Abstract
Background
This is a retrospective observational study evaluating the prevalence and clinical characteristics of spontaneous splenorenal shunt in liver cirrhosis.
Material/Methods
We included a total of 105 cirrhotic patients who were admitted to our hospital between June 2012 and December 2013 and underwent contrast-enhanced CT and/or MRI scans at admissions. Spontaneous splenorenal shunt was identified. Clinical and laboratory data were compared between cirrhotic patients with and without spontaneous splenorenal shunt.
Results
The prevalence of spontaneous splenorenal shunt was 10.5% (11/105). The prevalence of hepatic encephalopathy was higher in patients with spontaneous splenorenal shunt than in those without spontaneous splenorenal shunt (18.2% vs. 4.3%, p=0.062), but the difference between them was not statistically significant. The prevalence of acute upper-gastrointestinal bleeding was lower in patients with spontaneous splenorenal shunt than in those without spontaneous splenorenal shunt (0% vs. 18.1%, p=0.205), but the difference between them was not statistically significant. Patients with spontaneous splenorenal shunt had significantly higher Child-Pugh scores (9.50±1.65 vs. 7.43±2.02, p=0.002) and MELD scores (11.26±7.29 vs. 5.67±6.83, p=0.017) than those without spontaneous splenorenal shunt. In-hospital mortality was similar between them (0% vs. 4.3%, p=1.000).
Conclusions
Spontaneous splenorenal shunt might be associated with worse liver function in liver cirrhosis, but not with in-hospital mortality.
MeSH Keywords: Liver Cirrhosis, Prevalence, Risk Factors, Splenorenal Shunt, Survival
Background
Spontaneous formation of portosystemic shunt is one of classical signs of portal hypertension [1]. The major mechanism is that the blood flow is redirected from the high-pressure portal vessels to the low-pressure systemic vessels [2]. In nature, spontaneous portosystemic shunt is a compensation for portal hypertension [3,4]. However, great damage is caused by the overgrowth of portosystemic shunts. For example, gastro-esophageal varices, the most common type of spontaneous portosystemic shunt in cirrhotic portal hypertension, will result in life-threatening upper-gastrointestinal hemorrhage [5]. Large spontaneous portosystemic shunts increase the risk of persistent hepatic encephalopathy [6].
Splenorenal shunt refers to a communication between splenic vein and left renal vein [1]. It is a relatively uncommon type of spontaneous portosystemic shunt and is less recognized in clinical practice [7–9]. An early case-control study suggested that patients with large spontaneous splenorenal or gastrorenal shunt have a higher risk of large esophageal varices, but have a similar risk of variceal bleeding [10]. However, another early study found that only 10% of patients with spontaneous splenorenal or gastrorenal shunt had esophageal varices alone, but 48.8% of patients without spontaneous splenorenal or gastrorenal shunt had esophageal varices alone [11]. Most researchers agree that the risk of chronic hepatic encephalopathy is increased by the presence of spontaneous splenorenal shunt [10]. However, a recent study found that the grade of hepatic encephalopathy was similar between patients with and without spontaneous splenorenal shunt [12]. Due to substantial controversies among studies, more research is needed to explore the clinical significance of spontaneous splenorenal shunt in liver cirrhosis.
We performed the present retrospective observational study to evaluate the prevalence and clinical characteristics of spontaneous splenorenal shunt in liver cirrhosis.
Material and Methods
Patient selection
Inclusion criteria were: 1) patients admitted to our hospital between June 2012 and December 2013; 2) patients with a diagnosis of liver cirrhosis; and 3) patients who underwent contrast-enhanced CT and/or MRI scans at admissions. Exclusion criteria were: 1) patients with malignancy, including hepatocellular carcinoma; 2) patients who underwent splenectomy; and 3) patients who underwent surgical splenorenal shunt. Notably, some of them had been included in our previous studies [13–22]. The study protocol of this retrospective observational study was approved by the Medical Ethical Committee of our hospital. The approval number was k (2016)03. The requirement for written informed consent was waived due to the nature of this study.
