Abstract
Deregulation of mitotic spindle genes has been reported to contribute to the development and progression of malignant tumours. The aim of the present study was to explore the association between the expression profiles of Aurora kinases (AURKA, AURKB and AURKC), cytoskeleton-associated protein 5 (CKAP5), discs large-associated protein 5 (DLGAP5), kinesin-like protein 11 (KIF11), microtubule nucleation factor (TPX2), monopolar spindle 1 kinase (TTK), and β-tubulins (TUBB) and (TUBB3) genes and clinicopathological characteristics in human non-small cell lung carcinoma (NSCLC). Reverse transcription-quantitative polymerase chain reaction-based RNA gene expression profiles of 132 NSCLC and 44 adjacent wild-type tissues were generated, and Cox's proportional hazard regression was used to examine associations. With the exception of AURKC, all genes exhibited increased expression in NSCLC tissues. Of the 10 genes examined, only AURKA was significantly associated with prognosis in NSCLC. Multivariate Cox's regression analysis demonstrated that AURKA mRNA expression [hazard ratio (HR), 1.81; 95% confidence interval (CI), 1.16–2.84; P=0.009], age (HR, 1.03; 95% CI, 1.00–1.06; P=0.020), pathological tumour stage 2 (HR, 2.43; 95% CI, 1.16–5.10; P=0.019) and involvement of distal nodes (pathological node stage 2) (HR, 3.14; 95% CI, 1.24–7.99; P=0.016) were independent predictors of poor prognosis in patients with NSCLC. Poor prognosis of patients with increased AURKA expression suggests that those patients may benefit from surrogate therapy with AURKA inhibitors.
Keywords: mRNA expression, mitotic spindle genes, lung cancer, prognosis
Introduction
Lung cancer is the most common cause of cancer-associated mortality in the UK for both males and females (1), and >1/5 patients with cancer succumb to this malignancy worldwide (2). Non-small cell lung carcinoma (NSCLC) accounts for 80–85% of all cases of lung cancer, and develops through the accumulation of molecular alterations, which may serve as prognostic biomarkers for NSCLC outcome (3).
Mitotic spindle formation and the spindle checkpoint are critical for the maintenance of cell division and chromosome segregation (4). A number of mitotic spindle-associated proteins have been implicated in multiple malignancies, including lung cancer (5,6). Overexpression and gene amplification have been reported to contribute to the development and progression of malignant tumours for a number of mitotic spindle genes, including those involved in centrosome maturation [e.g., Aurora kinase (AURK)A, microtubule nucleation factor TPX2 (TPX2) and kinesin-like protein 11 (KIF11)] (7,8), microtubule formation [e.g., AURKA, cytoskeleton-associated protein 5 (CKAP5), tubulin β (TUBB) and TUBB3] (9–11), and chromosomal alignment and segregation [e.g., AURKA, AURKB, AURKC, discs large-associated protein 5 (DLGAP5) and TTK protein kinase (TTK)] (12–14). AURKA serves a central role in recruiting other mitotic spindle members (5). A number of previous studies conducted in lung cancer have investigated the prognostic value of various of the aforementioned genes, including TPX2 (15), AURKA (16–18) and AURKB (18–21); however, the prognostic value of AURKA and AURKB remains a matter of debate. No information on the potential prognostic significance in human NSCLC has yet been provided for DLGAP5, CKAP5 or TTK.
Personalised medicine relies on the utilisation of gene profiling (including expression, mutation and methylation) in combination with clinicopathological characteristics to provide an optimal management plan for the patient. Therefore, it is necessary to expand our efforts in investigating the association of particular molecular profiles with patient outcomes. The aim of the present study was to acquire a comprehensive expression profile of mitotic spindle-associated genes (AURKA, AURKB, AURKC, CKAP5, DLGAP5, KIF11, TPX2, TTK, TUBB and TUBB3) in NSCLC and to investigate the potential associations with clinicopathological characteristics and patient survival rates.
