Abstract
Epstein-Barr virus-associated gastric carcinoma (EBVaGC) is a distinct subtype of gastric cancer characterized by clinicopathological features including lymphoepithelioma-like histology. Aberrant expression of activation-induced cytidine deaminase (AID) as a genomic modulator was demonstrated through pathogen-related nuclear factor κB (NF-κB) signaling in Helicobacter pylori-associated gastric cancer. To elucidate whether or not AID expression is relevant to carcinogenesis in EBVaGC, immunohistochemical expression of AID and AID-regulatory factors between EBVaGC and EBV-non-associated gastric carcinoma (GC) were evaluated, each using 15 cases of GC with lymphoid stroma (GCLS) and other types of GC. Aberrant expression of AID, NF-κB and paired box 5 (PAX5) were significantly decreased in EBVaGC (0/11, 1/11 and 1/11) compared with in EBV-non-associated GC (7/19, 12/19 and 11/19) (P=0.025, 0.005 and 0.01, respectively); however, no significant difference in c-Myb proto-oncogene expression was identified. AID expression was also decreased in EBV-associated GCLS (0/10) compared with in EBV-non-associated GCLS (3/5). Unexpectedly, decreased expression of NF-κB and PAX5 was observed in GCLS (1/15 and 2/15) compared with in GC without LS (12/15 and 10/15) (P<0.001 and P=0.003, respectively). Decreased AID expression observed in EBVaGC is consistent with the reported molecular characterization of hypermethylation and rare somatic gene mutation in EBVaGC. Only PAX5 was identified to be significantly associated with venous invasion (P=0.022). The results of the present study suggest that pathogen-induced AID expression may be irrelevant to carcinogenesis of EBVaGC, whereas it contributes to carcinogenesis in certain types of EBV-non-associated GC.
Keywords: activation-induced cytidine deaminase, Epstein-Barr virus-associated gastric cancer, nuclear factor κB, paired box 5, c-Myb proto-oncogene
Introduction
Epstein-Barr virus (EBV), also known as human herpes virus 4, is one of the most common human viruses. The majority of individuals are infected during infancy or childhood, such that the majority of adults have been infected and have established lifelong latent infections (1). EBV was the first human oncovirus to be described, which was identified from a Burkitt's lymphoma cell line in 1964 (2). Subsequent studies revealed that EBV caused infectious mononucleosis and many different human malignancies including nasopharyngeal carcinoma, Hodgkin's lymphoma, extranodal natural killer/T-cell lymphoma, lymphoproliferative disorders in immunocompromised hosts and a number of types of gastric cancer (1).
EBV-associated gastric carcinoma (EBVaGC or EBV(+) GC) is a distinct subtype of gastric cancer that accounts for <10% of GC (gastric carcinoma). EBVaGC is defined by the monoclonal proliferation of carcinoma cells with latent EBV infection, as demonstrated using EBV-encoded small RNA (EBER) in situ hybridization (1). EBVaGC has characteristic clinicopathological features including a lymphoepithelioma-like histology and favorable prognosis (3). It has been histologically subdivided into two types: A lymphoepithelioma-like carcinoma (LELC) type and conventional type adenocarcinoma. The LELC type is also referred to as ‘GC with lymphoid stroma (GCLS)’. In total, >80% of GCLS cases have been identified to exhibit EBV(+) tumors (3,4).
A previous study highlighted the important role of activation-induced cytidine deaminase (AID), a nucleotide-editing enzyme that is essential for the somatic hypermutation and class-switch recombination of the immunoglobulin gene, as a genomic modulator in inflammation-associated cancer development in digestive organs including Helicobacter pylori-associated gastric cancer, hepatitis C virus-positive hepatocellular carcinoma and colitis-associated colon cancers (5). Consistent with the finding that a number of transcription factors, including nuclear factor-κB (NF-κB), mediate the expression of AID in B cells, the proinflammatory cytokine stimulation of numerous types of gastrointestinal epithelial cells, including gastric, colonic, hepatic and biliary epithelia, induced aberrant AID expression through the NF-κB signaling pathway (6). NF-κB is a key molecule in inflammation-associated carcinogenesis, and NF-κB activation in epithelial cells and malignant cells is involved in generating genomic instability through aberrant AID expression, cell growth, proliferation, survival, angiogenesis and epithelial-mesenchymal transition (7).
The expression and activity of AID are tightly regulated at the levels of transcription, post-transcription and enzymatic function. Four distinct DNA regions (regions I to IV) of the AID gene locus contain binding sites for multiple transcription factors. Region I functions as a promoter containing the binding site for NF-κB, a transcriptional activator. In B and non-B cells, enhancer elements in region II bind to the activators paired box 5 (PAX5) and transcription factor E2A, whereas silencer elements in region II bind to the repressor proteins c-Myb proto-oncogene (c-Myb) and transcription factor E2F in order to counter the activities of transcriptional activators (8).
