Abstract
Ovarian cancer is the most lethal type of gynecological cancer and is the fifth leading cause of cancer-associated mortality in females globally. The majority of patients with ovarian cancer suffer from recurrent, progressive disease, due to the acquisition of a resistance phenotype towards various conventional chemotherapy drugs. Although paclitaxel has been demonstrated to be effective against ovarian tumors, there have been reports of the development of a resistant phenotype against Taxol® treatment. The multidrug resistance (MDR)-1/P-glycoprotein has previously been demonstrated to be associated with the acquisition of paclitaxel resistance in certain ovarian tumors. Therefore, the screening of novel drug candidates able to target MDR-1 in ovarian cancer cells and increase the sensitivity to Taxol® is required in order to improve the treatment of this disease. In the present study, the underlying mechanisms by which the dietary flavonoid myricetin enhances the cytotoxic potential of paclitaxel in ovarian cancer cells, was investigated. It was observed that myricetin induced significant cytotoxicity in A2780 and OVCAR3 ovarian cancer cells, with the IC50 value obtained at 25 µM. Myricetin treatment also resulted in the induction of apoptosis in the two cell lines, accompanied by the modulation of certain pro- and anti-apoptotic markers. It was also determined that the pre-incubation of ovarian cancer cells with a lower dose of myricetin was able to increase the cytotoxicity of paclitaxel, due to the significant downregulation of MDR-1 in these cells.
Keywords: myricetin, apoptosis, ovarian cancer, chemoresistance
Introduction
Ovarian cancer is the most lethal type of gynecological cancer amongst females worldwide, responsible for ~125,000 cancer-associated mortalities annually (1,2). Ovarian cancer is the seventh most common type of cancer and the fifth leading cause of cancer-associated mortality amongst females globally (3,4). The incidence rate of this disease is high, with >225,000 females diagnosed with ovarian cancer annually (5,6). Improvements have been made in the treatment of this disease, including the combination of surgery, radiation and chemotherapy with paclitaxel, gemcitabine and cisplatin; however, the overall 5-year survival rate is <40% (7). Recently, various platinum-based treatment regimens have been administered for the treatment of ovarian cancer (8), but no improvement in patient survival has been observed (1) due to the acquisition of chemotherapeutic drug resistance and tumor recurrence.
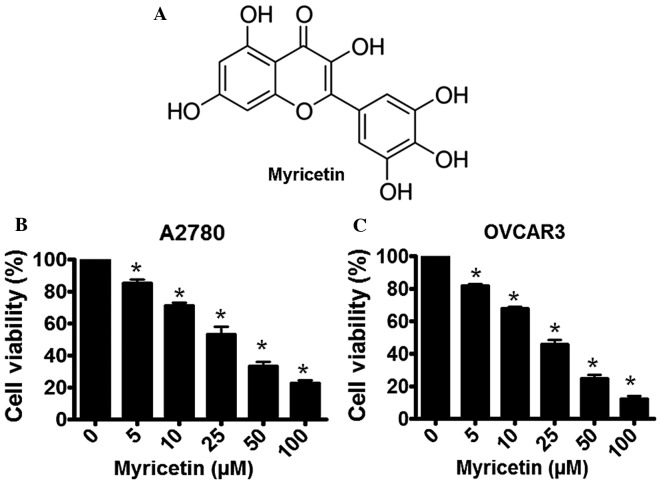
Flavonoids are naturally occurring phytochemicals that are abundant in the majority of fresh fruits, grains, green vegetables and certain traditional medicinal herbs (9,10). Almost all chemically synthesized drugs currently used for cancer therapy have a significant toxicity to normal cells (11); however, various naturally-occurring flavonoids have demonstrated selective cytotoxicity in types of human cancer cells, accompanied by minimal toxicity to normal cells (10). Myricetin (3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-4-chromenone; Fig. 1A) is a dietary flavonoid present in fresh fruits (such as grapes, oranges, berries), vegetables (including sweet potatoes, parsley, broad beans), herbs, tea and wine (12,13). The physiological availability of myricetin in certain natural products is significant, such as 24 mg/kg in grapes, 0.2–0.5 g/kg physical abundance in average food and 0.8–1.6 g/kg in green tea (12).
Figure 1.
