ABSTRACT
Campylobacter spp., especially Campylobacter jejuni and C. coli, are leading bacterial foodborne pathogens worldwide. In the United States, an estimated 0.8 million cases of campylobacteriosis occur annually, mostly involving C. jejuni. Campylobacteriosis is generally self-limiting, but in severe cases, treatment with antibiotics may be mandated. The increasing incidence of fluoroquinolone resistance in Campylobacter has rendered macrolides such as erythromycin and azithromycin the drugs of choice for human campylobacteriosis. The prevalence of macrolide resistance in C. jejuni remains low, but macrolide resistance can be common in C. coli. Substitutions in the 23S rRNA gene, specifically A2075G, and less frequently A2074C/G, remain the most common mechanism for high-level resistance to macrolides. In C. jejuni, resistance mediated by such substitutions is accompanied by a reduced ability to colonize chickens and other fitness costs, potentially contributing to the low incidence of macrolide resistance. Interestingly, similar fitness impacts have not been noted in C. coli. Also noteworthy is a novel mechanism first reported in 2014 for a C. coli isolate from China and mediated by erm(B) harbored on multidrug resistance genomic islands. The incidence of erm(B) appears to reflect clonal expansion of certain strains, and whole-genome sequencing has been critical to the elucidation of erm(B)-associated macrolide resistance in Campylobacter spp. With the exception of one report from Spain, erm(B)-mediated macrolide resistance has been restricted to Campylobacter spp., mostly C. coli, of animal and human origin from China. If erm(B)-mediated macrolide resistance does not confer fitness costs in C. jejuni, the range of this gene may expand in C. jejuni, threatening to compromise treatment effectiveness for severe campylobacteriosis cases.
KEYWORDS: Campylobacter, Campylobacter coli, Campylobacter jejuni, erm(B), erythromycin, macrolide, multidrug resistance, resistance
INTRODUCTION
In 2013, the Centers for Disease Control and Prevention listed fluoroquinolone- and macrolide-resistant Campylobacter as one of the serious antibiotic resistance threats to public health (1). Campylobacter spp. are major etiologic agents for human foodborne illness (campylobacteriosis), resulting in an estimated 0.8 million annual cases of disease in the United States alone (2–4). Campylobacter jejuni is responsible for about 90% of campylobacteriosis cases, with Campylobacter coli accounting for the majority of the remainder (2, 3). Poultry, contaminated water, and raw milk are the most frequently identified vehicles for illness, with most outbreaks attributed to the latter two (5–8). In addition to acute gastroenteritis, campylobacteriosis may result in severe autoimmune sequelae, including reactive arthritis and in about one of every 1,000 cases, Guillain-Barré syndrome (GBS). Campylobacteriosis is considered to be the most frequent antecedent for GBS (9, 10).
Antimicrobial treatment is not routinely recommended, as most human cases resolve on their own within 3 to 5 days, but it may be recommended for patients with unusually severe and prolonged symptoms, especially those with AIDS, the elderly, or other vulnerable categories (4, 11). Treatment with fluoroquinolones such as ciprofloxacin has been challenged by a high incidence of fluoroquinolone resistance among human isolates, which led to the 2005 ban of the fluoroquinolone enrofloxacin for use in poultry (12). Resistance to the fluoroquinolone ciprofloxacin among human clinical isolates continues to be frequently encountered, e.g., 21.6% of C. jejuni and 24.5% C. coli at the time of the 2005 ban versus 26.7% and 35.6% of C. jejuni and C. coli, respectively, in 2014 (13). In view of the continuing relatively high incidence of fluoroquinolone resistance in Campylobacter spp. from human cases, macrolides such as erythromycin and azithromycin are considered the drugs of choice for treatment of human campylobacteriosis (1, 4, 14).
Historically, the incidence of resistance to erythromycin and other macrolides has been low, especially in C. jejuni, even though there are several mechanisms by which Campylobacter can acquire resistance to these antimicrobial agents. Data from the most recent National Antimicrobial Resistance Monitoring System (NARMS) report indicate that the prevalence of erythromycin resistance (MIC ≥ 8 μg/ml) in C. jejuni has remained below 4% in human and chicken isolates since NARMS testing began in 1997 and 2001, respectively (13). Macrolide resistance was noted in 22% of C. jejuni isolates from hogs, but this was based on only nine isolates, and C. jejuni is generally uncommon in swine (16). In contrast to the stable, low prevalence of macrolide resistance in C. jejuni, an overall trend for increased prevalence of macrolide resistance (MIC ≥ 16 μg/ml) was noted in C. coli; erythromycin resistance in C. coli derived from humans more than tripled in 2014 compared to 2011 (10.3% versus 2.7%) and more than doubled in retail chicken isolates in the same time frame (11.4% versus 5.2%) (13). Analysis of C. coli from samples collected at slaughter revealed macrolide resistance in 21% of isolates from sows, 40% from market swine, 11% from chickens, and 6.7% from turkeys (13). Analysis of 678 C. jejuni isolates and 119 C. coli isolates from human cases in Spain indicated that the prevalence of erythromycin resistance (MIC ≥ 32 μg/ml) was at relatively low levels (3.8%) similar to those in the U.S. NARMS report and that most (28/30) of the resistant isolates were C. coli (17).
Macrolide resistance in C. jejuni and C. coli has been the focus of intense study due to its potential to compromise therapeutic effectiveness of macrolides, and several excellent reviews previously addressed resistance mechanisms and implications (14, 18–20). Nonetheless, more-recent investigations have expanded our understanding of the biological implications of macrolide resistance, especially in regard to associated fitness costs in C. jejuni (21–24). In addition, employment of whole-genome sequencing (WGS) has not only advanced understanding of the distribution of previously known resistance-associated mutations but, starting in 2014, also revealed the emergence of erm(B), a macrolide resistance determinant not previously detected in Campylobacter spp. (25). For these collective reasons, we considered the timing appropriate for an updated minireview of mechanisms and implications of macrolide resistance in this pathogen.
MECHANISMS OF RESISTANCE
Target mutations in 23S rRNA genes.
