Abstract
Background:
People with schizophrenia and other psychosis show increased proinflammatory and prooxidative status. However, the few studies that have specifically assessed oxidative and inflammatory markers in early onset psychosis (onset before age 18) have shown contradictory results.
Methods:
Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines for systematic reviews and meta-analyses were used to conduct a systematic literature search to detect studies comparing inflammatory and oxidative markers in early onset psychosis patients and healthy controls.
Results:
Seven studies met criteria for the qualitative analysis. Four studies met criteria for meta-analysis, comprising an overall sample of 261 early onset psychosis patients and 246 healthy controls. Six independent meta-analyses were performed for catalase, glutathione, glutathione peroxidase, superoxide dismutase, total antioxidant status, and cell/DNA oxidative damage. No significant differences were found between early onset psychosis patients and controls in any of the parameters assessed. Heterogeneity among studies was high. Qualitative analysis of individual studies showed an association of inflammatory and oxidative markers with clinical, cognitive, and neurobiological outcomes, especially in longitudinal assessments.
Conclusions:
Despite the lack of significant differences between early onset psychosis patients and controls in the oxidative markers assessed in the meta-analyses, results based on individual studies suggest that greater inflammation and oxidative stress might lead to poorer outcomes in patients with first episodes of early onset psychosis.
Keywords: schizophrenia, early onset psychosis, biomarker, meta-analysis, inflammatory
Introduction
The onset of psychosis is usually preceded by many years of multiple underlying abnormal biological processes (Kahn and Sommer, 2015). One is the presence of increased proinflammatory and prooxidative status compared with normally developing individuals (Song et al., 2009; Girgis et al., 2014; Muller, 2014). This imbalance seems to be present from the earliest stages of the disease (Doorduin et al., 2009; Mico et al., 2011; Miller et al., 2011; Di Nicola et al., 2013; Garcia-Bueno et al., 2014b; Kenk et al., 2015), even before onset of pharmacological treatment (Song et al., 2014). Inflammation and increased oxidative stress could constitute a common pathway between early genetic and/or environmental factors (such as prenatal infections, obstetric complications, hypoxia, or stress during pregnancy) and the development of psychosis (Miller et al., 2013; Bergink et al., 2014; Khandaker et al., 2015). Exposure to inflammation during critical periods of brain development may affect processes such as cell differentiation, survival, and function (Khandaker et al., 2013; Stolp, 2013) as well as contribute to sensitization of dopamine neurons (Aguilar-Valles et al., 2012), which may predispose patients to earlier onset of neurodevelopmental illnesses such as schizophrenia and other psychoses (Brown et al., 2004; Brown and Derkits, 2010).
Around 15% of patients with schizophrenia and other psychoses present with their first episode of psychosis before age 18 (early onset psychosis [EOP]) (Amminger et al., 2011). Patients with EOP seem to constitute a more developmentally impaired group and show greater negative symptom severity and increased risk for some negative outcomes than patients with later onset (Ballageer et al., 2005; Joa et al., 2009; Diaz-Caneja et al., 2015). Young people with first episodes of EOP constitute an especially suitable population for assessing the potential role of inflammatory and oxidative processes on the development of psychosis, given their relative homogeneity in terms of potential confounding factors such as previous exposure to medication and the impact of inflammatory and oxidative processes on neurodevelopment. Additionally, considering that inflammatory alterations seem to be associated with poorer clinical and functional outcomes in patients with first episode psychosis (FEP) (Garcia-Bueno et al., 2014a; Mondelli et al., 2015), information on the association between those alterations and clinical and neurobiological outcomes in EOP might be especially valuable, since it can provide support for targeted interventions with antioxidant and antiinflammatory strategies in this especially impaired subpopulation.
Two meta-analyses explored inflammatory (Miller et al., 2011) and oxidative markers (Flatow et al., 2013) in patients with schizophrenia and reported increased levels of proinflammatory cytokines (i.e., interleukin [IL] 1-beta, IL-6, IL-12, interferon gamma, tumor necrosis factor alpha, tumor growth factor beta) and reduced levels of antioxidants (total antioxidant status [TAS], catalase [CAT], plasma nitrites, and superoxide dismutase [SOD]) in patients with FEP. However, few studies have specifically assessed oxidative and inflammatory markers in EOP and they have shown contradictory results (Mico et al., 2011; Parellada et al., 2012).
Our goal was to review oxidative stress and inflammatory biomarkers at the peripheral level in subjects with first episodes of EOP and to explore the potential association of these parameters with clinical, cognitive, and other neurobiological measures, with a special focus on longitudinal outcomes.
Methods
Search Strategies
Using the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines, a systematic 2-step literature search was conducted to identify potentially relevant studies (Shamseer et al., 2015). First, a PubMed search from inception through October 2016 was performed to detect studies comparing inflammatory and oxidative markers in patients with first episodes of EOP and healthy controls.
The initial search covered the combination of 2 concepts: ‘early onset psychosis’ (OR ‘EOP’ OR ‘first-episode psychosis’ OR ‘FEP’ OR ‘early onset schizophrenia’ OR ‘first episode schizophrenia’ OR ‘early psychosis’ OR ‘early schizophrenia’) AND ‘inflammation’ OR ‘oxidation’ (OR ‘inflamm*’ OR ‘inflammatory’ OR ‘anti-inflammatory’ OR ‘cytokine’ OR ‘interleukin’ OR ‘interferon’ OR ‘tumor necrosis factor’ OR ‘oxidat*’ OR ‘oxidative’). Second, the reference lists of the selected articles were manually checked for any studies not identified by the computerized literature search.
Selection Criteria
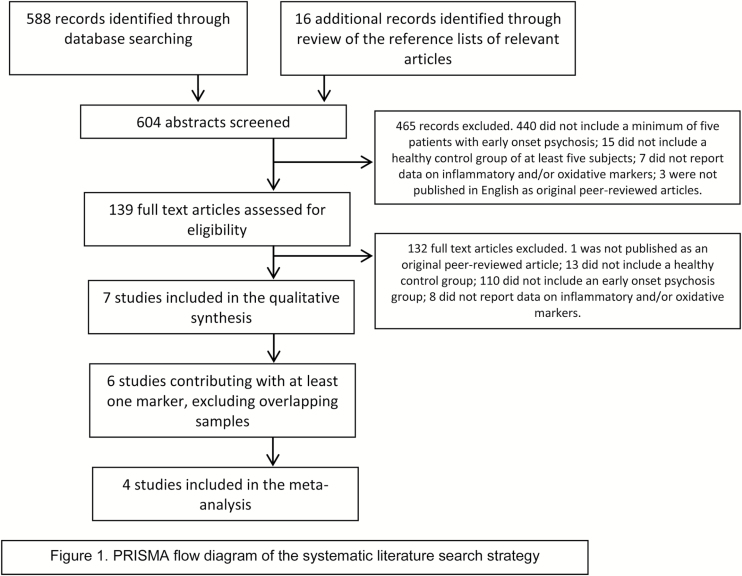
The initial literature search yielded 2468 studies. After removing duplicates, 604 potential studies were evaluated. All papers were double-screened in 4 phases with discrepancies resolved through discussion and consensus. In phase 1, the titles and abstracts of the retrieved papers were screened using the following hierarchical inclusion criteria: (1) they included patients with a diagnosis of EOP, early onset schizophrenia, FEP, early psychosis, or early schizophrenia; (2) they included a healthy control group; (3) they reported data on inflammatory and/or oxidative markers; (4) they included a minimum of 5 subjects in both the EOP and the control group; and (5) they were published in English as original peer-reviewed articles. Of the 604 studies, 139 fulfilled these inclusion criteria.
