The clinical, pathologic, and radiologic features of uncommon soft-tissue sarcomas arising in the abdomen and pelvis and the abdominal wall are reviewed, with emphasis on the radiologic features that aid in the differential diagnosis.
Abstract
Soft-tissue sarcomas occurring in the abdomen and pelvis are an uncommon but important group of malignancies. Recent changes to the World Health Organization classification of soft-tissue tumors include the movement of gastrointestinal stromal tumors (GISTs) into the soft-tissue tumor classification. GIST is the most common intraperitoneal sarcoma. Liposarcoma is the most common retroperitoneal sarcoma, and leiomyosarcoma is the second most common. GIST, liposarcoma, and leiomyosarcoma account for the majority of sarcomas encountered in the abdomen and pelvis and are discussed in part 1 of this article. Undifferentiated pleomorphic sarcoma (previously called malignant fibrous histiocytoma), dermatofibrosarcoma protuberans, solitary fibrous tumor, malignant peripheral nerve sheath tumor, rhabdomyosarcoma, extraskeletal chondro-osseous sarcomas, vascular sarcomas, and sarcomas of uncertain differentiation uncommonly arise in the abdomen and pelvis and the abdominal wall. Although these lesions are rare sarcomas and their imaging features overlap, familiarity with the locations where they occur and their imaging features is important so they can be diagnosed accurately. The anatomic location and clinical history are important factors in the differential diagnosis of these lesions because metastasis, more-common sarcomas, borderline fibroblastic proliferations (such as desmoid tumors), and endometriosis have imaging findings that overlap with those of these uncommon sarcomas. In this article, the clinical, pathologic, and imaging findings of uncommon soft-tissue sarcomas of the abdomen and pelvis and the abdominal wall are reviewed, with an emphasis on their differential diagnosis.
See Illumination by Frazier
SA-CME LEARNING OBJECTIVES
After completing this journal-based SA-CME activity, participants will be able to:
■ Discuss the classification of soft-tissue sarcomas.
■ Describe and illustrate the radiologic and pathologic features of undifferentiated pleomorphic sarcoma, dermatofibrosarcoma protuberans, solitary fibrous tumor, and MPNST.
■ Identify the imaging findings that are most important for establishing an accurate differential diagnosis for soft-tissue masses in the abdomen and pelvis.
Introduction
Soft-tissue tumors are a diverse group of tumors of mesenchymal or neuroectodermal origin with biologic behavior that ranges from benign to malignant. Benign tumors are extremely common and are encountered in daily clinical practice by physicians of all specialties. In contrast, sarcomas are malignant soft-tissue tumors that are rare, especially when compared with other cancers. The estimated number of new cancer cases in the United States in 2016 is 1 685 210. Of those, only 12 310 cases (0.7%) are soft-tissue sarcomas (1). Despite the rarity of soft- tissue sarcomas, they are an important group of tumors to understand and accurately recognize so they can be diagnosed as early as possible.
Soft-tissue sarcomas arise most commonly in the extremities, followed by the trunk wall, retroperitoneum, and head and neck (2). In part 1 of this article, we reviewed (a) the anatomic locations in which sarcomas arise in the abdomen and pelvis; (b) the updated World Health Organization classification of soft-tissue sarcomas, which included the movement of gastrointestinal stromal tumors (GISTs) into the classification; and (c) the most common soft-tissue sarcomas occurring in the abdomen and pelvis (Table) (3). Liposarcoma, leiomyosarcoma, and GIST are the three most common sarcomas in the abdomen and pelvis. Although the remaining soft-tissue sarcomas are not common in the abdomen and pelvis, it is important to have knowledge of them so they can be considered in the differential diagnosis of masses arising in the abdomen and pelvis and the abdominal wall.
Table 1.
Classification of Soft-Tissue Sarcomas Occurring in the Abdomen and Pelvis on the Basis of the 2013 HO Classification of Tumors of Soft Tissue
Note.—Reprinted from reference 3.
In the retroperitoneum, well-differentiated liposarcoma is the most common sarcoma. It is composed of mature fatty elements. Consequently, the imaging appearance of well-differentiated liposarcoma is readily suggestive of its diagnosis. Soft-tissue elements within a fatty retroperitoneal mass are suggestive that the mass is a dedifferentiated liposarcoma. Dedifferentiated liposarcoma may also be a completely solid soft-tissue mass, which makes the diagnosis more challenging. Leiomyosarcoma, GIST, and uncommon soft-tissue sarcomas such as undifferentiated pleomorphic sarcomas and malignant solitary fibrous tumors also manifest as a solid heterogeneous retroperitoneal mass.
In the peritoneal cavity, GIST is the most common sarcoma. Because most GISTs arise from the muscularis propria of the stomach and intestine, finding an attachment to the stomach or the intestine is suggestive of the diagnosis. If no attachment can be identified, GIST should still be considered in the differential diagnosis because GIST may be extraintestinal, or it may lose its pedicle of attachment and appear separate from the stomach or intestine. Dedifferentiated liposarcoma, leiomyosarcoma, and uncommon sarcomas are in the differential diagnosis after more common diseases such as metastasis, lymphoma, and borderline fibroblastic proliferations (eg, desmoid tumors) are considered.
Any soft-tissue sarcoma may arise in the abdominal wall. Such sarcomas may be deep seated, arising in the abdominal wall musculature, or superficial. Dermatofibrosarcoma protuberans is a superficial sarcoma that arises from the skin and may extend into the deeper layers of the abdominal wall when its development is advanced.
This article reviews the clinical, pathologic, and radiologic features of uncommon soft-tissue sarcomas arising in the abdomen and pelvis and the abdominal wall. These lesions are sarcomas that cannot be differentiated or classified, fibroblastic/myofibroblastic malignancies, MPNSTs, skeletal muscle sarcomas (rhabdomyosarcomas), vascular sarcomas, chondro-osseous sarcomas, and sarcomas of uncertain lineage.
Undifferentiated/Unclassified Sarcomas
Definition
Twenty percent of soft-tissue sarcomas show no lines of differentiation and are classified as undifferentiated sarcomas. These lesions are most often undifferentiated pleomorphic sarcoma (previously known as pleomorphic malignant fibrous histiocytoma).
Clinical and Pathologic Features
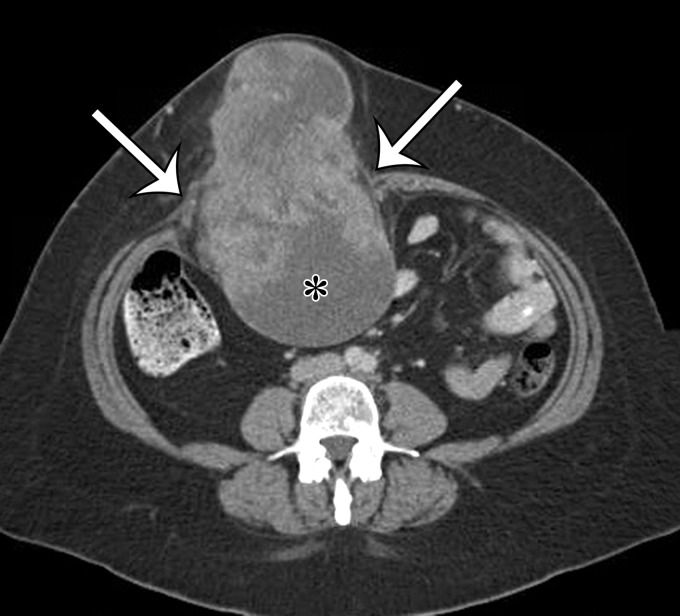
Undifferentiated pleomorphic sarcoma usually occurs in older adults and may arise at any location in the body. Men account for two-thirds of cases, and undifferentiated pleomorphic sarcoma is more common in white subjects (4). Undifferentiated pleomorphic sarcoma is the most common sarcoma to develop at sites of prior irradiation (5). Investigators have shown that most patients have received a radiation dose of 50 Gy or more, and the median time interval between radiation exposure and the development of a radiation-associated sarcoma is approximately 10 years (6). Undifferentiated pleomorphic sarcoma may also arise at sites of chronic ulceration (7). The extremities are the most common location. Approximately 15% of undifferentiated pleomorphic sarcomas arise in the abdomen and pelvis (8,9).
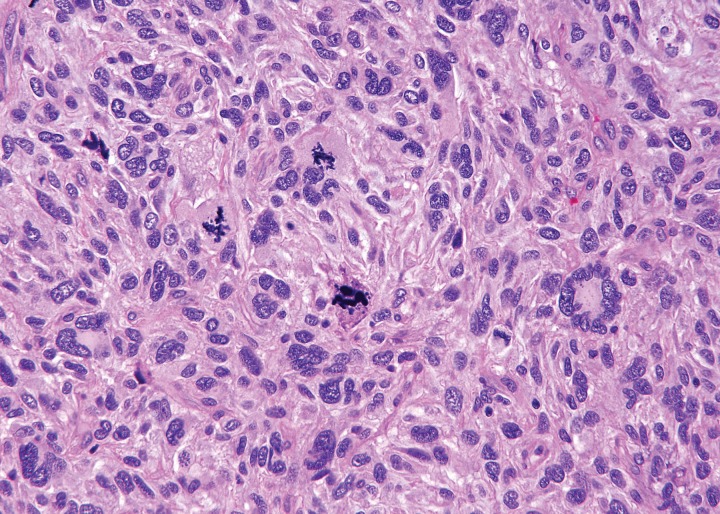
Clinical signs and symptoms of undifferentiated pleomorphic sarcoma are usually nonspecific. Fever, weight loss, and abdominal pain have been reported for those patients with intra-abdominal lesions. Many investigators have noted that a substantial proportion of undifferentiated pleomorphic sarcomas in the abdomen are dedifferentiated liposarcomas. At gross pathologic examination, undifferentiated pleomorphic sarcoma is typically a solitary large lesion, 5–10 cm in diameter, at the time of diagnosis (4). The lesion is multilobulated, with necrosis, degeneration, or hemorrhage on the cut surface. In some tumors, extensive hemorrhage can be present. Although the border of the tumor may appear well defined at gross pathologic examination, microscopic spread along muscle fibers and fascial planes is often present. Microscopically, tumor cells with variably pleomorphic nuclei are characteristic (Fig 1) (10). There is no characteristic immunohistochemical profile. Immunohistochemical analysis is used to help exclude other tumors that may have a pleomorphic appearance.
Figure 1.
Photomicrograph of undifferentiated pleomorphic sarcoma. The tumor is composed of pleomorphic undifferentiated cells showing high mitotic activity. (Hematoxylin-eosin stain; original magnification, ×100.)
Radiologic Features
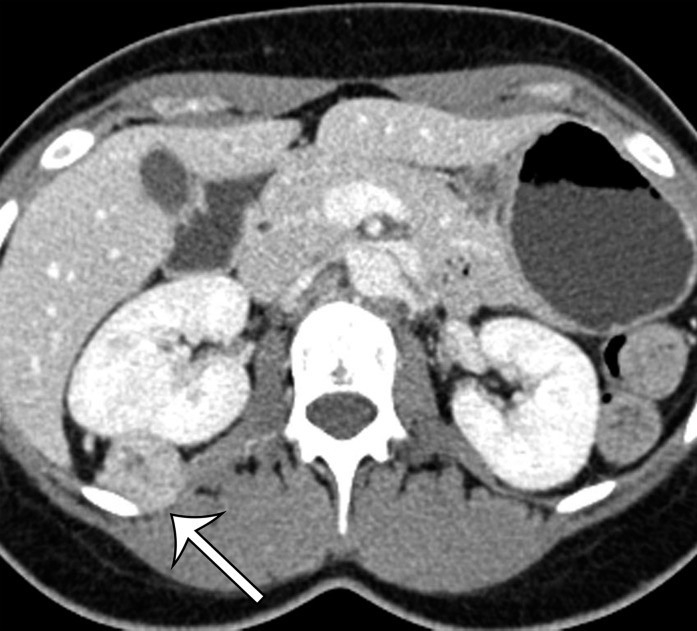
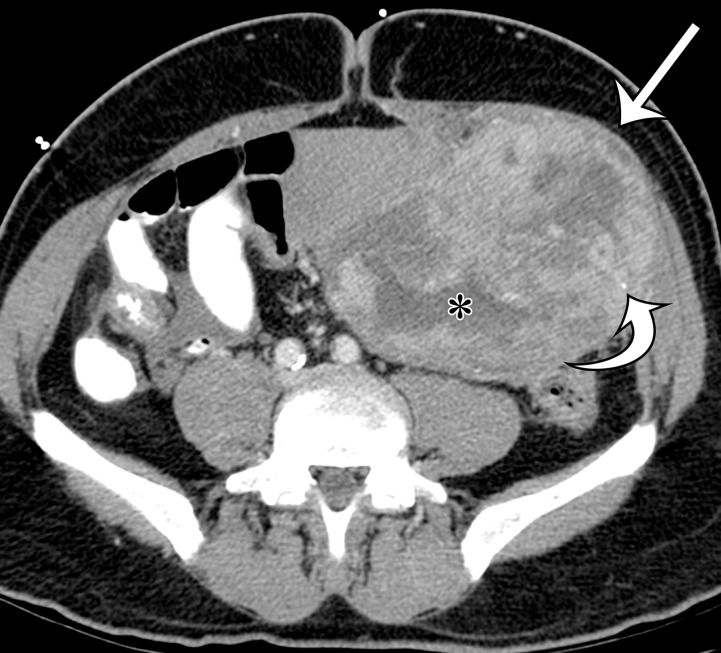
Undifferentiated pleomorphic sarcomas may arise at any soft-tissue site in the abdomen and pelvis. On CT images, these lesions are well-circumscribed, multinodular, or infiltrating masses of soft-tissue attenuation. Undifferentiated pleomorphic sarcomas are often large at the time of diagnosis and invade adjacent anatomic structures (Fig 2). Centrally within the tumor, areas of low-attenuation necrosis, hemorrhage, or myxoid change can be seen on CT images. The tumor may appear cystic with a rim of peripheral enhancement when marked intralesional hemorrhage is present (11). Calcification from osseous or chondroid metaplasia occurs in approximately 16% of cases (12). Calcifications may be small and punctate in a speckled pattern or coarse and chunky (9). After administration of intravenous contrast material, the enhancement pattern is variable, although regions of marked contrast enhancement and large intratumoral vessels may be seen. Hemoperitoneum from tumoral hemorrhage has been reported (13).