Materials and methods
Patients and samples
The present study was undertaken within the context of the Liverpool Lung Project (22). Appropriate ethical approval from the Liverpool Research Ethics Committee, ref 157/97, was obtained and all patients provided written informed consent. A total of 132 frozen surgical tumour samples, collected between January 1999 and December 2005 at Liverpool Heart and Chest Hospital (Liverpool, UK), were available from patients with primary NSCLC, 56 from adenocarcinoma (AdC) and 76 from squamous cell carcinoma of the lung (SqCCL). In addition, 44 paired non-tumour surgical lung samples (20 from patients with AdC and 24 from patients with SqCCL) were analysed. The median age of the patients was 67 years (range, 45–82 years); 56 of the patients were female and 77 were male. The majority of the specimens were of the pathological tumour (pT)2 stage (n=101), whereas the pT1 and pT3/4 groups comprised 19 and 12 patients, respectively. The HBEC-3KT cell line (23) used as a calibrator was provided by Professor John Minna and Professor Adi Gazdar.
RNA extraction and reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from primary lung tumour tissue (ten 20-µm thick sections per specimen) using a Direct-zol™ RNA MiniPrep kit (Zymo Research Corp., Irvine, CA, USA), according to the manufacturer's protocol. The quality and quantity of RNA were determined using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Inc., Wilmington, DE, USA) and 200 ng RNA was reverse transcribed using a High-Capacity cDNA Reverse Transcription kit (Thermo Fisher Scientific, Inc., Waltham, MA, USA), according to the manufacturer's protocol. Predesigned 6-carboxyfluorescein-labelled TaqMan Gene Expression Assays (Thermo Fisher Scientific, Inc.) were employed, according to the manufacturer's protocol, to analyse mRNA expression: AURKA, Hs01582072_m1; AURKB, Hs00945858_g1; AURKC, Hs00152930_m1; CKAP5, Hs01120723_m1; DLGAP5, Hs00207323_m1, KIF11, Hs00189698_m1; TPX2, Hs00201616_m1; TTK, Hs01009870_m1; TUBB, Hs00962419_g1; and TUBB3, Hs00964962_g1, with a 4,7,2′-trichloro-7′-phenyl-6-carboxyfluorescein-labelled β-actin (ACTB) TaqMan Gene Expression Assay (cat. no. 4326315E; Thermo Fisher Scientific, Inc.) serving as an endogenous control. RNA from human bronchial epithelial cells (HBEC-3KT) was used as technical calibrator. Three technical replicates were performed for every qPCR assay. Thermocycling conditions were 95°C for 10 min (activation), 45 cycles of 95°C for 15 sec (denaturation), 60°C for 1 min (annealing and extension)] on a Life Technologies StepOnePlus Real-Time PCR System. mRNA levels were expressed as relative quantification (RQ) values, which were calculated as RQ=2−ΔΔCq (24). Quantification cycle (Cq) values were determined using StepOne software (version 1.2; Thermo Fisher Scientific, Inc.) and normalised to the corresponding Cq value for the endogenous control ACTB, generating ΔCq values (ΔCq=Cq target-Cq ACTB). Sample ΔCq values were further normalised against an immortalised bronchial epithelial cell line HBEC-3KT (23) calibrator using the formula: ΔΔCq=(ΔCq sample-ΔCq HBEC-3KT).