In phase 2, full text articles were comprehensively reviewed. Studies were excluded if they met any of the following hierarchical exclusion criteria: (1) they were not published as original peer-reviewed articles; (2) they did not include a healthy control group; (3) they did not include a first-episode EOP group (to consider this group as ‘first episode’ ‘early onset psychosis group’, all patients included in the study should be experiencing a first episode of psychosis according to DSM-III, DSM-IV, or DSM-IV-TR criteria and should be 18 years old or younger at the onset of psychosis; including brief psychotic disorder, psychotic disorder not otherwise specified, schizophreniform disorder, schizophrenia, mania with psychotic symptoms, and depression with psychotic symptoms; none of the reviewed studies included patients with substance-induced psychosis); and (4) they did not report data (mean and SD) either on inflammatory and/or oxidative markers or data on the statistical comparison of inflammatory and/or oxidative markers between EOP patients and controls. Of the 139 studies, 7 met none of the exclusion criteria and were thus available for the qualitative analysis (Mico et al., 2011; Fraguas et al., 2012; Parellada et al., 2012; Martinez-Cengotitabengoa et al., 2014; Simsek et al., 2016b; Simsek et al., 2016a; Xu et al., 2016).
In phase 3, overlapping studies assessing the same inflammatory and/or oxidative markers in the same cohort were excluded to ensure that only independent samples were included in each of the meta-analyses. The following hierarchical criteria were used to determine the inclusion of overlapping studies: (1) study with the largest EOP group sample for the parameter of interest; (2) study with the largest control group sample for the parameter of interest; (3) most recent publication. Six of the 7 studies (Mico et al., 2011; Parellada et al., 2012; Martinez-Cengotitabengoa et al., 2014; Simsek et al., 2016a, 2016b; Xu et al., 2016) contributed with at least one marker and entered Phase 4.
Six separate meta-analyses were performed for inflammatory and/or oxidative markers with data available in at least 2 nonoverlapping studies (CAT, glutathione [GSH], glutathione peroxidase [GPx], SOD, TAS, and cell/DNA oxidative damage [as linoleic acid hydroperoxides or 8-oxo-2’-deoxyguanosine, whose data were analyzed as equivalent (Kanazawa et al., 2016]). Four studies contributed to the 6 independent meta-analyses (Mico et al., 2011; Parellada et al., 2012; Martinez-Cengotitabengoa et al., 2014; Simsek et al., 2016b). Figure 1 shows the PRISMA flow diagram of the systematic literature search strategy.
Figure 1.
PRISMA flow diagram of the systematic literature search strategy.
Additionally, in the 7 studies selected for qualitative analysis, we reviewed associations between inflammatory/oxidative markers and baseline and/or longitudinal clinical, cognitive, functional, and other neurobiological variables.
Quality Assessment
The quality of the 4 studies ultimately included in the meta-analyses was assessed using a checklist constructed specifically for this review, based on a previous meta-analysis on oxidative markers in schizophrenia (Flatow et al., 2013). Quality assessment was coded for each study on a scale of 0 to 10, with higher values meaning greater quality (details are given in supplementary Table 1).
Data Extraction
Data extracted from eligible studies after Phase 4 included: name of inflammatory and/or oxidative marker; first author; location and year of the study; description of the study design; fasting status at blood extraction (as yes/no); study quality; and number of EOP patients, number of controls, mean and SD for each inflammatory and/or oxidative marker (for both EOP patients and controls), and/or statistical comparison of each marker between EOP patients and controls. The same data were extracted from studies assessing markers with insufficient data for performing a meta-analysis.
Additionally, putative moderating data from EOP patients, controls, or both, as appropriate, were extracted for markers included in the meta-analyses: mean age, sex (as percentage of male), diagnosis (as percentage of schizophrenia), mean body mass index (BMI), tobacco users (as percentage), cannabis use/abuse (as percentage), substance use/abuse (as percentage), exclusion of medical comorbidity (as yes/no), exclusion of NSAID/antiinflammatory drugs (as yes/no), mean duration of untreated psychosis, mean duration of untreated illness, antipsychotic naïve (as percentage), antipsychotic dose (as chlorpromazine equivalents), antipsychotic monotherapy (as yes/no), and antipsychotic name (if monotherapy).
Statistical Analysis
Data were entered into an electronic database and analyzed with a quantitative meta-analytical approach using Comprehensive Meta-Analysis Software version 3 (Biostat, Inc., Englewood, NJ).
Standardized mean differences (SMD) based on Hedges’s adjusted g (SMD of -0.2 was considered small, -0.5 medium, and -0.8 large) and its 95% CIs were calculated for every individual marker. Based on the known heterogeneity of markers, we expected that the estimates would also vary substantially between studies. Random-effects models assume that the true effect size varies from one study to the next and, accordingly, are more conservative than fixed effect models. Therefore, random effects models by DerSimonian and Laird were used (DerSimonian and Laird, 1986).
Statistical heterogeneity was assessed through visual inspection of forest plots and using the Q statistic (a magnitude of heterogeneity) and the I2 statistic (a measure of the proportion of variance in summary effect size attributable to heterogeneity) (Lipsey and Wilson, 2000). Publication bias was assessed by visually inspecting funnel plots and using the Duval and Tweedie trim-and-fill method to estimate an effect size corrected for publication bias (Duval and Tweedie, 2000).
Meta-regression using a random-effects model with unrestricted maximum likelihood was used to test effects of moderators on effect estimates. The slope of meta-regression line, β coefficient: direct (+) or inverse (−), indicates the strength of the relationship between moderator and outcome. Meta-regressions were planned when at least 5 studies were available for the moderator variable. Since the final number of included studies was lower, no meta-regressions could be performed.
To limit the risk of false positive (type I) errors arising from multiple comparisons, we adjusted the P value by dividing it by the number of meta-analyses conducted (corrected P = .0083 [0.05/6]).
For markers with a single study contributing data, SMD with 95% CI were also calculated and included in a separate table.
Results
Inflammatory and Oxidative Markers in EOP
Seven studies reported data on oxidative stress or inflammatory markers in EOP compared with healthy controls (Mico et al., 2011; Fraguas et al., 2012; Parellada et al., 2012; Martinez-Cengotitabengoa et al., 2014; Simsek et al., 2016b; Simsek et al., 2016a; Xu et al., 2016). Details of these 7 studies are given in Table 1.
