S et al. Chemical genomics approach to identify genes associated with sensitivity to rapamycin in the fission yeast Schizosaccharomyces pombe. Genes Cells 2015;20:292–309 [DOI] [PubMed] [Google Scholar]
- Entian KD, Zimmermann FK. Glycolytic enzymes and intermediates in carbon catabolite repression mutants of Saccharomyces cerevisiae. Mol Gen Genet 1980;177:345–50 [DOI] [PubMed] [Google Scholar]
- Estill M, Kerwin-Iosue CL, Wykoff DD. Dissection of the PHO pathway in Schizosaccharomyces pombe using epistasis and the alternate repressor adenine. Curr Genet 2015;61:175–83 [DOI] [PubMed] [Google Scholar]
- Forsburg SL. Comparison of Schizosaccharomyces pombe expression systems. Nucleic Acids Res 1993;21:2955–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal A, Simanis V. Characterization of ypa1 and ypa2, the Schizosaccharomyces pombe orthologs of the peptidyl proyl isomerases that activate PP2A, reveals a role for Ypa2p in the regulation of cytokinesis. Genetics 2012;190:1235–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta DR, Paul SK, Oowatari Y et al. Complex formation, phosphorylation, and localization of protein kinase A of Schizosaccharomyces pombe upon glucose starvation. Biosci Biotech Bioch 2011;75:1456–65 [DOI] [PubMed] [Google Scholar]
- Harkness TA, Arnason TG. A simplified method for measuring secreted invertase activity in Saccharomyces cerevisiae. Biochem Pharmacol 2014;03; DOI: 10.4172/2167-0501.1000151 [DOI] [Google Scholar]
- Hoffman CS. Glucose sensing via the protein kinase A pathway in Schizosaccharomyces pombe. Biochem Soc T 2005;33:257–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman CS, Wood V, Fantes PA. An ancient yeast for young geneticists: a primer on the Schizosaccharomyces pombe model system. Genetics 2015;201:403–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaspers HTA, van Steveninck J. Transport-associated phosphorylation of 2-deoxy-d-glucose in Saccharomyces fragilis. BBA-Biomembranes 1975;406:370–85 [DOI] [PubMed] [Google Scholar]
- Johnson BF. Lysis of yeast cell walls induced by 2-deoxyglucose at their sites of glucan synthesis. J Bacteriol 1968;95:1169–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanter-Smoler G, Dahlkvist A, Sunnerhagen P. Improved method for rapid transformation of intact Schizosaccharomyces pombe cells. BioTechniques 1994;16:798–800 [PubMed] [Google Scholar]
- Kato H, Kira S, Kawamukai M. The transcription factors Atf1 and Pcr1 are essential for transcriptional induction of the extracellular maltase Agl1 in fission yeast. PLoS One 2013;8:e80572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kig C, Turkel S, Temizkan G. Isolation and characterization of glucose derepressed invertase mutants from Schizosaccharomyces pombe. Biosci Biotech Bioch 2005;69:2475–8 [DOI] [PubMed] [Google Scholar]
- Kim D-U, Hayles J, Kim D et al. Analysis of a genome-wide set of gene deletions in the fission yeast Schizosaccharomyces pombe. Nat Biotechnol 2010;28:617–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krátký Z, Biely P, Bauer Š. Mechanism of 2-Deoxy-d-glucose inhibition of cell-wall polysaccharide and glycoprotein biosyntheses in Saccharomyces cerevisiae. Eur J Biochem 1975;54:459–67 [DOI] [PubMed] [Google Scholar]
- Lobo Z, Maitra PK. Resistance to 2-deoxyglucose in yeast: a direct selection of mutants lacking glucose-phosphorylating enzymes. Mol Gen Genet 1977;157:297–300 [DOI] [PubMed] [Google Scholar]
- Madrid M, Fernández-Zapata J, Sánchez-Mir L et al. Role of the fission yeast cell integrity MAPK pathway in response to glucose limitation. BMC Microbiol 2013;13:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrid M, Soto T, Franco A et al. A cooperative role for Atf1 and Pap1 in the detoxification of the oxidative stress induced by glucose deprivation in Schizosaccharomyces pombe. J Biol Chem 2004;279:41594–602 [DOI] [PubMed] [Google Scholar]
- Madrid M, Soto T, Khong HK et al. Stress-induced response, localization, and regulation of the Pmk1 cell integrity pathway in Schizosaccharomyces pombe. J Biol Chem 2006;281:2033–43 [DOI] [PubMed] [Google Scholar]