Statistical analysis
Gene expression in tumour and adjacent wild-type tissues were compared using the Wilcoxon non-parametric test. The study characteristics were examined using descriptive statistics. Categorical variables were compared using a χ2 test and continuous variables were examined using a Mann-Whitney U test. Overall survival time was calculated from the date of surgery to the date of mortality or last follow-up date. Overexpression for a tumour sample was designated as >95% reference interval [mean ± (2x standard deviation)] of wild-type tissues. Postoperative univariate survival analysis was explored using Kaplan-Meier estimator curves for all the categorical predictors. Tests of equality across strata were also conducted to evaluate the suitability of including potential predictors in the final multivariate model. For the categorical variables, a log-rank test of equality across strata was used, and a univariate Cox's proportional hazard regression was used to analyse continuous variables to examine the differences in survival rate. Variables with P<0.25 in the univariate analysis were selected for inclusion in the final multivariate model as previously suggested (25). A multivariate Cox's proportional hazard model was used to examine the association between mRNA expression and other relevant prognostic factors. All statistical analyses were performed using IBM® SPSS® statistical software (version 22.0; IBM SPSS, Armonk, NY, USA) and Stata® (version 13.1; StataCorp LLC, College Station, TX, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Gene expression analysis
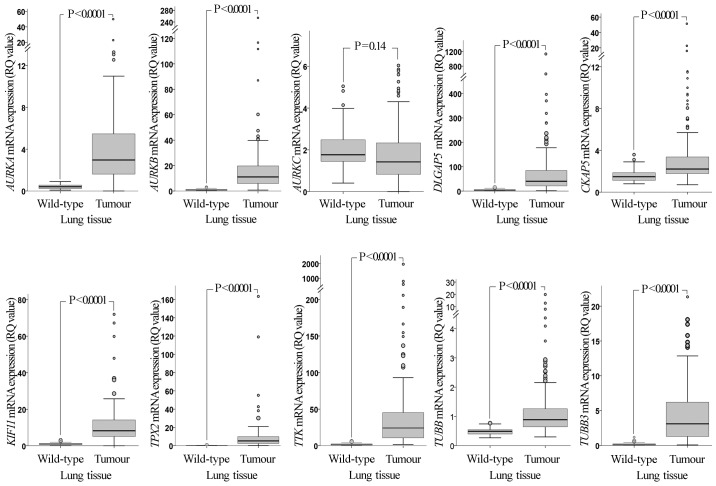
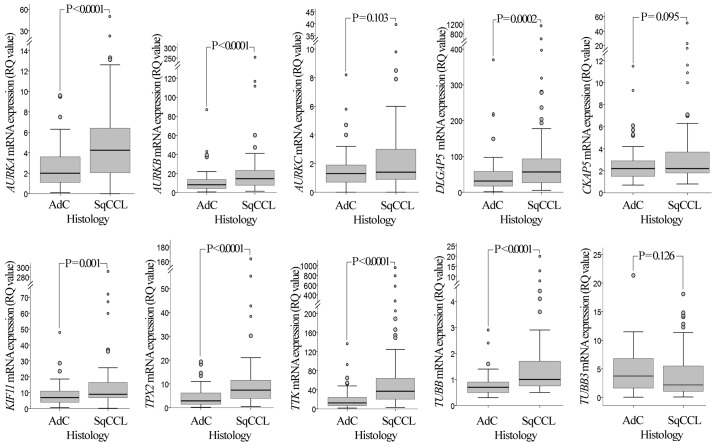
RT-qPCR analysis revealed that, with the exception of AURKC, the mRNA expression levels of all the genes examined in the present study (AURKA, AURKB, AURKC, DLGAP5, CKAP5, KIF11, TPX2, TTK, TUBB and TUBB3) were significantly upregulated in NSCLC tissues compared with those in wild-type adjacent lung tissues (P<0.0001; Fig. 1). Comparison between histology types (Fig. 2) revealed that the mRNA expression of seven genes was significantly increased in SqCCL compared with that in AdC tissues (P<0.001 for AURKA, AURKB, DLGAP5, TPX2, TTK and TUBB; P=0.001 for KIF11).
Figure 1.
Comparative mRNA expression of the examined genes in NSCLC tissues and adjacent normal tissues. The mRNA expression levels of the genes in NSCLC tumours are significantly higher than those in adjacent normal tissues, with the exception of AURKC. P-values were calculated using a Mann-Whitney U test and adjusted for multiple comparisons by Bonferroni correction. RQ values were calculated using RNA from the non-tumorigenic immortalised human bronchial epithelial cell line HBEC-3KT as a calibrator. Larger circles represent outlier values (>1.5x interquartile range); smaller circles represent extreme values (>3x interquartile range). NSCLC, non-small cell lung cancer; RQ, relative quantification; AURK, Aurora kinase; DLGAP5, discs large-associated protein 5; CKAP5, cytoskeleton-associated protein 5; KIF11, kinesin-like protein 11; TPX2, microtubule nucleation factor TPX2; TTK, TTK protein kinase; TUBB, tubulin β.