Table 1.
Characteristics of the Seven Studies Assessing Independent Oxidative and/or Inflammatory Data Identified in the Systematic Review
| Author and Year | Cohort | Biomarkers assessed | Included in the meta-analysis | N (EOP) | Age (mean), EOP | % Male, EOP | % Tobacco users, EOP | % SCZ, EOP | % AP naïve, EOP | DUP, EOP | N (C) | Age (mean), C | % Male, C | % Tobacco users, C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fraguas et al., 2012 | Spain-CAFEPS | GSH | No | 48 | 16.0 | 72.9 | 27.1 | 56.3 | na | <6 months | 56 | 15.4 | 66.1 | 1.8 |
| Martínez- Cengotitabengoa et al., 2014 | Spain-CAFEPS | GSH, TAS | Yes | 105 | 15.5 | 67.6 | 30.5 | 36.2 | 2.9 | <6 months | 97 | 15.2 | 64.9 | 6.2 |
| Mico et al., 2011 | Spain-CAFEPS | CAT, GPx, SOD, Oxidative damahe (LOOH) | Yes | 102 | 15.6 | 66.7 | na | 48 | na | <6 months | 95 | 15.3 | 64.2 | na |
| Parellada et al., 2012 | Spain-HGUGM | CAT, GPx, GSH, SOD, TAS, Hcy, TBARS, Uric acid, Albumin, Vit. B12, Vit. A, Vit. E, Transferrin | Yes | 34 | 15.8 | 67.6 | na | 41.2 | na | na | 34 | 12.8 | 94.3 | na |
| Simsek et al., 2016a | Turkey-Dicle | GPx, SOD, Oxidative damage (8-oxo-dG), CoQ | Yes | 20 | 14.5 | 40 | na | 100 | na | na | 20 | 14.4 | 40 | na |
| Simsek et al., 2016b | Turkey-Dicle | IL-2, IL-4, IL-6, IL-10, TNF-alpha, IFN-gamma, IL-17 alpha | No | 30 | 14.7 | 43.3 | na | na | na | na | 26 | 14.5 | 33.3 | na |
| Xu et al., 2016 | China- Beijing | IL-18, IL-18R | No | 14 | 14.4 | 50.0 | na | 100 | na | na | 13 | 13.5 | 53.8 | na |
Abbreviations: 8-oxo-dG, 8-oxo-2’-deoxyguanosine; AP, antipsychotic drug; C, control subjects; CAT, catalase; CoQ, coenzyme Q; DUP, duration of untreated psychosis; EOP, early onset psychosis; GPx, glutathione peroxidase; GSH, glutathione; Hcy, homocysteine (plasma); IFN-gamma, Interferon gamma; IL, interleukin; LOOH, linoleic acid hydroperoxides; na, not avalilable; SCZ, schizophrenia; SOD, superoxide dismutase activity; TAS, total antioxidant status; TBARS, thiobarbituric acid reactive substances; TNF-alpha, tumor necrosis factor alpha.
Meta-Analysis
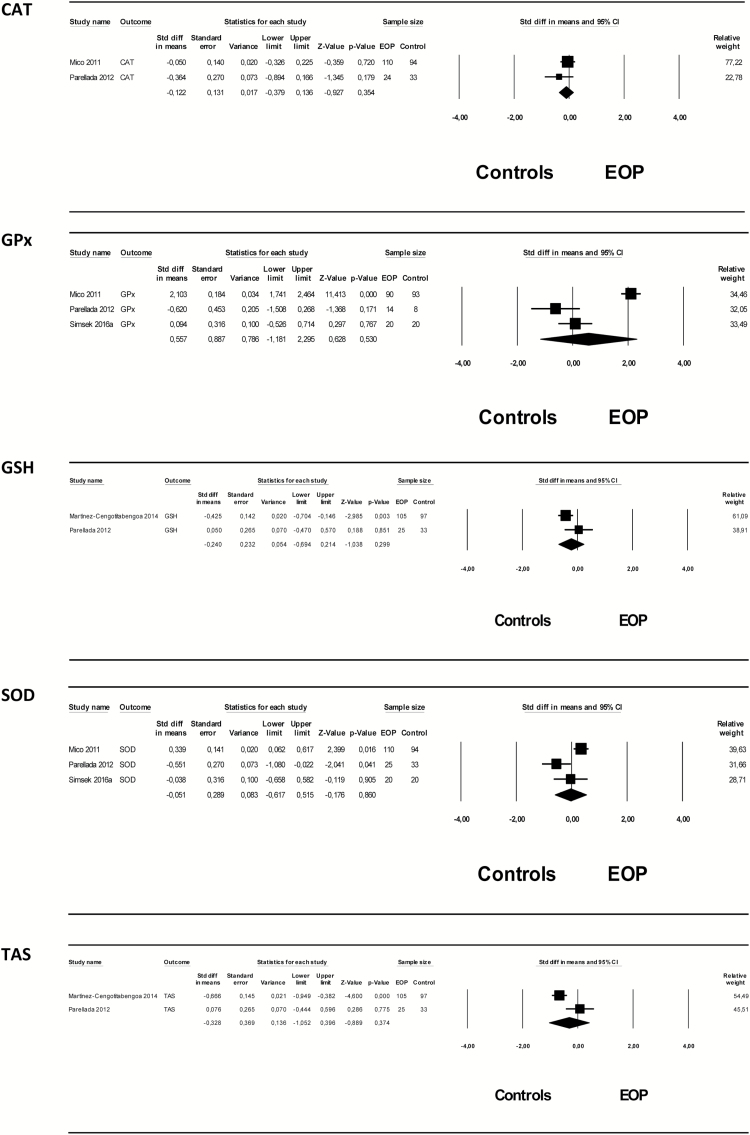
A total of 6 markers were addressed by 2 or more studies: CAT, GSH, GPx, SOD, TAS, and cell/DNA oxidative damage. Therefore, 6 meta-analyses were performed assessing these oxidative markers from 4 studies, comprising an overall sample of 261 EOP patients and 246 healthy controls, were performed. The mean age of EOP patients and controls ranged from 14.5 to 15.8 years and from 14.4 to 15.3 years, respectively. The percentage of male EOP patients and controls ranged from 40% to 67.6% and from 40% to 94.3%, respectively. Of the 261 EOP patients, 121 (46.4%) had a diagnosis of schizophrenia.