Honjo et al (9) described pathogen-induced AID expression in gastric cancer (caused by H. pylori), adult T cell leukemia/lymphoma (caused by human T lymphotrophic virus 1), hepatoma (caused by hepatitis C virus) and Burkitt's lymphoma (caused by EBV), but not in classical Hodgkin's lymphoma (caused by EBV) (9). However, to the best of our knowledge, aberrant AID expression in EBVaGC has not yet been elucidated. The majority of EBVaGCs exhibit marked lymphoid stroma surrounding cancer cells, which suggests pathogen-induced chronic inflammation. Chronic inflammation, regardless of the infectious agent involved, serves important roles in the development of various types of cancer, particularly in digestive organs (5). Therefore, the aim of the present study was to determine whether the inflammation-associated cancer development theory through aberrant AID expression via a pathogen-related NF-κB signal also applies to EBVaGC and GCLS.
In order to elucidate the question of whether or not EBV-induced AID expression is a causative factor of carcinogenesis in EBVaGC, the aberrant expression of AID and AID-regulatory factors including NF-κB (activator), PAX5 (activator) and c-Myb (repressor) were immunohistochemically evaluated and compared between EBVaGC and EBV-non-associated GC, and also between GCLS and GC without LS.
Materials and methods
Tumor samples
Formalin-fixed paraffin-embedded (FFPE) tumor samples were obtained from 30 patients who had undergone surgical resection at Tottori University Hospital between January 2005 and December 2014, and who were histopathologically diagnosed with GCLS (n=15; 14 males and 1 female) or age-sex-stage-matched other gastric cancers (GC without LS) (n=15; 14 males and 1 female). The mean age ± standard deviation (SD) was 64.7±9.4 years (range, 45–77 years). The tumor location was defined as the upper third, middle third and lower third of the tissue or remnant. All samples were histopathologically classified according to the Japanese Classification of Gastric Carcinoma (10). The present study was approved by the Institutional Ethics Committee of Tottori University (Yonago, Japan; no. 2483).
Evaluation of surrounding mucosal inflammation
The grading of gastritis in the non-cancerous mucosa adjacent to a tumor was evaluated and scored according to the updated Sydney System (USS) including H. pylori infection (11). Gimenez staining was used to detect H. pylori.
Immunohistochemical staining of AID, NF-κB, c-Myb, PAX5 and latent membrane protein 1 (LMP-1)
Immuno-histochemical staining was performed using the following primary antibodies: Anti-AID (#39-2500; dilution, 1:200; Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA), NF-κB (#ab86299, polyclonal; dilution, 1:100; Abcam, Cambridge, MA, USA), PAX5 (#M7307; dilution, 1:30; Dako; Agilent Technologies, Inc., Santa Clara, CA, USA), c-Myb (#LS-C49755; dilution, 1:150; LifeSpan BioSciences, Inc., Seattle, WA, USA) and LMP-1 (#M0897; dilution, 1:100; Dako; Agilent Technologies, Inc.). Sections were cut to a thickness of 4 µm, deparaffinized in xylene and dehydrated in descending dilutions of ethanol. Antigen retrieval was performed using a pressure cooker at 120°C with citrate buffer (pH 6.0) for 10 min (AID and c-Myb) or with Target Retrieval Solution (pH 9.0; Dako; Agilent Technologies, Inc.) for 20 min (NF-κB, PAX5 and LMP-1). Endogenous peroxidase activity was blocked by a 5-min incubation at room temperature with 3% hydrogen peroxidase. Sections were incubated with primary antibodies and antibody diluents with background-reducing components (Dako; Agilent Technologies, Inc.) at room temperature for 120 min (AID, NF-κB and PAX5) or at 4°C overnight (c-Myb and LMP-1). Horseradish peroxidase (HRP) -conjugated goat anti-mouse IgG (EnVision™+/HRP; #K4000; prediluted; AID, NF-κB, PAX5 and LMP-1) or goat anti-rabbit IgG (EnVision™+/HRP; #K4002; prediluted, c-Myb) was applied as the secondary antibody (both from Dako; Agilent Technologies, Inc.). Sections were incubated with secondary antibody at room temperature for 30 min prior to washing in PBS. Diaminobenzidine was used as the chromogen. Sections were counterstained with Mayer's hematoxylin and eosin.
Immunostaining densities were assessed by pathologists using the ECLIPSE 80i light microscope and NIS Elements D 3.2 imaging software (both from Nikon Corporation, Tokyo, Japan) and the H-score method, as described previously (12): H-score = (% tumor cells unstained × 0) + (% tumor cells weakly stained × 1) + (% tumor cells moderately stained × 2) + (% tumor cells strongly stained × 3). H-scores ranged between 0 (100% negative tumor cells) and 300 (100% strongly positive tumor cells).