Figure 2.
mRNA expression of AURKA, AURKB, AURKC, DLGAP5, CKAP5, KIF11, TPX2, TTK, TUBB and TUBB3 genes in SqCCL and AdC of the lung. Comparison between histology types demonstrated that the mRNA expression levels of the AURKA, AURKB, DLGAP5, KIF11, TPX2, TTK and TUBB genes in SqCCL tumours are significantly higher than those in AdC tumours. P-values were calculated using a Mann-Whitney U test and adjusted for multiple comparisons by Bonferroni correction. RQ values were calculated using RNA from the non-tumorigenic immortalised human bronchial epithelial cell line HBEC-3KT as a calibrator. Larger circles represent outlier values (>1.5x interquartile range); smaller circles represent extreme values (>3x interquartile range). AURK, Aurora kinase; DLGAP5, discs large-associated protein 5; CKAP5, cytoskeleton-associated protein 5; KIF11, kinesin-like protein 11; TPX2, microtubule nucleation factor TPX2; TTK, TTK protein kinase; TUBB, tubulin β; SqCCL, squamous cell carcinoma of the lung; AdC, adenocarcinoma; RQ, relative quantification.
Survival analysis
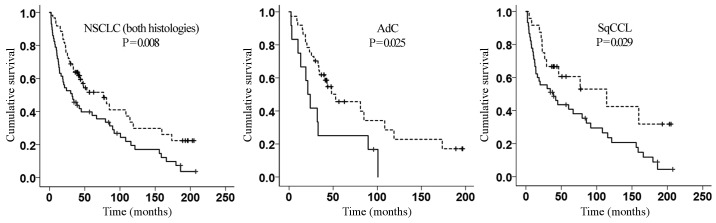
There was no association between the mRNA expression of any of the genes evaluated with age, sex, pathological stage or nodal status (Table I). Potential associations between the expression level of the target genes and overall survival rate were examined. In univariate analysis, pathological stage, nodal status and AURKA mRNA expression were predictors of overall survival rate (Table II). Most importantly, multivariate analysis demonstrated that AURKA mRNA expression [hazard ratio (HR), 1.81; 95% confidence interval (CI) 1.16–2.84; P=0.009] independently predicts poor prognosis in patients with NSCLC upon adjusting for age, pT2 and involvement of distal nodes (pathological node stage 2) (Table II). This observation was consistent with the Kaplan-Meier estimator curve (Fig. 3). The association with prognosis remained significant even when SqCCL and AdC tissues were tested separately (P=0.025 and P=0.029, respectively; Fig. 3).
Table I.
Clinicopathological characteristics of the study patients in association with AURKA mRNA expression profile.
| Clinicopathological characteristic | Total number of patients (%) 124 (100) | High expression of Aurora-A mRNA (n=59) | Low expression of Aurora-A mRNA (n=65) | P-value |
|---|---|---|---|---|
| Mean age, years (standard deviation) | 66.5 (8.5) | 65.9 (8.5) | 67.5 (8.5) | 0.223a |
| Gender | 0.180b | |||
| Male | 70 (56.5) | 37 (52.9) | 33 (47.1) | |
| Female | 54 (43.5) | 22 (40.7) | 32 (59.3) | |
| Histology | <0.001b | |||
| Adenocarcinoma | 52 (41.9) | 13 (25.0) | 39 (75.0) | |
| Squamous cell carcinoma | 72 (58.1) | 46 (63.9) | 26 (36.1) | |
| Tumour stage | 0.513b | |||
| 1 | 19 (15.3) | 7 (36.8) | 12 (63.2) | |
| 2 | 91 (73.3) | 45 (49.5) | 46 (50.6) | |
| ≥3 | 12 (9.6) | 7 (58.3) | 5 (41.7) | |
| Nodal status | 0.975b | |||
| 0 | 68 (54.8) | 32 (47.1) | 36 (52.9) | |
| 1 | 38 (30.6) | 18 (47.4) | 20 (52.6) | |
| 2 | 18 (14.6) | 9 (50.0) | 9 (50.0) |
Mann-Whitney U test
χ2 test. AURK, Aurora kinase.