No significant differences were found between EOP patients and healthy controls in any of the oxidative parameters assessed. Heterogeneity among studies was high, with I2 values >50%, except for CAT (with I2 of 5.3), (details of the results of the meta-analyses are shown in Table 2 and Figure 2). Due to the small numbers of studies included for each parameter, no meta-regression analyses could be performed. Separate meta-analyses based on 2 papers (Mico et al., 2011; Simsek et al., 2016b) providing specific data on GPx and cell/DNA oxidative damage in EOP patients with a follow-up diagnosis of schizophrenia as compared with controls did not yield significant results either (SMD=1.34 (95% CI: -1.08 to 3.76), P=.279 for GPx, and SMD=1.37 (95% CI:-1.43 to 4,18), P=.338 for cell/DNA oxidative damage). The quality of the included studies was in general low (supplementary Table 1).
Table 2.
Main Results of the Meta-Analysis
| Random model | Effect size | Heterogeneity | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Marker | N studies | N EOP | N control | SMD (95% CI) | Z | P | Q | P (Q) |
I
squared |
Tau
squared |
| CAT | 2 | 134 | 127 | -0.122 (-0.379 to 0.136) |
-0.927 | .354 | 1.056 | 0.304 | 5.316 | 0.003 |
| GPx* | 3 | 124 | 121 | 0.557 (-1.181 to 2.295) |
0.628 | .530 | 51.359 | 0.000 | 96.106 | 2.248 |
| GSH | 2 | 130 | 130 | -0.240 (-0.694 to 0.214) |
-1.038 | .299 | 2.489 | 0.115 | 59.826 | 0.067 |
| Oxidative damage± | 2 | 94 | 102 | 1.641 (-1.688 to 4.971) |
0.966 | .334 | 71.513 | 0.000 | 98.602 | 5.690 |
| SOD* | 3 | 155 | 147 | -0.051 (-0.617 to 0.515) |
-0.176 | .860 | 8.830 | 0.012 | 77.350 | 0.190 |
| TAS | 2 | 130 | 130 | -0.328 (-1.052 to 0.396) |
-0.889 | .374 | 6.023 | 0.014 | 83.397 | 0.229 |
±Oxidative damage includes two different measures: LOOH and 8-oxo-dG.
*Publication bias for GPx and SOD: Duval and Tweedie trim-and-fill, adjusted SMD= 0,557, (95% CI -1,181 to 2,295), adjusted Q= 51,359 for GPx; adjusted SMD= -0,051, (95% CI 0,617 to 0,515), adjusted Q=8,830 for SOD.
Abbreviations: 8-oxo-dG, 8-oxo-2’-deoxyguanosine; CAT, Catalase; EOP, Early Onset Psychosis; GPx, Glutathione Peroxidase; GSH, Glutathione; LOOH, Linoleic acid Hydroperoxides; SMD, standard mean difference; SOD, Superoxide Dismutase activity; TAS, Total Antioxidant Status.
Figure 2.
Forest plot of main results of the meta-analyses. CAT, catalase; GPx, glutathione peroxidase; GSH, glutathione; SOD, superoxide dismutase activity; TAS, total antioxidant status.
Other Inflammatory and Oxidative Markers
SMD between EOP and controls for markers with insufficient data for performing a meta-analysis are detailed in Table 3. Significant differences were found between EOP and controls in levels of IL 18 receptor (IL-18R1) (Xu et al., 2016) and homocysteine (Parellada et al., 2012). No significant differences were found in the rest of the parameters assessed: albumin, thiobarbituric acid reactive substances, transferrin, uric acid, vitamin A, vitamin B12, vitamin E (Parellada et al., 2012), coenzyme Q (Simsek et al., 2016b), interferon-gamma, IL-2, IL-4, IL-6, IL-10, IL-17 alpha, or tumor necrosis factor-alpha (Simsek et al., 2016a).
Table 3.
Effect Size between EOP Patients and Controls for Markers Assessed by a Single Study
| Marker | Study | N EOP patients | N controls | SMD (95% CI) | Z* | P |
|---|---|---|---|---|---|---|
| Albumin | Parellada et al., 2012 | 34 | 34 | 0.195 (-0.325 to 0.717) | 0.737 | .461 |
| CoQ | Simsek et al., 2016b | 20 | 20 | -0.055 (-0.675 to 0.565) | -0.174 | .862 |
| Hcy | Parellada et al., 2012 | 34 | 34 | 1.620 (0.990 to 2.249) | 5.042 | <.001 |
| IFN-gamma | Simsek et al., 2016a | 34 | 34 | 0.151 (-0.375 to 0.677) | 0.562 | .574 |
| IL-10 | Simsek et al., 2016a | 30 | 26 | -0.534 (-1.069 to 1.557) | -1.959 | .050 |
| IL-17 alpha | Simsek et al., 2016a | 30 | 26 | 0.447 (-0.084 to 0.979)) | 1.649 | .099 |
| IL-18 | Xu et al., 2016 | 14 | 13 | 0.368 (-0.393 to 1.130) | 0.948 | .343 |
| IL-18R | Xu et al., 2016 | 14 | 13 | -1.191 (-2.010 to -0.372) | -2.849 | .004 |
| IL-2 | Simsek et al., 2016a | 30 | 26 | 0.515 (-0.019 to 1.048) | 1.890 | .059 |
| IL-4 | Simsek et al., 2016a | 30 | 26 | 0.073 (-0.452 to 0.598) | 0.272 | .786 |
| IL-6 | Simsek et al., 2016a | 30 | 26 | 0.194 (-0.332 to 0.721) | 0.723 | .470 |
| TBARS | Parellada et al., 2012 | 34 | 34 | 0.049 (-0470 to 0.569) | 0.186 | .852 |
| TNF-alpha | Simsek et al., 2016a | 30 | 26 | 0.301 (-0.227 to 0.829) | 1.117 | .264 |
| Transferrin | Parellada et al., 2012 | 34 | 34 | 0.402 (-0.123 to 0.926) | 0.150 | .134 |
| Uric acid | Parellada et al., 2012 | 34 | 34 | 0.378 (-0.146 to 0.902) | 1.413 | .158 |
| Vitamin A | Parellada et al., 2012 | 34 | 34 | 0.381 (-0.143 to 0.906) | 1.426 | .154 |
| Vitamin B12 | Parellada et al., 2012 | 34 | 34 | -0.347 (-0.817 to 0.176) | -1.300 | .194 |
| Vitamin E | Parellada et al., 2012 | 34 | 34 | 0.112 (-0.408 to 0.632) | 0.423 | .673 |
Abbreviations: CoQ, coenzyme Q; EOP, early onset psychosis; Hcy, homocysteine (plasma); IFN-gamma, interferon gamma; IL, interleukin; SMD, standard mean difference; TBARS, thiobarbituric acid reactive substances; TNF-alpha, tumor necrosis factor alpha.
Significant P values are bold.
* A positive value means that marker is higher in EOP patients than in controls.
