Abstract
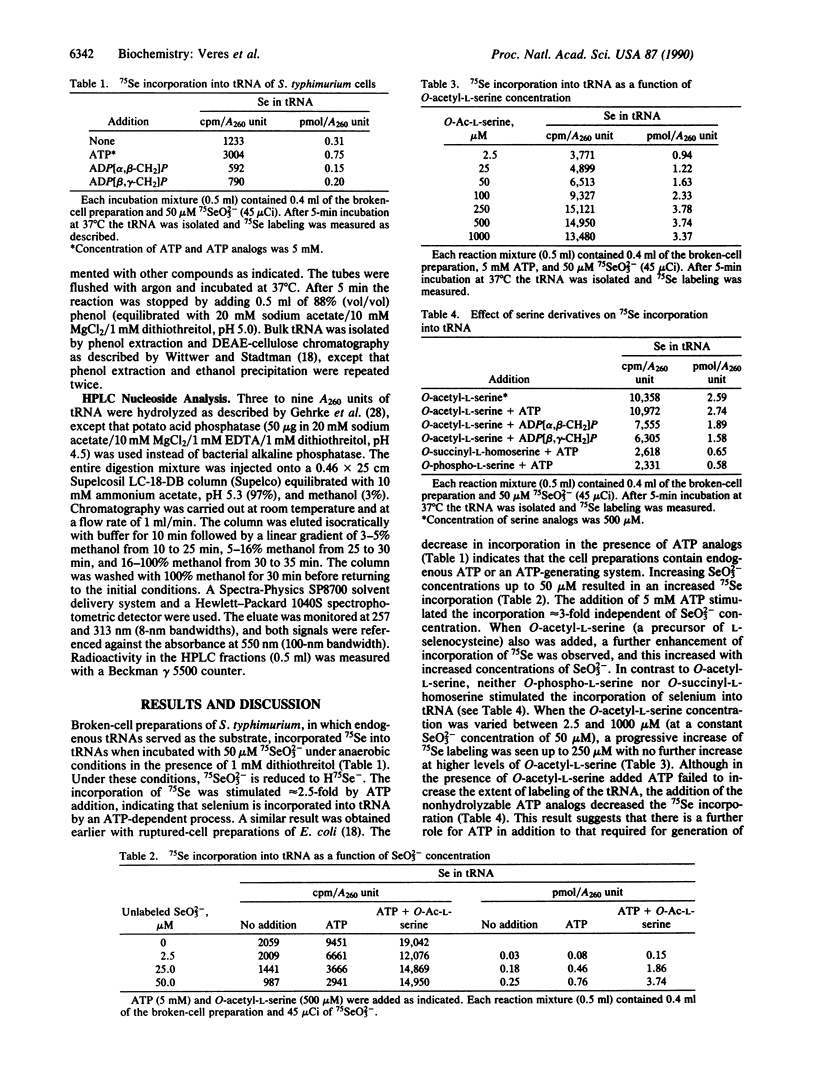
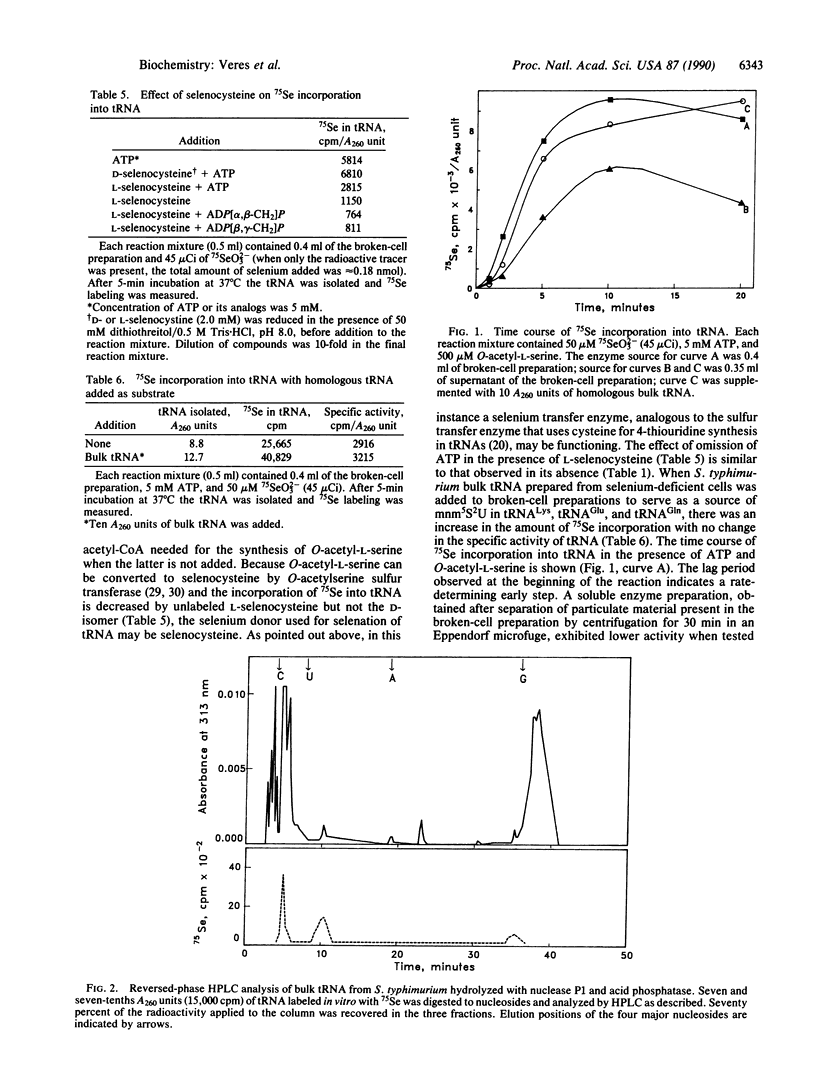
Broken-cell preparations of Salmonella typhimurium rapidly incorporated 75Se from 75SeO3(2-) into tRNA by an ATP-dependent process. Selenium incorporation in the presence of 50 microM 75SeO3(2-) (0.8-1 pmol per A260 unit) was enhanced by the selenocysteine precursor, O-acetyl-L-serine (to 3.7 pmol per A260 unit). This increase in incorporation was a function of O-acetyl-L-serine concentration. Neither O-acetyl-L-homoserine nor O-phospho-L-serine stimulated the incorporation of selenium into tRNA. The incorporation of 75Se from 75SeO3(2-) was decreased by adding L-selenocysteine but not by adding the D isomer. When homologous bulk tRNA was added to the broken-cell preparations, an increased rate of 75Se labeling was observed. The supernatant fraction of the broken-cell preparation contained all of the enzymes required for this process. Reversed-phase HPLC analysis of labeled bulk tRNA digested to nucleosides showed the presence of a labeled compound that coeluted with authentic 5-methylaminomethyl-2-selenouridine.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrell J. W., Kaufman E. E., Lipsett M. N. The biosynthesis of 4-thiouridylate. Separation and purification of two enzymes in the transfer ribonucleic acid-sulfurtransferase system. J Biol Chem. 1971 Jan 25;246(2):294–301. [PubMed] [Google Scholar]
- Axley M. J., Stadtman T. C. Selenium metabolism and selenium-dependent enzymes in microorganisms. Annu Rev Nutr. 1989;9:127–137. doi: 10.1146/annurev.nu.09.070189.001015. [DOI] [PubMed] [Google Scholar]
- Chakraburtty K., Steinschneider A., Case R. V., Mehler A. H. Primary structure of tRNA-Lys of E. coli B. Nucleic Acids Res. 1975 Nov;2(11):2069–2075. doi: 10.1093/nar/2.11.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. S., Stadtman T. C. Selenium-containing tRNAs from Clostridium sticklandii: cochromatography of one species with L-prolyl-tRNA. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1403–1407. doi: 10.1073/pnas.77.3.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching W. M., Alzner-DeWeerd B., Stadtman T. C. A selenium-containing nucleoside at the first position of the anticodon in seleno-tRNAGlu from Clostridium sticklandii. Proc Natl Acad Sci U S A. 1985 Jan;82(2):347–350. doi: 10.1073/pnas.82.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching W. M. Characterization of selenium-containing tRNAGlu from Clostridium sticklandii. Arch Biochem Biophys. 1986 Jan;244(1):137–146. doi: 10.1016/0003-9861(86)90102-5. [DOI] [PubMed] [Google Scholar]