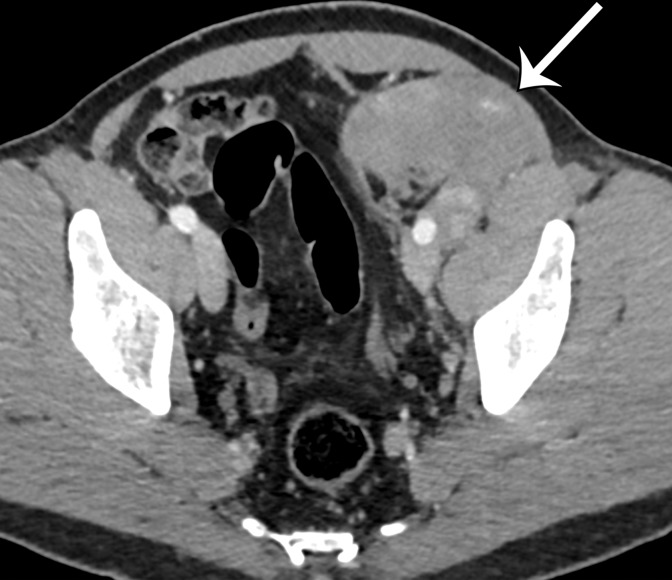
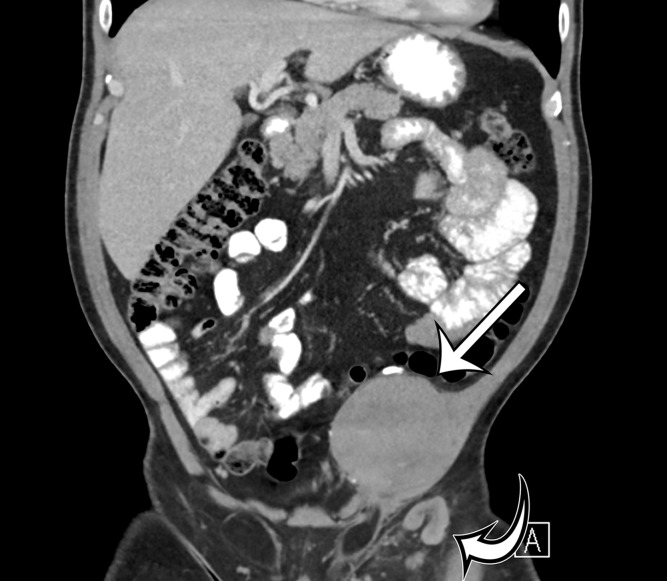
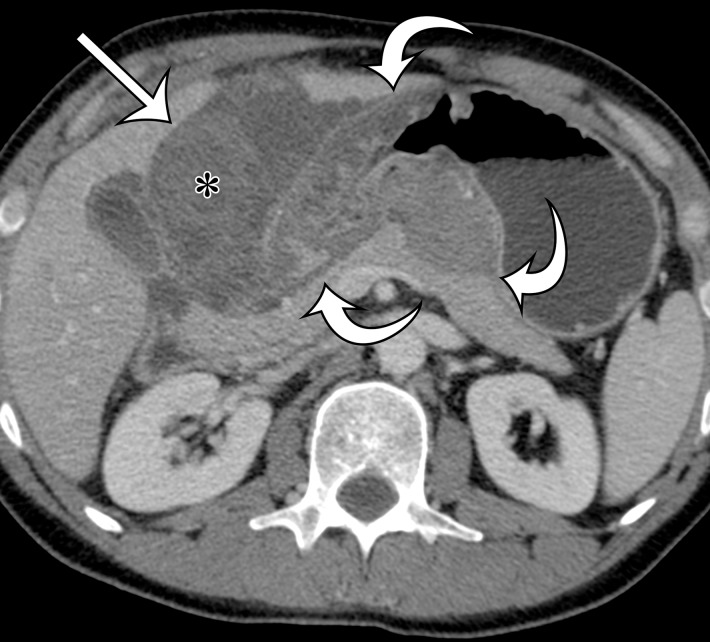
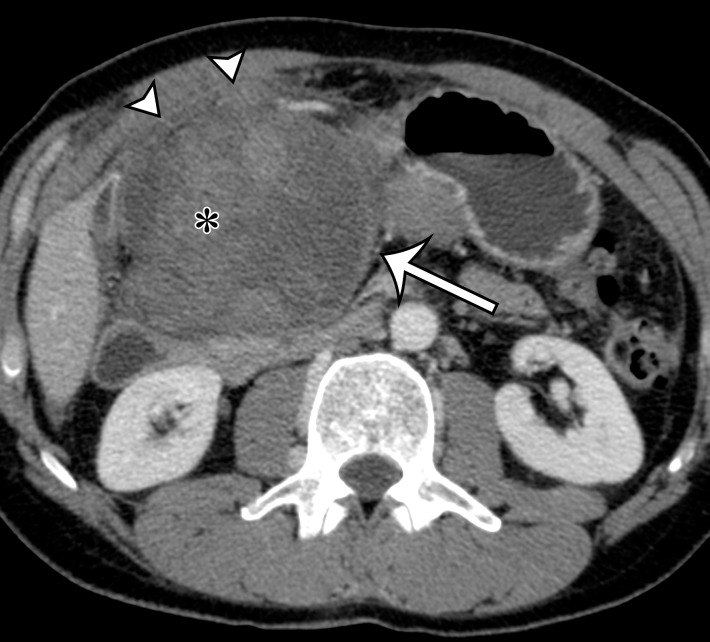
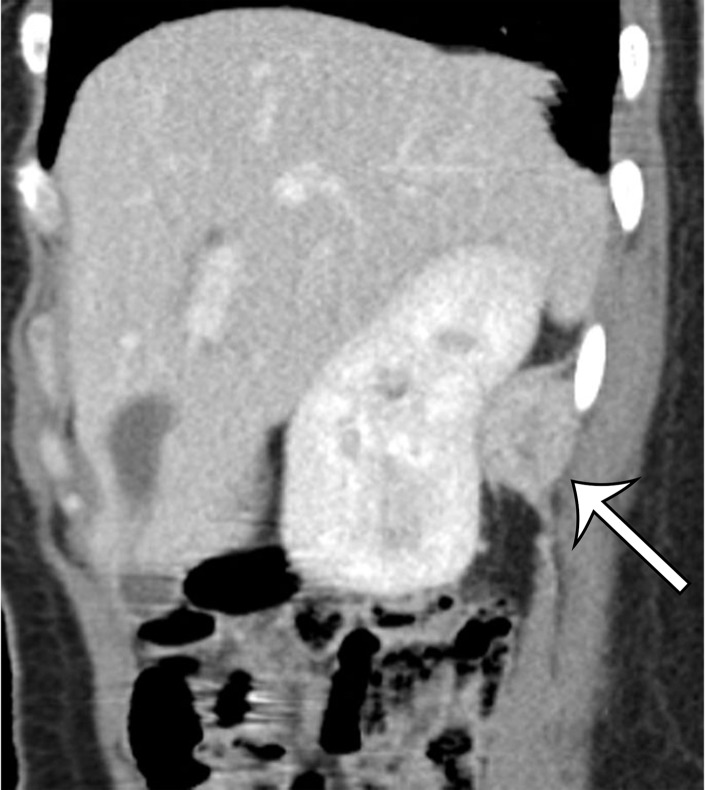
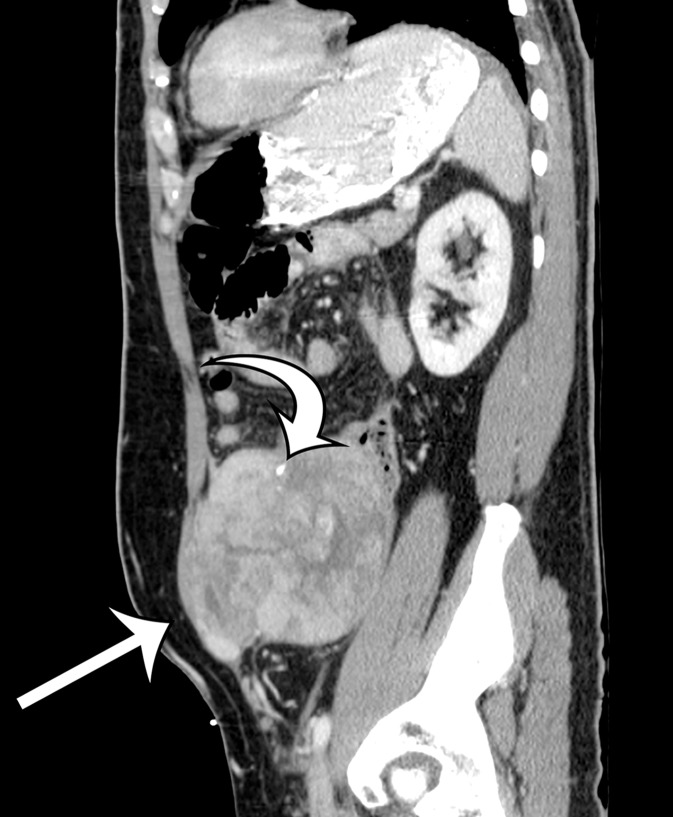
Figure 2a.
Radiologic features of undifferentiated pleomorphic sarcoma in two patients. (a, b) Undifferentiated pleomorphic sarcoma in a 57-year-old man with left leg swelling: Axial (a) and coronal (b) contrast-enhanced CT images show mostly homogeneous enhancement of a well-circumscribed soft-tissue mass (straight arrow) invading the left anterolateral abdominal wall and obliterating the left external iliac vein. A reactive lymph node (curved arrow on b) is depicted in the left inguinal region. (c–e) Undifferentiated pleomorphic sarcoma in a 64-year-old man with vague right upper quadrant pain. (c) Axial contrast-enhanced CT image shows areas of low attenuation reflecting necrosis (*) within a sharply marginated, heterogeneously enhancing mass (arrow). (d, e) Axial half-Fourier acquisition single-shot turbo spin-echo (HASTE; Siemens, Erlangen, Germany) (d) and equilibrium phase contrast-enhanced T1-weighted fat-suppressed (e) MR images of the same patient as in c better show infiltration into the adjacent liver (arrow), as well as areas of necrosis (*).
Figure 2b.
Radiologic features of undifferentiated pleomorphic sarcoma in two patients. (a, b) Undifferentiated pleomorphic sarcoma in a 57-year-old man with left leg swelling: Axial (a) and coronal (b) contrast-enhanced CT images show mostly homogeneous enhancement of a well-circumscribed soft-tissue mass (straight arrow) invading the left anterolateral abdominal wall and obliterating the left external iliac vein. A reactive lymph node (curved arrow on b) is depicted in the left inguinal region. (c–e) Undifferentiated pleomorphic sarcoma in a 64-year-old man with vague right upper quadrant pain. (c) Axial contrast-enhanced CT image shows areas of low attenuation reflecting necrosis (*) within a sharply marginated, heterogeneously enhancing mass (arrow). (d, e) Axial half-Fourier acquisition single-shot turbo spin-echo (HASTE; Siemens, Erlangen, Germany) (d) and equilibrium phase contrast-enhanced T1-weighted fat-suppressed (e) MR images of the same patient as in c better show infiltration into the adjacent liver (arrow), as well as areas of necrosis (*).
Figure 2c.
Radiologic features of undifferentiated pleomorphic sarcoma in two patients. (a, b) Undifferentiated pleomorphic sarcoma in a 57-year-old man with left leg swelling: Axial (a) and coronal (b) contrast-enhanced CT images show mostly homogeneous enhancement of a well-circumscribed soft-tissue mass (straight arrow) invading the left anterolateral abdominal wall and obliterating the left external iliac vein. A reactive lymph node (curved arrow on b) is depicted in the left inguinal region. (c–e) Undifferentiated pleomorphic sarcoma in a 64-year-old man with vague right upper quadrant pain. (c) Axial contrast-enhanced CT image shows areas of low attenuation reflecting necrosis (*) within a sharply marginated, heterogeneously enhancing mass (arrow). (d, e) Axial half-Fourier acquisition single-shot turbo spin-echo (HASTE; Siemens, Erlangen, Germany) (d) and equilibrium phase contrast-enhanced T1-weighted fat-suppressed (e) MR images of the same patient as in c better show infiltration into the adjacent liver (arrow), as well as areas of necrosis (*).
Figure 2d.
Radiologic features of undifferentiated pleomorphic sarcoma in two patients. (a, b) Undifferentiated pleomorphic sarcoma in a 57-year-old man with left leg swelling: Axial (a) and coronal (b) contrast-enhanced CT images show mostly homogeneous enhancement of a well-circumscribed soft-tissue mass (straight arrow) invading the left anterolateral abdominal wall and obliterating the left external iliac vein. A reactive lymph node (curved arrow on b) is depicted in the left inguinal region. (c–e) Undifferentiated pleomorphic sarcoma in a 64-year-old man with vague right upper quadrant pain. (c) Axial contrast-enhanced CT image shows areas of low attenuation reflecting necrosis (*) within a sharply marginated, heterogeneously enhancing mass (arrow). (d, e) Axial half-Fourier acquisition single-shot turbo spin-echo (HASTE; Siemens, Erlangen, Germany) (d) and equilibrium phase contrast-enhanced T1-weighted fat-suppressed (e) MR images of the same patient as in c better show infiltration into the adjacent liver (arrow), as well as areas of necrosis (*).
Figure 2e.
Radiologic features of undifferentiated pleomorphic sarcoma in two patients. (a, b) Undifferentiated pleomorphic sarcoma in a 57-year-old man with left leg swelling: Axial (a) and coronal (b) contrast-enhanced CT images show mostly homogeneous enhancement of a well-circumscribed soft-tissue mass (straight arrow) invading the left anterolateral abdominal wall and obliterating the left external iliac vein. A reactive lymph node (curved arrow on b) is depicted in the left inguinal region. (c–e) Undifferentiated pleomorphic sarcoma in a 64-year-old man with vague right upper quadrant pain. (c) Axial contrast-enhanced CT image shows areas of low attenuation reflecting necrosis (*) within a sharply marginated, heterogeneously enhancing mass (arrow). (d, e) Axial half-Fourier acquisition single-shot turbo spin-echo (HASTE; Siemens, Erlangen, Germany) (d) and equilibrium phase contrast-enhanced T1-weighted fat-suppressed (e) MR images of the same patient as in c better show infiltration into the adjacent liver (arrow), as well as areas of necrosis (*).
Recurrent and Metastatic Disease
Undifferentiated pleomorphic sarcoma may recur locally or as metastatic disease. The lesion usually recurs within 12–24 months after the initial diagnosis (4). Lung, bone, and liver are the most common sites of distant metastasis. Similar to the metastases from other sarcomas, metastases to the lymph nodes from undifferentiated pleomorphic sarcoma are rare.
Fibroblastic/Myofibroblastic Tumors
The sarcomas classified as fibroblastic/myofibroblastic tumors display histologic, immunohistochemical, and ultrastructural features of fibroblasts or myofibroblasts, as well as elements of an extracellular matrix such as collagen. In this section, we cover dermatofibrosarcoma protuberans and solitary fibrous tumor.
Dermatofibrosarcoma Protuberans
Definition.—Dermatofibrosarcoma protuberans is a locally aggressive superficial neoplasm arising in the dermis or subcutis.
Clinical and Pathologic Features.—Dermatofibrosarcoma protuberans most often occurs in young to middle-aged adults and is more common in blacks and in women. The increased occurrence in blacks is most exaggerated in the 45–49-year-old group, in which dermatofibrosarcoma protuberans is 3.5 times more common compared with other racial groups (14). The trunk wall (50%) and proximal extremities are the most common locations.
Dermatofibrosarcoma protuberans grows slowly and indolently during several years before beginning a rapid-growth phase. During the rapid-growth phase, the tumor develops its characteristic protuberant appearance. Progression to fibrosarcoma occurs in 10%–15% of cases (15,16). Patients come to medical attention because of an enlarging dermal mass. At gross pathologic examination, dermatofibrosarcoma protuberans is a nodular or plaquelike mass that may contain a protuberant cutaneous component. Histologically, dermatofibrosarcoma protuberans is typically infiltrative in the subcutaneous fat and is composed of uniform fibroblasts often arranged in whorling patterns. Development of a fascicular histologic appearance, often with an increased mitotic rate, indicates fibrosarcomatous transformation. The tumor cells are positive for CD34, which can be lost in fibrosarcomatous transformation (16).
Radiologic Features.—Fig 317,18,19On CT and MR images, dermatofibrosarcoma protuberans is in the skin and may extend into the subcutaneous fat, with occasional deep lesions. Dermatofibrosarcoma protuberans demonstrates soft-tissue attenuation on CT images, with variable enhancement after intravenous contrast material administration (Fig 3) (17). At MR imaging, the lesion is isointense or hyperintense relative to skeletal muscle on T1-weighted MR images and is isointense or hyperintense relative to the adjacent fat on T2-weighted MR images (18). Fat-suppressed T2-weighted sequences or short inversion time inversion-recovery sequences are helpful in the identification of the extent of the lesion because of its high signal intensity on MR images obtained with these sequences (19). Linear extensions into the skin and satellite nodules may also be seen.
Figure 3a.
Dermal and deep dermatofibrosarcoma protuberans in two patients. (a–c) Dermatofibrosarcoma protuberans in a 61-year-old woman with a painful, rapidly enlarging mass on her anterior abdominal wall. (a, b) Axial (a) and sagittal (b) contrast-enhanced CT images show a well-circumscribed multinodular soft-tissue–attenuation mass extending from the skin surface into the subcutaneous fat (arrows), without involvement of the anterior abdominal wall musculature. (c) Photograph of the resected specimen shows the dermal mass protruding from the skin, with central ulceration (arrow) at the skin surface. (Scale is in centimeters.) (d) Dermatofibrosarcoma protuberans in a 40-year-old man with a painful right lower chest wall mass: Axial contrast-enhanced CT image shows a heterogeneous subcutaneous mass inseparable from the anterolateral abdominal wall musculature (arrows).