Association of Inflammatory and Oxidative Markers with Clinical, Cognitive, and Neurobiological Outcomes
Five studies reported data on the relationship between inflammatory and oxidative markers with clinical (Mico et al., 2011; Simsek et al., 2016a, 2016b), cognitive (Martinez-Cengotitabengoa et al., 2014), and neuroimaging (Fraguas et al., 2012) outcomes. Three studies explored the concurrent association between baseline oxidative and inflammatory markers and psychotic symptomatology (Mico et al., 2011; Simsek et al., 2016a, 2016b). A significant negative correlation was found between levels of IL-4 and IL-10 and negative symptoms in one of the studies (Simsek et al., 2016a) (details are shown on Supplementary Table 2). No significant associations were found between any baseline oxidative markers and clinical measures. Two studies found a significant association between baseline oxidative markers and gray matter loss (Fraguas et al., 2012) and cognitive outcomes (Martinez-Cengotitabengoa et al., 2014) at 2-year follow-up (supplementary Table 2).
Discussion
The meta-analyses of first episodes of EOP and 6 antioxidant markers did not yield any significant results, with great heterogeneity among studies, and even opposite effects found for some markers in different studies. These contradictory findings might be due to some extent to great differences among samples, including differing assessment and analytical methods and clinical factors that might alter the levels of oxidative parameters, such as different exposure to antipsychotics (Kriisa et al., 2016) or diagnosis. Additionally, most of the studies provided insufficient information or did not control for well-known confounding factors such as BMI or substance or tobacco use.
We did not replicate the findings of reduced antioxidant markers in adult-onset FEP from a previous meta-analysis based on 44 studies (Flatow et al., 2013). This could be due to the fact that all studies included in our meta-analyses assessed oxidative parameters in plasma in early onset of FEP, while most significant findings (except for TAS) in the previous meta-analysis were based on red blood cell markers in adult-onset FEP (Flatow et al., 2013). Moreover, even if all patients included in our meta-analysis were experiencing a first episode of EOP, there was moderate clinical heterogeneity. Clinical status may have affected state-related markers, such as TAS, CAT, and plasma nitrites, thereby affecting our findings. The finding of increased homocysteine and decreased IL-18R1 in patients with EOP is in agreement with previous findings in adult-onset psychosis (Kale et al., 2010; Palladino et al., 2012; Flatow et al., 2013). Both markers could be involved in the pathophysiology of schizophrenia through their role in brain immune modulation, neuroinflammation, and neurodegeneration (Mattson and Shea, 2003; Alboni et al., 2011).
Increased levels of peripheral homocysteine have been associated with serum levels of complement component 4 (C4) (Li et al., 2015). The strongest genetic association described so far for schizophrenia affects the major histocompatibility complex locus (Stefansson et al., 2009; Schizophrenia-Working-Group-of-the-Psychiatric-Genomics-Consortium, 2014). A recent genetic study suggests that this association might be due to genetic variation in C4 activity (Sekar et al., 2016), which could underlie unbalanced neuroinflammation processes with excessive stimulation of microglia (Bloomfield et al., 2016), leading to abnormal pruning of synapses during adolescence and early adulthood in people with psychosis. It could be speculated that increased levels of homocysteine could therefore indirectly reflect increased levels of C4. However, given that these findings are based on a single study, they should be appraised with caution, and future studies assessing the same markers are warranted.
Even though the meta-analyses did not find differences between EOP patients and controls in any of the markers assessed, some individual studies revealed an association of some oxidative and inflammatory parameters with some clinical dimensions at the first episode and longitudinal outcomes of the illness. Although these associations could not be specifically addressed in the meta-analyses, this suggests that greater inflammation and oxidative stress might lead to disturbed neural plasticity and increased neurodegeneration (Marin, 2016), which could be associated with poorer outcomes in patients with FEP (Garcia-Bueno et al., 2014a). However, it should be noted that most of the putative associations with clinical variables assessed in cross-sectional studies at the first episode of an EOP were not significant, with only 2 cohorts finding a significant association between baseline inflammatory or oxidative stress biomarker levels and concurrent clinical or cognitive measures (Mico et al., 2011; Martinez-Cengotitabengoa et al., 2014; Simsek et al., 2016a), while findings of a significant association between baseline levels of antioxidants and gray matter loss and cognitive performance at follow-up were based on a single cohort (Martinez-Cengotitabengoa et al., 2014; Fraguas et al., 2016). This highlights the need for further studies on the issue, especially on the potential effect of alterations in these biological parameters on longitudinal outcomes. Relatively small variance in symptomatic and functional variables among patients at the first episode might preclude finding significant associations that become evident during follow-up, facilitating the identification of subpopulations.
Results for some antioxidant and antiinflammatory agents in the management of schizophrenia are promising (Sommer et al., 2014). Interventions on the inflammatory and/or oxidative level seem to have a neuroprotective role (Keller et al., 2013), comparable to the neuroprotective effect of cognitive therapy (Eack et al., 2010; Thorsen et al., 2014) or physical exercise (Vakhrusheva et al., 2016). However, antioxidant and antiinflammatory strategies for the treatment of psychosis still give conflicting results, with little evidence for their efficacy in the management of some psychopathological dimensions such as cognition (Buoli and Altamura, 2015). The use of average values of inflammatory and oxidative markers for heterogeneous populations instead of using biomarkers to stratify patients may be one reason. Considering the intrinsic heterogeneity of psychotic disorders and the failure to identify biological markers for psychiatric disorders despite substantial effort (Singh and Rose, 2009), alterations in inflammatory and oxidative markers could help identify subpopulations among EOP patients with different biological underpinnings that might also impact their prognosis (Fillman et al., 2014). That could, in turn, guide patient stratification and targeted interventions. This points to the need for stratification-based therapeutic strategies, as suggested by a recent trial assessing an antiinflammatory agent for management of treatment-resistant depression. The trial did not detect any significant efficacy in the complete sample but did find it to be efficacious in the subgroup of patients with high baseline levels of inflammatory markers (Raison et al., 2013). Along those lines, a placebo-controlled RCT evaluating the efficacy of N-Acetylcysteine (NAC) in adult-onset FEP did not detect significant differences in any of the primary or secondary outcome measures between the placebo- and NAC-treated groups. However, NAC was found to significantly improve positive psychotic symptoms in the subgroup of NAC-treated patients with increased oxidative stress (increased levels of GPx) at baseline (Do, 2016). Stratification of patients based on baseline inflammatory or oxidative parameters, in accordance with personalized or precision medicine, could be especially indicated in EOP, where current treatment strategies fail to satisfactorily address some dimensions of the illness (Fraguas et al., 2016).