Campylobacter spp. may evade macrolide binding by alteration of the antimicrobial's 23S rRNA target at position 2074 or 2075. The substitutions A2075G, A2074G, A2074C, and the more rarely encountered A2074T have been shown to confer high-level resistance (>512 μg/ml) to erythromycin when present in all three copies of the 23S rRNA gene in C. jejuni and C. coli (14, 17, 20, 26, 27). High-level resistance also requires an intact CmeABC efflux system as will be discussed in detail below (14, 26, 27). The ribosomal substitutions may not always occur in all three copies of the gene, resulting in a lower level of resistance (14, 19, 28). Additionally, isolates may have different substitutions on different copies of the 23S rRNA gene. For example, one C. jejuni strain with an erythromycin MIC of >128 μg/ml harbored A2074C and A2075G substitutions in different copies of the 23S rRNA gene (28).
WGS analysis of 114 Campylobacter isolates from the United States identified 52 isolates (46%), including 17 C. jejuni isolates and 35 C. coli isolates, that were resistant to erythromycin; all but one harbored the A2075G substitution in the 23S rRNA gene with the remaining isolate, a C. jejuni isolate, harboring A2074T, suggesting that A2075G remains the most prevalent genetic event conferring high-level resistance to erythromycin (29). All erythromycin-resistant isolates were coresistant to azithromycin and vice versa, with the exception of one strain found to be azithromycin resistant but erythromycin susceptible and harboring an amino acid substitution (A86E) in ribosomal protein L22 (29).
Target mutations in ribosomal proteins.
In the absence of mutations in 23S rRNA genes, mutations in rplD and rplV (ribosomal proteins L4 and L22, respectively) are associated with a lower level of resistance to macrolides (erythromycin MIC, 32 μg/ml) compared to isolates with ribosomal substitutions that exhibit erythromycin MICs of >512 μg/ml (19, 24, 30). Strains with L4 and L22 mutations developed high-level resistance (>256 μg/ml) upon acquisition of mutations in the 23S rRNA gene (30). Numerous macrolide resistance-conferring substitutions and insertions in these ribosomal proteins have been recorded. For instance, various substitutions (e.g., G74D, G67V, R72I, and A71D), and a glycine insertion at position 60 were noted in L4 (14, 26, 30, 31). For L22, A88E and G86E have been noted as well as a nine-base duplication at position 292 of rplV and insertions at position 86 or 98 (14, 26, 30).
Ribosomal methylation encoded by erm(B).
For many years, base substitutions in the 23S rRNA sequence were the only mechanism known to specifically mediate high-level macrolide resistance in Campylobacter spp. (14). However, in 2014, a ribosomal methylase encoded by erm(B) was reported for the first time in Campylobacter spp., in C. coli strain ZC113 of swine origin in China (25). Erm(B) dimethylates a single adenine in the 23S rRNA gene, leading to decreased binding of macrolides (32). C. coli ZC113 and the majority of other erm(B)-harboring strains are constitutively resistant to macrolides and also express erm(B) constitutively (33). However, a small number of erm(B)-harboring strains were found to be susceptible to erythromycin; such strains expressed erm(B) and became resistant to macrolides with MICs as high as strains constitutively expressing erm(B) upon preincubation with erythromycin or clindamycin (33). Interestingly, the majority of erm(B)-harboring strains appear to harbor deletions in the erm(B) regulatory region, which may account for their constitutive resistance to macrolides (33). It is tempting to speculate that such deletion derivatives have been selected upon exposure to macrolides, e.g., in animal production.
In C. coli ZC113, erm(B) was harbored by a chromosomal multidrug resistance genomic island (MDRGI) composed of 17 open reading frames (ORFs), 8 of which encoded antimicrobial resistance determinants (25). Besides erm(B), the MDRGI included a truncated tet(O) and the aminoglycoside resistance cassette aadE-sat4-aphA3, previously found in conjunction with erm(B) in Enterococcus (25, 34). The MDRGI-associated erm(B) showed 100% identity with erm(B) of Gram-positive bacteria such as Streptococcus suis, Enterococcus faecium, and Lactobacillus plantarum, and most other MDRGI ORFs exhibited high homology with counterparts in Gram-positive bacteria, suggesting a Gram-positive origin for the erm(B)-harboring island in Campylobacter (25, 35, 36).
Subsequent reports have identified erm(B) in several additional isolates (25, 37–39). Multilocus sequence typing (MLST) analysis of 58 erm(B)-harboring C. coli isolates from China identified 30 different sequence types, many of which clustered into one clonal complex (CC 828) (36). erm(B)-positive isolates from another study also mostly clustered within CC 828 (40). erm(B)-harboring isolates exhibited high levels of erythromycin resistance (MIC, 512 μg/ml) with the exception of two of the five erm(B)-positive C. jejuni isolates identified thus far (MIC, 16 μg/ml) (25, 37, 41). Interestingly, three erm(B)-positive C. coli isolates also harbored the A2075G substitution in the 23S rRNA gene (39).
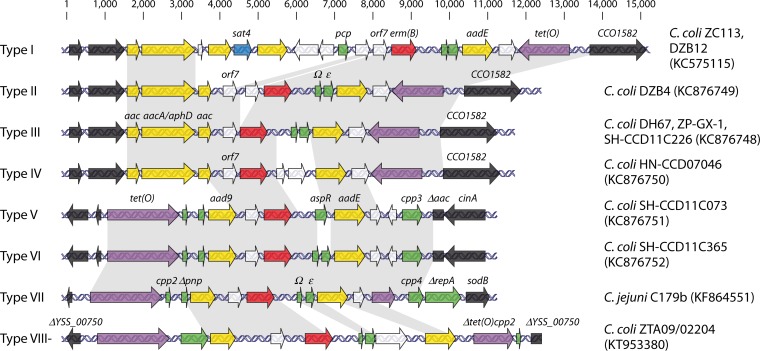
In all investigated cases, erm(B) was harbored by an MDRGI, frequently (57%) on the chromosome or on plasmids of different sizes (35–39). Sequence analysis has revealed at least eight different erm(B) genomic organizations, with the arrangement in the first reported erm(B)-harboring strain, C. coli ZC113, being designated as type I (Fig. 1) (36, 37). Various insertion sites were identified for different MDRGIs, but erm(B) was highly conserved (>98% identity at the nucleotide sequence level) among all eight MDRGIs (Fig. 1) (37, 38). The most divergent Erm(B) (type III MDRGI) had only four amino acid substitutions compared to Erm(B) in the type I MDRGI. The lone erm(B)-positive strain reported outside China (MDRGI type VIII) had only one amino acid substitution in Erm(B), while erm(B) in the type VII MDRGI of C. jejuni C179b (accession no. KF864551) had one nucleotide substitution compared to its type I homolog, which did not result in an amino acid change (37, 38).