Figure 3b.
Dermal and deep dermatofibrosarcoma protuberans in two patients. (a–c) Dermatofibrosarcoma protuberans in a 61-year-old woman with a painful, rapidly enlarging mass on her anterior abdominal wall. (a, b) Axial (a) and sagittal (b) contrast-enhanced CT images show a well-circumscribed multinodular soft-tissue–attenuation mass extending from the skin surface into the subcutaneous fat (arrows), without involvement of the anterior abdominal wall musculature. (c) Photograph of the resected specimen shows the dermal mass protruding from the skin, with central ulceration (arrow) at the skin surface. (Scale is in centimeters.) (d) Dermatofibrosarcoma protuberans in a 40-year-old man with a painful right lower chest wall mass: Axial contrast-enhanced CT image shows a heterogeneous subcutaneous mass inseparable from the anterolateral abdominal wall musculature (arrows).
Figure 3c.
Dermal and deep dermatofibrosarcoma protuberans in two patients. (a–c) Dermatofibrosarcoma protuberans in a 61-year-old woman with a painful, rapidly enlarging mass on her anterior abdominal wall. (a, b) Axial (a) and sagittal (b) contrast-enhanced CT images show a well-circumscribed multinodular soft-tissue–attenuation mass extending from the skin surface into the subcutaneous fat (arrows), without involvement of the anterior abdominal wall musculature. (c) Photograph of the resected specimen shows the dermal mass protruding from the skin, with central ulceration (arrow) at the skin surface. (Scale is in centimeters.) (d) Dermatofibrosarcoma protuberans in a 40-year-old man with a painful right lower chest wall mass: Axial contrast-enhanced CT image shows a heterogeneous subcutaneous mass inseparable from the anterolateral abdominal wall musculature (arrows).
Figure 3d.
Dermal and deep dermatofibrosarcoma protuberans in two patients. (a–c) Dermatofibrosarcoma protuberans in a 61-year-old woman with a painful, rapidly enlarging mass on her anterior abdominal wall. (a, b) Axial (a) and sagittal (b) contrast-enhanced CT images show a well-circumscribed multinodular soft-tissue–attenuation mass extending from the skin surface into the subcutaneous fat (arrows), without involvement of the anterior abdominal wall musculature. (c) Photograph of the resected specimen shows the dermal mass protruding from the skin, with central ulceration (arrow) at the skin surface. (Scale is in centimeters.) (d) Dermatofibrosarcoma protuberans in a 40-year-old man with a painful right lower chest wall mass: Axial contrast-enhanced CT image shows a heterogeneous subcutaneous mass inseparable from the anterolateral abdominal wall musculature (arrows).
Recurrent and Metastatic Disease.—Dermatofibrosarcoma protuberans has a high local recurrence rate, up to 45% if the lesion is not excised with a wide margin (≥3 cm) (20). Those lesions resected with wide margins have a 10%–20% chance of recurrence. The recurrence rates after Mohs surgery are lower because of the ability to estimate the location of microscopic tumor with this technique (21). Distant lung metastases from dermatofibrosarcoma protuberans are not common but may occur with fibrosarcomatous transformation.
Solitary Fibrous Tumor
Definition.—Solitary fibrous tumor (including tumors previously called hemangiopericytomas) is a fibroblastic tumor that often has a prominent hemangiopericytoma-like vascular pattern.
Clinical and Pathologic Features.—Solitary fibrous tumor typically arises in deep soft tissues, such as those of the thigh, retroperitoneum, pelvis, and pleura. Solitary fibrous tumor has no gender predilection and is most common in adults aged 20–70 years (15). Clinically, the lesion is a slow-growing painless mass. Less than 5% of patients may present with hypoglycemia (Doege-Potter syndrome) (22). The biologic behavior of the majority of solitary fibrous tumors is unpredictable, but frankly sarcomatous examples are also recognized. At gross pathologic examination, solitary fibrous tumor often appears as a circumscribed firm mass, which is pale gray to yellowish at sectioning. Hemorrhage and necrosis may be present. Histologically, the tumors can be hypercellular or hypocellular with areas of dense collagen and hyalinization. A mitotic rate of more than four mitoses per 10 high-power fields has been considered a sign for risk of malignant behavior (23).
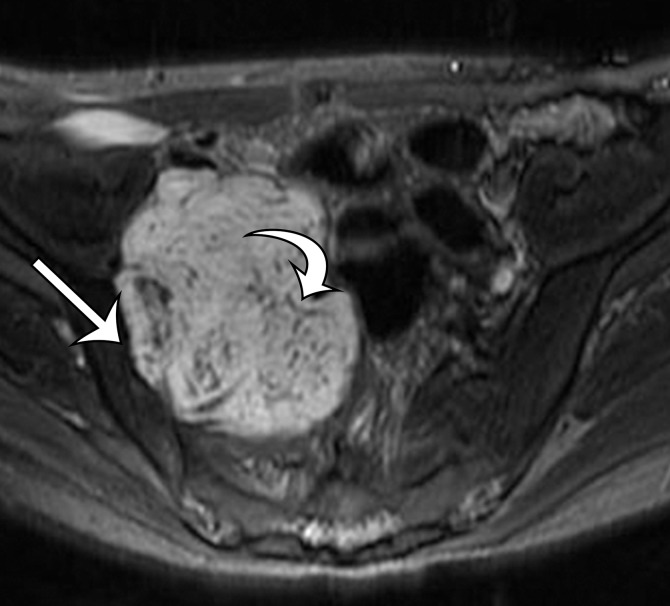
Radiologic Features.—On CT and MR images, solitary fibrous tumor may be single or may form multiple masses. Solitary fibrous tumor is seen as a sharply marginated round or oval mass. After administration of intravenous contrast material, large and often tortuous vessels may be seen along the periphery of solitary fibrous tumor on arterial phase contrast-enhanced images (Fig 4) (24). The tumor may show intense enhancement in the arterial phase and persistence of enhancement in the portal venous and delayed phases of contrast enhancement (25,26). The pattern of enhancement has been correlated to highly vascular cellular areas within the tumor (24). Cystic areas of degeneration and intratumoral necrosis may be present, such that the lesion resembles a GIST or leiomyosarcoma.
Figure 4a.
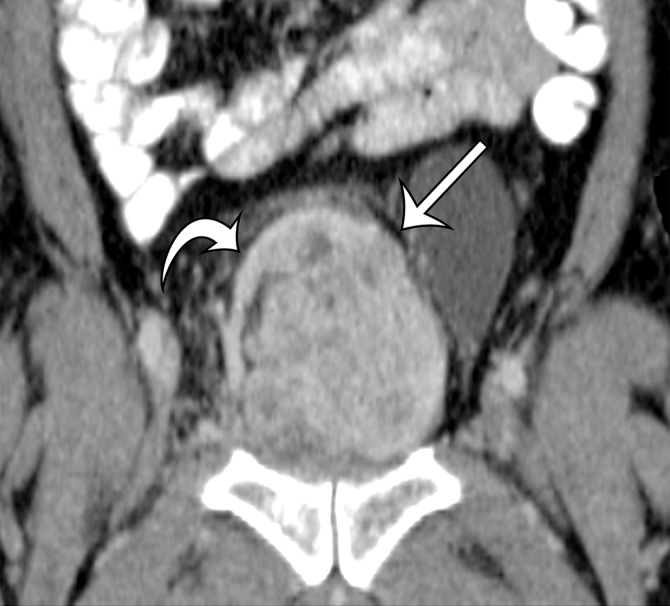
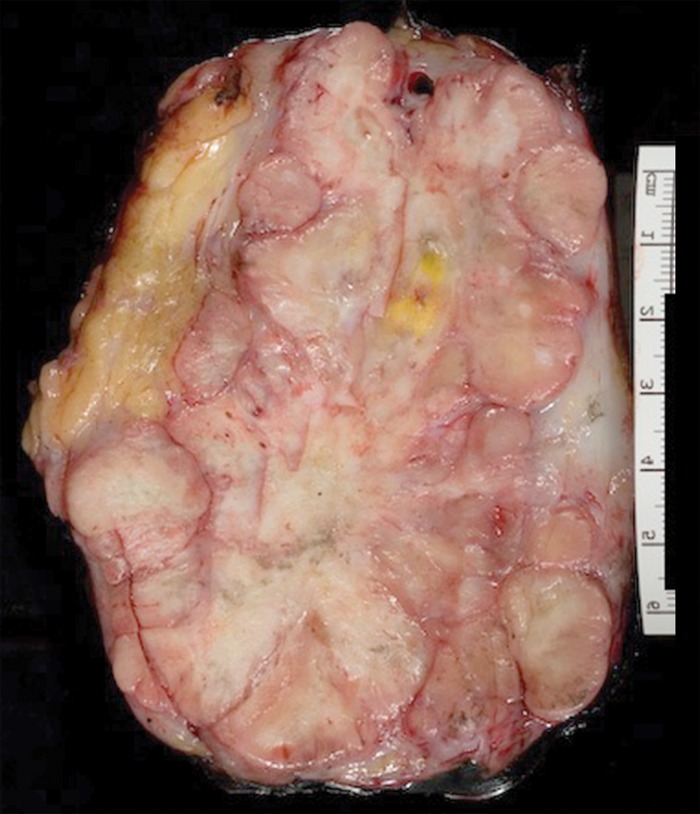
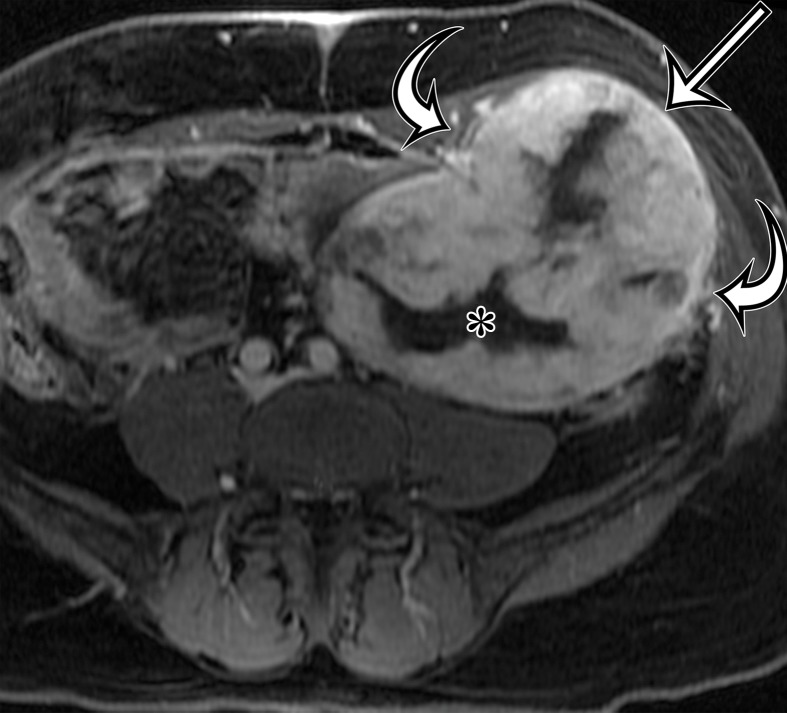
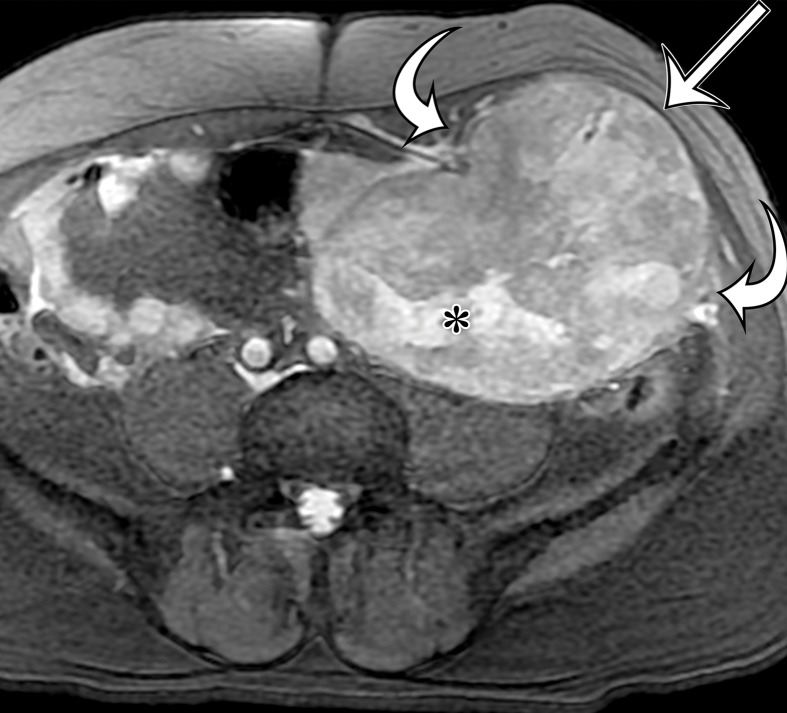
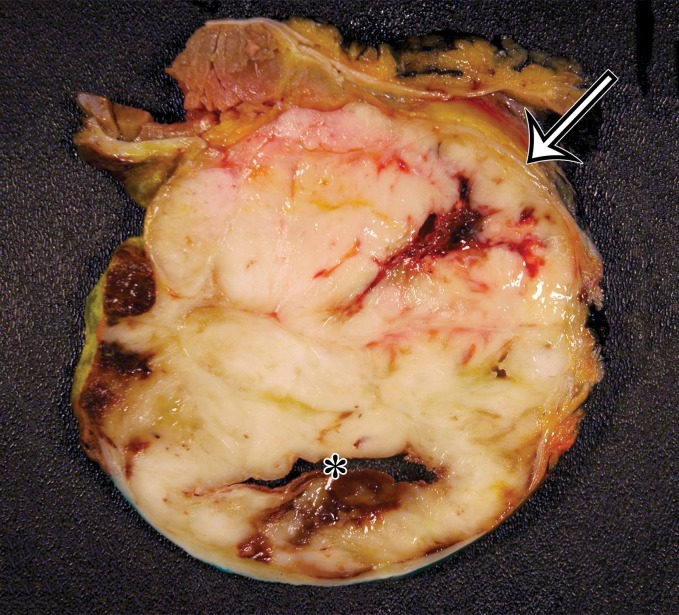
Solitary fibrous tumor in a 69-year-old man with long-term lower urinary tract symptoms and back pain radiating to the right leg. (a, b) Axial (a) and coronal (b) contrast-enhanced CT images show marked heterogeneous enhancement of a sharply marginated lobulated solid mass (straight arrow) in the right pelvis. A prominent peripheral blood vessel (curved arrow) drapes over the superior margin of the mass. (c) Photograph of the cut surface of the resected specimen shows the encapsulated pink-tan soft-tissue mass. (Scale is in centimeters.)
Figure 4b.
Solitary fibrous tumor in a 69-year-old man with long-term lower urinary tract symptoms and back pain radiating to the right leg. (a, b) Axial (a) and coronal (b) contrast-enhanced CT images show marked heterogeneous enhancement of a sharply marginated lobulated solid mass (straight arrow) in the right pelvis. A prominent peripheral blood vessel (curved arrow) drapes over the superior margin of the mass. (c) Photograph of the cut surface of the resected specimen shows the encapsulated pink-tan soft-tissue mass. (Scale is in centimeters.)
Figure 4c.
Solitary fibrous tumor in a 69-year-old man with long-term lower urinary tract symptoms and back pain radiating to the right leg. (a, b) Axial (a) and coronal (b) contrast-enhanced CT images show marked heterogeneous enhancement of a sharply marginated lobulated solid mass (straight arrow) in the right pelvis. A prominent peripheral blood vessel (curved arrow) drapes over the superior margin of the mass. (c) Photograph of the cut surface of the resected specimen shows the encapsulated pink-tan soft-tissue mass. (Scale is in centimeters.)