This work was subject to several caveats. First, despite the extensive systematic literature review and the large number of papers initially identified, few articles could be included in the meta-analyses, and there was great heterogeneity among them. Furthermore, quality of the retrieved papers was not optimal, and most studies did not control for confounding factors such as BMI, tobacco, or substance use. This limits our capability to extrapolate the results of this meta-analysis and highlights the need for further studies on the issue, especially concerning inflammatory markers. Second, separate information based on diagnostic subgroups was not available for all the studies included in the meta-analyses. In this sense, we were only able to assess GPx and cell/DNA oxidative damage in EOP patients with a follow-up diagnosis of schizophrenia, with no significant differences between early onset schizophrenia subjects and controls. Considering that inflammatory and oxidative levels and their neurobiological impact might differ among EOP subpopulations (Mico et al., 2011; Fraguas et al., 2012; Altamura et al., 2014), future studies should assess the potential role of clinical diagnosis on this issue. Third, the impact of developmental stage on inflammatory and oxidative status in normally developing individuals is still understudied. The lack of replication of some of the previous findings in adults could be thus related to differential aspects of the inflammatory and oxidative mechanisms in younger people (Tamura et al., 2006). Studies assessing the impact of developmental stage on levels of inflammatory and oxidative parameters both in clinical and nonclinical populations are needed to appropriately appraise the potential role of these alterations in patients with EOP. Finally, although individuals with psychosis show increased cytokine levels in both peripheral blood and cerebrospinal fluid (Miller et al., 2011), we only assessed peripheral biomarkers and these do not necessarily reflect the inflammatory and oxidative state of the brain. However, recent studies have found that cerebrospinal fluid levels of markers like IL-6 are significantly correlated with the plasma levels in patients with recent-onset schizophrenia (Coughlin et al., 2016), suggesting that peripheral and central inflammatory systems probably operate in parallel in a synchronized way (Fernandes et al., 2016).
Regardless of these limitations, this is to the best of our knowledge the first meta-analysis to specifically assess oxidative markers in patients with first episodes of EOP. We did not find consistent differences between EOP patients and healthy controls in any of the assessed parameters in the meta-analyses, possibly due to relative heterogeneity in the methods used and lack of consideration for potentially relevant factors such as diagnosis. In one of the cohorts assessed we did find a significant association between reduced levels of antioxidants and longitudinal outcomes, such as increased left frontal, left parietal, and left total gray matter loss and poorer cognitive performance at 2-year follow-up (Fraguas et al., 2012; Martinez-Cengotitabengoa et al., 2014). This supports the role of oxidative alterations in the pathophysiology of EOP, or maybe in the pathophysiology of a subtype of psychosis (Fillman et al., 2014). That is, even if no differences might be detected in some parameters between EOP patients and controls, oxidative alterations might delineate a subgroup within EOP patients that shows poorer outcomes. This could lead to a personalized or precision clinical approach, help stratify patients, and have direct therapeutic implications by guiding targeted interventions with promising strategies such as antioxidants or antiinflammatory agents.
Supplementary Material
Supplementary data are available at International Journal of Neuropsychopharmacology online.
Funding
This work was supported by the Spanish Ministry of Economy and Competitiveness, Instituto de Salud Carlos III, cofinanced by ERDF Funds from the European Commission, “A way of making Europe,” CIBERSAM, Madrid Regional Government (S2010/BMD-2422 AGES), European Union Structural Funds, and European Union Seventh Framework Programme under grant agreements FP7-HEALTH-2009-2.2.1-2-241909 (Project EU-GEI), FP7-HEALTH-2009-2.2.1-3-242114 (Project OPTiMISE), FP7- HEALTH-2013-2.2.1-2-603196 (Project PSYSCAN), and FP7-HEALTH-2013-2.2.1-2-602478 (Project METSY); Fundación Alicia Koplowitz and Fundación Mutua Madrileña, and ERA-NET NEURON (Network of European Funding for Neuroscience Research).
Statement of Interest
David Fraguas has been a consultant and/or has received fees from AstraZeneca, Bristol-Myers-Squibb, Janssen, Lundbeck, Otsuka, and Pfizer and has received grant support from Instituto de Salud Carlos III (ISCIII), Spanish Ministry of Economy and Competitiveness. Covadonga M. Diaz-Caneja and has previously held a “Río Hortega” grant from ISCIIII and a grant from the Alicia Koplowitz Foundation. Alberto Rodríguez-Quiroga has previously held a “Río Hortega” grant from ISCIII. Celso Arango has been a consultant to or has received honoraria or grants from Abbott, Amgen, AstraZeneca, Bristol-Myers Squibb, Caja Navarra, CIBERSAM, Fundación Alicia Koplowitz, Instituto de Salud Carlos III, Janssen-Cilag, Lundbeck, Merck, Ministerio de Ciencia e Innovación, Ministerio de Sanidad, Ministerio de Economía y Competitividad, Mutua Madrileña, Otsuka, Pfizer, Roche, Servier, Shire, Takeda, and Schering-Plough.
Supplementary Material
Acknowledgments
We thank Beatriz Sorce for her support in the editing of the manuscript.
References
- Aguilar-Valles A, Jung S, Poole S, Flores C, Luheshi GN. (2012) Leptin and interleukin-6 alter the function of mesolimbic dopamine neurons in a rodent model of prenatal inflammation. Psychoneuroendocrinology 37:956–969. [DOI] [PubMed] [Google Scholar]
- Alboni S, Montanari C, Benatti C, Blom JM, Simone ML, Brunello N, Caggia F, Guidotti G, Marcondes MC, Sanchez-Alavez M, Conti B, Tascedda F. (2011) Constitutive and LPS-regulated expression of interleukin-18 receptor beta variants in the mouse brain. Brain Behav Immun 25:483–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altamura AC, Buoli M, Pozzoli S. (2014) Role of immunological factors in the pathophysiology and diagnosis of bipolar disorder: comparison with schizophrenia. Psychiatry Clin Neurosci 68:21–36. [DOI] [PubMed] [Google Scholar]
- Amminger GP, Henry LP, Harrigan SM, Harris MG, Alvarez-Jimenez M, Herrman H, Jackson HJ, McGorry PD. (2011) Outcome in early-onset schizophrenia revisited: findings from the Early Psychosis Prevention and Intervention Centre long-term follow-up study. Schizophr Res 131:112–119. [DOI] [PubMed] [Google Scholar]
- Ballageer T, Malla A, Manchanda R, Takhar J, Haricharan R. (2005) Is adolescent-onset first-episode psychosis different from adult onset? J Am Acad Child Adolesc Psychiatry 44:782–789. [DOI] [PubMed] [Google Scholar]