FIG 1.
The genetic environment of erm(B)-harboring MDRGIs in Campylobacter spp. Shaded regions indicate areas with greater than 98% identity. The erm(B) gene is shown in red, aminoglycoside resistance genes are shown in yellow, the tetracycline resistance gene tet(O) is shown in purple, genes with predicted functions are shown in green, and genes encoding hypothetical proteins are shown in white with bordering regions shown in black in accordance with previously published MDRGIs (25, 36, 37). The comparisons were performed using Geneious (version 9.1.4) (58). Deltas indicate deletions in the corresponding genes.
Detection of erm(B) remains uncommon in Campylobacter, and as of this writing, erm(B) seems to be largely confined to China, with only one erm(B)-positive strain reported elsewhere (Spain) (37). WGS analysis of 114 C. jejuni and C. coli isolates from the NARMS collection failed to identify erm(B) among any of the 52 macrolide-resistant isolates (29). It is not clear why reports for erm(B)-harboring strains have been largely absent from nations other than China. China's antimicrobial use may have conferred unique selective pressures on Campylobacter spp. there. While antimicrobial use is difficult to measure, China has been estimated to be responsible for 23% of the global antimicrobial use, with the United States estimated at 13% (40). It is also conceivable that erm(B)-harboring Campylobacter strains arose in China via horizontal gene transfer from Gram-positive microbes cooccurring with Campylobacter, e.g., in swine, in response to animal husbandry attributes that might be more common in animal production in China than elsewhere.
Even in China, however, incidence of erm(B)-harboring strains appears to be low. For instance, only 58 of 1,554 (3.7%) C. jejuni and C. coli isolates from animal and human cases were positive for erm(B) by PCR (36). It is noteworthy that most (53/58) of these erm(B)-harboring isolates were C. coli obtained in 2011 and 2012, with only 5 isolates from previous years (2007 to 2009), suggesting that erm(B) may be expanding (36). The earliest erm(B)-positive Campylobacter isolate was C. jejuni cj94473, isolated from a case of human diarrheal disease in China in 1994 and exhibiting intermediate (MIC, 16 μg/ml) resistance to erythromycin (41). Another PCR analysis of 858 C. jejuni and C. coli isolates from human diarrheal cases, chicken, and swine from China identified only 30 (3.5%) that were erm(B) positive; all were C. coli, with a substantial fraction (13/30 [43%]) originating from human diarrheal cases (39). To the best of the authors' knowledge, fitness impacts of these MDRGIs in Campylobacter have not yet been described.
The sole erm(B)-positive strain reported outside China may warrant special attention. This strain, C. coli ZTA09/02204, harbored erm(B) on an MDRGI (type VIII) that was genetically different enough from that of C. coli ZC113 (Fig. 1) to prompt the speculation that it may have originated independently (37). The type VIII MDRGI harbors 12 ORFs, including 5 antimicrobial resistance (AMR) genes with an intact tet(O), in contrast to the type I MDRGI from C. coli ZC113 which contained 17 ORFs with 8 AMR genes and a truncated tet(O) (Fig. 1) (25, 37). Especially interesting is the high (99%) similarity of the type VIII MDRGI to a region on the previously characterized plasmid pN29710-1, identified in C. coli from retail chicken in the United States and harboring the recently identified aminoglycoside resistance gene aph(2″)-Ig (42). In the type VIII MDRGI, a cluster of genes, including erm(B), an omega transcriptional repressor, a toxin-antitoxin system, and aadE (Fig. 1), may have been inserted between aad9 and the truncated tet(O) of pN29710-1 (37).
Multidrug efflux pumps.
Macrolide resistance mediated by specific mutations or horizontally acquired determinants such as discussed above operates over and above baseline resistance levels conferred by innate efflux systems such as the CmeABC efflux pump. CmeABC is a member of the resistance-nodulation-division (RND) efflux transporter family; it harbors the typical structural motif of 12 transmembrane α-helices and mediates efflux of various compounds (14, 43–46). CmeABC inactivation resulted in up to a 64-fold decrease in the erythromycin MIC of C. jejuni harboring the A2075G substitution in the 23S rRNA gene (43, 46, 47). The extent to which CmeABC inactivation may decrease erythromycin MICs of erm(B)-harboring strains remains to be determined.
Mutations in the regulatory region of cmeABC may affect the MIC of macrolide-resistant isolates, and recently, a C. jejuni variant with enhanced macrolide resistance and harboring a single A-to-G substitution in the CmeR-binding site of cmeABC (resistance-enhancing CmeABC [RE-CmeABC]) was identified (48). CmeR represses cmeABC transcription by binding to an inverted repeat in the cmeR-cmeA intergenic region (49, 50). Mutations in the CmeR-binding site have been shown to result in overexpression of CmeABC (51, 52), and indeed one RE-CmeABC variant, which was identified using WGS, exhibited a fivefold increase in cmeABC transcript levels (48). Such enhanced-resistance variants may be expanding within Campylobacter. The prevalence of RE-CmeABC among chicken- and swine-derived C. jejuni and C. coli increased from 7% to 20% between 2012 and 2014 (48). It is noteworthy that RE-CmeABC was noticeably more common in C. jejuni than C. coli, possibly suggesting a fitness advantage in C. jejuni (48).
Fitness costs.