Table II.
Univariate and multivariate Cox's proportional hazard regression analyses of potential predictors of overall survival among the study patients.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Covariates | HR (95% CI) | P-value | HR (95% CI) | P-value |
| AURKA mRNA | 1.79 (1.16–2.77) | 0.009 | 1.81 (1.16–2.84) | 0.009 |
| Age | 1.02 (1.00–1.05) | 0.066 | 1.03 (1.00–1.06) | 0.020 |
| Tumour stage | ||||
| 1 | Reference | Reference | Reference | Reference |
| 2 | 2.82 (1.35–5.86) | 0.006 | 2.43 (1.16–5.10) | 0.019 |
| ≥3 | 3.80 (1.42–10.15) | 0.008 | 1.39 (0.38–5.09) | 0.623 |
| Nodal status | ||||
| 0 | Reference | Reference | Reference | Reference |
| 1 | 1.62 (1.03–2.55) | 0.037 | 1.45 (0.90–2.34) | 0.128 |
| 2 | 2.55 (1.35–4.84) | 0.004 | 3.14 (1.24–7.99) | 0.016 |
HR, hazard ratio; CI, confidence interval; AURK, Aurora kinase.
Figure 3.
Kaplan-Meier estimator curves of cumulative survival of patients with NSCLC dichotomised by 95% reference interval of AURKA mRNA expression in wild-type tissues. The P-values were calculated using a log-rank (Mantel-Cox) test. Increased AURKA expression (unbroken line) is associated with decreased survival time. The correlation between AURKA expression and cumulative survival is significant in AdC and SqCCL. Decreased AURKA expression (broken line) is associated with increased survival time. NSCLC, non-small cell lung cancer; AdC, adenocarcinoma; SqCCL, squamous cell carcinoma of the lung.
Discussion
Spindle formation is a key process for cell proliferation (8). It is well known that spindle assembly aberrations lead to aneuploidy and are extensively involved in the development of cancer (26). Thus, it was hypothesised that the expression of genes associated with this process may be indicative of the aggressiveness of a tumour and therefore may exhibit prognostic value.
In the present study, the mRNA expression of the AURKA, AURKB, AURKC, CKAP5, DLGAP5, KIF11, TPX2, TTK, TUBB and TUBB3 genes was investigated in a large cohort of human NSCLC tissues, and potential associations between expression profiles and clinicopathological characteristics, including survival rates, were evaluated. All genes, with the exception of AURKC, were overexpressed in the malignant tissues in comparison with adjacent wild-type tissues. These results possibly reflect the requirement for increased mitotic spindle genes expression to cope with the increased replication rate of cancer cells (27,28). However, the important clinical question is whether the overexpression of any of these genes is able to confer a selective advantage on cancer cells and increase their invasive properties. The results of the present study confirm that up-regulation of mitotic spindle genes is a common abnormality in NSCLC and further support a role for the maintenance of a tumorigenic phenotype (5,29). The results of the present study demonstrated that, of the 10 genes examined, only AURKA overexpression was associated with poor prognosis, which suggests that this gene has a particular contribution to a more aggressive phenotype. It is notable that multivariate Cox's regression analysis identified AURKA mRNA expression as an independent predictor of poor prognosis in patients with NSCLC.