Recurrent and Metastatic Disease.—Solitary fibrous tumors in the retroperitoneum and pelvis tend to behave aggressively compared with those located in the extremities. Local recurrence and distant metastases to the lung, bone, and liver may be seen (27).
Malignant Peripheral Nerve Sheath Tumor
Definition
MPNST is typically a high-grade sarcoma that usually arises in a neurofibroma or a nerve trunk, often in patients with neurofibromatosis type 1 (15). MPNST was previously known as neurofibrosarcoma, malignant schwannoma, and neurogenic sarcoma.
Clinical and Pathologic Features
Approximately 50% of MPNSTs occur in patients with neurofibromatosis type 1, 10% of MPNSTs are radiation induced, and the remainder of them occur sporadically (28). The mean age at diagnosis is 35 years in the general population, compared with 26 years in patients with neurofibromatosis type 1 (28,29). MPNST is seen in a variety of locations, including the head and neck and the extremities. The paraspinal regions of the retroperitoneum are the most common location in the abdomen and pelvis. MPNST is most often asymptomatic, although some patients may report neurologic symptoms in the distribution of the affected nerve (29).
At gross pathologic examination, MPNST often forms an elongated fusiform mass with the ends, representing the nerve of origin, usually transformed into neurofibroma (30). MPNST may be truly encapsulated, reflecting the origin from a nerve trunk. Necrosis, hemorrhage, and cystic change may be present on the cut surface. At histopathologic examination, the tumor is typically composed of spindle cells to pleomorphic cells that demonstrate nuclear atypia and high mitotic activity (28). Divergent differentiation, such as rhabdomyosarcomatous, angiosarcomatous, or chondro-osseous differentiation, may be seen in an MPNST.
Radiologic Features
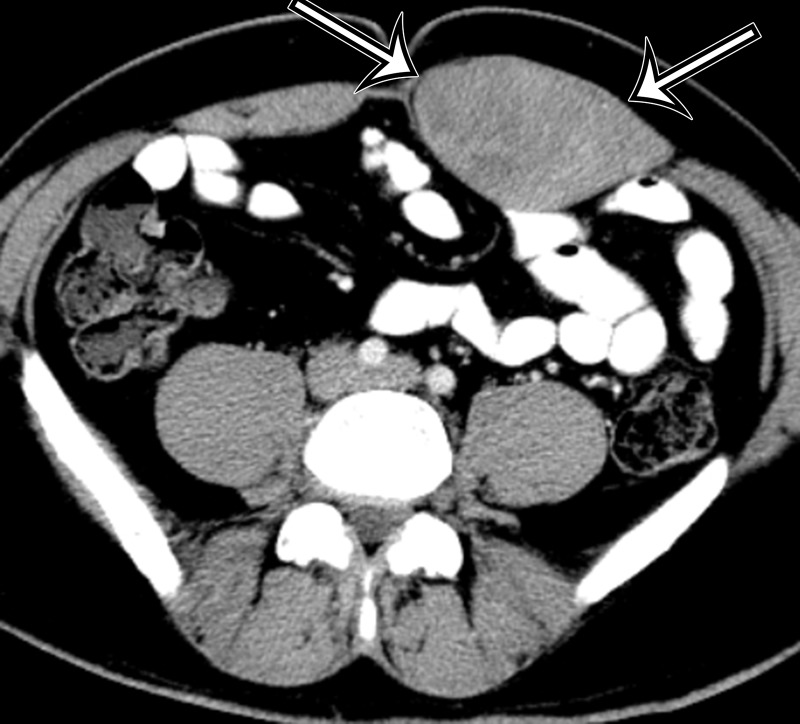
On CT and MR images, MPNST is characteristically a large aggressive tumor with well-defined or ill-defined margins. MPNST is typically heterogeneous on both nonenhanced and intravenous contrast material–enhanced CT and MR images because of necrosis within the tumor (31). MPNST may invade and infiltrate into adjacent organs and tissues and erode adjacent bone (Fig 5). In patients with neurofibromatosis type 1, it may be difficult to differentiate MPNST from coexisting plexiform neurofibromas because both have similar imaging features, including bone erosion and heterogeneity (32).
Figure 5a.
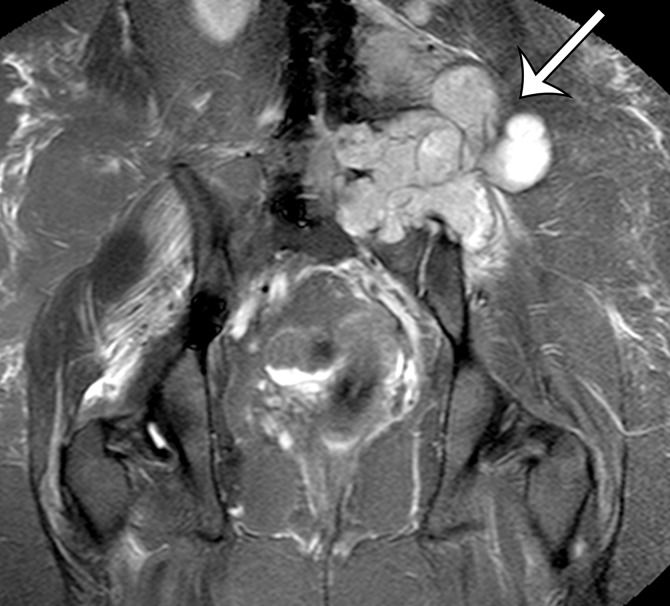
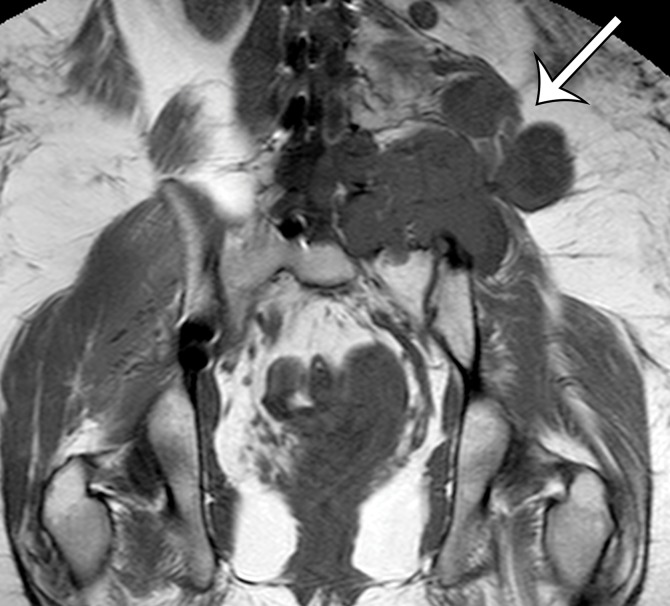
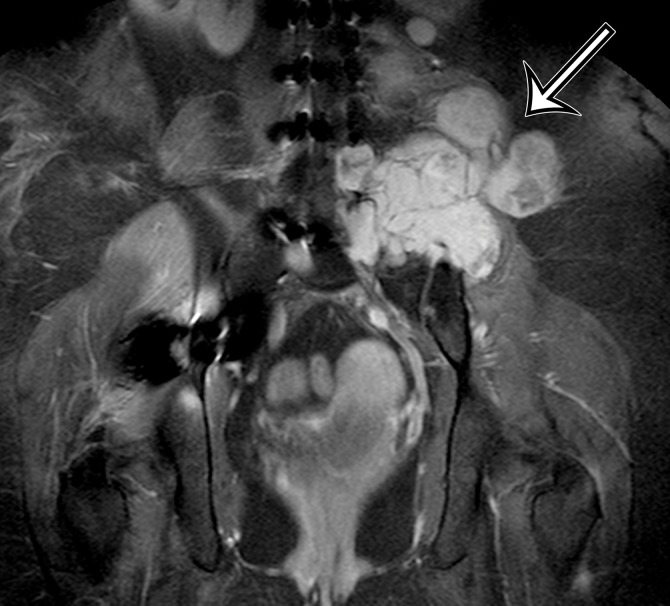
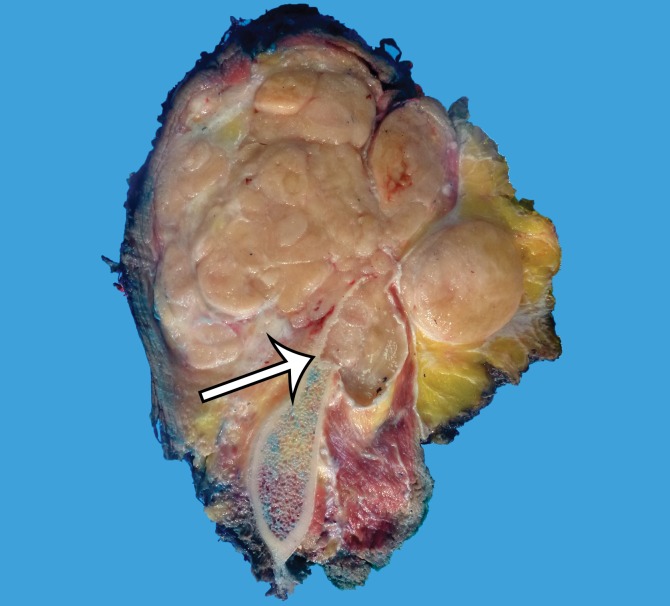
MPNST in a 34-year-old woman with low back pain radiating to the left lower extremity. (a–c) Coronal T2-weighted inversion-recovery (a), T1-weighted (b), and contrast-enhanced T1-weighted fat-suppressed (c) MR images show a heterogeneous lobulated left paraspinal mass (arrow) invading the L5 vertebral body medially, the sacrum inferomedially, and the iliac wing inferolaterally. The mass is T1 hypo- to isointense (arrow on b) and T2 hyperintense (arrow on a) relative to skeletal muscle, with avid heterogeneous enhancement (arrow on c). (d) Photograph of the cut surface of the resected specimen shows a multinodular pale yellow–tan mass invading the iliac wing (arrow).
Figure 5b.
MPNST in a 34-year-old woman with low back pain radiating to the left lower extremity. (a–c) Coronal T2-weighted inversion-recovery (a), T1-weighted (b), and contrast-enhanced T1-weighted fat-suppressed (c) MR images show a heterogeneous lobulated left paraspinal mass (arrow) invading the L5 vertebral body medially, the sacrum inferomedially, and the iliac wing inferolaterally. The mass is T1 hypo- to isointense (arrow on b) and T2 hyperintense (arrow on a) relative to skeletal muscle, with avid heterogeneous enhancement (arrow on c). (d) Photograph of the cut surface of the resected specimen shows a multinodular pale yellow–tan mass invading the iliac wing (arrow).
Figure 5c.
MPNST in a 34-year-old woman with low back pain radiating to the left lower extremity. (a–c) Coronal T2-weighted inversion-recovery (a), T1-weighted (b), and contrast-enhanced T1-weighted fat-suppressed (c) MR images show a heterogeneous lobulated left paraspinal mass (arrow) invading the L5 vertebral body medially, the sacrum inferomedially, and the iliac wing inferolaterally. The mass is T1 hypo- to isointense (arrow on b) and T2 hyperintense (arrow on a) relative to skeletal muscle, with avid heterogeneous enhancement (arrow on c). (d) Photograph of the cut surface of the resected specimen shows a multinodular pale yellow–tan mass invading the iliac wing (arrow).
Figure 5d.
MPNST in a 34-year-old woman with low back pain radiating to the left lower extremity. (a–c) Coronal T2-weighted inversion-recovery (a), T1-weighted (b), and contrast-enhanced T1-weighted fat-suppressed (c) MR images show a heterogeneous lobulated left paraspinal mass (arrow) invading the L5 vertebral body medially, the sacrum inferomedially, and the iliac wing inferolaterally. The mass is T1 hypo- to isointense (arrow on b) and T2 hyperintense (arrow on a) relative to skeletal muscle, with avid heterogeneous enhancement (arrow on c). (d) Photograph of the cut surface of the resected specimen shows a multinodular pale yellow–tan mass invading the iliac wing (arrow).
Recurrent and Metastatic Disease
MPNSTs are highly aggressive sarcomas with poor survival rates. Forty percent of patients have local recurrence, and 40%–60% of patients have metastatic disease within 1 year after the initial resection (33). The lung is the most common site for metastatic disease. Liver, adrenal gland, brain, and bone metastases may also occur. Occasionally, lymph node metastases may be seen.
Rhabdomyosarcoma
Definition
Rhabdomyosarcomas are malignant tumors with exclusively skeletal muscle differentiation. They are the most common sarcomas of childhood and are rare in adults. It should be noted that many other tumors, such as dedifferentiated liposarcoma, MPNST, and uterine carcinosarcoma, can contain rhabdomyosarcomatous elements and are more common than true rhabdomyosarcomas in adults.
Clinical and Pathologic Features
The WHO recognizes four distinct subtypes of rhabdomyosarcoma: embryonal, alveolar, pleomorphic, and spindle cell/sclerosing (15). Almost all embryonal rhabdomyosarcomas and the majority of alveolar rhabdomyosarcomas are seen in pediatric and young adult populations and are beyond the scope of this review. If found in the abdomen and pelvis of an adult, embryonal and alveolar rhabdomyosarcomas arise in the paratesticular soft tissues, perineum, and retroperitoneum (34).
Pleomorphic rhabdomyosarcoma is the variant that is seen almost exclusively in adults. It occurs in older adults, with a mean age at diagnosis of 51 years (10). By definition, tumor cells should express desmin, as well as myogenic transcription factors MyoD1 or myogenin, or both (10). Spindle cell/sclerosing rhabdomyosarcoma is also uncommon. In adults, it arises in the deep soft tissues of the neck, paratesticular region, retroperitoneum, and external genitalia (35). Microscopically, spindle cell/sclerosing rhabdomyosarcomas are composed of elongated spindle-shaped cells in a dense collagenous stroma. The immunohistochemical profile of spindle cell/sclerosing rhabdomyosarcomas should support skeletal muscle differentiation, to distinguish them from other sarcomas (36).
Radiologic Features
Cross-sectional imaging data with regard to adult abdominal and pelvic rhabdomyosarcomas are limited, reflecting the rarity of these tumors. Regardless of the histopathologic subtype, rhabdomyosarcomas of the perineal regions (vulva in women and paratesticular soft tissues of men) are reported to be infiltrating masses with poorly defined borders (34,37,38). In the retroperitoneum, these lesions are large masses at the time of clinical presentation, similar to other retroperitoneal sarcomas. On CT images, rhabdomyosarcomas are predominantly isoattenuating to skeletal muscle. They may have areas of degeneration and intratumoral hemorrhage that create regions of hypoattenuation within the tumor. At MR imaging, the lesions are isointense to skeletal muscle on T1-weighted MR images and demonstrate high signal intensity on T2-weighted MR images (34). Calcification is not a feature of rhabdomyosarcoma.
Recurrent and Metastatic Disease
Lymphadenopathy is an important feature of metastatic disease in rhabdomyosarcoma. In those patients with abdominal and pelvic lesions, the retroperitoneum is a common site for nodal disease, which may be present at the initial clinical presentation or may be discovered as a site of recurrence (34,37). The lung is the most common site for distant metastatic disease.
Vascular Sarcomas
Vascular sarcomas are only briefly mentioned because of their exceedingly rare occurrence in the soft tissues of the abdomen and pelvis. This group of tumors includes epithelioid hemangioendothelioma, angiosarcoma, and Kaposi sarcoma. Epithelioid hemangioendothelioma is a low-grade malignancy that is usually seen in the extremities but can occur in any anatomic location. In the abdomen, the lesion is most often seen in the liver. Angiosarcomas of the soft tissues are more common in the extremities. In the abdomen and pelvis, angiosarcomas of the soft tissues may rarely arise in the soft tissues of the deep retroperitoneum, and some cases may involve the peritoneum diffusely, simulating mesothelioma (39). Visceral angiosarcomas occur in the liver and spleen.