Myricetin induces cytotoxicity in human ovarian cancer cells, as determined by an MTT assay. (A) The chemical structure of myricetin. (B) The effect of various concentrations of myricetin (0–100 µM) on A2780 cells. (C) The effect of various concentrations of myricetin (0–100 µM) on OVCAR3 cells. The results are presented as the mean ± standard deviation of three independent experiments (n=3). *P<0.05, control vs. myricetin-treated cells.
The therapeutic potential of myricetin has previously been established and it is considered to have various pathophysiological properties, including antioxidant, cytoprotective, antiviral, antimicrobial, antiplatelet and anticancer properties (12,14). Myricetin has also been indicated to be significantly effective in the treatment of various types of cancers, including prostate cancer, hepatocellular carcinoma, gastric cancer and human squamous cell carcinoma (12,15–18). Myricetin may also increase the chemotherapeutic potential of certain anticancer drugs by sensitizing the target cancer cells to chemotherapy (19,20). As the currently available conventional chemotherapeutic agents have not been observed to improve the overall survival rate of patients with ovarian cancer, novel drug regimens are required in order to combat the disease. In the present study, the anticancer effects of myricetin were evaluated in the A2780 and OVCAR3 ovarian cancer cell lines. It was also investigated whether myricetin is able to sensitize these ovarian cancer cells to the frequently used chemotherapeutic drugs paclitaxel and cisplatin.
Materials and methods
Cell culture and maintenance
The A2780 and OVCAR3 human ovarian cancer cells were purchased from ATCC (Manassas, VA, USA) and cultured in RPMI-1640 medium (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) supplemented with 10% fetal bovine serum (Sigma-Aldrich; Merck KGaA), penicillin (100 U/ml; Sigma-Aldrich; Merck KGaA) and streptomycin (100 µg/ml; Sigma-Aldrich; Merck KGaA), at 37°C in a humidified chamber.
Cell viability assay
Cell viability was determined spectrophotometrically using an MTT assay. A2780 and OVCAR3 cells were seeded into 96-well plates at a density of 1×104 cells/ml. The cells were cultured to ~70% confluency and exposed to various concentrations of myricetin (0, 5, 10, 25, 50 and 100 µM; Sigma-Aldrich; Merck KGaA) for 48 h, at 37°C, under the described cell culture conditions. Following treatment, MTT (Sigma-Alrich; Merck KGaA) solution was added to each well at a concentration of 2 mg/ml and incubated at 37°C for 2 h. The formazan that formed was dissolved using DMSO and the absorbance was measured at 570 nm using a microplate reader and Multiskan EX software, v3.4 (Multiskan EX, Lab systems, Helsinki, Finland).
Apoptosis assay
Role of apoptosis in myricetin-induced cytotoxicity in A2780 and OVCAR3 cells was determined using a single stranded-DNA Apoptosis ELISA kit (EMD Millipore, Billerica, MA, USA) (21). Cultured A2780 and OVCAR3 cells (1×104 cells/ml) were treated with 25 µM myricetin for 48 h at 37°C, and induction of apoptosis in untreated (control) and myricetin-treated cells were determined according to the manufacturer's protocol. The extent of apoptosis was determined from the absorbance measured at 405 nm using a microplate reader and Multiskan EX software, v3.4 (Multiskan EX, Lab systems).
Evaluation of cell migration using a Boyden chamber assay
The migratory properties of A2780 and OVCAR3 cells in the presence and absence of myricetin were determined using a Boyden chamber assay with track-etched polyethylene terephthalate membranes and an 8.0-µm diameter pore size (Corning Incorporated, Corning, NY, USA). A2780 and OVCAR3 cells (1×104 cells/ml) were treated with various concentrations of myricetin (0, 10 and 25 µM) for 48 h under the described cell culture conditions and the migration assay was performed according to the manufacturer's protocol.