The overexpression of AURKA in NSCLC has been demonstrated previously (16,17). Consistent with these previous studies, it was observed in the present study that AURKA mRNA overexpression was increased in SqCCL compared with that in AdC tissue. However, the prognostic value of AURKA in lung cancer has not yet been established. In contrast to the study of Tang et al (30), the prognostic significance of AURKA expression in the present study appears to hold true for both histological subtypes. There is a lack of consensus on this issue, with previous studies debating on the prognostic significance of AURKA in SqCCL (17,18). Furthermore, perimembrane immunohistochemical staining was demonstrated to be a marked predictor of poor prognosis in patients with SqCCL, but not in patients with AdC (16), whereas microarray data analysis demonstrated that AURKA mRNA overexpression is associated with poor prognosis in patients with AdC, but not in patients with SqCCL (30). The reported differences are possibly due to dissimilarities in the study design, measurement of AURKA expression and the small study size, which decreases statistical significance. It is imperative that a large multicentre study is undertaken to determine a definitive explanation of these discrepancies.
AURKA overexpression may serve an important role in cancer aggressiveness through a range of underlying molecular mechanisms. Elevated levels of AURKA perturb mitotic spindle formation and therefore cytokinesis due to centrosome amplification, leading to chromosomal instability and consequently aneuploidy or polyploidy (5,31). AURKA overexpression also inactivates several tumour-suppressor genes, including p53 (32). The association between AURKA overexpression and p53 mutation, as well as advanced tumour grade and advanced cancer stage, was also reported in patients with hepatocellular carcinoma (33), and with clinically aggressive disease and decreased survival rates in patients with ovarian cancer (34). These AURKA-associated events (the perturbation of spindle formation and inactivation of tumour-suppressor genes by elevated AURKA expression) may explain the association identified between up-regulated AURKA expression and poor outcome of patients with NSCLC. Nonetheless, the hypothesis that up-regulated AURKA expression contributes to a poor survival outcome in lung cancer has been debated, presumably because NSCLC represents a set of heterogeneous malignancies, with various outcomes, even among those with the same clinicopathological features (35). The results of the present study provide evidence to support the prognostic role of AURKA expression in patients with NSCLC and highlight the requirement for a large multicentre clinical study which will take into consideration further parameters, including therapeutic regimens. Most importantly, the results of the present study suggest that NSCLC patients may benefit from therapy with AURKA inhibitors and this requires validation in a prospective clinical study.
Acknowledgements
The Liverpool Lung Project is funded by the Roy Castle Lung Cancer Foundation (Liverpool, UK). The present study was also supported through a PhD studentship awarded to A.S.K. Al-Khafaji (grant no. SL25) by the University of Baghdad (Baghdad, Iraq).
References
- 1.Field JK, Devaraj A, Duffy SW, Baldwin DR. CT screening for lung cancer: Is the evidence strong enough? Lung Cancer. 2016;91:29–35. doi: 10.1016/j.lungcan.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Lacroix L, Commo F, Soria JC. Gene expression profiling of non-small-cell lung cancer. Expert Rev Mol Diagn. 2008;8:167–178. doi: 10.1586/14737159.8.2.167. [DOI] [PubMed] [Google Scholar]
- 4.Wassmann K, Benezra R. Mitotic checkpoints: From yeast to cancer. Curr Opin Genet Dev. 2001;11:83–90. doi: 10.1016/S0959-437X(00)00161-1. [DOI] [PubMed] [Google Scholar]