- Bergink V, Gibney SM, Drexhage HA. (2014) Autoimmunity, inflammation, and psychosis: a search for peripheral markers. Biol Psychiatry 75:324–331. [DOI] [PubMed] [Google Scholar]
- Bloomfield PS, Selvaraj S, Veronese M, Rizzo G, Bertoldo A, Owen DR, Bloomfield MA, Bonoldi I, Kalk N, Turkheimer F, McGuire P, de Paola V, Howes OD. (2016) Microglial activity in people at ultra high risk of psychosis and in schizophrenia: an [(11)C]PBR28 PET brain imaging study. Am J Psychiatry 173:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Derkits EJ. (2010) Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry 167:261–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Hooton J, Schaefer CA, Zhang H, Petkova E, Babulas V, Perrin M, Gorman JM, Susser ES. (2004) Elevated maternal interleukin-8 levels and risk of schizophrenia in adult offspring. Am J Psychiatry 161:889–895. [DOI] [PubMed] [Google Scholar]
- Buoli M, Altamura AC. (2015) May non-antipsychotic drugs improve cognition of schizophrenia patients? Pharmacopsychiatry 48:41–50. [DOI] [PubMed] [Google Scholar]
- Coughlin JM, Wang Y, Ambinder EB, Ward RE, Minn I, Vranesic M, Kim PK, Ford CN, Higgs C, Hayes LN, Schretlen DJ, Dannals RF, Kassiou M, Sawa A, Pomper MG. (2016) In vivo markers of inflammatory response in recent-onset schizophrenia: a combined study using [(11)C]DPA-713 PET and analysis of CSF and plasma. Transl Psychiatry 6:e777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188. [DOI] [PubMed] [Google Scholar]
- Di Nicola M, Cattaneo A, Hepgul N, Di Forti M, Aitchison KJ, Janiri L, Murray RM, Dazzan P, Pariante CM, Mondelli V. (2013) Serum and gene expression profile of cytokines in first-episode psychosis. Brain Behav Immun 31:90–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Caneja CM, Pina-Camacho L, Rodriguez-Quiroga A, Fraguas D, Parellada M, Arango C. (2015) Predictors of outcome in early-onset psychosis: a systematic review. NPJ Schizophr 1:14005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do K. (2016) Oxidative stress as one core mechanism in schizophrenia pathophysiology and impact of antioxidant N-acetyl-cysteine in a clinical trial with early psychosis patients. In: ACNP 55th Annual Meeting, pp S1–S115: Neuropsychopharmacology. [Google Scholar]
- Doorduin J, de Vries EF, Willemsen AT, de Groot JC, Dierckx RA, Klein HC. (2009) Neuroinflammation in schizophrenia-related psychosis: a PET study. J Nucl Med 50:1801–1807. [DOI] [PubMed] [Google Scholar]
- Duval S, Tweedie R. (2000) Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 56:455–463. [DOI] [PubMed] [Google Scholar]
- Eack SM, Hogarty GE, Cho RY, Prasad KM, Greenwald DP, Hogarty SS, Keshavan MS. (2010) Neuroprotective effects of cognitive enhancement therapy against gray matter loss in early schizophrenia: results from a 2-year randomized controlled trial. Arch Gen Psychiatry 67:674–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes BS, Steiner J, Bernstein HG, Dodd S, Pasco JA, Dean OM, Nardin P, Goncalves CA, Berk M. (2016) C-reactive protein is increased in schizophrenia but is not altered by antipsychotics: meta-analysis and implications. Mol Psychiatry 21:554–564. [DOI] [PubMed] [Google Scholar]
- Fillman SG, Sinclair D, Fung SJ, Webster MJ, Shannon Weickert C. (2014) Markers of inflammation and stress distinguish subsets of individuals with schizophrenia and bipolar disorder. Transl Psychiatry 4:e365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatow J, Buckley P, Miller BJ. (2013) Meta-analysis of oxidative stress in schizophrenia. Biol Psychiatry 74:400–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraguas D, Diaz-Caneja CM, State MW, O’Donovan MC, Gur RE, Arango C. (2016) Mental disorders of known aetiology and precision medicine in psychiatry: a promising but neglected alliance. Psychol Med:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraguas D, Gonzalez-Pinto A, Mico JA, Reig S, Parellada M, Martinez-Cengotitabengoa M, Castro-Fornieles J, Rapado-Castro M, Baeza I, Janssen J, Desco M, Leza JC, Arango C. (2012) Decreased glutathione levels predict loss of brain volume in children and adolescents with first-episode psychosis in a two-year longitudinal study. Schizophr Res 137:58–65. [DOI] [PubMed] [Google Scholar]
- Garcia-Bueno B, Bioque M, MacDowell KS, Santabarbara J, Martinez-Cengotitabengoa M, Moreno C, Saiz PA, Berrocoso E, Gasso P, Fe Barcones M, Gonzalez-Pinto A, Parellada M, Bobes J, Mico JA, Bernardo M, Leza JC. (2014a) Pro-/antiinflammatory dysregulation in early psychosis: results from a 1-year follow-up study. Int J Neuropsychopharmacol 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Bueno B, Bioque M, Mac-Dowell KS, Barcones MF, Martinez-Cengotitabengoa M, Pina-Camacho L, Rodriguez-Jimenez R, Saiz PA, Castro C, Lafuente A, Santabarbara J, Gonzalez-Pinto A, Parellada M, Rubio G, Garcia-Portilla MP, Mico JA, Bernardo M, Leza JC. (2014b) Pro-/anti-inflammatory dysregulation in patients with first episode of psychosis: toward an integrative inflammatory hypothesis of schizophrenia. Schizophr Bull 40:376–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girgis RR, Kumar SS, Brown AS. (2014) The cytokine model of schizophrenia: emerging therapeutic strategies. Biol Psychiatry 75:292–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joa I, Johannessen JO, Langeveld J, Friis S, Melle I, Opjordsmoen S, Simonsen E, Vaglum P, McGlashan T, Larsen TK. (2009) Baseline profiles of adolescent vs. adult-onset first-episode psychosis in an early detection program. Acta Psychiatr Scand 119:494–500. [DOI] [PubMed] [Google Scholar]
- Kahn RS, Sommer IE. (2015) The neurobiology and treatment of first-episode schizophrenia. Mol Psychiatry 20:84–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kale A, Naphade N, Sapkale S, Kamaraju M, Pillai A, Joshi S, Mahadik S. (2010) Reduced folic acid, vitamin B12 and docosahexaenoic acid and increased homocysteine and cortisol in never-medicated schizophrenia patients: implications for altered one-carbon metabolism. Psychiatry Res 175:47–53. [DOI] [PubMed] [Google Scholar]