Western blot analysis
Cultured (at 37°C) A2780 and OVCAR3 cells were treated with 25 µM of myricetin. Total protein from the cell extracts was isolated in ice-cold lysis buffer consisting of 150 mM NaCl, 20 mM Tris-HCl, 1% NP-40, 20 µg/ml leupeptin, 20 µg/ml aprotinin, 1 mM ortho-vanadate and 2 mM PMSF, pH 7.4. After treatment, cells were incubated in the lysis buffer at 4°C for 30 min and the cell suspension was subsequently centrifuged at 2,000 × g for 15 min at 4°C. Protein concentration was estimated using a Bradford reagent (Sigma-Alrich; Merck KGaA). Protein from each cell sample (~50 µg) was loaded into each well and separated by 10% SDS-PAGE, and then transferred to a polyvinylidene difluoride membrane (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The membrane was blocked in Super Block blocking buffer (Thermo Fisher Scientific, Inc., Waltham, MA, USA) for 1 h at room temperature and subsequently incubated with various primary antibodies, including rabbit polyclonal anti-Bax (dilution, 1:500; N20, #sc-493; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), rabbit polyclonal anti-Bcl-2 (dilution, 1:500; N-19, sc-7382; Santa Cruz Biotechnology, Inc.), rabbit monoclonal anti-cleaved caspase-3 (Asp175) antibody (dilution, 1:1,000; #9661; Cell Signaling Technology, Inc., Danvers, MA, USA), mouse monoclonal anti-Mdr-1 antibody (dilution, 1:500; G-1; sc-13131, Santa Cruz Biotechnology, Inc.). After blocking membranes were washed three times in 0.25% TBST buffer for 10 min and incubated with secondary antibodies, including goat anti-mouse immunoglobulin G (IgG)-horseradish peroxidase (HRP; #sc-2005; Santa Cruz Biotechnology, Inc.) and mouse anti-rabbit IgG-HRP (#sc-2357; Santa Cruz Biotechnology, Inc.) for 30 min at room temperature. The secondary antibodies were used at a dilution of 1:10,000. Images of the membranes were captured using the Super Signal ULTRA Chemiluminescent Substrate (Pierce; Thermo Fisher Scientific, Inc.) using a KODAK Image Station 4000 (Kodak, Rochester, NY, USA).
Statistical analysis
The data were analyzed using GraphPad Prism version 4.0 (GraphPad Software, Inc., La Jolla, CA, USA). All data are presented as the mean ± standard deviation. Statistically significant differences between the groups were determined using a paired Student's two-tailed t-test. P<0.05 was considered to indicate a statistically significant result.
Results
Myricetin induces cytotoxicity in ovarian cancer cells
Treatment of the A2780 and OVCAR3 human ovarian cancer cells with various concentration of myricetin resulted in the induction of cytotoxicity. Following a 48-h incubation, a gradual loss of cell viability was observed in myricetin-treated A2780 cells, with the IC50 concentration determined to be ~25 µM (Fig. 1B). A similar inhibition of cellular growth was observed in myricetin-treated OVCAR3 cells following a 48-h incubation period, with the IC50 concentration calculated as ~25 µM (Fig. 1C).
Myricetin induces apoptosis in ovarian cancer cells
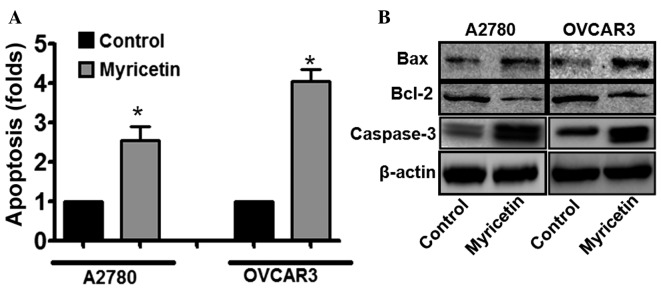
To elucidate the mechanisms underlying myricetin-induced cytotoxicity in ovarian cancer cells, the involvement of apoptosis in myricetin-treated cells was investigated. The induction of apoptosis was evaluated using the single-stranded DNA Apoptosis ELISA kit. Myricetin treatment was observed to induce apoptosis in A2780 and OVCAR3 cells. In A2780 cells treated with 25 µM myricetin, an ~2.5-fold increase in the apoptotic signal was observed, compared with untreated cells, whereas under similar treatment conditions, the apoptotic signal was increased by ~4-fold in OVCAR3 cells compared with untreated cells (*P<0.05, control vs. myricetin-treated cells; Fig. 2A). The results suggest that apoptosis is involved in myricetin-induced cytotoxicity in certain ovarian cancer cells.
Figure 2.
Myricetin induces apoptosis in human ovarian cancer cells. (A) Cultured A2780 and OVCAR3 cells were pre-treated with myricetin and degree of apoptosis was determined using an apoptosis detection kit. The results are presented as the mean ± standard deviation of three independent experiments (n=3). *P<0.05, control vs. myricetin-treated cells. (B) Western blot analyses representing the relative expression levels of BAX, Bcl-2 and cleaved caspase-3 in the control cells and myricetin-treated A2780 and OVCAR3 cells. The results are presented as the mean ± standard deviation of three independent experiments. Bcl-2, B-cell lymphoma-2; BAX, Bcl-2-associated X-protein.