- 5.Fu J, Bian M, Jiang Q, Zhang C. Roles of aurora kinases in mitosis and tumorigenesis. Mol Cancer Res. 2007;5:1–10. doi: 10.1158/1541-7786.MCR-06-0208. [DOI] [PubMed] [Google Scholar]
- 6.Martens-de Kemp SR, Nagel R, Stigter-van Walsum M, van der Meulen IH, van Beusechem VW, Braakhuis BJ, Brakenhoff RH. Functional genetic screens identify genes essential for tumor cell survival in head and neck and lung cancer. Clin Cancer Res. 2013;19:1994–2003. doi: 10.1158/1078-0432.CCR-12-2539. [DOI] [PubMed] [Google Scholar]
- 7.Sankaran S, Parvin JD. Centrosome function in normal and tumor cells. J Cell Biochem. 2006;99:1240–1250. doi: 10.1002/jcb.21003. [DOI] [PubMed] [Google Scholar]
- 8.Tanenbaum ME, Medema RH. Mechanisms of centrosome separation and bipolar spindle assembly. Dev Cell. 2010;19:797–806. doi: 10.1016/j.devcel.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 9.Royle SJ. The role of clathrin in mitotic spindle organisation. J Cell Sci. 2012;125:19–28. doi: 10.1242/jcs.094607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manning AL, Compton DA. Structural and regulatory roles of nonmotor spindle proteins. Curr Opin Cell Biol. 2008;20:101–106. doi: 10.1016/j.ceb.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leandro-Garcia LJ, Leskelä S, Landa I, Montero-Conde C, López-Jiménez E, Letón R, Cascón A, Robledo M, Rodríguez-Antona C. Tumoral and tissue-specific expression of the major human beta-tubulin isotypes. Cytoskeleton (Hoboken) 2010;67:214–223. doi: 10.1002/cm.20436. [DOI] [PubMed] [Google Scholar]
- 12.Jelluma N, Brenkman AB, van den Broek NJ, Cruijsen CW, van Osch MH, Lens SM, Medema RH, Kops GJ. Mps1 phosphorylates Borealin to control Aurora B activity and chromosome alignment. Cell. 2008;132:233–246. doi: 10.1016/j.cell.2007.11.046. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen HG, Makitalo M, Yang D, Chinnappan D, St Hilaire C, Ravid K. Deregulated Aurora-B induced tetraploidy promotes tumorigenesis. FASEB J. 2009;23:2741–2748. doi: 10.1096/fj.09-130963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Slattery SD, Mancini MA, Brinkley BR, Hall RM. Aurora-C kinase supports mitotic progression in the absence of Aurora-B. Cell Cycle. 2009;8:2984–2994. doi: 10.4161/cc.8.18.9591. [DOI] [PubMed] [Google Scholar]
- 15.Kadara H, Behrens C, Yuan P, Solis L, Liu D, Gu X, Minna JD, Lee JJ, Kim E, Hong WK, et al. A five-gene and corresponding protein signature for stage-I lung adenocarcinoma prognosis. Clin Cancer Res. 2011;17:1490–1501. doi: 10.1158/1078-0432.CCR-10-2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ogawa E, Takenaka K, Katakura H, Adachi M, Otake Y, Toda Y, Kotani H, Manabe T, Wada H, Tanaka F. Perimembrane Aurora-A expression is a significant prognostic factor in correlation with proliferative activity in non-small-cell lung cancer (NSCLC) Ann Surg Oncol. 2008;15:547–554. doi: 10.1245/s10434-007-9653-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lo Iacono M, Monica V, Saviozzi S, Ceppi P, Bracco E, Papotti M, Scagliotti GV. Aurora kinase A expression is associated with lung cancer histological-subtypes and with tumor de-differentiation. J Transl Med. 2011;9:100. doi: 10.1186/1479-5876-9-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takeshita M, Koga T, Takayama K, Kouso H, Nishimura-Ikeda Y, Yoshino I, Maehara Y, Nakanishi Y, Sueishi K. CHFR expression is preferentially impaired in smoking-related squamous cell carcinoma of the lung, and the diminished expression significantly harms outcomes. Int J Cancer. 2008;123:1623–1630. doi: 10.1002/ijc.23673. [DOI] [PubMed] [Google Scholar]
- 19.Vischioni B, Oudejans JJ, Vos W, Rodriguez JA, Giaccone G. Frequent overexpression of aurora B kinase, a novel drug target, in non-small cell lung carcinoma patients. Mol Cancer Ther. 2006;5:2905–2913. doi: 10.1158/1535-7163.MCT-06-0301. [DOI] [PubMed] [Google Scholar]