Chondro-osseous Sarcomas
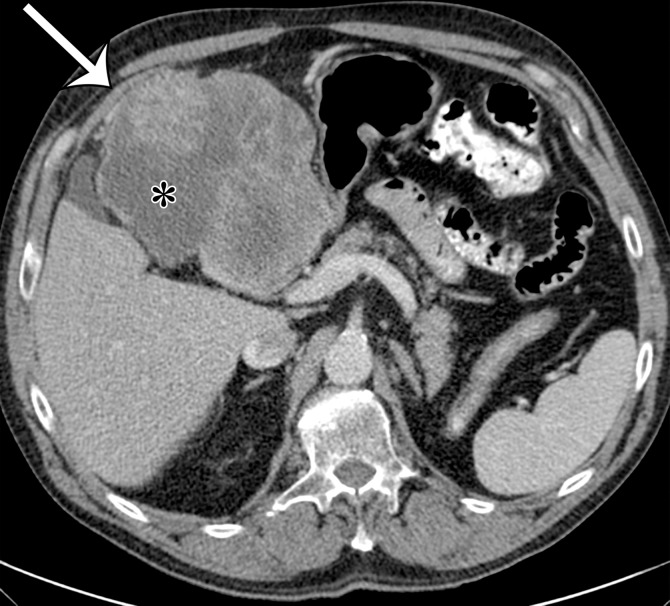
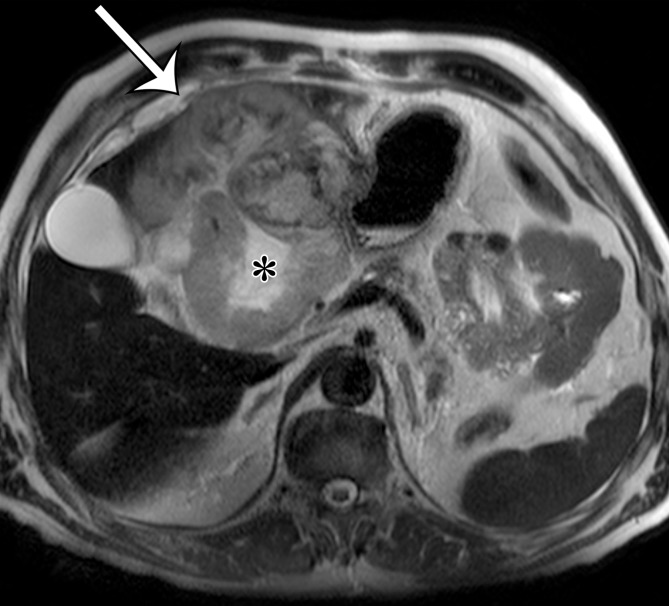
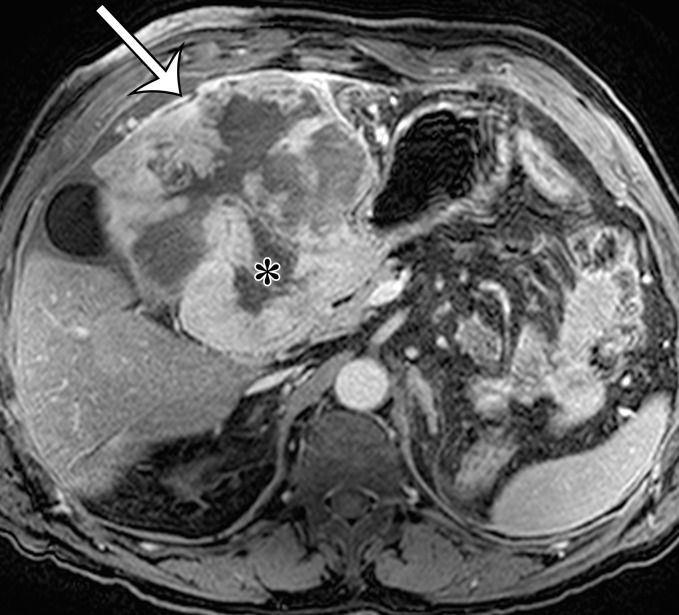
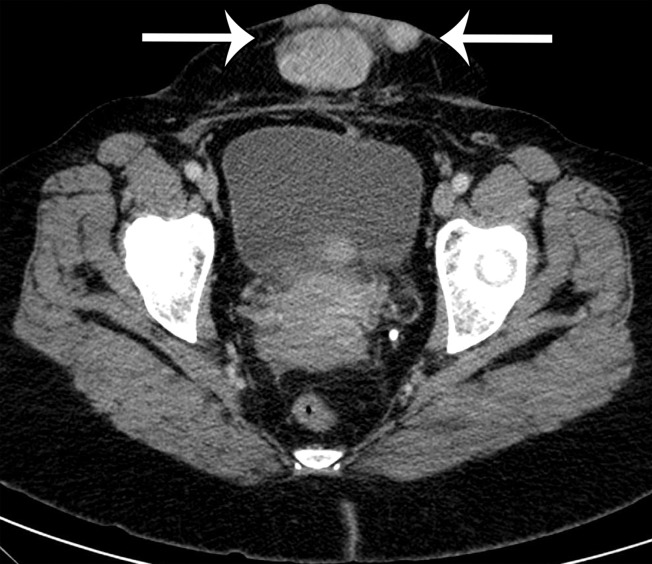
Extraskeletal osteosarcoma is a soft-tissue sarcoma composed of malignant cells that produce osteoid matrix. Extraskeletal osteosarcoma occurs predominantly in older patients and may be associated with irradiation in at least 10% of cases (40). Extraskeletal osteosarcoma may be seen in the retroperitoneum but is more common in the thigh and buttocks. Histologically, the tumors are usually high grade, with pleomorphism and variable amounts of osteoid, bone, and cartilage. The imaging diagnosis is based on observing a chondroid or osseous matrix within a retroperitoneal soft-tissue mass at CT (Figs 6, 7). Osteosarcomatous differentiation can also be seen in dedifferentiated liposarcoma and MPNST (41,42).
Figure 6a.
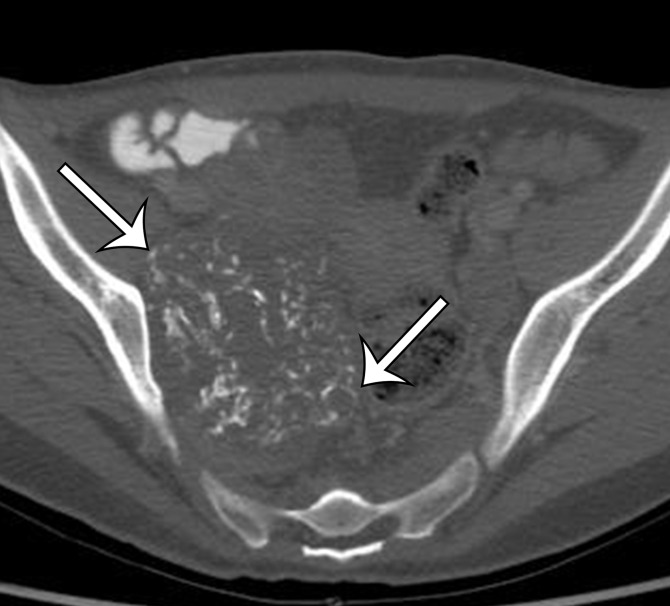
Soft-tissue osteosarcoma in a 59-year-old man with right flank pain. (a) Axial CT image (bone window) shows dense calcification (straight arrow) and an osteoid matrix (curved arrow) in a lobulated right pelvic mass. (b) Axial T2-weighted fat-suppressed MR image shows central areas of markedly T2-hyperintense necrosis (*). (c) Axial contrast-enhanced T1-weighted fat-suppressed MR image shows enhancement of the periphery (arrow) of the mass.
Figure 7a.
Chondrosarcoma in a 21-year-old woman with right lower quadrant pain. (a, b) Axial CT images obtained with soft-tissue window (a) and bone window (b) show a sharply marginated soft-tissue–attenuation mass closely approximating the right iliac wing (arrow on a), with chondroid-type calcifications in rings and arcs (arrows on b). (c, d) Axial T1-weighted (c) and T2-weighted (d) MR images better show the well-defined margins with scalloping of the iliac wing, but without medullary infiltration (straight arrow). Note the hypointense foci of chondroid calcifications (curved arrow).
Figure 6b.
Soft-tissue osteosarcoma in a 59-year-old man with right flank pain. (a) Axial CT image (bone window) shows dense calcification (straight arrow) and an osteoid matrix (curved arrow) in a lobulated right pelvic mass. (b) Axial T2-weighted fat-suppressed MR image shows central areas of markedly T2-hyperintense necrosis (*). (c) Axial contrast-enhanced T1-weighted fat-suppressed MR image shows enhancement of the periphery (arrow) of the mass.
Figure 6c.
Soft-tissue osteosarcoma in a 59-year-old man with right flank pain. (a) Axial CT image (bone window) shows dense calcification (straight arrow) and an osteoid matrix (curved arrow) in a lobulated right pelvic mass. (b) Axial T2-weighted fat-suppressed MR image shows central areas of markedly T2-hyperintense necrosis (*). (c) Axial contrast-enhanced T1-weighted fat-suppressed MR image shows enhancement of the periphery (arrow) of the mass.
Figure 7b.
Chondrosarcoma in a 21-year-old woman with right lower quadrant pain. (a, b) Axial CT images obtained with soft-tissue window (a) and bone window (b) show a sharply marginated soft-tissue–attenuation mass closely approximating the right iliac wing (arrow on a), with chondroid-type calcifications in rings and arcs (arrows on b). (c, d) Axial T1-weighted (c) and T2-weighted (d) MR images better show the well-defined margins with scalloping of the iliac wing, but without medullary infiltration (straight arrow). Note the hypointense foci of chondroid calcifications (curved arrow).
Figure 7c.
Chondrosarcoma in a 21-year-old woman with right lower quadrant pain. (a, b) Axial CT images obtained with soft-tissue window (a) and bone window (b) show a sharply marginated soft-tissue–attenuation mass closely approximating the right iliac wing (arrow on a), with chondroid-type calcifications in rings and arcs (arrows on b). (c, d) Axial T1-weighted (c) and T2-weighted (d) MR images better show the well-defined margins with scalloping of the iliac wing, but without medullary infiltration (straight arrow). Note the hypointense foci of chondroid calcifications (curved arrow).
Figure 7d.
Chondrosarcoma in a 21-year-old woman with right lower quadrant pain. (a, b) Axial CT images obtained with soft-tissue window (a) and bone window (b) show a sharply marginated soft-tissue–attenuation mass closely approximating the right iliac wing (arrow on a), with chondroid-type calcifications in rings and arcs (arrows on b). (c, d) Axial T1-weighted (c) and T2-weighted (d) MR images better show the well-defined margins with scalloping of the iliac wing, but without medullary infiltration (straight arrow). Note the hypointense foci of chondroid calcifications (curved arrow).
Sarcomas of Uncertain Differentiation
The sarcomas of uncertain differentiation are grouped together because they have unknown lineage. The WHO classification includes synovial sarcoma (Fig 8), epithelioid sarcoma, alveolar soft part sarcoma (Fig 9), clear cell sarcoma of soft tissue, extraskeletal myxoid chondrosarcoma, extraskeletal Ewing sarcoma, desmoplastic small round cell tumor, extrarenal rhabdoid tumor, and malignant perivascular epithelioid cell tumor (also called “PEComa”) (Fig 10) (15). Each of these sarcomas has distinctive clinical and pathologic features and genetic alterations. These sarcomas represent less than 1% of all soft-tissue sarcomas. The imaging features of these malignancies are nonspecific, and they may occur in the retroperitoneum and abdominal wall and, very rarely, in the peritoneal cavity.
Figure 8a.
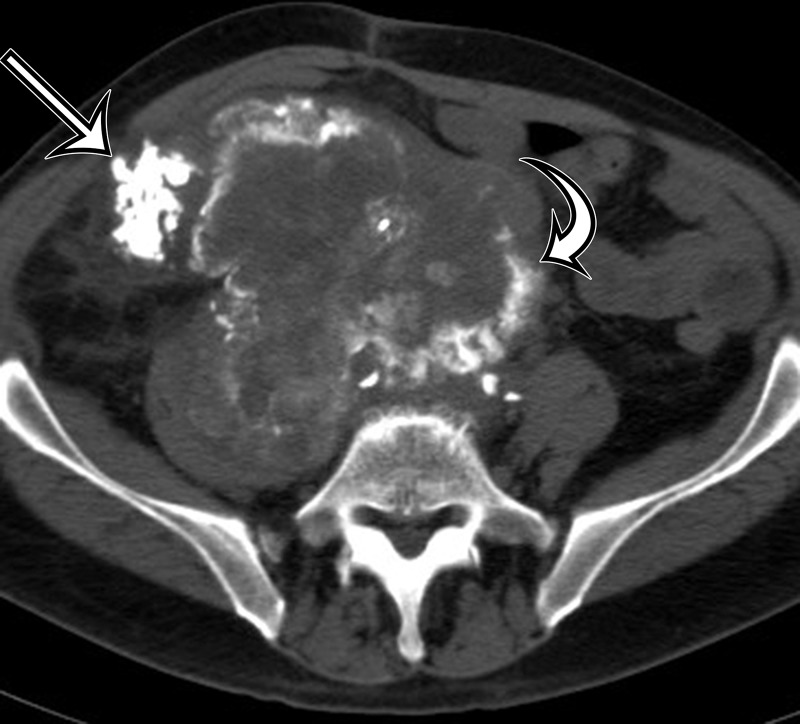
Synovial sarcoma in two different patients. (a) Synovial sarcoma in a 59-year-old woman who presented with rapid enlargement of a palpable painless mass: Axial contrast-enhanced CT image shows a well-circumscribed heterogeneously enhancing mass within the right anterior abdominal wall (arrows), with cystic components (*). (b, c) Synovial sarcoma in a previously healthy 36-year-old man with new onset of intractable vomiting: Axial contrast-enhanced CT images (c obtained at a lower level than b) show a large heterogeneous mass (straight arrow) infiltrating along the gastrohepatic ligament and circumferentially invading the gastric antrum and lesser curvature (curved arrows on b), with mass effect on the liver, gallbladder, duodenum, and pancreas. Areas of nonenhancing hyperattenuation are consistent with intratumoral (*) and intraperitoneal (arrowheads on c) hemorrhage.
Figure 9a.
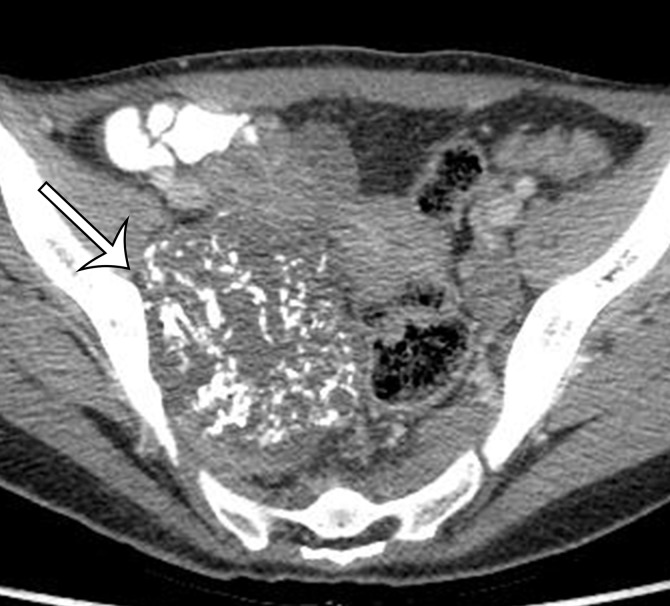
Alveolar soft part sarcoma in a 45-year-old woman with back pain. Axial (a) and sagittal (b) contrast-enhanced CT images show a well-circumscribed enhancing soft-tissue mass (arrow) that is inseparable from the posterior abdominal wall, with mass effect on the posterior right kidney.
Figure 10a.