Data collection
Our study group consistently collected clinical and laboratory data from the electronic medical charts. The accuracy of clinical and laboratory data for the present study was further validated by 2 investigators (XQ and HD). The primary data included age, sex, etiology of liver cirrhosis, history of diabetes, major clinical presentations at their admissions (i.e., ascites, hepatic encephalopathy, and acute upper-gastrointestinal bleeding), presence and severity of esophageal varices at endoscopy, red blood cell, hemoglobin, white blood cell, platelet count, total bilirubin, albumin, alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, gamma-glutamyl transpeptidase, blood urea nitrogen, creatinine, potassium, sodium, prothrombin time, activated partial thromboplastin time, and international normalized ratio. Child-Pugh score and class and model for end-stage of liver disease (MELD) score were calculated. In-hospital death was also recorded.
The imaging data was evaluated by 1 investigator (XQ), who discussed uncertain cases with other investigators. Presence of spontaneous splenorenal shunt was identified by consecutively tracing the origins of splenic vein and left renal vein and their confluence on the axial contrast-enhanced CT and/or MRI scans. Notably, in the cases with spontaneous splenorenal shunt, multiple planes of CT and/or MRI scans should be studied due to the presence of tortuous collateral circulation. Additionally, the maximum diameters of the spleen, splenic vein, and main portal vein were measured.
Data analysis
Patient characteristics were summarized by the mean with standard deviation and the median with range for continuous variables and the frequency (percentage) for categorical variables. Patients were divided into 2 groups according to the presence of spontaneous splenorenal shunt. The data regarding the clinical symptoms, laboratory tests, and images were compared between patients with and without spontaneous splenorenal shunt. Continuous data were compared by using the independent sample t test, and categorical data were compared by using the chi-square or Fisher’s exact tests. P value<0.05 was considered statistically significant. All statistical analyses were performed by the SPSS statistical software version 16.0.0.
Results
Patients
Overall, 113 cirrhotic patients underwent contrast-enhanced CT and/or MRI scans. Among them, 91 patients underwent contrast-enhanced CT scans alone and 22 patients underwent contrast-enhanced MRI scans alone.
Because 8 patients who underwent splenectomy were excluded, 105 patients were finally included. Among them, 85 patients underwent contrast-enhanced CT scans alone, and 20 patients underwent contrast-enhanced MRI scans alone. Patient characteristics are shown in Table 1. A majority of included patients were male, had hepatitis virus- or alcohol-related liver cirrhosis, and had Child-Pugh class A and B. In-hospital mortality was 3.8% (4/105).
Table 1.
Patient characteristics.
| N | Mean or frequency (percentage) | Std. deviation | Median | Minimum | Maximum | |