Furthermore, it was also revealed that the expression of the pro-apoptotic protein B-cell lymphoma-2 (Bcl-2)-associated X-protein (BAX) was significantly upregulated, and the expression of the anti-apoptotic protein Bcl-2 was downregulated, in myricetin-treated ovarian cancer cells compared with untreated cells (*P<0.05, control vs. myricetin-treated cells; Fig. 2B). Cleaved caspase-3 is central to the intrinsic apoptotic signaling pathway, and was also revealed to be upregulated in myricetin-treated cells compared with untreated cells (*P<0.05, control vs. myricetin-treated cells). Therefore, the results suggested that apoptosis has an important role in myricetin-induced cell death.
Myricetin inhibits the migratory properties of ovarian cancer cells
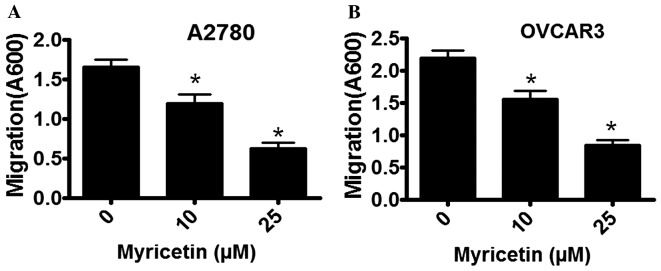
The migratory properties of A2780 and OVCAR3 cells were determined in the absence and presence of myricetin using a Boyden Chamber assay. Cell migration was observed to be markedly inhibited by myricetin, in a dose-dependent manner. In A2780 cells that were treated with 10 µM myricetin the extent of migration was inhibited by ~18%, whereas in the presence of 25 µM myricetin migratory activity was inhibited by ~60% compared with untreated cells (*P<0.05, control vs. myricetin-treated cells; Fig. 3A). A similar inhibitory pattern was observed in myricetin-treated OVCAR3 cells. In the presence of 10 µM myricetin, OVCAR3 cell migration was inhibited by ~28%, and in the presence of 25 µM myricetin it was inhibited by ~65% compared with untreated cells (*P<0.05, control vs. myricetin-treated cells).
Figure 3.
Myricetin inhibits the migratory capacity of ovarian cancer cells, as determined using a Boyden Chamber assay. The extent of migration was determined by staining the Boyden Chambers with crystal violet and measuring the absorbance at a wavelength of 600 nm. (A) The effect of myricetin on the migration of A2780 cells. (B) The effect of myricetin on OVCAR3 cells. The results are presented as the mean ± standard deviation of three independent experiments. *P<0.05, control vs. myricetin-treated cells.
Myricetin enhances the chemotherapeutic potential of paclitaxel in ovarian cancer cells by targeting MDR-1
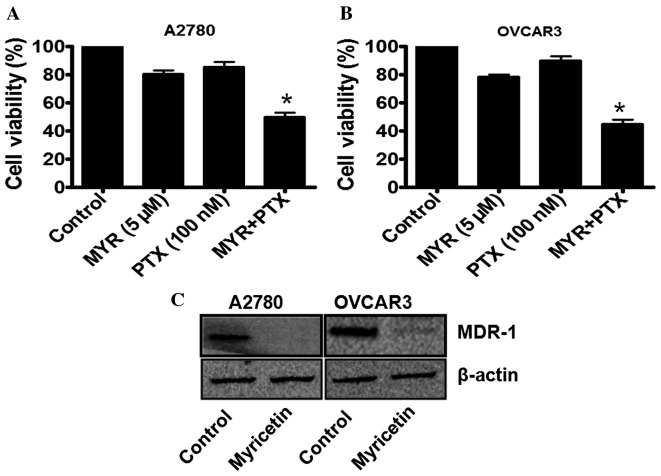
In order to investigate whether myricetin is able to enhance the chemotherapeutic potential of paclitaxel (PTX), the ovarian cancer cells were treated with a sub-lethal concentration of myricetin (5 µM) for 48 h, and then incubated with a sub-lethal concentration of paclitaxel (100 nM). As previously demonstrated (Fig. 1), 5 µM myricetin induces <25% inhibition of cell viability in A2780 and OVCAR3 cells. By contrast, 100 nM paclitaxel was not observed to induce significant cytotoxicity in the two cell types. However, when the myricetin-treated cells were further incubated with 100 nM paclitaxel, a marked reduction in cell viability was observed (Fig. 4A and B). The combination therapy of myricetin and paclitaxel was, therefore, effective in these cell lines, causing an ~50% loss of cell viability (Fig. 4A and B).