Malignant perivascular epithelioid cell tumor in a 56-year-old man with crampy left lower quadrant pain. (a, b) Axial (a) and sagittal (b) contrast-enhanced CT images show a heterogeneously enhancing soft-tissue mass inseparable from the anterior abdominal wall musculature (straight arrow), with central low-attenuation areas of necrosis (* on a) and punctate calcifications (curved arrow). (c, d) Axial T2-weighted fat-suppressed (c) and contrast-enhanced T1-weighted (d) MR images show heterogeneous T2 hyperintensity and avid enhancement within the cellular portions (straight arrow), with more focal hyperintensity and lack of enhancement of the necrotic portions (*). Abdominal wall invasion (curved arrows) is more apparent on the MR images. (e) Photograph of the cut surface of the resected specimen shows corresponding areas of necrosis and hemorrhage (*) within the predominantly solid lobulated mass. Mass is inseparable from the abdominal wall (arrow).
Figure 8b.
Synovial sarcoma in two different patients. (a) Synovial sarcoma in a 59-year-old woman who presented with rapid enlargement of a palpable painless mass: Axial contrast-enhanced CT image shows a well-circumscribed heterogeneously enhancing mass within the right anterior abdominal wall (arrows), with cystic components (*). (b, c) Synovial sarcoma in a previously healthy 36-year-old man with new onset of intractable vomiting: Axial contrast-enhanced CT images (c obtained at a lower level than b) show a large heterogeneous mass (straight arrow) infiltrating along the gastrohepatic ligament and circumferentially invading the gastric antrum and lesser curvature (curved arrows on b), with mass effect on the liver, gallbladder, duodenum, and pancreas. Areas of nonenhancing hyperattenuation are consistent with intratumoral (*) and intraperitoneal (arrowheads on c) hemorrhage.
Figure 8c.
Synovial sarcoma in two different patients. (a) Synovial sarcoma in a 59-year-old woman who presented with rapid enlargement of a palpable painless mass: Axial contrast-enhanced CT image shows a well-circumscribed heterogeneously enhancing mass within the right anterior abdominal wall (arrows), with cystic components (*). (b, c) Synovial sarcoma in a previously healthy 36-year-old man with new onset of intractable vomiting: Axial contrast-enhanced CT images (c obtained at a lower level than b) show a large heterogeneous mass (straight arrow) infiltrating along the gastrohepatic ligament and circumferentially invading the gastric antrum and lesser curvature (curved arrows on b), with mass effect on the liver, gallbladder, duodenum, and pancreas. Areas of nonenhancing hyperattenuation are consistent with intratumoral (*) and intraperitoneal (arrowheads on c) hemorrhage.
Figure 9b.
Alveolar soft part sarcoma in a 45-year-old woman with back pain. Axial (a) and sagittal (b) contrast-enhanced CT images show a well-circumscribed enhancing soft-tissue mass (arrow) that is inseparable from the posterior abdominal wall, with mass effect on the posterior right kidney.
Figure 10b.
Malignant perivascular epithelioid cell tumor in a 56-year-old man with crampy left lower quadrant pain. (a, b) Axial (a) and sagittal (b) contrast-enhanced CT images show a heterogeneously enhancing soft-tissue mass inseparable from the anterior abdominal wall musculature (straight arrow), with central low-attenuation areas of necrosis (* on a) and punctate calcifications (curved arrow). (c, d) Axial T2-weighted fat-suppressed (c) and contrast-enhanced T1-weighted (d) MR images show heterogeneous T2 hyperintensity and avid enhancement within the cellular portions (straight arrow), with more focal hyperintensity and lack of enhancement of the necrotic portions (*). Abdominal wall invasion (curved arrows) is more apparent on the MR images. (e) Photograph of the cut surface of the resected specimen shows corresponding areas of necrosis and hemorrhage (*) within the predominantly solid lobulated mass. Mass is inseparable from the abdominal wall (arrow).
Figure 10c.
Malignant perivascular epithelioid cell tumor in a 56-year-old man with crampy left lower quadrant pain. (a, b) Axial (a) and sagittal (b) contrast-enhanced CT images show a heterogeneously enhancing soft-tissue mass inseparable from the anterior abdominal wall musculature (straight arrow), with central low-attenuation areas of necrosis (* on a) and punctate calcifications (curved arrow). (c, d) Axial T2-weighted fat-suppressed (c) and contrast-enhanced T1-weighted (d) MR images show heterogeneous T2 hyperintensity and avid enhancement within the cellular portions (straight arrow), with more focal hyperintensity and lack of enhancement of the necrotic portions (*). Abdominal wall invasion (curved arrows) is more apparent on the MR images. (e) Photograph of the cut surface of the resected specimen shows corresponding areas of necrosis and hemorrhage (*) within the predominantly solid lobulated mass. Mass is inseparable from the abdominal wall (arrow).
Figure 10d.
Malignant perivascular epithelioid cell tumor in a 56-year-old man with crampy left lower quadrant pain. (a, b) Axial (a) and sagittal (b) contrast-enhanced CT images show a heterogeneously enhancing soft-tissue mass inseparable from the anterior abdominal wall musculature (straight arrow), with central low-attenuation areas of necrosis (* on a) and punctate calcifications (curved arrow). (c, d) Axial T2-weighted fat-suppressed (c) and contrast-enhanced T1-weighted (d) MR images show heterogeneous T2 hyperintensity and avid enhancement within the cellular portions (straight arrow), with more focal hyperintensity and lack of enhancement of the necrotic portions (*). Abdominal wall invasion (curved arrows) is more apparent on the MR images. (e) Photograph of the cut surface of the resected specimen shows corresponding areas of necrosis and hemorrhage (*) within the predominantly solid lobulated mass. Mass is inseparable from the abdominal wall (arrow).
Figure 10e.
Malignant perivascular epithelioid cell tumor in a 56-year-old man with crampy left lower quadrant pain. (a, b) Axial (a) and sagittal (b) contrast-enhanced CT images show a heterogeneously enhancing soft-tissue mass inseparable from the anterior abdominal wall musculature (straight arrow), with central low-attenuation areas of necrosis (* on a) and punctate calcifications (curved arrow). (c, d) Axial T2-weighted fat-suppressed (c) and contrast-enhanced T1-weighted (d) MR images show heterogeneous T2 hyperintensity and avid enhancement within the cellular portions (straight arrow), with more focal hyperintensity and lack of enhancement of the necrotic portions (*). Abdominal wall invasion (curved arrows) is more apparent on the MR images. (e) Photograph of the cut surface of the resected specimen shows corresponding areas of necrosis and hemorrhage (*) within the predominantly solid lobulated mass. Mass is inseparable from the abdominal wall (arrow).
Differential Diagnosis
In part 1 of this article, we discussed the differential diagnosis of soft-tissue masses of the abdomen and pelvis that contain fat, cystic components, myxoid material, and calcification (3). These differential considerations are most helpful with the most commonly occurring soft-tissue sarcomas. The uncommon soft-tissue sarcomas discussed in this article have less-specific features and thus require a broader differential diagnosis and, in almost all cases, biopsy to establish the diagnosis.
In general, in a patient with no known primary malignancy, a soft-tissue mass in the retroperitoneum that is not associated with a retroperitoneal organ (adrenal gland, kidney, pancreas, or the retroperitoneal portions of the gastrointestinal tract) should be considered a sarcoma until proven otherwise. If a patient has a known primary malignancy, metastatic disease may be seen as single or multiple soft-tissue nodules or masses in the retroperitoneum or involving retroperitoneal organs and the psoas muscle. Lymphoma may manifest as a primary retroperitoneal mass. Unlike many of the sarcomas, most lymphomas demonstrate homogeneous CT attenuation and homogeneous MR signal intensity, because they lack degeneration and/or necrosis. The exception is high-grade lymphomas. Splenomegaly and regional or distant adenopathy may also be present to support a diagnosis of lymphoma. Inflammatory processes such as abscesses or phlegmons can simulate tumors. Clinical findings of infection are generally present to help distinguish these inflammatory processes from malignancies. Retroperitoneal hemorrhage can also be masslike. However, early hemorrhage will demonstrate high attenuation typical of blood on CT images obtained without administration of intravenous contrast material. Also, retroperitoneal hemorrhage tends to spread along the fascial planes of the retroperitoneal compartments, rather than being round or oval in configuration.
Intraperitoneal soft-tissue masses have a similar differential diagnosis to that of retroperitoneal soft-tissue masses except that it may be more difficult to determine if the mass is arising from the soft tissues or the gastrointestinal tract. GIST is the most common intraperitoneal sarcoma, and its diagnosis can be suggested when a well-defined mass is arising from the bowel wall. GIST may also have an intraluminal component. Epithelial malignancies of the gastrointestinal tract, namely, adenocarcinomas of the colon, stomach, and small intestine, are mucosal in origin and will produce a luminal mass or mucosal irregularity. Gastrointestinal adenocarcinomas tend to be infiltrative lesions that concentrically thicken the wall of the gastrointestinal tract and produce a serosal margin that is poorly defined and spiculated. These patterns are not seen in GISTs or other soft-tissue sarcomas. Furthermore, regional adenopathy is a common feature in adenocarcinomas, whereas it is uncommon in sarcomas. Similar to gastrointestinal adenocarcinomas, malignant neuroendocrine tumors and carcinoids of the gastrointestinal tract will have an abnormality in the involved segment of bowel, either a focal mass or wall thickening. Other intraperitoneal lesions that are considerations in the differential diagnosis include metastatic disease, lymphoma, desmoid tumor (also called mesenteric fibromatosis), and sclerosing mesenteritis. Rarely, benign soft-tissue and neural tumors may be indistinguishable from sarcomas.
Special differential diagnostic considerations are given to those lesions arising in the abdominal wall. The differential diagnosis for skin lesions such as dermatofibrosarcoma protuberans includes cutaneous and subcutaneous metastasis, nerve sheath tumors, sebaceous cysts, hemangiomas, and other primary skin or skin appendage tumors of the trunk wall. In many cases, the clinical context and the appearance of the lesion at clinical examination are diagnostic.
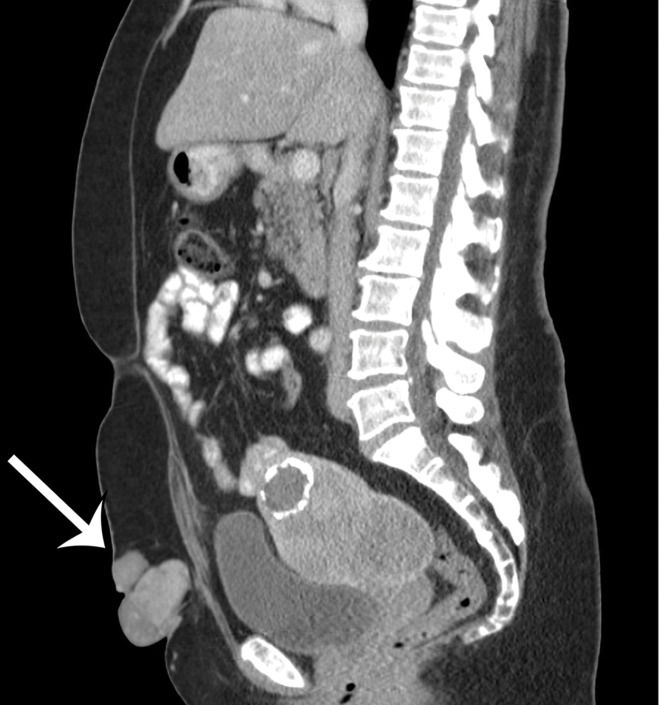
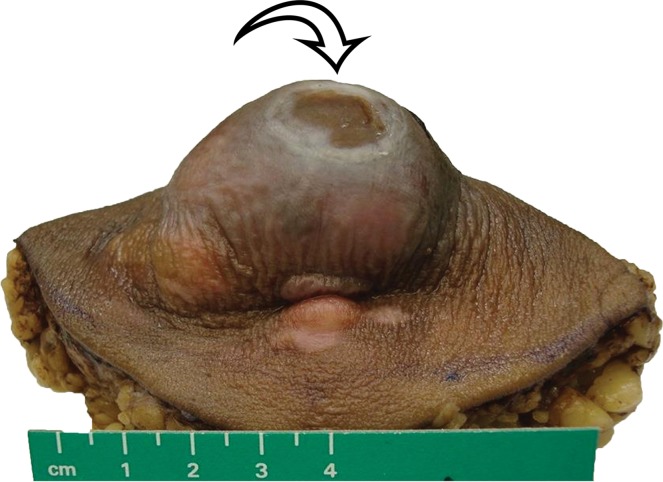
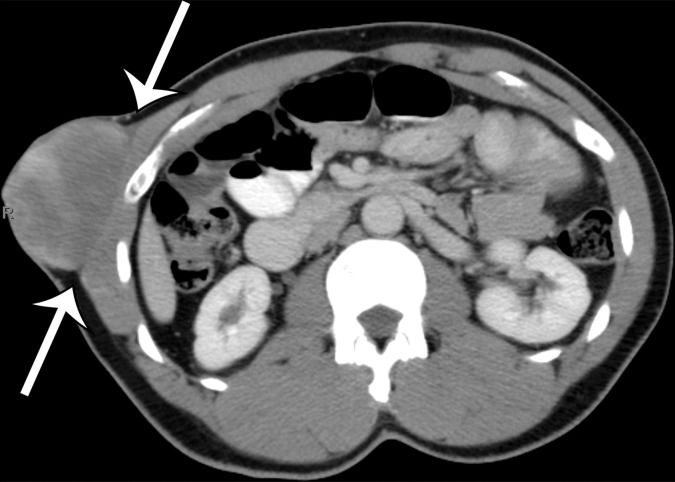
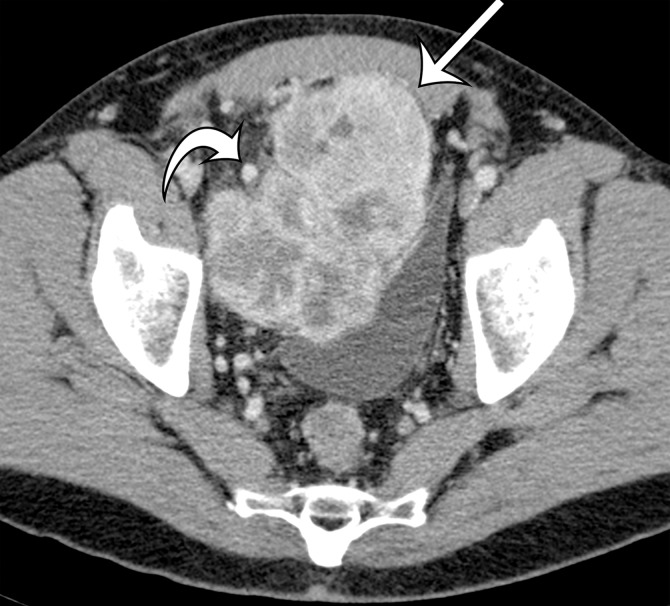
The differential diagnosis for deep lesions of the abdominal wall includes desmoid tumor, endometriosis, and metastatic disease. Other masses in the abdominal wall such as lipoma, hematoma, abscess, and hernia do not cause diagnostic dilemmas because they are generally easily diagnosed clinically and confirmed by the imaging findings. Desmoid tumors (also called fibromatosis) of the abdominal wall may be more difficult to differentiate from sarcomas. Desmoid tumors are benign lesions that do not metastasize but may recur locally. On CT images, desmoid tumors are classically well-defined homogeneous lesions that are isoattenuating to skeletal muscle, with little or minimal enhancement after intravenous contrast material administration. On MR images, the signal intensity is often more heterogeneous, depending on the amount of collagenous or myxoid stroma within the lesion (Fig 11). Those desmoid tumors with abundant myxoid stroma demonstrate high signal intensity on T2-weighted MR images (43). Most desmoid tumors characteristically show avid enhancement on arterial phase MR images obtained after administration of intravenous gadolinium-based contrast material. Endometriosis in the abdominal wall is almost exclusively associated with prior pelvic surgery and occurs in locations where surgical incisions were made, such as cesarean section scars or laparoscopic port sites. On CT images, abdominal wall endometriosis is a solid enhancing mass. At MR imaging, abdominal wall endometriosis is heterogeneous in signal intensity on T1- and T2-weighted MR images and may enhance after administration of intravenous gadolinium-based contrast material (Fig 12) (44). Abdominal wall endometriosis may contain cystic areas, areas of T1 hyperintensity caused by blood products, or areas of T1 and T2 hypointensity caused by hemosiderin.