|---|---|---|---|---|---|---|
| Age (years) | 105 | 55.21 | 13.01 | 55.19 | 22.14 | 85.46 |
| Sex (Male/Female) | 105 | 71 (67.6%)/34 (32.4%) | ||||
| Etiology of liver diseases – n. | 105 | |||||
| – Hepatitis B virus alone | 32 (30.5%) | |||||
| – Hepatitis C virus alone | 7 (6.7%) | |||||
| – Hepatitis B + C virus | 3 (2.9%) | |||||
| – Alcohol | 30 (28.6%) | |||||
| – Hepatitis B virus + Alcohol | 7 (6.7%) | |||||
| – Hepatitis C virus + Alcohol | 1 (1%) | |||||
| – Autoimmunity | 5 (4.8%) | |||||
| – Drug-related | 2 (1.9%) | |||||
| – Unknown | 18 (17.1%) | |||||
| Diabetes – n. | 105 | 13 (12.4%) | ||||
| Ascites – n. | 105 | 59 (56.2%) | ||||
| Hepatic encephalopathy – n. | 104 | 6 (5.8%) | ||||
| Acute upper-gastrointestinal bleeding – n. | 105 | 17 (16.2%) | ||||
| Esophageal varices at endoscopy – n. | 42 | |||||
| – No | 10 (23.8%) | |||||
| – Mild | 2 (4.8%) | |||||
| – Moderate | 9 (21.4%) | |||||
| – Severe | 21 (50%) | |||||
| Gastric varices at endoscopy – n. | 42 | 24 (57.1%) | ||||
| Hemoglobin (g/L) | 102 | 106.18 | 30.30 | 107.00 | 42.00 | 170.00 |
| White blood cell count (109/L) | 102 | 4.96 | 3.21 | 4.15 | 1.50 | 20.50 |
| Platelet count (109/L) | 102 | 92.97 | 76.65 | 73.50 | 11.00 | 545.00 |
| Total bilirubin (umol/L) | 104 | 48.44 | 71.87 | 23.80 | 5.10 | 436.50 |
| Albumin (g/L) | 103 | 32.33 | 6.72 | 32.20 | 11.70 | 44.30 |
| Alanine aminotransferase (U/L) | 104 | 51.61 | 63.08 | 33.00 | 8.00 | 429.00 |
| Aspartate aminotransferase (U/L) | 104 | 74.13 | 98.12 | 47.00 | 10.00 | 889.00 |
| Alkaline phosphatase (U/L) | 104 | 117.67 | 80.09 | 92.00 | 34.00 | 524.40 |
| Gamma-glutamyl transpeptidase (U/L) | 104 | 159.73 | 221.69 | 68.50 | 12.00 | 1130.00 |
| Blood urea nitrogen (mmol/L) | 102 | 5.80 | 2.60 | 5.26 | 1.73 | 17.18 |
| Creatinine (umol/L) | 102 | 59.42 | 21.61 | 57.00 | 29.00 | 151.00 |
| Potassium (mmol/L) | 103 | 4.04 | 0.47 | 4.00 | 3.01 | 5.43 |
| Sodium (mmol/L) | 103 | 138.02 | 6.50 | 139.20 | 83.00 | 144.50 |
| Prothrombin time (seconds) | 103 | 16.34 | 6.55 | 14.80 | 11.40 | 62.80 |
| Activated partial thromboplastin time (seconds) | 103 | 45.13 | 16.53 | 42.10 | 29.90 | 180.00 |
| International normalized ratio | 103 | 1.35 | 0.84 | 1.16 | 0.77 | 7.96 |
| Serum ammonia (umol/L) | 53 | 46.04 | 32.59 | 35 | 9 | 127 |
| Child-Pugh score | 100 | 7.64 | 2.07 | 8.00 | 5.00 | 12.00 |
| Child-Pugh class A/B/C | 100 | 37 (37%)/40 (40%)/23 (23%) | ||||
| MELD score | 100 | 6.23 | 7.05 | 4.86 | (5.20) | 34.52 |
| Maximum diameter of spleen (mm) | 105 | 140.27 | 30.77 | 138.20 | 83.80 | 240.90 |
| Maximum diameter of splenic vein (mm) | 105 | 10.68 | 3.83 | 10.30 | 4.30 | 29.60 |
| Maximum diameter of main portal vein (mm) | 105 | 18.34 | 5.18 | 18.00 | 0.00 | 31.00 |
| In-hospital mortality | 105 | 4 (3.8%) |
The prevalence of spontaneous splenorenal shunt was 10.5% (11/105) (Figures 1, 2). Patient characteristics at admission were compared between patients with and without spontaneous splenorenal shunt (Table 2).
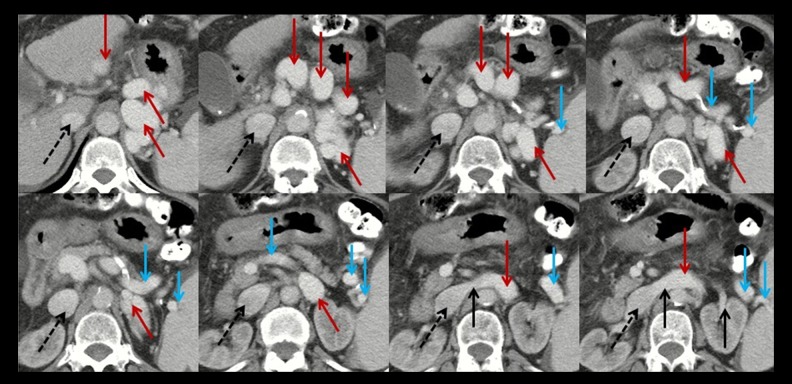
Figure 1.