- Ching W. M. Occurrence of selenium-containing tRNAs in mouse leukemia cells. Proc Natl Acad Sci U S A. 1984 May;81(10):3010–3013. doi: 10.1073/pnas.81.10.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching W. M., Stadtman T. C. Selenium-containing tRNAGlu from Clostridium sticklandii: correlation of aminoacylation with selenium content. Proc Natl Acad Sci U S A. 1982 Jan;79(2):374–377. doi: 10.1073/pnas.79.2.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching W. M., Wittwer A. J., Tsai L., Stadtman T. C. Distribution of two selenonucleosides among the selenium-containing tRNAs from Methanococcus vannielii. Proc Natl Acad Sci U S A. 1984 Jan;81(1):57–60. doi: 10.1073/pnas.81.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone J. E., Del Río R. M., Davis J. N., Stadtman T. C. Chemical characterization of the selenoprotein component of clostridial glycine reductase: identification of selenocysteine as the organoselenium moiety. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2659–2663. doi: 10.1073/pnas.73.8.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMoll E., Shive W. Determination of the metabolic origin of the sulfur atom in thiamin of Escherichia coli by mass spectrometry. Biochem Biophys Res Commun. 1985 Oct 15;132(1):217–222. doi: 10.1016/0006-291x(85)91010-1. [DOI] [PubMed] [Google Scholar]
- Forstrom J. W., Zakowski J. J., Tappel A. L. Identification of the catalytic site of rat liver glutathione peroxidase as selenocysteine. Biochemistry. 1978 Jun 27;17(13):2639–2644. doi: 10.1021/bi00606a028. [DOI] [PubMed] [Google Scholar]
- Gauss D. H., Sprinzl M. Compilation of tRNA sequences. Nucleic Acids Res. 1983 Jan 11;11(1):r1–53. [PMC free article] [PubMed] [Google Scholar]
- Gehrke C. W., Kuo K. C., McCune R. A., Gerhardt K. O., Agris P. F. Quantitative enzymatic hydrolysis of tRNAs: reversed-phase high-performance liquid chromatography of tRNA nucleosides. J Chromatogr. 1982 Jul 9;230(2):297–308. [PubMed] [Google Scholar]
- Jones J. B., Dilworth G. L., Stadtman T. C. Occurrence of selenocysteine in the selenium-dependent formate dehydrogenase of Methanococcus vannielii. Arch Biochem Biophys. 1979 Jul;195(2):255–260. doi: 10.1016/0003-9861(79)90351-5. [DOI] [PubMed] [Google Scholar]
- Kramer G. F., Ames B. N. Isolation and characterization of a selenium metabolism mutant of Salmonella typhimurium. J Bacteriol. 1988 Feb;170(2):736–743. doi: 10.1128/jb.170.2.736-743.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinfelder W., Forchhammer K., Veprek B., Zehelein E., Böck A. In vitro synthesis of selenocysteinyl-tRNA(UCA) from seryl-tRNA(UCA): involvement and characterization of the selD gene product. Proc Natl Acad Sci U S A. 1990 Jan;87(2):543–547. doi: 10.1073/pnas.87.2.