The finding that macrolide resistance is much more common in C. coli than C. jejuni prompts the hypothesis that fitness costs associated with substitutions in the 23S rRNA macrolide target keep the prevalence low in C. jejuni. In vitro competitive fitness assays have in fact indicated markedly impaired fitness associated with erythromycin resistance for certain strains of C. jejuni during growth in laboratory media (21, 23, 24, 53). Erythromycin-susceptible strains of C. jejuni and their isogenic erythromycin-resistant mutants (harboring either the A2074G or A2075G 23S rRNA substitution) had equal ability to colonize chickens when inoculated as monocultures, but in mixtures and in the absence of erythromycin, the erythromycin-resistant mutants were readily outcompeted by their parental strain counterparts (21, 24). Interestingly, such fitness costs in bird colonization were not noted with macrolide-resistant mutants of C. coli (21).
The impaired ability of erythromycin-resistant C. jejuni to colonize chickens may account for the overall low prevalence of erythromycin resistance in C. jejuni from human disease, for which chicken is a major vehicle. It also raises questions not only about the mechanisms underlying the observed differences in fitness impacts between C. jejuni and C. coli but also about mechanisms that allow stable, high-level erythromycin resistance in certain C. jejuni strains (19, 22). Compensatory mutations in such strains may counteract the fitness impacts. One study employed inoculation of chickens with mixtures of a C. jejuni strain and an isogenic erythromycin-resistant mutant harboring A2074G in the 23S rRNA gene. A spontaneous colonization-proficient derivative of the erythromycin-resistant mutant was recovered from the inoculated birds and harbored a potentially compensatory C2551G substitution in the 23S rRNA gene in addition to the original A2074G substitution, retaining resistance to erythromycin (21). In another study, erythromycin-resistant mutants selected in vitro upon exposure to increasing levels of macrolides were found to harbor numerous mutations in addition to 23S rRNA substitutions (30). WGS analysis is expected to further elucidate possible compensatory mechanisms in C. jejuni that harbor specific substitutions in 23S rRNA and also exhibit stable, high-level resistance to macrolides.
C. jejuni strains with amino acid substitutions in L4 and L22 also exhibited downregulation of motility and energy metabolism genes; in addition, they had slower growth kinetics and became outcompeted in poultry hosts (24, 30). It is possible that the physiological impacts of L4 and L22 mutations that may precede those in 23S rRNA may contribute to the observed fitness impacts of macrolide resistance in C. jejuni (30). However, the roles of ribosomal proteins in macrolide resistance and fitness costs remain to be further elucidated. While some studies identified substitutions in the ribosomal proteins in conjunction with those in the 23S rRNA gene, others did not find such associations (19, 28–30, 41, 54).
The potential impacts of erm(B) on Campylobacter's ability to colonize animals or other adaptations remain to be determined. Additionally, while the increasing prevalence of RE-CmeABC in C. jejuni may indicate a fitness advantage, experimental fitness assessments of these strains have not been reported.
It should be kept in mind that mutations conferring antimicrobial resistance do not always affect Campylobacter fitness in a negative way. Consider fluoroquinolone resistance of C. jejuni, mediated by a C257T substitution in gyrA (55). Although growth kinetics, motility, and ability to colonize chickens were similar between the parental strain and isogenic resistant mutants in monoculture, when inoculated as mixed cultures, the resistant mutants frequently outcompeted their susceptible parental counterparts, even in the absence of ciprofloxacin (14, 55). This contrasts with macrolide resistance where, as discussed above, resistant C. jejuni mutants were outcompeted by their susceptible counterparts.
Dissemination of macrolide resistance.
Mutations in the 23S rRNA gene as well as those in rplV and rplD can be transferred from erythromycin-resistant to erythromycin-susceptible Campylobacter strains by natural transformation (19, 31, 56). Interestingly, turkey-derived erythromycin-susceptible C. coli strains were more efficiently transformed to erythromycin resistance than C. coli from swine, and the transformation frequency was significantly higher at 42°C, the body temperature of poultry, than at 25°C (56, 57). Certain strains of Campylobacter have fragmented 23S rRNA stemming from posttranscriptional excision of intervening sequences (IVS) (58). Analysis of erythromycin-resistant C. coli from turkeys revealed that, in addition to harboring the A2075G substitution in the 23S rRNA gene, these strains also tended to harbor IVS in all three 23S rRNA genes (59). Both the 23S rRNA A2075G mutation and IVS were transferrable to erythromycin-susceptible, IVS-free C. coli by natural transformation, rendering the latter resistant to erythromycin (59). It remains to be determined whether the higher prevalence of A2075G-mediated erythromycin resistance in C. coli, in comparison to C. jejuni, may reflect differences in the frequency of IVS and 23S rRNA fragmentation in these two species.
erm(B) is also transferable via natural transformation. Even though most of the reported erm(B)-harboring isolates have been C. coli, with only five erm(B)-positive C. jejuni strains reported thus far (38, 41), erm(B)-mediated resistance was transferable from C. coli to C. jejuni by natural transformation with total genomic DNA (25, 36). The type VII erm(B)-carrying MDRGI in the chromosome of C. jejuni C179b (Fig. 1) was also transferable via natural transformation into erythromycin-susceptible C. jejuni (38). Along with macrolide resistance, C. jejuni transformants acquired additional AMR genes harbored in the MDRGI, gaining resistance to the corresponding antimicrobials, including lincosamides, tetracycline, ciprofloxacin, and gentamicin (25, 36). Finally, the RE-CmeABC mutation could also be disseminated via natural transformation, with transformants exhibiting up to a 32-fold increase in erythromycin MIC (from 0.5 μg/ml to 16 μg/ml) (48).
Whole-genome sequencing.