Axial contrast-enhanced computed tomography at the portal vein phase in a 63-year-old female patient with spontaneous splenorenal shunt (HXY). Black dashed arrows represent the inferior vena cava; black solid arrows represent the left renal vein; blue solid arrows represent the splenic vein; red solid arrows represent the communication between splenic vein and left renal vein.
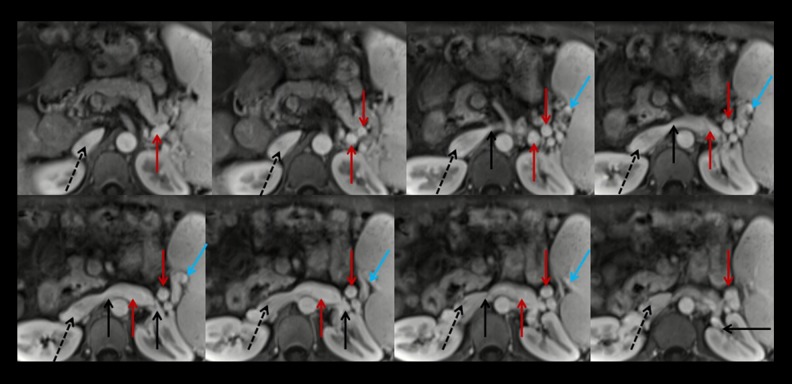
Figure 2.
Axial contrast-enhanced MRI scans at the portal vein phase in a 44-year-old male patient with spontaneous splenorenal shunt (MQS). Black dashed arrows represent the inferior vena cava; black solid arrows represent the left renal vein; blue solid arrows represent the splenic vein; red solid arrows represent the communication between splenic vein and left renal vein.
Table 2.
Comparison of characteristics between patients with and without splenorenal shunt.
| Variables | With splenorenal shunt (n=11) | Without splenorenal shunt (n=94) | P value | ||||
|---|---|---|---|---|---|---|---|
| N | Mean or frequency (percentage) | Std. deviation | N | Mean or frequency (percentage) | Std. deviation | ||
| Age (years) | 11 | 56.89 | 12.98 | 94 | 55.02 | 13.07 | 0.653 |
| Sex (Male/Female) | 11 | 6 (54.5%)/5 (45.5%) | 94 | 65 (69.1%)/29 (30.9%) | 0.327 | ||
| Etiology of liver diseases – n. | 11 | 94 | 0.059 | ||||
| – Hepatitis B virus alone | 4 (36.4%) | 28 (29.8%) | |||||
| – Hepatitis C virus alone | 0 (0%) | 7 (7.4%) | |||||
| – Hepatitis B + C virus | 0 (0%) | 3 (3.2%) | |||||
| – Alcohol | 0 (0%) | 27 (28.7%) | |||||
| – Hepatitis B virus + Alcohol | 3 (27.3%) | 7 (7.4%) | |||||
| – Hepatitis C virus + Alcohol | 0 (0%) | 1 (1.1%) | |||||
| – Autoimmunity | 3 (27.3%) | 2 (2.2%) | |||||
| – Drug-related | 0 (0%) | 2 (2.2%) | |||||
| – Unknown | 1 (9.1%) | 17 (18.1%) | |||||
| Diabetes – n. | 11 | 2 (18.2%) | 94 | 11 (11.7%) | 0.537 | ||
| Ascites – n. | 11 | 7 (63.6%) | 94 | 52 (55.3%) | 0.599 | ||
| Hepatic encephalopathy – n. | 11 | 2 (18.2%) | 94 | 4 (4.3%) | 0.062 | ||
| Acute upper-gastrointestinal bleeding – n. | 11 | 0 (0%) | 94 | 17 (18.1%) | 0.205 | ||
| Esophageal varices at endoscopy – n. | 3 | 39 | 0.066 | ||||
| – No | 0 (0%) | 10 (25.6%) | |||||
| – Mild | 1 (33.3%) | 1 (2.6%) | |||||
| – Moderate | 0 (0%) | 9 (23.1%) | |||||
| – Severe | 2 (66.7%) | 19 (48.7%) | |||||
| Gastric varices at endoscopy – n. | 3 | 3 (100%) | 39 | 21 (53.8%) | 0.247 | ||
| Hemoglobin (g/L) | 10 | 89.90 | 24.53 | 92 | 107.95 | 30.45 | 0.073 |
| White blood cell count (109/L) | 10 | 5.19 | 4.25 | 92 | 4.94 | 3.10 | 0.814 |
| Platelet count (109/L) | 10 | 90.50 | 60.55 | 92 | 93.24 | 78.47 | 0.915 |