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinfelder W., Forchhammer K., Zinoni F., Sawers G., Mandrand-Berthelot M. A., Böck A. Escherichia coli genes whose products are involved in selenium metabolism. J Bacteriol. 1988 Feb;170(2):540–546. doi: 10.1128/jb.170.2.540-546.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryals J., Hsu R. Y., Lipsett M. N., Bremer H. Isolation of single-site Escherichia coli mutants deficient in thiamine and 4-thiouridine syntheses: identification of a nuvC mutant. J Bacteriol. 1982 Aug;151(2):899–904. doi: 10.1128/jb.151.2.899-904.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtman T. C., Davis J. N., Zehelein E., Böck A. Biochemical and genetic analysis of Salmonella typhimurium and Escherichia coli mutants defective in specific incorporation of selenium into formate dehydrogenase and tRNAs. Biofactors. 1989 Mar;2(1):35–44. [PubMed] [Google Scholar]
- Stadtman T. C. Some selenium-dependent biochemical processes. Adv Enzymol Relat Areas Mol Biol. 1979;48:1–28. doi: 10.1002/9780470122938.ch1. [DOI] [PubMed] [Google Scholar]
- Sullivan M. A., Cannon J. F., Webb F. H., Bock R. M. Antisuppressor mutation in Escherichia coli defective in biosynthesis of 5-methylaminomethyl-2-thiouridine. J Bacteriol. 1985 Jan;161(1):368–376. doi: 10.1128/jb.161.1.368-376.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H., Soda K. Selenocysteine. Methods Enzymol. 1987;143:240–243. doi: 10.1016/0076-6879(87)43045-0. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Wittwer A. J., Ching W. M. Selenium-containing tRNA(Glu) and tRNA(Lys) from Escherichia coli: purification, codon specificity and translational activity. Biofactors. 1989 Mar;2(1):27–34. [PubMed] [Google Scholar]
- Wittwer A. J. Specific incorporation of selenium into lysine- and glutamate- accepting tRNAs from Escherichia coli. J Biol Chem. 1983 Jul 25;258(14):8637–8641. [PubMed] [Google Scholar]
- Wittwer A. J., Stadtman T. C. Biosynthesis of 5-methylaminomethyl-2-selenouridine, a naturally occurring nucleoside in Escherichia coli tRNA. Arch Biochem Biophys. 1986 Aug 1;248(2):540–550. doi: 10.1016/0003-9861(86)90507-2. [DOI] [PubMed] [Google Scholar]
- Wittwer A. J., Tsai L., Ching W. M., Stadtman T. C. Identification and synthesis of a naturally occurring selenonucleoside in bacterial tRNAs: 5-[(methylamino)methyl]-2-selenouridine. Biochemistry. 1984 Sep 25;23(20):4650–4655. doi: 10.1021/bi00315a021. [DOI] [PubMed] [Google Scholar]
- Yamazaki S. A selenium-containing hydrogenase from Methanococcus vannielii. Identification of the selenium moiety as a selenocysteine residue. J Biol Chem. 1982 Jul 25;257(14):7926–7929. [PubMed] [Google Scholar]