Figure 4.
Myricetin enhances the efficacy of paclitaxel in ovarian cancer cells by targeting MDR-1. (A and B) Cultured A2780 and OVCAR3 cells were treated with 5 µM myricetin for 48 h, and then treated with 100 nM paclitaxel for a further 48 h. Cell viability was assessed using an MTT assay. (C) The effect of myricetin on MDR-1 expression in A2780 and OVCAR3 cells. MYR, myricetin; PTX, paclitaxel; MDR-1, multidrug resistance protein 1.
As MDR-1 expression in ovarian cancer cells has been indicated to be associated with paclitaxel-resistance, the status of MDR-1 in the myricetin-treated cells was further investigated using western blot analysis. It was observed that, in the myricetin-treated A2780 and OVCAR3 cells, MDR-1 expression was significantly downregulated compared with untreated cells (*P<0.05, control vs. myricetin-treated cells; Fig. 4C), which may be associated with the enhancement of paclitaxel efficacy in certain ovarian cancer cells.
Discussion
Amongst all the gynecological malignancies, ovarian cancer is responsible for the majority of these types of cancer-associated mortalities globally, often due to a late-stage diagnosis and a poor prognosis (1–8). The currently available conventional therapies for ovarian cancer include surgery, or combined chemotherapy with cisplatin, paclitaxel and various other drugs (5–7). Although there has been an improvement in the overall survival rate in recent years the overall 5-year survival-rate is <40% (7), and the majority of patients with ovarian cancer suffer from a recurrent, progressive disease due to the acquisition of a resistance phenotype towards the conventional chemotherapeutic agents (1,6,7). Due to the heterogeneous nature of ovarian tumors (1), the development of defined and novel therapeutic regimens for this disease has been a challenge. Previous clinical studies have demonstrated that numerous chemotherapy drugs, including 5-fluorouracil, cyclophosphamide, dactinomycin and vincristine, have failed to inhibit tumor growth and progression in patients with ovarian cancer (22,23). However, members of the taxane group of drugs, including paclitaxel, which acts as a mitotic poison and targets the cellular microtubule network, administered as monotherapy or in combination with cisplatin have emerged as a potentially effective therapeutic regimen against ovarian cancer (23,24).
Previous studies have indicated that MDR1/P-glycoprotein has an important role in the exclusion of drugs from tumor cells (24,25). MDR-1 is a 170-kDa membrane-localized phospho-glycoprotein encoded by the MDR1 gene, which acts as an ATP-dependent efflux pump and is associated with the decreased accumulation of chemotherapy drugs within cancer cells (26,27). Numerous studies have revealed that the overexpression of MDR-1 in aggressive ovarian cancer cells is associated with the development of resistance to paclitaxel treatment (28,29). Therefore, it is essential to screen novel drug candidates that may target MDR-1 in ovarian cancer cells, and also to enhance the chemotherapeutic potential of currently available anticancer drugs.