The massive increase in WGS data for Campylobacter spp. (25, 29, 42, 60–63) has yielded a rich resource that can be mined to identify determinants associated with resistance to macrolides and other antimicrobials. The earlier-discussed WGS analysis of 114 C. jejuni and C. coli isolates from humans, retail meats, and food animals in the United States revealed that detection of specific AMR genes could accurately predict the corresponding resistance phenotype. For example, resistance to tetracycline and fluoroquinolones perfectly correlated with the presence of tet(O) or mutations in gyrA, respectively (29). WGS was also critical in the identification of erm(B) in Campylobacter and in analysis of the contents and insertional sites of the erm(B)-harboring MDRGIs in Campylobacter (25, 37–39). However, differences in the levels of macrolide resistance among isolates meeting the resistance threshold, e.g., ≥8 μg/ml and ≥16 μg/ml for C. jejuni and C. coli, respectively (64), may not be readily predictable based on the presence or absence of specific resistance mutations or determinants, as has also been recognized for other bacterial pathogens (65). Further integration of WGS data and phenotypic macrolide resistance assessments may yield WGS signatures with the capacity to predict differences in levels of macrolide resistance in Campylobacter spp. beyond the resistance threshold.
CONCLUSIONS
Conserved (primarily A2075G) substitutions in domain V of the 23S rRNA gene still represent the most commonly encountered mechanism for macrolide resistance in C. jejuni and C. coli. The accompanying fitness costs in C. jejuni, especially in colonization of chickens, may be responsible for the relatively low prevalence of macrolide resistance in this species. For reasons that remain to be elucidated, similar fitness costs in colonization have not been observed with C. coli, which also exhibits markedly higher prevalence of macrolide resistance, especially among isolates from food and animal sources. The issue of erythromycin resistance in C. jejuni is intriguing; in spite of the documented fitness costs, stable erythromycin-resistant strains and poultry flocks colonized by such strains have been reported (19, 66–70). Fitness costs in C. jejuni may be dependent on the strain or alleviated by compensatory mutations. Increasing availability and use of WGS data are expected to make major contributions in the further elucidation of these issues.
Even though thus far erm(B) remains primarily confined to C. coli from China, it has been shown to be transferrable via natural transformation to C. jejuni, the species responsible for the majority of cases of campylobacteriosis in humans, and it was recently also identified outside China (Spain). Further clonal expansion of erm(B)-harboring strains and infiltration of erm(B) into C. jejuni or C. coli populations in other regions can compromise the clinical effectiveness of macrolides for severe campylobacteriosis. Dissemination of the newly described resistance-enhancing CmeABC mutations (RE-CmeABC) may also have public health implications, especially if they emerge in C. jejuni strains already harboring 23S rRNA point mutations or erm(B).
ACKNOWLEDGMENTS
This study was partially supported by USDA-NIFA grant 2011-2936/2011-51110-31050.
We thank all members of our laboratory for feedback and support.
REFERENCES
- 1.Centers for Disease Control and Prevention. 2013. Antibiotic resistance threats in the United States, 2013. Centers for Disease Control and Prevention, Atlanta, GA: https://www.cdc.gov/drugresistance/threat-report-2013/. [Google Scholar]
- 2.Scallan E, Hoekstra RM, Mahon BE, Jones TF, Griffin PM. 2015. An assessment of the human health impact of seven leading foodborne pathogens in the United States using disability adjusted life years. Epidemiol Infect 143:2795–2804. doi: 10.1017/S0950268814003185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson M-A, Roy SL, Jones JL, Griffin PM. 2011. Foodborne illness acquired in the United States–major pathogens. Emerg Infect Dis 17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 2014. Campylobacter, general information. Centers for Disease Control and Prevention, Atlanta, GA: https://www.cdc.gov/foodsafety/diseases/campylobacter/index.html. [Google Scholar]
- 5.Taylor EV, Herman KM, Ailes EC, Fitzgerald C, Yoder JS, Mahon BE, Tauxe RV. 2013. Common source outbreaks of Campylobacter infection in the USA, 1997-2008. Epidemiol Infect 141:987–996. doi: 10.1017/S0950268812001744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hermans D, Pasmans F, Messens W, Martel A, Van Immerseel F, Rasschaert G, Heyndrickx M, Van Deun K, Haesebrouck F. 2012. Poultry as a host for the zoonotic pathogen Campylobacter jejuni. Vector-Borne Zoonotic Dis 12:89–98. doi: 10.1089/vbz.2011.0676. [DOI] [PubMed] [Google Scholar]
- 7.Gillespie IA, O'Brien SJ, Frost JA, Adak GK, Horby P, Swan AV, Painter MJ, Neal KR. 2002. A case-case comparison of Campylobacter coli and Campylobacter jejuni infection: a tool for generating hypotheses. Emerg Infect Dis 8:937–942. doi: 10.3201/eid0809.010817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Humphrey T, O'Brien S, Madsen M. 2007. Campylobacters as zoonotic pathogens: a food production perspective. Int J Food Microbiol 117:237–257. doi: 10.1016/j.ijfoodmicro.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Yuki N, Hartung H-P. 2012. Guillain-Barré syndrome. N Engl J Med 366:2294–2304. doi: 10.1056/NEJMra1114525. [DOI] [PubMed] [Google Scholar]
- 10.Yang B, Lian Y, Liu Y, Wu B-Y, Duan R-S. 2016. A retrospective analysis of possible triggers of Guillain–Barre syndrome. J Neuroimmunol 293:17–21. doi: 10.1016/j.jneuroim.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Ruiz-Palacios GM. 2007. The health burden of Campylobacter infection and the impact of antimicrobial resistance: playing chicken. Clin Infect Dis 44:701–703. doi: 10.1086/509936. [DOI] [PubMed] [Google Scholar]
- 12.US Food and Drug Administration. 2014. Withdrawal of enrofloxacin for poultry. US Food and Drug Administration, Silver Spring, MD: https://www.fda.gov/AnimalVeterinary/SafetyHealth/RecallsWithdrawals/ucm042004.htm. [Google Scholar]
- 13.US Food and Drug Administration. 2014. NARMS integrated report: 2014. The National Antimicrobial Resistance Monitoring System (NARMS): enteric bacteria. US Food and Drug Administration, Silver Spring, MD: https://www.fda.gov/downloads/AnimalVeterinary/SafetyHealth/AntimicrobialResistance/NationalAntimicrobialResistanceMonitoringSystem/UCM528861.pdf. [Google Scholar]
- 14.Luangtongkum T, Jeon B, Han J, Plummer P, Logue CM, Zhang Q. 2009. Antibiotic resistance in Campylobacter: emergence, transmission and persistence. Future Microbiol 4:189–200. doi: 10.2217/17460913.4.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reference deleted.