| Total bilirubin (umol/L) | 11 | 52.56 | 31.01 | 93 | 47.95 | 75.34 | 0.842 |
| Albumin (g/L) | 11 | 25.70 | 5.92 | 92 | 33.12 | 6.39 | <0.001 |
| Alanine aminotransferase (U/L) | 11 | 56.09 | 70.28 | 93 | 51.08 | 62.57 | 0.804 |
| Aspartate aminotransferase (U/L) | 11 | 91.09 | 59.43 | 93 | 72.12 | 101.77 | 0.547 |
| Alkaline phosphatase (U/L) | 11 | 182.85 | 134.31 | 93 | 109.96 | 68.21 | 0.004 |
| Gamma-glutamyl transpeptidase (U/L) | 11 | 218.27 | 254.72 | 93 | 152.81 | 217.97 | 0.357 |
| Blood urea nitrogen (mmol/L) | 10 | 6.98 | 3.73 | 92 | 5.68 | 2.45 | 0.134 |
| Creatinine (umol/L) | 10 | 71.75 | 38.85 | 92 | 58.08 | 18.73 | 0.057 |
| Potassium (mmol/L) | 10 | 3.89 | 0.50 | 93 | 4.05 | 0.46 | 0.298 |
| Sodium (mmol/L) | 10 | 136.91 | 4.09 | 93 | 138.14 | 6.71 | 0.573 |
| Prothrombin time (seconds) | 10 | 18.88 | 4.94 | 93 | 16.06 | 6.66 | 0.198 |
| Activated partial thromboplastin time (seconds) | 10 | 49.23 | 13.81 | 93 | 44.68 | 16.80 | 0.411 |
| International normalized ratio | 10 | 1.61 | 0.55 | 93 | 1.32 | 0.86 | 0.309 |
| Serum ammonia (umol/L) | 6 | 60.50 | 27.19 | 47 | 44.19 | 33.01 | 0.698 |
| Child-Pugh score | 10 | 9.50 | 1.65 | 90 | 7.43 | 2.02 | 0.002 |
| Child-Pugh class A/B/C | 10 | 0 (0%)/ 3 (30%)/ 7 (70%) | 90 | 37 (41.1%)/37 (41.1%)/16 (17.8%) | 0.001 | ||
| MELD score | 10 | 11.26 | 7.29 | 90 | 5.67 | 6.83 | 0.017 |
| Maximum diameter of spleen (mm) | 11 | 147.71 | 25.84 | 94 | 139.40 | 31.30 | 0.399 |
| Maximum diameter of splenic vein (mm) | 11 | 10.43 | 1.79 | 94 | 10.71 | 4.01 | 0.816 |
| Maximum diameter of main portal vein (mm) | 11 | 12.30 | 5.35 | 94 | 19.05 | 4.70 | <0.001 |
| In-hospital mortality | 11 | 0 (0%) | 94 | 4 (4.3%) | 1.000 | ||
Clinical symptoms
The prevalence of hepatic encephalopathy was higher in patients with spontaneous splenorenal shunt than in those without (18.2% vs. 4.3%), but the difference between them was not statistically significant (p=0.062).
None of the patients with spontaneous splenorenal shunt presented with acute upper-gastrointestinal bleeding, but 18.1% of patients without spontaneous splenorenal shunt presented with acute upper-gastrointestinal bleeding; however, the difference between them was not statistically significant (p=0.205).
All of the patients with spontaneous splenorenal shunt who underwent upper-gastrointestinal endoscopy had esophageal varices and 84.4% of patients without spontaneous splenorenal shunt who underwent upper-gastrointestinal endoscopy had esophageal varices, but the difference between them was not statistically significant (p=0.066).