Figure 11a.
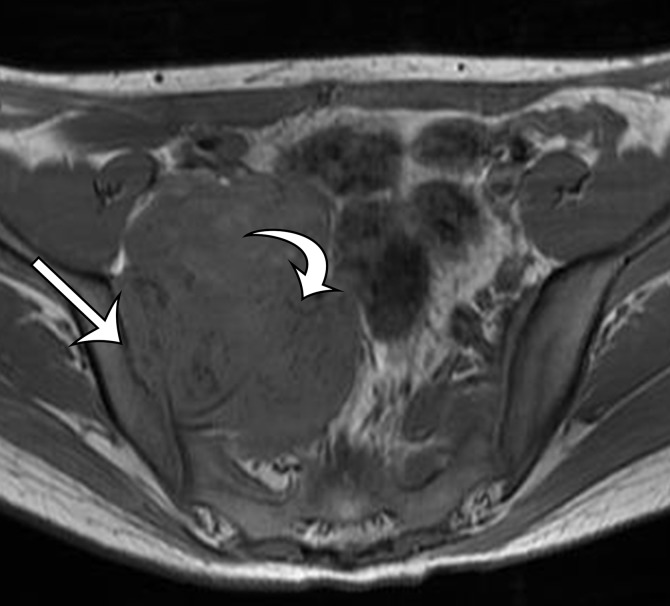
Desmoid tumor incidentally identified in the anterior abdominal wall of a 43-year-old man. (a) Axial contrast-enhanced CT image shows heterogeneous expansion of the left rectus muscle, with a suspected mass in the medial aspect of the muscle (arrows). (b, c) Axial T2-weighted fat-suppressed (b) and contrast-enhanced T1-weighted (c) MR images obtained to better characterize the lesion show a heterogeneous predominantly hyperintense solid mass (arrows on b) with heterogeneous enhancement (arrows on c).
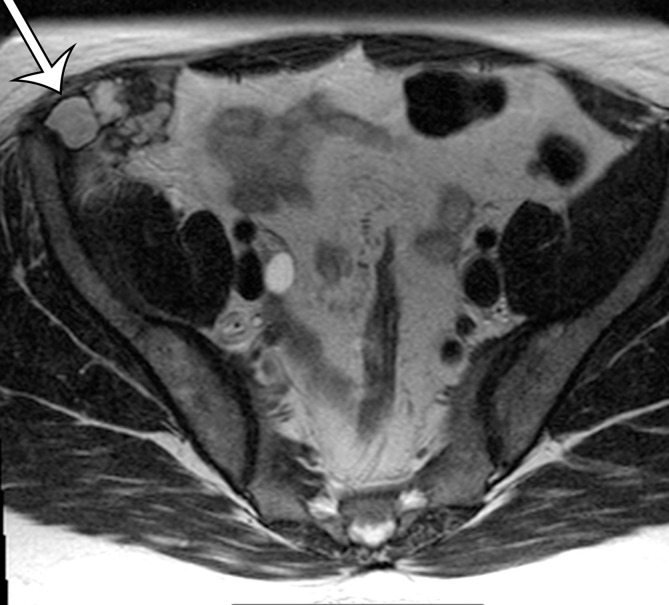
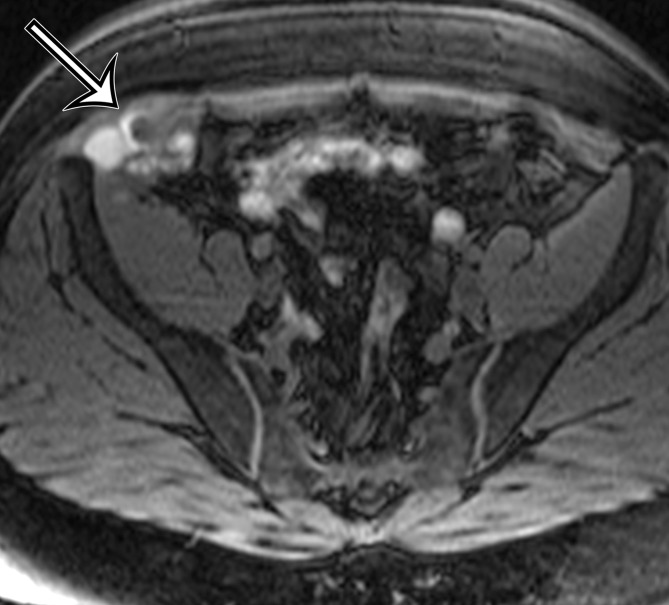
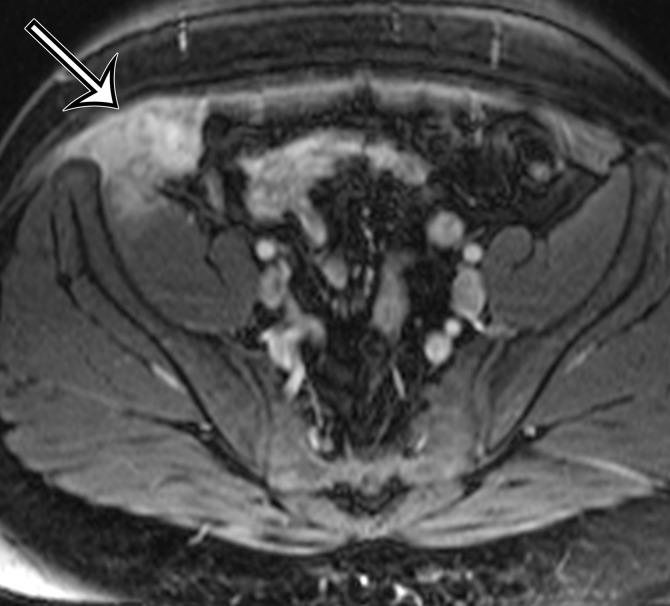
Figure 12a.
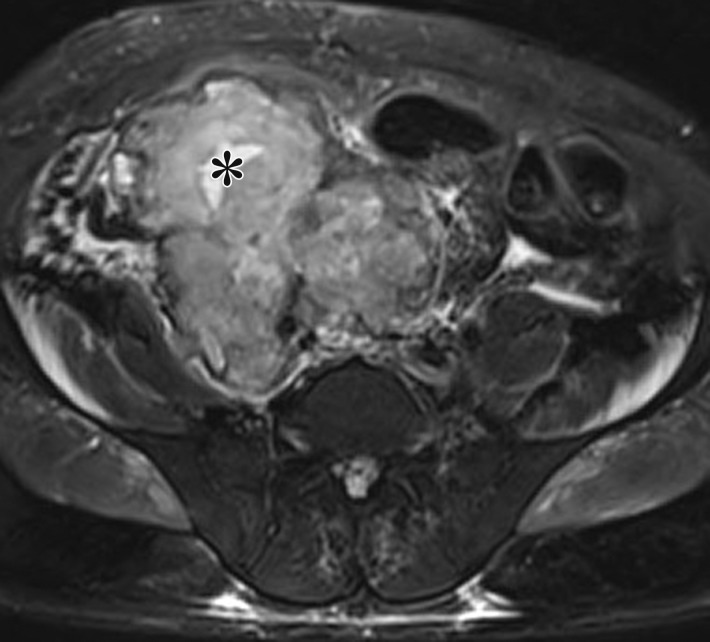
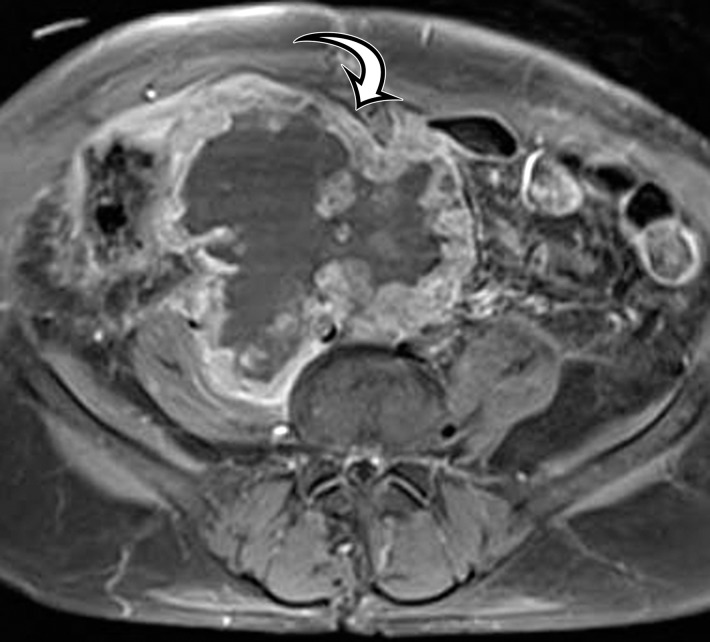
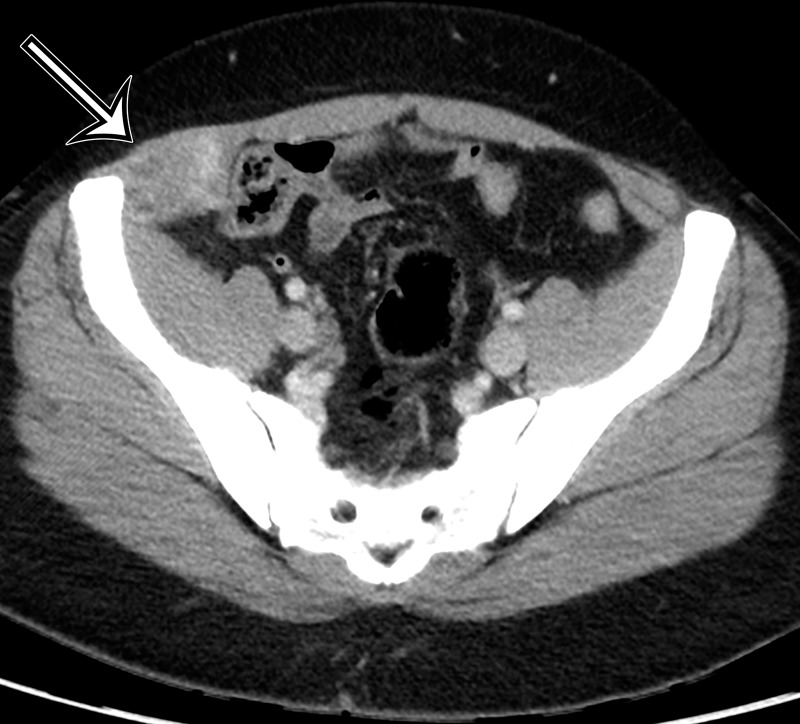
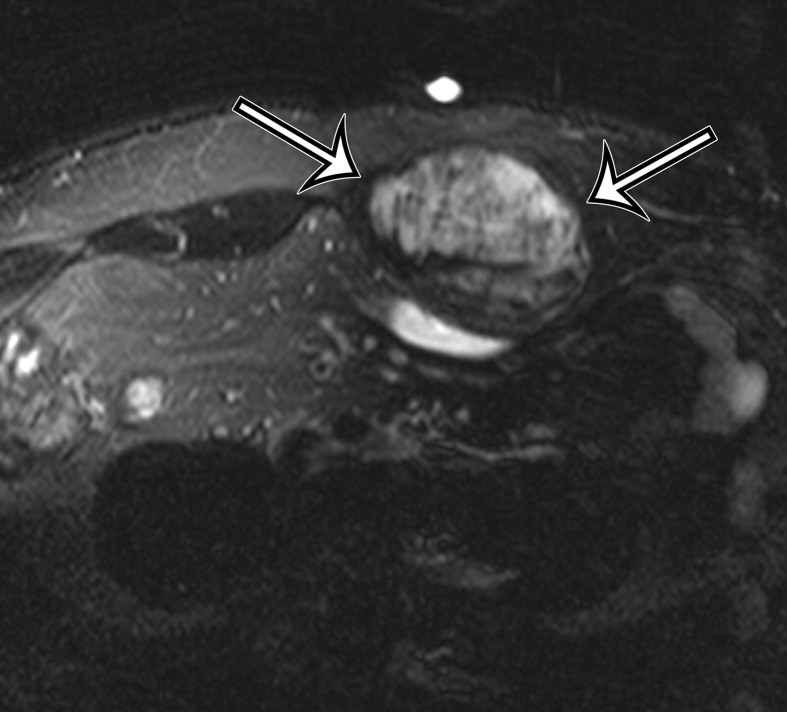
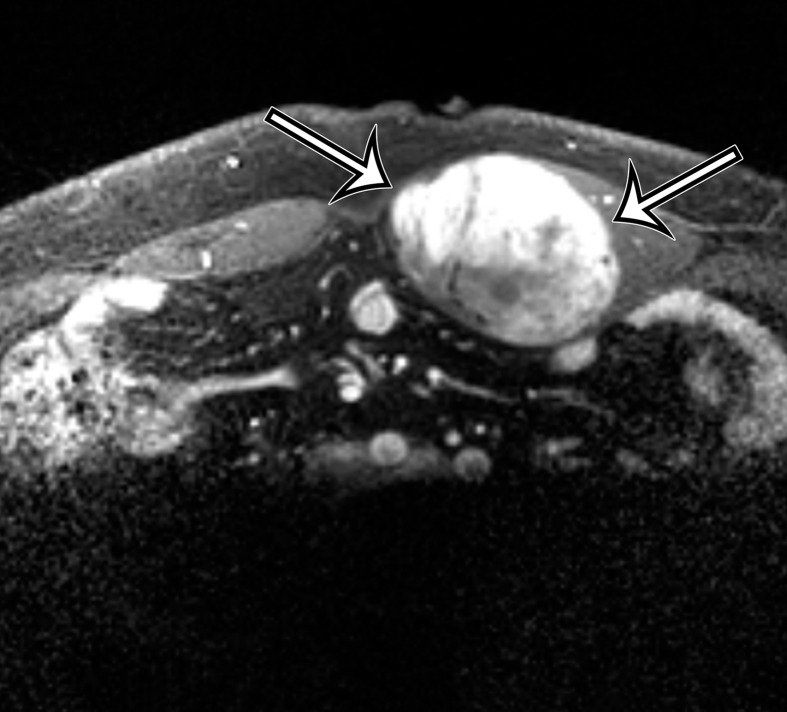
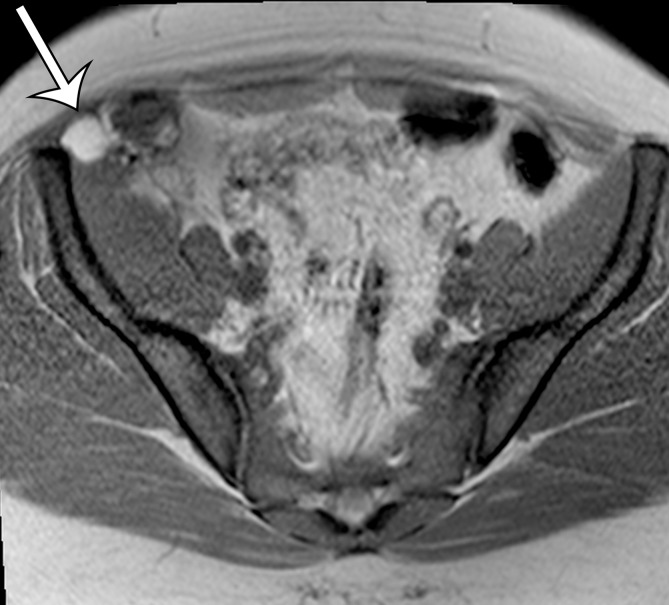
Endometriosis in the abdominal wall of a 43-year-old woman with worsening pain in the right lower quadrant that had begun 4 months after laparoscopic hysterectomy for menorrhagia. (a) Axial contrast-enhanced CT image shows a heterogeneously enhancing mass (arrow) in the right lateral abdominal wall musculature, adjacent to the anterior iliac wing. (b, c) T1-weighted (b) and T2-weighted (c) MR images show heterogeneous signal intensity within the multiloculated mass, with increased T1 and T2 signal intensity in a lateral loculated component (arrow). (d, e) T1-weighted fat-suppressed MR images obtained before (d) and after (e) the administration of gadolinium-based contrast material better show the intrinsic T1 hyperintensity (arrow on d) and heterogeneous enhancement (arrow on e).