In the current study, it was demonstrated that the naturally occurring dietary flavonoid myricetin efficiently inhibits the proliferation of A2780 and OVCAR3 ovarian cancer cells, with the simultaneous induction of apoptosis. It was also observed that myricetin is able to inhibit the migratory capacity of ovarian cancer cells. Following an evaluation of the cytotoxic effect of myricetin in ovarian cancer cells, it was investigated whether myricetin enhances the sensitivity of ovarian cancer cells to paclitaxel treatment. It was observed that paclitaxel activity was significantly increased in the cells that were pre-treated with a sub-lethal concentration of myricetin. The results suggest that myricetin is able to enhance the chemotherapeutic potential of paclitaxel, in addition to increasing the efficacy of paclitaxel at lower concentrations. In order to investigate the underlying mechanisms by which myricetin increases the cytotoxic potential of paclitaxel, the status of MDR-1 in myricetin-treated cells was determined. Treatment of A2780 and OVCAR3 cells with myricetin induced a marked downregulation of MDR-1, which may explain the enhancement of paclitaxel efficacy in ovarian cancer cells. In conclusion, the results suggest that that myricetin monotherapy may be a potentially effective therapeutic agent for the treatment of patients with ovarian cancer.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Vecchione A, Belletti B, Lovat F, Volinia S, Chiappetta G, Giglio S, Sonego M, Cirombella R, Onesti EC, Pellegrini P, et al. A microRNA signature defines chemoresistance in ovarian cancer through modulation of angiogenesis; Proc Natl Acad Sci USA; 2013; pp. 9845–9850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jayson GC, Kohn EC, Kitchener HC, Ledermann JA. Ovarian cancer. Lancet. 2014;384:1376–1388. doi: 10.1016/S0140-6736(13)62146-7. [DOI] [PubMed] [Google Scholar]
- 4.Nam MS, Jung DB, Seo KH, Kim BI, Kim JH, Kim JH, Kim B, Baek NI, Kim SH. Apoptotic effect of sanggenol L via caspase activation and inhibition of NF-kB signaling in ovarian cancer cells. Phytother Res. 2016;30:90–96. doi: 10.1002/ptr.5505. [DOI] [PubMed] [Google Scholar]
- 5.Ivanov S, Ivanov S, Khadzhiolov N. Ovarian tumours-accuracy of frozen section diagnosis. Akush Ginekol (Sofiia) 2005;44:11–13. (In Bulgarian) [PubMed] [Google Scholar]
- 6.Saika K, Sobue T. Cancer statistics in the world. Gan To Kagaku Ryoho. 2013;40:2475–2480. (In Japanese) [PubMed] [Google Scholar]
- 7.Edwards SJ, Barton S, Thurgar E, Trevor N. Topotecan, pegylated liposomal doxorubicin hydrochloride, paclitaxel, trabectedin and gemcitabine for advanced recurrent or refractory ovarian cancer: A systematic review and economic evaluation. Health Technol Assess. 2015;19:1–480. doi: 10.3310/hta19070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bookman MA, Brady MF, McGuire WP, Harper PG, Alberts DS, Friedlander M, Colombo N, Fowler JM, Argenta PA, de Geest K, et al. Evaluation of new platinum-based treatment regimens in advanced-stage ovarian cancer: A Phase III Trial of the gynecologic cancer intergroup. J Clin Oncol. 2009;27:1419–1425. doi: 10.1200/JCO.2008.19.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sak K. Site-specific anticancer effects of dietary flavonoid quercetin. Nutr Cancer. 2014;66:177–193. doi: 10.1080/01635581.2014.864418. [DOI] [PubMed] [Google Scholar]
- 10.Sak K. Cytotoxicity of dietary flavonoids on different human cancer types. Pharmacogn Rev. 2014;8:122–146. doi: 10.4103/0973-7847.134247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta S, Afaq F, Mukhtar H. Selective growth-inhibitory, cell-cycle deregulatory and apoptotic response of apigenin in normal versus human prostate carcinoma cells. Biochem Biophys Res Commun. 2001;287:914–920. doi: 10.1006/bbrc.2001.5672. [DOI] [PubMed] [Google Scholar]
- 12.Devi KP, Rajavel T, Habtemariam S, Nabavi SF, Nabavi SM. Molecular mechanisms underlying anticancer effects of myricetin. Life Sci. 2015;142:19–25. doi: 10.1016/j.lfs.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Harnly JM, Doherty RF, Beecher GR, Holden JM, Haytowitz DB, Bhagwat S, Gebhardt S. Flavonoid content of U.S. fruits, vegetables, and nuts. J Agric Food Chem. 2006;54:9966–9977. doi: 10.1021/jf061478a. [DOI] [PubMed] [Google Scholar]