- 16.Tyson GH, Tate HP, Abbott J, Tran T-T, Kabera C, Crarey E, Young S, McDermott PF, Sprague G, Campbell M, Adeyemo O, Browne-Silva J, Myers M, Thitaram S, Zhao S. 2016. Molecular subtyping and source attribution of Campylobacter isolated from food animals. J Food Prot 79:1891–1897. doi: 10.4315/0362-028X.JFP-16-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perez-Boto D, Lopez-Portoles JA, Simon C, Valdezate S, Echeita MA. 2010. Study of the molecular mechanisms involved in high-level macrolide resistance of Spanish Campylobacter jejuni and Campylobacter coli strains. J Antimicrob Chemother 65:2083–2088. doi: 10.1093/jac/dkq268. [DOI] [PubMed] [Google Scholar]
- 18.Gibreel A, Taylor DE. 2006. Macrolide resistance in Campylobacter jejuni and Campylobacter coli. J Antimicrob Chemother 58:243–255. doi: 10.1093/jac/dkl210. [DOI] [PubMed] [Google Scholar]
- 19.Gibreel A, Kos VN, Keelan M, Trieber CA, Levesque S, Michaud S, Taylor DE. 2005. Macrolide resistance in Campylobacter jejuni and Campylobacter coli: molecular mechanism and stability of the resistance phenotype. Antimicrob Agents Chemother 49:2753–2759. doi: 10.1128/AAC.49.7.2753-2759.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Payot S, Bolla J-M, Corcoran D, Fanning S, Mégraud F, Zhang Q. 2006. Mechanisms of fluoroquinolone and macrolide resistance in Campylobacter spp. Microbes Infect 8:1967–1971. doi: 10.1016/j.micinf.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 21.Zeitouni S, Collin O, Andraud M, Ermel G, Kempf I. 2012. Fitness of macrolide resistant Campylobacter coli and Campylobacter jejuni. Microb Drug Resist 18:101–108. doi: 10.1089/mdr.2011.0188. [DOI] [PubMed] [Google Scholar]
- 22.Hao H, Dai M, Wang Y, Peng D, Liu Z, Yuan Z. 2009. 23S rRNA mutation A2074C conferring high-level macrolide resistance and fitness cost in Campylobacter jejuni. Microb Drug Resist 15:239–244. doi: 10.1089/mdr.2009.0008. [DOI] [PubMed] [Google Scholar]
- 23.Han F, Pu S, Wang F, Meng J, Ge B. 2009. Fitness cost of macrolide resistance in Campylobacter jejuni. Int J Antimicrob Agents 34:462–466. doi: 10.1016/j.ijantimicag.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 24.Luangtongkum T, Shen Z, Seng VW, Sahin O, Jeon B, Liu P, Zhang Q. 2012. Impaired fitness and transmission of macrolide-resistant Campylobacter jejuni in its natural host. Antimicrob Agents Chemother 56:1300–1308. doi: 10.1128/AAC.05516-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qin S, Wang Y, Zhang Q, Zhang M, Deng F, Shen Z, Wu C, Wang S, Zhang J, Shen J. 2014. Report of ribosomal RNA methylase gene erm(B) in multidrug-resistant Campylobacter coli. J Antimicrob Chemother 69:964–968. doi: 10.1093/jac/dkt492. [DOI] [PubMed] [Google Scholar]
- 26.Caldwell DB, Wang Y, Lin J. 2008. Development, stability, and molecular mechanisms of macrolide resistance in Campylobacter jejuni. Antimicrob Agents Chemother 52:3947–3954. doi: 10.1128/AAC.00450-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin J, Yan M, Sahin O, Pereira S, Chang Y-J, Zhang Q. 2007. Effect of macrolide usage on emergence of erythromycin-resistant Campylobacter isolates in chickens. Antimicrob Agents Chemother 51:1678–1686. doi: 10.1128/AAC.01411-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vacher S, Menard A, Bernard E, Santos A, Megraud F. 2005. Detection of mutations associated with macrolide resistance in thermophilic Campylobacter spp. by real-time PCR. Microb Drug Resist 11:40–47. doi: 10.1089/mdr.2005.11.40. [DOI] [PubMed] [Google Scholar]
- 29.Zhao S, Tyson GH, Chen Y, Li C, Mukherjee S, Young S, Lam C, Folster JP, Whichard JM, McDermott PF. 2015. Whole-genome sequencing analysis accurately predicts antimicrobial resistance phenotypes in Campylobacter spp. Appl Environ Microbiol 82:459–466. doi: 10.1128/AEM.02873-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hao H, Yuan Z, Shen Z, Han J, Sahin O, Liu P, Zhang Q. 2013. Mutational and transcriptomic changes involved in the development of macrolide resistance in Campylobacter jejuni. Antimicrob Agents Chemother 57:1369–1378. doi: 10.1128/AAC.01927-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cagliero C, Mouline C, Cloeckaert A, Payot S. 2006. Synergy between efflux pump CmeABC and modifications in ribosomal proteins L4 and L22 in conferring macrolide resistance in Campylobacter jejuni and Campylobacter coli. Antimicrob Agents Chemother 50:3893–3896. doi: 10.1128/AAC.00616-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leclercq R. 2002. Mechanisms of resistance to macrolides and lincosamides: nature of the resistance elements and their clinical implications. Clin Infect Dis 34:482–492. doi: 10.1086/324626. [DOI] [PubMed] [Google Scholar]
- 33.Deng F, Shen J, Zhang M, Wu C, Zhang Q. 2015. Constitutive and inducible expression of the rRNA methylase gene erm(B) in Campylobacter. Antimicrob Agents Chemother 59:6661–6664. doi: 10.1128/AAC.01103-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Werner G, Hildebrandt B, Witte W. 2003. Linkage of erm(B) and aadE-sat4-aphA-3 in multiple-resistant Enterococcus faecium isolates of different ecological origins. Microb Drug Resist 9(Suppl 1):S9–S16. doi: 10.1089/107662903322541847. [DOI] [PubMed] [Google Scholar]