Laboratory tests
Patients with spontaneous splenorenal shunt had significantly lower albumin levels than those without spontaneous splenorenal shunt (p<0.001).
Patients with spontaneous splenorenal shunt had significantly higher alkaline phosphatase levels than those without spontaneous splenorenal shunt (p=0.004).
Patients with spontaneous splenorenal shunt had significantly higher Child-Pugh (p=0.002) and MELD (p=0.017) scores than those without spontaneous splenorenal shunt, and the proportion of Child-Pugh class was significantly different between them (p=0.001).
Images
The maximum diameter of the main portal vein was significantly smaller in patients with spontaneous splenorenal shunt than in those without spontaneous splenorenal shunt (p<0.001).
Discussion
In this retrospective study, we explored the prevalence and clinical characteristics of spontaneous splenorenal shunt in liver cirrhosis. Tarantino and Maruyama also performed 2 similar studies on the implications of spontaneous splenorenal shunt in liver cirrhosis [12,23]. In the 2 studies, splenorenal shunt was evaluated by Doppler ultrasound. In Tarantino’s study, hepatocellular carcinoma was not excluded. By comparison, the major feature of our study was that we carefully checked the images on the axial contrast-enhanced CT and MRI scans to identify the presence of spontaneous splenorenal shunt; therefore, our findings might be more objective and accurate. Several additional characteristics of the present study are: 1) the clinical and laboratory data were collected by our study group and validated by 2 investigators; 2) the data on diameters of spleen, splenic vein, and main portal vein were also collected; and 3) hepatocellular carcinoma was excluded.
A major finding of our study was that the presence of spontaneous splenorenal shunt was associated with worse liver function (i.e., higher Child-Pugh and MELD scores) in liver cirrhosis. This association was primarily due to a significantly lower albumin level in cirrhotic patients with spontaneous splenorenal shunt. In addition, we should acknowledge that cirrhotic patients with spontaneous splenorenal shunt had higher total bilirubin, creatinine, and international normalized ratio and higher prevalence of hepatic encephalopathy and ascites. Our findings are similar to the findings of Maruyama et al. that hepatic decompensation was more frequently observed in patients with splenorenal shunts, but are in contrast to the findings of Tarantino et al. that Child-Pugh classification did not predict splenorenal shunts (odds ratio: 1.145, 95% confidence intervals: 0.77–1.51, p=0.66). Further research using repeated validation is needed.
We could not determine the order of spontaneous splenorenal shunt and liver dysfunction. If it is true that the occurrence of spontaneous splenorenal shunt results in the deterioration of liver function, the potential mechanism may be as follows: hepatic perfusion is largely reduced in patients with spontaneous splenorenal shunt, thereby leading to the liver function abnormality. If the perspective that the development of spontaneous splenorenal shunt is secondary to the deterioration of liver function is supported, the potential mechanism may be as follows: portal pressure is largely elevated in cirrhotic patients with poor liver function, thereby inducing the development of spontaneous splenorenal shunt. Certainly, we should never neglect a synergistic effect between spontaneous splenorenal shunt and liver dysfunction.
Our study did not find any significant association between spontaneous splenorenal shunt and in-hospital death. This is primarily explained by the small number of patients included and the low in-hospital mortality. Additionally, information regarding long-term outcome was not available in our study. Therefore, additional work is needed to establish whether spontaneous splenorenal shunt influences the long-term survival of cirrhotic patients. Recently, Maruyama et al. examined the clinical effect of splenorenal shunt and short gastric vein on long-term outcomes in patients with cirrhosis. They found that the 1-, 3-, and 5-year cumulative survival rates were 80%, 66.6%, and 58.3% in patients with splenorenal shunt; 94.1%, 87.4%, and 72.8% in those with short gastric vein; and 88.3% 73.1%, and 58%, respectively, in those without splenorenal shunt or short gastric vein. The difference among the 3 groups was not statistically significant (p=0.2). It appears that patients with short gastric veins have the best survival, followed by patients without splenorenal shunt or short gastric vein and patients with splenorenal shunt.