- 14.Ong CK, Nee S, Rambaut A, Bernard HU, Harvey PH. Elucidating the population histories and transmission dynamics of papillomaviruses using phylogenetic trees. J Mol Evol. 1997;44:199–206. doi: 10.1007/PL00006136. [DOI] [PubMed] [Google Scholar]
- 15.Boam T. Anti-androgenic effects of flavonols in prostate cancer. Ecancermedicalscience. 2015;9:585. doi: 10.3332/ecancer.2015.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng J, Chen X, Wang Y, Du Y, Sun Q, Zang W, Zhao G. Myricetin inhibits proliferation and induces apoptosis and cell cycle arrest in gastric cancer cells. Mol Cell Biochem. 2015;408:163–170. doi: 10.1007/s11010-015-2492-1. [DOI] [PubMed] [Google Scholar]
- 17.Iyer SC, Gopal A, Halagowder D. Myricetin induces apoptosis by inhibiting P21 activated kinase 1 (PAK1) signaling cascade in hepatocellular carcinoma. Mol Cell Biochem. 2015;407:223–237. doi: 10.1007/s11010-015-2471-6. [DOI] [PubMed] [Google Scholar]
- 18.Maggioni D, Nicolini G, Rigolio R, Biffi L, Pignataro L, Gaini R, Garavello W. Myricetin and naringenin inhibit human squamous cell carcinoma proliferation and migration in vitro. Nutr Cancer. 2014;66:1257–1267. doi: 10.1080/01635581.2014.951732. [DOI] [PubMed] [Google Scholar]
- 19.Huang H, Chen AY, Ye X, Li B, Rojanasakul Y, Rankin GO, Chen YC. Myricetin inhibits proliferation of cisplatin-resistant cancer cells through a p53-dependent apoptotic pathway. Int J Oncol. 2015;47:1494–1502. doi: 10.3892/ijo.2015.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yi JL, Shi S, Shen YL, Wang L, Chen HY, Zhu J, Ding Y. Myricetin and methyl eugenol combination enhances the anticancer activity, cell cycle arrest and apoptosis induction of cis-platin against HeLa cervical cancer cell lines. Int J Clin Exp Pathol. 2015;8:1116–1127. [PMC free article] [PubMed] [Google Scholar]
- 21.Balint K, Xiao M, Pinnix CC, Soma A, Veres I, Juhasz I, Brown EJ, Capobianco AJ, Herlyn M, Liu ZJ. Activation of Notch1 signaling is required for beta-catenin-mediated human primary melanoma progression. J Clin Invest. 2005;115:3166–3176. doi: 10.1172/JCI25001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tavassoli FA, Norris HJ. Sertoli tumors of the ovary. A clinicopathologic study of 28 cases with ultrastructural observations. Cancer. 1980;46:2281–2297. doi: 10.1002/1097-0142(19801115)46:10<2281::AID-CNCR2820461028>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 23.Burton ER, Brady M, Homesley HD, Rose PG, Nakamura T, Kesterson JP, Rotmensch J, Thigpen J Tate, Van Le L. A phase II study of paclitaxel for the treatment of ovarian stromal tumors: An NRG oncology/gynecologic oncology group study. Gynecol Oncol. 2016;140:48–52. doi: 10.1016/j.ygyno.2015.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, Clarke-Pearson DL, Davidson M. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med. 1996;334:1–6. doi: 10.1056/NEJM199601043340101. [DOI] [PubMed] [Google Scholar]
- 25.Szakács G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5:219–234. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- 26.Alvarez M, Paull K, Monks A, Hose C, Lee JS, Weinstein J, Grever M, Bates S, Fojo T. Generation of a drug resistance profile by quantitation of mdr-1/P-glycoprotein in the cell lines of the national cancer institute anticancer drug screen. J Clin Invest. 1995;95:2205–2214. doi: 10.1172/JCI117910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen H, Hao J, Wang L, Li Y. Coexpression of invasive markers (uPA, CD44) and multiple drug-resistance proteins (MDR1, MRP2) is correlated with epithelial ovarian cancer progression. Br J Cancer. 2009;101:432–440. doi: 10.1038/sj.bjc.6605185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang B, Li S, Meng X, Shang H, Guan Y. Inhibition of mdr1 by G-quadruplex oligonucleotides and reversal of paclitaxel resistance in human ovarian cancer cells. Tumour Biol. 2015;36:6433–6443. doi: 10.1007/s13277-015-3333-2. [DOI] [PubMed] [Google Scholar]
- 29.Zhang H, Wang J, Cai K, Jiang L, Zhou D, Yang C, Chen J, Chen D, Dou J. Downregulation of gene MDR1 by shRNA to reverse multidrug-resistance of ovarian cancer A2780 cells. J Cancer Res Ther. 2012;8:226–231. doi: 10.4103/0973-1482.98975. [DOI] [PubMed] [Google Scholar]