- Kanazawa K, Sakamoto M, Ishigaki Y, Aihara Y, Hashimoto T, Mizuno M. (2016) Lipid peroxides as endogenous oxidants forming 8-oxo-guanosine and lipid-soluble antioxidants as suppressing agents. J Clin Biochem Nutr 59:16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller WR, Kum LM, Wehring HJ, Koola MM, Buchanan RW, Kelly DL. (2013) A review of anti-inflammatory agents for symptoms of schizophrenia. J Psychopharmacol 27:337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenk M, Selvanathan T, Rao N, Suridjan I, Rusjan P, Remington G, Meyer JH, Wilson AA, Houle S, Mizrahi R. (2015) Imaging neuroinflammation in gray and white matter in schizophrenia: an in-vivo PET study with [18F]-FEPPA. Schizophr Bull 41:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandaker GM, Zimbron J, Lewis G, Jones PB. (2013) Prenatal maternal infection, neurodevelopment and adult schizophrenia: a systematic review of population-based studies. Psychol Med 43:239–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandaker GM, Cousins L, Deakin J, Lennox BR, Yolken R, Jones PB. (2015) Inflammation and immunity in schizophrenia: implications for pathophysiology and treatment. Lancet Psychiatry 2:258–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriisa K, Haring L, Vasar E, Koido K, Janno S, Vasar V, Zilmer K, Zilmer M. (2016) Antipsychotic treatment reduces indices of oxidative stress in first-episode psychosis patients. Oxid Med Cell Longev 2016:9616593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Chen Y, Li J, Yang X, Zhang H, Qin X, Hu Y, Mo Z. (2015) Serum Homocysteine concentration is significantly associated with inflammatory/immune factors. PLoS One 10:e0138099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsey M, Wilson D. (2000) Practical meta-analysis. Thousand Oaks, CA: Sage Publications. [Google Scholar]
- Marin O. (2016) Developmental timing and critical windows for the treatment of psychiatric disorders. Nat Med 22:1229–1238. [DOI] [PubMed] [Google Scholar]
- Martinez-Cengotitabengoa M, Mico JA, Arango C, Castro-Fornieles J, Graell M, Paya B, Leza JC, Zorrilla I, Parellada M, Lopez MP, Baeza I, Moreno C, Rapado-Castro M, Gonzalez-Pinto A. (2014) Basal low antioxidant capacity correlates with cognitive deficits in early onset psychosis. A 2-year follow-up study. Schizophr Res 156:23–29. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Shea TB. (2003) Folate and homocysteine metabolism in neural plasticity and neurodegenerative disorders. Trends Neurosci 26:137–146. [DOI] [PubMed] [Google Scholar]
- Mico JA, Rojas-Corrales MO, Gibert-Rahola J, Parellada M, Moreno D, Fraguas D, Graell M, Gil J, Irazusta J, Castro-Fornieles J, Soutullo C, Arango C, Otero S, Navarro A, Baeza I, Martinez-Cengotitabengoa M, Gonzalez-Pinto A. (2011) Reduced antioxidant defense in early onset first-episode psychosis: a case-control study. BMC Psychiatry 11:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. (2011) Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry 70:663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BJ, Culpepper N, Rapaport MH, Buckley P. (2013) Prenatal inflammation and neurodevelopment in schizophrenia: a review of human studies. Prog Neuropsychopharmacol Biol Psychiatry 42:92–100. [DOI] [PubMed] [Google Scholar]
- Mondelli V, Ciufolini S, Belvederi Murri M, Bonaccorso S, Di Forti M, Giordano A, Marques TR, Zunszain PA, Morgan C, Murray RM, Pariante CM, Dazzan P. (2015) Cortisol and inflammatory biomarkers predict poor treatment response in first episode psychosis. Schizophr Bull 41:1162–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller N. (2014) Immunology of schizophrenia. Neuroimmunomodulation 21:109–116. [DOI] [PubMed] [Google Scholar]
- Palladino I, Salani F, Ciaramella A, Rubino IA, Caltagirone C, Fagioli S, Spalletta G, Bossu P. (2012) Elevated levels of circulating IL-18BP and perturbed regulation of IL-18 in schizophrenia. J Neuroinflammation 9:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parellada M, Moreno C, Mac-Dowell K, Leza JC, Giraldez M, Bailon C, Castro C, Miranda-Azpiazu P, Fraguas D, Arango C. (2012) Plasma antioxidant capacity is reduced in Asperger syndrome. J Psychiatr Res 46:394–401. [DOI] [PubMed] [Google Scholar]
- Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, Haroon E, Miller AH. (2013) A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry 70:31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schizophrenia-Working-Group-of-the-Psychiatric-Genomics-Consortium (2014) Biological insights from 108 schizophrenia-associated genetic loci. Nature 511:421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekar A, Bialas AR, de Rivera H, Davis A, Hammond TR, Kamitaki N, Tooley K, Presumey J, Baum M, Van Doren V, Genovese G, Rose SA, Handsaker RE, Daly MJ, Carroll MC, Stevens B, McCarroll SA. (2016) Schizophrenia risk from complex variation of complement component 4. Nature 530:177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA. (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 349:g7647. [DOI] [PubMed] [Google Scholar]
- Simsek S, Yildirim V, Cim A, Kaya S. (2016a) Serum IL-4 and IL-10 levels correlate with the symptoms of the drug-naive adolescents with first episode, early onset schizophrenia. J Child Adolesc Psychopharmacol 26:721–726. [DOI] [PubMed] [Google Scholar]
- Simsek S, Gencoglan S, Yuksel T, Kaplan I, Alaca R, Aktas H. (2016b) Oxidative stress and DNA damage in untreated first-episode psychosis in adolescents. Neuropsychobiology 73:92–97. [DOI] [PubMed] [Google Scholar]
- Singh I, Rose N. (2009) Biomarkers in psychiatry. Nature 460:202–207. [DOI] [PubMed] [Google Scholar]
- Sommer IE, van Westrhenen R, Begemann MJ, de Witte LD, Leucht S, Kahn RS. (2014) Efficacy of anti-inflammatory agents to improve symptoms in patients with schizophrenia: an update. Schizophr Bull 40:181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Fan X, Li X, Zhang W, Gao J, Zhao J, Harrington A, Ziedonis D, Lv L. (2014) Changes in pro-inflammatory cytokines and body weight during 6-month risperidone treatment in drug naive, first-episode schizophrenia. Psychopharmacology (Berl) 231:319–325. [DOI] [PubMed] [Google Scholar]
- Song XQ, Lv LX, Li WQ, Hao YH, Zhao JP. (2009) The interaction of nuclear factor-kappa B and cytokines is associated with schizophrenia. Biol Psychiatry 65:481–488. [DOI] [PubMed] [Google Scholar]
- Stefansson H, et al. (2009) Common variants conferring risk of schizophrenia. Nature 460:744–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolp HB. (2013) Neuropoietic cytokines in normal brain development and neurodevelopmental disorders. Mol Cell Neurosci 53:63–68. [DOI] [PubMed] [Google Scholar]
- Tamura S, Tsukahara H, Ueno M, Maeda M, Kawakami H, Sekine K, Mayumi M. (2006) Evaluation of a urinary multi-parameter biomarker set for oxidative stress in children, adolescents and young adults. Free Radic Res 40:1198–1205. [DOI] [PubMed] [Google Scholar]
- Thorsen AL, Johansson K, Loberg EM. (2014) Neurobiology of cognitive remediation therapy for schizophrenia: a systematic review. Front Psychiatry 5:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakhrusheva J, Marino B, Stroup TS, Kimhy D. (2016) Aerobic exercise in people with schizophrenia: neural and neurocognitive benefits. Curr Behav Neurosci Rep 3:165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Yue W, Shugart YY, Yuan J, Wang G, Wang HZ, Lehrman B, Zhang F, Zhang D. (2016) Potential involvement of the interleukin-18 pathway in schizophrenia. J Psychiatr Res 74:10–16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.