Another finding of our study was a very low risk of acute upper-gastrointestinal bleeding in cirrhotic patients with spontaneous splenorenal shunt. This finding is readily explained by the fact that spontaneous splenorenal shunt reduces the portal pressure, thereby preventing development of variceal bleeding. It is seemingly contradictory that the prevalence of esophageal varices at endoscopy was higher in cirrhotic patients with spontaneous splenorenal shunt than in those without spontaneous splenorenal shunt. However, it should be noted that only a low proportion of cirrhotic patients underwent endoscopic examinations. Thus, the potential selection bias should be emphasized.
Several additional limitations of our study should be acknowledged. First, angiography was not regularly performed due to its invasiveness. Second, due to the retrospective nature of our study, not all included patients had relevant clinical and laboratory data. Third, the data at previous admissions were not collected.
Conclusions
In conclusion, the presence of spontaneous splenorenal shunt indicates more severe liver damage. Additionally, it might be associated with a higher prevalence of hepatic encephalopathy, but a lower prevalence of acute upper-gastrointestinal bleeding. Considering its clinical significance, physicians should pay more attention to the presence of spontaneous splenorenal shunt. Further well-designed prospective studies are warranted to explore the clinical implications of spontaneous splenorenal shunt in liver cirrhosis.
Footnotes
This work was partially presented as a poster at the Asian Pacific Association for the Study of Liver (APASL) Single Topic Conference (STC) 2016 – 6th HBV Conference
Conflict of interest
None.
Source of support: This study was partially supported by the grants from the National Natural Science Foundation of China (no. 81500474), Natural Science Foundation of Liaoning Province (no. 2015020409), and China Postdoctoral Science Foundation (2015M582886) for Dr. Xingshun Qi
References
- 1.Arora A, Rajesh S, Meenakshi YS, et al. Spectrum of hepatofugal collateral pathways in portal hypertension: an illustrated radiological review. Insights Imaging. 2015;6(5):559–72. doi: 10.1007/s13244-015-0419-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zardi EM, Uwechie V, Caccavo D, et al. Portosystemic shunts in a large cohort of patients with liver cirrhosis: detection rate and clinical relevance. J Gastroenterol. 2009;44(1):76–83. doi: 10.1007/s00535-008-2279-1. [DOI] [PubMed] [Google Scholar]
- 3.Hsieh JS, Wang JY, Huang CJ, et al. Effect of spontaneous portosystemic shunts on hemorrhage from esophagogastric varices. World J Surg. 2004;28(1):23–28. doi: 10.1007/s00268-003-7068-7. [DOI] [PubMed] [Google Scholar]
- 4.Qi X, Ye C, Hou Y, Guo X. A large spontaneous intrahepatic portosystemic shunt in a cirrhotic patient. Intractable Rare Dis Res. 2016;5(1):58–60. doi: 10.5582/irdr.2016.01000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biecker E. Portal hypertension and gastrointestinal bleeding: Diagnosis, prevention and management. World J Gastroenterol. 2013;19(31):5035–50. doi: 10.3748/wjg.v19.i31.5035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riggio O, Efrati C, Catalano C, et al. High prevalence of spontaneous portal-systemic shunts in persistent hepatic encephalopathy: A case-control study. Hepatology. 2005;42(5):1158–65. doi: 10.1002/hep.20905. [DOI] [PubMed] [Google Scholar]
- 7.Culafic D, Perisic M, Vojinovic-Culafic V, et al. Spontaneous splenorenal shunt in a patient with liver cirrhosis and hypertrophic caudal lobe. J Gastrointestin Liver Dis. 2006;15(3):289–92. [PubMed] [Google Scholar]
- 8.Iannello S, Libertini L, Martini R, et al. A large spontaneous splenorenal shunt in a patient with liver cirrhosis and uncomplicated portal hypertension. Dig Dis. 1999;17(4):248–55. doi: 10.1159/000016944. [DOI] [PubMed] [Google Scholar]