- 20.Smith SL, Bowers NL, Betticher DC, Gautschi O, Ratschiller D, Hoban PR, Booton R, Santibáñez-Koref MF, Heighway J. Overexpression of aurora B kinase (AURKB) in primary non-small cell lung carcinoma is frequent, generally driven from one allele, and correlates with the level of genetic instability. Br J Cancer. 2005;93:719–729. doi: 10.1038/sj.bjc.6602779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perumal D, Singh S, Yoder SJ, Bloom GC, Chellappan SP. A novel five gene signature derived from stem-like side population cells predicts overall and recurrence-free survival in NSCLC. PLoS One. 2012;7:e43589. doi: 10.1371/journal.pone.0043589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cassidy A, Myles JP, van Tongeren M, Page RD, Liloglou T, Duffy SW, Field JK. The LLP risk model: An individual risk prediction model for lung cancer. Br J Cancer. 2008;98:270–276. doi: 10.1038/sj.bjc.6604158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sato M, Vaughan MB, Girard L, Peyton M, Lee W, Shames DS, Ramirez RD, Sunaga N, Gazdar AF, Shay JW, Minna JD. Multiple oncogenic changes (K-RAS(V12), p53 knockdown, mutant EGFRs, p16 bypass, telomerase) are not sufficient to confer a full malignant phenotype on human bronchial epithelial cells. Cancer Res. 2006;66:2116–2128. doi: 10.1158/0008-5472.CAN-05-2521. [DOI] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source Code Biol Med. 2008;3:17. doi: 10.1186/1751-0473-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cimini D, Degrassi F. Aneuploidy: A matter of bad connections. Trends Cell Biol. 2005;15:442–451. doi: 10.1016/j.tcb.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 27.Vader G, Lens SM. The aurora kinase family in cell division and cancer. Biochim Biophys Acta. 2008;1786:60–72. doi: 10.1016/j.bbcan.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Garrido G, Vernos I. Non-centrosomal TPX2-dependent regulation of the aurora a kinase: Functional implications for healthy and pathological cell division. Front Oncol. 2016;6:88. doi: 10.3389/fonc.2016.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eymin B, Gazzeri S. Role of cell cycle regulators in lung carcinogenesis. Cell Adh Migr. 2010;4:114–123. doi: 10.4161/cam.4.1.10977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang H, Xiao G, Behrens C, Schiller J, Allen J, Chow CW, Suraokar M, Corvalan A, Mao J, White MA, et al. A 12-gene set predicts survival benefits from adjuvant chemotherapy in non-small cell lung cancer patients. Clin Cancer Res. 2013;19:1577–1586. doi: 10.1158/1078-0432.CCR-12-2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marumoto T, Zhang D, Saya H. Aurora-A-a guardian of poles. Nat Rev Cancer. 2005;5:42–50. doi: 10.1038/nrc1526. [DOI] [PubMed] [Google Scholar]
- 32.Katayama H, Sasai K, Kawai H, Yuan ZM, Bondaruk J, Suzuki F, Fujii S, Arlinghaus RB, Czerniak BA, Sen S. Phosphorylation by aurora kinase A induces Mdm2-mediated destabilization and inhibition of p53. Nat Genet. 2004;36:55–62. doi: 10.1038/ng1279. [DOI] [PubMed] [Google Scholar]
- 33.Jeng YM, Peng SY, Lin CY, Hsu HC. Overexpression and amplification of Aurora-A in hepatocellular carcinoma. Clin Cancer Res. 2004;10:2065–2071. doi: 10.1158/1078-0432.CCR-1057-03. [DOI] [PubMed] [Google Scholar]
- 34.Lassmann S, Shen Y, Jütting U, Wiehle P, Walch A, Gitsch G, Hasenburg A, Werner M. Predictive value of Aurora-A/STK15 expression for late stage epithelial ovarian cancer patients treated by adjuvant chemotherapy. Clin Cancer Res. 2007;13:4083–4091. doi: 10.1158/1078-0432.CCR-06-2775. [DOI] [PubMed] [Google Scholar]
- 35.Chen Z, Fillmore CM, Hammerman PS, Kim CF, Wong KK. Non-small-cell lung cancers: A heterogeneous set of diseases. Nat Rev Cancer. 2014;14:535–546. doi: 10.1038/nrc3775. [DOI] [PMC free article] [PubMed] [Google Scholar]