Figure 11b.
Desmoid tumor incidentally identified in the anterior abdominal wall of a 43-year-old man. (a) Axial contrast-enhanced CT image shows heterogeneous expansion of the left rectus muscle, with a suspected mass in the medial aspect of the muscle (arrows). (b, c) Axial T2-weighted fat-suppressed (b) and contrast-enhanced T1-weighted (c) MR images obtained to better characterize the lesion show a heterogeneous predominantly hyperintense solid mass (arrows on b) with heterogeneous enhancement (arrows on c).
Figure 11c.
Desmoid tumor incidentally identified in the anterior abdominal wall of a 43-year-old man. (a) Axial contrast-enhanced CT image shows heterogeneous expansion of the left rectus muscle, with a suspected mass in the medial aspect of the muscle (arrows). (b, c) Axial T2-weighted fat-suppressed (b) and contrast-enhanced T1-weighted (c) MR images obtained to better characterize the lesion show a heterogeneous predominantly hyperintense solid mass (arrows on b) with heterogeneous enhancement (arrows on c).
Figure 12b.
Endometriosis in the abdominal wall of a 43-year-old woman with worsening pain in the right lower quadrant that had begun 4 months after laparoscopic hysterectomy for menorrhagia. (a) Axial contrast-enhanced CT image shows a heterogeneously enhancing mass (arrow) in the right lateral abdominal wall musculature, adjacent to the anterior iliac wing. (b, c) T1-weighted (b) and T2-weighted (c) MR images show heterogeneous signal intensity within the multiloculated mass, with increased T1 and T2 signal intensity in a lateral loculated component (arrow). (d, e) T1-weighted fat-suppressed MR images obtained before (d) and after (e) the administration of gadolinium-based contrast material better show the intrinsic T1 hyperintensity (arrow on d) and heterogeneous enhancement (arrow on e).
Figure 12c.
Endometriosis in the abdominal wall of a 43-year-old woman with worsening pain in the right lower quadrant that had begun 4 months after laparoscopic hysterectomy for menorrhagia. (a) Axial contrast-enhanced CT image shows a heterogeneously enhancing mass (arrow) in the right lateral abdominal wall musculature, adjacent to the anterior iliac wing. (b, c) T1-weighted (b) and T2-weighted (c) MR images show heterogeneous signal intensity within the multiloculated mass, with increased T1 and T2 signal intensity in a lateral loculated component (arrow). (d, e) T1-weighted fat-suppressed MR images obtained before (d) and after (e) the administration of gadolinium-based contrast material better show the intrinsic T1 hyperintensity (arrow on d) and heterogeneous enhancement (arrow on e).
Figure 12d.
Endometriosis in the abdominal wall of a 43-year-old woman with worsening pain in the right lower quadrant that had begun 4 months after laparoscopic hysterectomy for menorrhagia. (a) Axial contrast-enhanced CT image shows a heterogeneously enhancing mass (arrow) in the right lateral abdominal wall musculature, adjacent to the anterior iliac wing. (b, c) T1-weighted (b) and T2-weighted (c) MR images show heterogeneous signal intensity within the multiloculated mass, with increased T1 and T2 signal intensity in a lateral loculated component (arrow). (d, e) T1-weighted fat-suppressed MR images obtained before (d) and after (e) the administration of gadolinium-based contrast material better show the intrinsic T1 hyperintensity (arrow on d) and heterogeneous enhancement (arrow on e).
Figure 12e.
Endometriosis in the abdominal wall of a 43-year-old woman with worsening pain in the right lower quadrant that had begun 4 months after laparoscopic hysterectomy for menorrhagia. (a) Axial contrast-enhanced CT image shows a heterogeneously enhancing mass (arrow) in the right lateral abdominal wall musculature, adjacent to the anterior iliac wing. (b, c) T1-weighted (b) and T2-weighted (c) MR images show heterogeneous signal intensity within the multiloculated mass, with increased T1 and T2 signal intensity in a lateral loculated component (arrow). (d, e) T1-weighted fat-suppressed MR images obtained before (d) and after (e) the administration of gadolinium-based contrast material better show the intrinsic T1 hyperintensity (arrow on d) and heterogeneous enhancement (arrow on e).
Conclusion
Knowing the spectrum of common and uncommon soft-tissue sarcomas arising in the abdomen and pelvis and the abdominal wall is important to establish an accurate working differential diagnosis, to determine an early diagnosis. Liposarcoma, leiomyosarcoma, and GIST are the most common sarcomas of the abdomen and pelvis. Less commonly, undifferentiated pleomorphic sarcoma, solitary fibrous tumor, and MPNST may be found in the retroperitoneum and pelvis.
Supported by the American Institute for Radiologic Pathology, the Joint Pathology Center, and the Uniformed Services University of the Health Sciences. The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of Defense or the U.S. Government.
For this journal-based SA-CME activity, the authors, editor, and reviewers have disclosed no relevant relationships.
Abbreviations:
- GIST
- gastrointestinal stromal tumor
- MPNST
- malignant peripheral nerve sheath tumor
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.Cormier JN, Pollock RE. Soft tissue sarcomas. CA Cancer J Clin 2004;54(2):94–109. [DOI] [PubMed] [Google Scholar]
- 3.Levy AD, Manning MA, Al-Refaie WB, Miettinen MM. Soft-tissue sarcomas of the abdomen and pelvis: radiologic-pathologic features, part 1—common sarcomas. RadioGraphics 2017;37(2):462–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldblum JR, Folpe AL, Weiss SW. Undifferentiated pleomorphic sarcoma. In: Goldblum JR, Folpe AL, Weiss SW, eds. Enzinger and Weiss’s soft tissue tumors. 6th ed. Philadelphia, Pa: Saunders/Elsevier, 2013; 421–442. [Google Scholar]
- 5.Goette DK, Deffer TA. Postirradiation malignant fibrous histiocytoma. Arch Dermatol 1985;121(4):535–538. [PubMed] [Google Scholar]
- 6.Gladdy RA, Qin LX, Moraco N, et al. Do radiation-associated soft tissue sarcomas have the same prognosis as sporadic soft tissue sarcomas? J Clin Oncol 2010;28(12):2064–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fletcher CD. Soft tissue sarcomas apparently arising in chronic tropical ulcers. Histopathology 1987;11(5):501–510. [DOI] [PubMed] [Google Scholar]
- 8.Atmatzidis KS, Pavlidis TE, Galanis IN, Papaziogas BT, Papaziogas TB. Malignant fibrous histiocytoma of the abdominal cavity: report of a case. Surg Today 2003;33(10):794–796. [DOI] [PubMed] [Google Scholar]
- 9.Goldman SM, Hartman DS, Weiss SW. The varied radiographic manifestations of retroperitoneal malignant fibrous histiocytoma revealed through 27 cases. J Urol 1986;135(1):33–38. [DOI] [PubMed] [Google Scholar]
- 10.Goldblum JR. An approach to pleomorphic sarcomas: can we subclassify, and does it matter? Mod Pathol 2014;27(suppl 1):S39–S46. [DOI] [PubMed] [Google Scholar]
- 11.Karki B, Xu YK, Wu YK, Zhang WW. Primary malignant fibrous histiocytoma of the abdominal cavity: CT findings and pathological correlation. World J Radiol 2012;4(4):151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ko SF, Wan YL, Lee TY, Ng SH, Lin JW, Chen WJ. CT features of calcifications in abdominal malignant fibrous histiocytoma. Clin Imaging 1998;22(6):408–413. [DOI] [PubMed] [Google Scholar]
- 13.Kweon JH, Choi CS, Im CJ, Seo GS, Choi SC. Malignant fibrous histiocytoma arising from the omentum presenting as hemoperitoneum. Gut Liver 2010;4(2):241–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kreicher KL, Kurlander DE, Gittleman HR, Barnholtz-Sloan JS, Bordeaux JS. Incidence and survival of primary dermatofibrosarcoma protuberans in the United States. Dermatol Surg 2016;42(suppl 1):S24–S31. [DOI] [PubMed] [Google Scholar]
- 15.Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F, eds. WHO classification of tumours of soft tissue and bone. 4th ed. Lyon, France: IARC, 2013; 10–11. [Google Scholar]
- 16.Mentzel T, Beham A, Katenkamp D, Dei Tos AP, Fletcher CD. Fibrosarcomatous (“high-grade”) dermatofibrosarcoma protuberans: clinicopathologic and immunohistochemical study of a series of 41 cases with emphasis on prognostic significance. Am J Surg Pathol 1998;22(5):576–587. [DOI] [PubMed] [Google Scholar]
- 17.Li X, Zhang W, Xiao L, et al. Computed tomographic and pathological findings of dermatofibrosarcoma protuberans. J Comput Assist Tomogr 2012;36(4):462–468. [DOI] [PubMed] [Google Scholar]
- 18.Kransdorf MJ, Meis-Kindblom JM. Dermatofibrosarcoma protuberans: radiologic appearance. AJR Am J Roentgenol 1994;163(2):391–394. [DOI] [PubMed] [Google Scholar]
- 19.Torreggiani WC, Al-Ismail K, Munk PL, Nicolaou S, O’Connell JX, Knowling MA. Dermatofibrosarcoma protuberans: MR imaging features. AJR Am J Roentgenol 2002;178(4):989–993. [DOI] [PubMed] [Google Scholar]
- 20.Gloster HM, Jr. Dermatofibrosarcoma protuberans. J Am Acad Dermatol 1996;35(3 pt 1):355–374; quiz 375–376. [DOI] [PubMed] [Google Scholar]
- 21.Ratner D, Thomas CO, Johnson TM, et al. Mohs micrographic surgery for the treatment of dermatofibrosarcoma protuberans: results of a multiinstitutional series with an analysis of the extent of microscopic spread. J Am Acad Dermatol 1997;37(4):600–613. [DOI] [PubMed] [Google Scholar]
- 22.Chamberlain MH, Taggart DP. Solitary fibrous tumor associated with hypoglycemia: an example of the Doege-Potter syndrome. J Thorac Cardiovasc Surg 2000;119(1):185–187. [DOI] [PubMed] [Google Scholar]
- 23.Hasegawa T, Matsuno Y, Shimoda T, Hasegawa F, Sano T, Hirohashi S. Extrathoracic solitary fibrous tumors: their histological variability and potentially aggressive behavior. Hum Pathol 1999;30(12):1464–1473. [DOI] [PubMed] [Google Scholar]
- 24.Tian TT, Wu JT, Hu XH, et al. Imaging findings of solitary fibrous tumor in the abdomen and pelvis. Abdom Imaging 2014;39(6):1323–1329. [DOI] [PubMed] [Google Scholar]
- 25.Ginat DT, Bokhari A, Bhatt S, Dogra V. Imaging features of solitary fibrous tumors. AJR Am J Roentgenol 2011;196(3):487–495. [DOI] [PubMed] [Google Scholar]
- 26.Shanbhogue AK, Prasad SR, Takahashi N, Vikram R, Zaheer A, Sandrasegaran K. Somatic and visceral solitary fibrous tumors in the abdomen and pelvis: cross-sectional imaging spectrum. RadioGraphics 2011;31(2):393–408. [DOI] [PubMed] [Google Scholar]
- 27.Vallat-Decouvelaere AV, Dry SM, Fletcher CD. Atypical and malignant solitary fibrous tumors in extrathoracic locations: evidence of their comparability to intra-thoracic tumors. Am J Surg Pathol 1998;22(12):1501–1511. [DOI] [PubMed] [Google Scholar]
- 28.Ducatman BS, Scheithauer BW, Piepgras DG, Reiman HM, Ilstrup DM. Malignant peripheral nerve sheath tumors: a clinicopathologic study of 120 cases. Cancer 1986;57(10):2006–2021. [DOI] [PubMed] [Google Scholar]
- 29.King AA, Debaun MR, Riccardi VM, Gutmann DH. Malignant peripheral nerve sheath tumors in neurofibromatosis 1. Am J Med Genet 2000;93(5):388–392. [PubMed] [Google Scholar]
- 30.Scheithauer BW, Woodruff JM, Erlandson RA. Tumors of the peripheral nervous system. Washington, DC: Armed Forces Institute of Pathology, 1999. [Google Scholar]
- 31.Levy AD, Patel N, Dow N, Abbott RM, Miettinen M, Sobin LH. Abdominal neoplasms in patients with neurofibromatosis type 1: radiologic-pathologic correlation. RadioGraphics 2005;25(2):455–480. [DOI] [PubMed] [Google Scholar]
- 32.Levine E, Huntrakoon M, Wetzel LH. Malignant nerve-sheath neoplasms in neurofibromatosis: distinction from benign tumors by using imaging techniques. AJR Am J Roentgenol 1987;149(5):1059–1064. [DOI] [PubMed] [Google Scholar]
- 33.Goldblum JR, Folpe AL, Weiss SW. Malignant peripheral nerve sheath tumor. In: Goldblum JR, Folpe AL, Weiss SW, eds. Enzinger and Weiss’s soft tissue tumors. 6th ed. Philadelphia, Pa: Saunders/Elsevier, 2013; 855–879. [Google Scholar]
- 34.Allen SD, Moskovic EC, Fisher C, Thomas JM. Adult rhabdomyosarcoma: cross-sectional imaging findings including histopathologic correlation. AJR Am J Roentgenol 2007;189(2):371–377. [DOI] [PubMed] [Google Scholar]
- 35.Furlong MA, Mentzel T, Fanburg-Smith JC. Pleomorphic rhabdomyosarcoma in adults: a clinicopathologic study of 38 cases with emphasis on morphologic variants and recent skeletal muscle-specific markers. Mod Pathol 2001;14(6):595–603. [DOI] [PubMed] [Google Scholar]
- 36.Nascimento AF, Fletcher CD. Spindle cell rhabdomyosarcoma in adults. Am J Surg Pathol 2005;29(8):1106–1113. [PubMed] [Google Scholar]
- 37.Ferrari A, Bisogno G, Casanova M, et al. Paratesticular rhabdomyosarcoma: report from the Italian and German Cooperative Group. J Clin Oncol 2002;20(2):449–455. [DOI] [PubMed] [Google Scholar]
- 38.Munechika H, Cohan RH, Dunnick NR. Non-seminomatous testicular tumors: effect of lesion side on CT detection of lymph node metastasis. Comput Med Imaging Graph 1988;12(6):343–348. [DOI] [PubMed] [Google Scholar]
- 39.Meis-Kindblom JM, Kindblom LG. Angiosarcoma of soft tissue: a study of 80 cases. Am J Surg Pathol 1998;22(6):683–697. [DOI] [PubMed] [Google Scholar]
- 40.Orta L, Suprun U, Goldfarb A, Bleiweiss I, Jaffer S. Radiation-associated extraskeletal osteosarcoma of the chest wall. Arch Pathol Lab Med 2006;130(2):198–200. [DOI] [PubMed] [Google Scholar]
- 41.Hamdan A, Toman J, Taylor S, Keller A. Nuclear imaging of an extraskeletal retroperitoneal osteosarcoma: respective contribution of 18FDG-PET and (99m)Tc oxidronate (2005:1b). Eur Radiol 2005;15(4):840–844. [DOI] [PubMed] [Google Scholar]
- 42.Jaipuria J, Kumar A, Rao AS, Kataria SP. Large retroperitoneal low-grade extraskeletal osteosarcoma. BMJ Case Rep 2014;2014:bcr-2014-203745. doi:10.1136/bcr-2014-203745. Published online March 22, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levy AD, Rimola J, Mehrotra AK, Sobin LH. Benign fibrous tumors and tumorlike lesions of the mesentery: radiologic-pathologic correlation. RadioGraphics 2006;26(1):245–264. [DOI] [PubMed] [Google Scholar]
- 44.Busard MP, Mijatovic V, van Kuijk C, Hompes PG, van Waesberghe JH. Appearance of abdominal wall endometriosis on MR imaging. Eur Radiol 2010;20(5):1267–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]