- 35.Qin S, Wang Y, Zhang Q, Chen X, Shen Z, Deng F, Wu C, Shen J. 2012. Identification of a novel genomic island conferring resistance to multiple aminoglycoside antibiotics in Campylobacter coli. Antimicrob Agents Chemother 56:5332–5339. doi: 10.1128/AAC.00809-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y, Zhang M, Deng F, Shen Z, Wu C, Zhang J, Zhang Q, Shen J. 2014. Emergence of multidrug-resistant Campylobacter species isolates with a horizontally acquired rRNA methylase. Antimicrob Agents Chemother 58:5405–5412. doi: 10.1128/AAC.03039-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Florez-Cuadrado D, Ugarte-Ruiz M, Quesada A, Palomo G, Domínguez L, Porrero MC. 2016. Description of an erm(B)-carrying Campylobacter coli isolate in Europe. J Antimicrob Chemother 71:841–843. doi: 10.1093/jac/dkv383. [DOI] [PubMed] [Google Scholar]
- 38.Deng F, Wang Y, Zhang Y, Shen Z. 2015. Characterization of the genetic environment of the ribosomal RNA methylase gene erm(B) in Campylobacter jejuni. J Antimicrob Chemother 70:613–615. doi: 10.1093/jac/dku418. [DOI] [PubMed] [Google Scholar]
- 39.Zhang A, Song L, Liang H, Gu Y, Zhang C, Liu X, Zhang J, Zhang M. 2016. Molecular subtyping and erythromycin resistance of Campylobacter in China. J Appl Microbiol 121:287–293. doi: 10.1111/jam.13135. [DOI] [PubMed] [Google Scholar]
- 40.Van Boeckel TP, Brower C, Gilbert M, Grenfell BT, Levin SA, Robinson TP, Teillant A, Laxminarayan R. 2015. Global trends in antimicrobial use in food animals. Proc Natl Acad Sci U S A 112:5649–5654. doi: 10.1073/pnas.1503141112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou J, Zhang M, Yang W, Fang Y, Wang G, Hou F. 2016. A seventeen-year observation of the antimicrobial susceptibility of clinical Campylobacter jejuni and the molecular mechanisms of erythromycin-resistant isolates in Beijing, China. Int J Infect Dis 42:28–33. doi: 10.1016/j.ijid.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 42.Chen Y, Mukherjee S, Hoffmann M, Kotewicz ML, Young S, Abbott J, Luo Y, Davidson MK, Allard M, McDermott PF, Zhao S. 2013. Whole-genome sequencing of gentamicin-resistant Campylobacter coli isolated from U.S. retail meats reveals novel plasmid-mediated aminoglycoside resistance genes. Antimicrob Agents Chemother 57:5398–5405. doi: 10.1128/AAC.00669-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pumbwe L, Piddock L. 2002. Identification and molecular characterisation of CmeB, a Campylobacter jejuni multidrug efflux pump. FEMS Microbiol Lett 206:185–189. doi: 10.1111/j.1574-6968.2002.tb11007.x. [DOI] [PubMed] [Google Scholar]
- 44.Mamelli L, Amoros JP, Pagès JM, Bolla JM. 2003. A phenylalanine-arginine β-naphthylamide sensitive multidrug efflux pump involved in intrinsic and acquired resistance of Campylobacter to macrolides. Int J Antimicrob Agents 22:237–241. doi: 10.1016/S0924-8579(03)00199-7. [DOI] [PubMed] [Google Scholar]
- 45.Tseng TT, Gratwick KS, Kollman J, Park D, Nies DH, Goffeau A, Saier MH. 1999. The RND permease superfamily: an ancient, ubiquitous and diverse family that includes human disease and development proteins. J Mol Microbiol Biotechnol 1:107–125. [PubMed] [Google Scholar]
- 46.Guo B, Lin J, Reynolds DL, Zhang Q. 2010. Contribution of the multidrug efflux transporter CmeABC to antibiotic resistance in different Campylobacter species. Foodborne Pathog Dis 7:77–83. doi: 10.1089/fpd.2009.0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gibreel A, Wetsch NM, Taylor DE. 2007. Contribution of the CmeABC efflux pump to macrolide and tetracycline resistance in Campylobacter jejuni. Antimicrob Agents Chemother 51:3212–3216. doi: 10.1128/AAC.01592-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yao H, Shen Z, Wang Y, Deng F, Liu D, Naren G, Dai L, Su C-C, Wang B, Wang S, Wu C, Yu EW, Zhang Q, Shen J. 2016. Emergence of a potent multidrug efflux pump variant that enhances Campylobacter resistance to multiple antibiotics. mBio 7:e01543-16. doi: 10.1128/mBio.01543-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin J, Akiba M, Sahin O, Zhang Q. 2005. CmeR functions as a transcriptional repressor for the multidrug efflux pump CmeABC in Campylobacter jejuni. Antimicrob Agents Chemother 49:1067–1075. doi: 10.1128/AAC.49.3.1067-1075.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guo B, Wang Y, Shi F, Barton Y-W, Plummer P, Reynolds DL, Nettleton D, Grinnage-Pulley T, Lin J, Zhang Q. 2008. CmeR functions as a pleiotropic regulator and is required for optimal colonization of Campylobacter jejuni in vivo. J Bacteriol 190:1879–1890. doi: 10.1128/JB.01796-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cagliero C, Maurel M-C, Cloeckaert A, Payot S. 2007. Regulation of the expression of the CmeABC efflux pump in Campylobacter jejuni: identification of a point mutation abolishing the binding of the CmeR repressor in an in vitro-selected multidrug-resistant mutant. FEMS Microbiol Lett 267:89–94. doi: 10.1111/j.1574-6968.2006.00558.x. [DOI] [PubMed] [Google Scholar]
- 52.Grinnage-Pulley T, Zhang Q. 2015. Genetic basis and functional consequences of differential expression of the CmeABC efflux pump in Campylobacter jejuni isolates. PLoS One 10:e0131534. doi: 10.1371/journal.pone.0131534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ohno H, Wachino JI, Saito R, Jin W, Yamada K, Kimura K, Arakawa Y. 2016. A highly macrolide-resistant Campylobacter jejuni strain with rare A2074T mutations in 23S rRNA genes. Antimicrob Agents Chemother 60:2580–2581. doi: 10.1128/AAC.02822-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ladely SR, Meinersmann RJ, Englen MD, Fedorka-Cray PJ, Harrison MA. 2009. 23S rRNA gene mutations contributing to macrolide resistance in Campylobacter jejuni and Campylobacter coli. Foodborne Pathog Dis 6:91–98. doi: 10.1089/fpd.2008.0098. [DOI] [PubMed] [Google Scholar]