- Marchler-Bauer A, Derbyshire MK, Gonzales NR et al. CDD: NCBI's conserved domain database. Nucleic Acids Res 2015;43:D222–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney RR, Chandrashekarappa DG, Zhang BB et al. Genetic analysis of resistance and sensitivity to 2-deoxyglucose in Saccharomyces cerevisiae. Genetics 2014;198:635–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megnet R. Effect of 2-deoxyglucose on Schizosaccharomyces pombe. J Bacteriol 1965;90:1032–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta SV, Patil VB, Velmurugan S et al. Std1, a gene involved in glucose transport in Schizosaccharomyces pombe. J Bacteriol 1998;180:674–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milbradt B, Höfer M. Glucose-transport-deficient mutants of Schizosaccharomyces pombe: Phenotype, genetics and use for genetic complementation. Microbiology 1994;140:2617–23 [DOI] [PubMed] [Google Scholar]
- Monahan BJ, Villén J, Marguerat S et al. Fission yeast SWI/SNF and RSC complexes show compositional and functional differences from budding yeast. Nat Struct Mol Biol 2008;15:873–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno S, Klar A, Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Method Enzymol 1991;194:795–823 [DOI] [PubMed] [Google Scholar]
- Moreno S, Ruíz T, Sánchez Y et al. Subcellular localization and glycoprotein nature of the invertase from the fission yeast Schizosaccharomyces pombe. Arch Microbiol 1985;142:370–4 [DOI] [PubMed] [Google Scholar]
- Neigeborn L, Carlson M. Mutations causing constitutive invertase synthesis in yeast: genetic interactions with snf mutations. Genetics 1987;115:247–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell AF, McCartney RR, Chandrashekarappa DG et al. 2-Deoxyglucose impairs Saccharomyces cerevisiae growth by stimulating Snf1-regulated and α-arrestin-mediated trafficking of hexose transporters 1 and 3. Mol Cell Biol 2015;35:939–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelicano H, Martin DS, Xu R-H et al. Glycolysis inhibition for anticancer treatment. Oncogene 2006;25:4633–46 [DOI] [PubMed] [Google Scholar]
- Raez LE, Papadopoulos K, Ricart AD et al. A phase I dose-escalation trial of 2-deoxy-d-glucose alone or combined with docetaxel in patients with advanced solid tumors. Cancer Chemoth Pharm 2013;71:523–30 [DOI] [PubMed] [Google Scholar]
- Rallis C, López-Maury L, Georgescu T et al. Systematic screen for mutants resistant to TORC1 inhibition in fission yeast reveals genes involved in cellular ageing and growth. Biol Open 2014;3:161–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralser M, Wamelink MM, Struys EA et al. A catabolic block does not sufficiently explain how 2-deoxy-D-glucose inhibits cell growth. P Natl Acad Sci USA 2008;105:17807–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randez-Gil F, Blasco A, Prieto JA et al. DOGR1 and DOGR2: two genes from Saccharomyces cerevisiae that confer 2-deoxyglucose resistance when overexpressed. Yeast 1995;11:1233–40 [DOI] [PubMed] [Google Scholar]
- Randez-Gil F, Prieto JA, Sanz P. The expression of a specific 2-deoxyglucose-6P phosphatase prevents catabolite repression mediated by 2-deoxyglucose in yeast. Curr Genet 1995;28:101–7 [DOI] [PubMed] [Google Scholar]
- Roberts CWM, Orkin SH. The SWI/SNF complex — chromatin and cancer. Nat Rev Cancer 2004;4:133–42 [DOI] [PubMed] [Google Scholar]
- Roux AE, Leroux A, Alaamery MA et al. Pro-aging effects of glucose signaling through a g protein-coupled glucose receptor in fission yeast. PLoS Genet 2009;5:e1000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz P, Randez-Gil F, Prieto JA. Molecular characterization of a gene that confers 2-deoxyglucose resistance in yeast. Yeast 1994;10:1195–202 [DOI] [PubMed] [Google Scholar]
- Schweingruber ME, Edenharter E. Thiamin regulates agglutination and zygote formation in Schizosaccharomyces pombe. Curr Genet 1990;17:191–4 [DOI] [PubMed] [Google Scholar]
- Tanaka N, Ohuchi N, Mukai Y et al. Isolation and characterization of an invertase and its repressor genes from Schizosaccharomyces pombe. Biochem Bioph Res Co 1998;245:246–53 [DOI] [PubMed] [Google Scholar]
- Vachon L, Wood J, Kwon E-JG et al. Functional characterization of fission yeast transcription factors by overexpression analysis. Genetics 2013;194:873–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Slegtenhorst M, Mustafa A, Henske EP. Pas1, a G1 cyclin, regulates amino acid uptake and rescues a delay in G1 arrest in Tsc1 and Tsc2 mutants in Schizosaccharomyces pombe. Hum Mol Genet 2005;14:2851–8 [DOI] [PubMed] [Google Scholar]
- Wood V, Harris MA, McDowall MD et al. PomBase: a comprehensive online resource for fission yeast. Nucleic Acids Res 2012;40:D695–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann FK, Scheel I. Mutants of Saccharomyces cerevisiae resistant to carbon catabolite repression. Mol Gen Genet 1977;154:75–82 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data are available at FEMSYR online.