- 9.Cacciapaglia F, Vadacca M, Coppolino G, et al. Spontaneous splenorenal shunt in a patient with antiphospholipid syndrome: The first case reported. Lupus. 2007;16(1):56–58. doi: 10.1177/0961203306072390. [DOI] [PubMed] [Google Scholar]
- 10.Ohnishi K, Sato S, Saito M, et al. Clinical and portal hemodynamic features in cirrhotic patients having a large spontaneous splenorenal and/or gastrorenal shunt. Am J Gastroenterol. 1986;81(6):450–55. [PubMed] [Google Scholar]
- 11.Hayashishita N, Meguro T, Saga A, et al. Clinical evaluation of spontaneous splenorenal or gastrorenal shunt in patients with liver cirrhosis. J Gastroenterol Hepatol. 1989;4(Suppl 1):231–34. [PubMed] [Google Scholar]
- 12.Tarantino G, Citro V, Conca P, et al. What are the implications of the spontaneous spleno-renal shunts in liver cirrhosis? BMC Gastroenterol. 2009;9:89. doi: 10.1186/1471-230X-9-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qi X, Han G, Ye C, et al. Splenectomy causes 10-fold increased risk of portal venous system thrombosis in liver cirrhosis patients. Med Sci Monit. 2016;22:2528–50. doi: 10.12659/MSM.898866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peng Y, Qi X, Tang S, et al. Child-Pugh, MELD, and ALBI scores for predicting the in-hospital mortality in cirrhotic patients with acute-on-chronic liver failure. Expert Rev Gastroenterol Hepatol. 2016;10(8):971–80. doi: 10.1080/17474124.2016.1177788. [DOI] [PubMed] [Google Scholar]
- 15.Zou D, Qi X, Zhu C, et al. Albumin-bilirubin score for predicting the in-hospital mortality of acute upper gastrointestinal bleeding in liver cirrhosis: A retrospective study. Turk J Gastroenterol. 2016;27(2):180–86. doi: 10.5152/tjg.2016.15502. [DOI] [PubMed] [Google Scholar]
- 16.Zhang X, Qi X, De Stefano V, et al. Epidemiology, risk factors, and in-hospital mortality of venous thromboembolism in liver cirrhosis: A single-center retrospective observational study. Med Sci Monit. 2016;22:969–76. doi: 10.12659/MSM.896153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qi X, Li H, Chen J, et al. Serum liver fibrosis markers for predicting the presence of gastroesophageal varices in liver cirrhosis: A retrospective cross-sectional study. Gastroenterol Res Pract. 2015;2015:274534. doi: 10.1155/2015/274534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deng H, Qi X, Peng Y, et al. Diagnostic accuracy of APRI, AAR, FIB-4, FI, and king scores for diagnosis of esophageal varices in liver cirrhosis: A retrospective study. Med Sci Monit. 2015;21:3961–77. doi: 10.12659/MSM.895005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Qi X, Deng H, et al. Association of conventional haemostasis and coagulation tests with the risk of acute upper gastrointestinal bleeding in liver cirrhosis: A retrospective study. Gastroenterol Rep (Oxf) 2015 doi: 10.1093/gastro/gov059. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dai J, Qi X, Peng Y, et al. Association between D-dimer level and portal venous system thrombosis in liver cirrhosis: A retrospective observational study. Int J Clin Exp Med. 2015;8(9):15296–301. [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu C, Qi X, Li H, et al. Correlation of serum liver fibrosis markers with severity of liver dysfunction in liver cirrhosis: A retrospective cross-sectional study. Int J Clin Exp Med. 2015;8(4):5989–98. [PMC free article] [PubMed] [Google Scholar]
- 22.Peng Y, Qi X, Dai J, et al. Child-Pugh versus MELD score for predicting the in-hospital mortality of acute upper gastrointestinal bleeding in liver cirrhosis. Int J Clin Exp Med. 2015;8(1):751–57. [PMC free article] [PubMed] [Google Scholar]
- 23.Maruyama H, Kondo T, Kiyono S, et al. Influence of splenorenal shunt on long-term outcomes in cirrhosis. Scand J Gastroenterol. 2015;50(5):593–600. doi: 10.3109/00365521.2014.1003401. [DOI] [PubMed] [Google Scholar]