- 55.Luo N, Pereira S, Sahin O, Lin J, Huang S, Michel L, Zhang Q. 2005. Enhanced in vivo fitness of fluoroquinolone-resistant Campylobacter jejuni in the absence of antibiotic selection pressure. Proc Natl Acad Sci U S A 102:541–546. doi: 10.1073/pnas.0408966102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim J-S, Carver DK, Kathariou S. 2006. Natural transformation-mediated transfer of erythromycin resistance in Campylobacter coli strains from turkeys and swine. Appl Environ Microbiol 72:1316–1321. doi: 10.1128/AEM.72.2.1316-1321.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim J-S, Kim J-W, Kathariou S. 2008. Differential effects of temperature on natural transformation to erythromycin and nalidixic acid resistance in Campylobacter coli. Appl Environ Microbiol 74:6121–6125. doi: 10.1128/AEM.01075-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Trust TJ, Logan SM, Gustafson CE, Romaniuk PJ, Kim NW, Chan VL, Ragan MA, Guerry P, Gutell RR. 1994. Phylogenetic and molecular characterization of a 23S rRNA gene positions the genus Campylobacter in the epsilon subdivision of the Proteobacteria and shows that the presence of transcribed spacers is common in Campylobacter spp. J Bacteriol 176:4597–4609. doi: 10.1128/jb.176.15.4597-4609.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chan K, Miller WG, Mandrell RE, Kathariou S. 2007. The absence of intervening sequences in 23S rRNA genes of Campylobacter coli isolates from turkeys is a unique attribute of a cluster of related strains which also lack resistance to erythromycin. Appl Environ Microbiol 73:1208–1214. doi: 10.1128/AEM.01995-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weis AM, Huang BC, Storey DB, Kong N, Chen P, Arabyan N, Gilpin B, Mason C, Townsend AK, Smith WA, Byrne BA, Taff CC, Weimer BC. 2017. Large-scale release of Campylobacter draft genomes: resources for food safety and public health from the 100K Pathogen Genome Project. Genome Announc 5:e00925-16. doi: 10.1128/genomeA.00925-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dutta V, Altermann E, Olson J, Wray GA, Siletzky RM, Kathariou S. 2016. Whole-genome sequences of agricultural, host-associated Campylobacter coli and Campylobacter jejuni strains. Genome Announc 4:e00833-16. doi: 10.1128/genomeA.00833-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Miller WG, Huynh S, Parker CT, Niedermeyer JA. 2016. Complete genome sequences of multidrug-resistant Campylobacter jejuni strain 14980A (turkey feces) and Campylobacter coli strain 14983A (housefly from a turkey farm), harboring a novel gentamicin resistance mobile element. Genome Announc 4:e01175-16. doi: 10.1128/genomeA.01175-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weis AM, Clothier KA, Huang BC, Kong N, Weimer BC. 2016. Draft genome sequences of Campylobacter jejuni strains that cause abortion in livestock. Genome Announc 4:e01324-16. doi: 10.1128/genomeA.01324-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Centers for Disease Control and Prevention. 2016. National Antimicrobial Resistance Monitoring System (NARMS): 2014 human isolates surveillance report. Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 65.Ellington MJ, Ekelund O, Aarestrup FM, Canton R, Doumith M, Giske C, Grundman H, Hasman H, Holden M, Hopkins KL, Iredell J, Kahlmeter G, Köser CU, MacGowan A, Mevius D, Mulvey M, Naas T, Peto T, Rolain J-M, Samuelsen Ø, Woodford N. 2017. The role of whole genome sequencing (WGS) in antimicrobial susceptibility testing of bacteria: report from the EUCAST Subcommittee. Clin Microbiol Infect 23:2–22. doi: 10.1016/j.cmi.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 66.Bolinger H, Kirchner M, Chandrashekhar K, Miller W, Niedermeyer J, Carver D, Kathariou S. 2016. Investigation of erythromycin-resistant Campylobacter jejuni from turkey farms in North Carolina, abstr P2-192. 2016 IAFP Annu Meet. International Association for Food Protection, Des Moines, IA. [Google Scholar]
- 67.Luangtongkum T, Morishita TYT, Ison AJ, Huang S, McDermott PF, Zhang Q. 2006. Effect of conventional and organic production practices on the prevalence and antimicrobial resistance of Campylobacter spp. in poultry. Appl Environ Microbiol 72:3600–3607. doi: 10.1128/AEM.72.5.3600-3607.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen X, Naren GW, Wu CM, Wang Y, Dai L, Xia LN, Luo PJ, Zhang Q, Shen JZ. 2010. Prevalence and antimicrobial resistance of Campylobacter isolates in broilers from China. Vet Microbiol 144:133–139. doi: 10.1016/j.vetmic.2009.12.035. [DOI] [PubMed] [Google Scholar]
- 69.Frediani-Wolf V, Stephan R. 2003. Resistance patterns of Campylobacter spp. strains isolated from poultry carcasses in a big Swiss poultry slaughterhouse. Int J Food Microbiol 89:233–240. doi: 10.1016/S0168-1605(03)00152-1. [DOI] [PubMed] [Google Scholar]
- 70.Jacobs-Reitsma WF, Koenraad P, Bolder N, Mulder R. 1994. In vitro susceptibility of Campylobacter and Salmonella isolates from broilers to quinolones, ampicillin, tetracycline, and erythromycin. Vet Q 16:206–208. doi: 10.1080/01652176.1994.9694450. [DOI] [PubMed] [Google Scholar]