For AID, lymphocytes from germinal centers in lymphoid follicles were used as internal positive controls because they were primarily activated B cells and were strongly stained throughout the specimens. H-scores were calculated for AID, NF-κB, PAX5 and c-Myb for each sample. The following threshold values were adopted: 105 for AID, 125 for NF-κB, 70 for PAX5 and 100 for c-Myb.
In situ hybridization (ISH) of EBER1
ISH was performed using a fluorescein isothiocyanate-labeled probe complementary to an EBER1 sequence as described previously (13). Sections of FFPE tissue (thickness, 4 µm) were used for ISH and were imaged using a light microscope.
Statistical analysis
Statistical analyses were performed using Fisher's exact test, χ2 test, Mann-Whitney U test and Kaplan-Meier survival estimates using SPSS software (version 23.0.0.0; IBM SPSS, Armonk, NY, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Clinicopathological findings
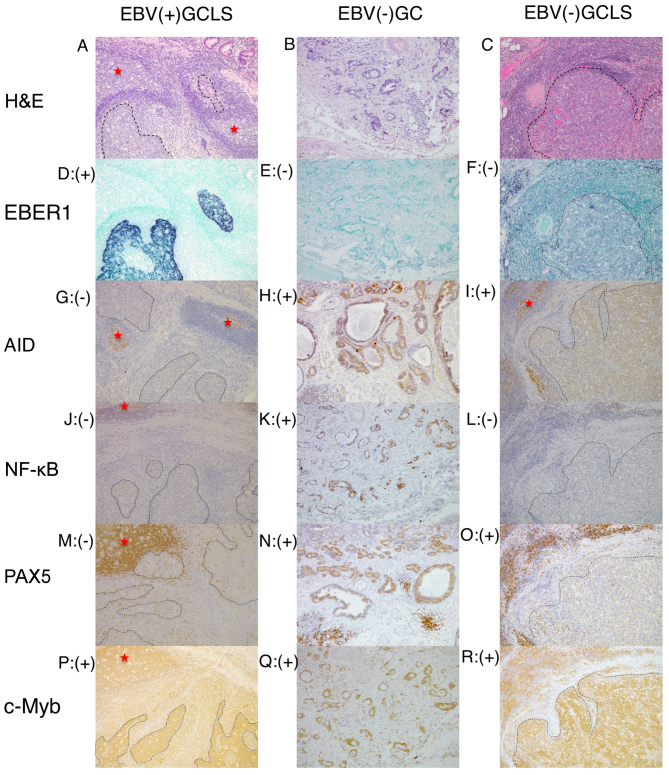
Representative histology, ISH and immunostaining images of EBV(+) GCLS, EBV(−) GC without LS and EBV(−) GCLS are presented in Fig. 1. Clinicopathological data are presented in Tables I–III.
Figure 1.
Representative histology, ISH and immunostaining images of EBV(+) GCLS, EBV(−) GC without LS and EBV(−) GCLS. H&E staining of (A) EBV(+) GCLS, (B) EBV(−) GC without LS and (C) EBV(−) GCLS. EBER1 ISH of (D) EBV(+) GCLS, (E) EBV(−) GC without LS and (F) EBV(−) GCLS. AID immunostaining of (G) EBV(+) GCLS, (H) EBV(−) GC without LS and (I) EBV(−) GCLS. NF-κB immunostaining of (J) EBV(+) GCLS, (K) EBV(−) GC without LS and (L) EBV(−) GCLS. PAX5 immunostaining of (M) EBV(+) GCLS, (N) EBV(−) GC without LS and (O) EBV(−) GCLS. c-Myb immunostaining of (P) EBV(+) GCLS, (Q) EBV(−) GC without LS and (R) EBV(−) GCLS. Magnification, ×100. (G, I) Red stars indicate AID expression as the positive internal control observed in the lymphoid cells of the germinal center. ISH, in situ hybridization; EBV, Epstein-Barr virus; GCLS, GC with LS; GC, gastric carcinoma; LS, lymphoid stroma; H&E, hematoxylin and eosin; EBER1, EBV-encoded small RNA 1; AID, activation-induced cytidine deaminase; NF-κB, nuclear factor κB; PAX5, paired box 5; c-Myb, c-Myb proto-oncogene.
Table I.
Comparative analysis of clinical and histopathological characteristics between EBV(+) and EBV(−) GC.
| Case | Sex | Age | T | ly | v | N | M | Stage | Histo-pathological classification | Location | State | Survival time, days | EBER1 | EBV-LMP-1 | AID H-score | NF-κB H-score | PAX5 H-score | c-Myb H-score | Mean USSa (Helico-bacter pylori) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EBV(+) GC | |||||||||||||||||||
| 1 | Male | 48 | 1b | 1 | 0 | 0 | 0 | IA | GCLS | UT | NED | 3,469 | + | − | 20 | 190 | 0 | 70 | 1.6 (−) |
| 2 | Male | 68 | 3 | 1 | 1 | 0 | 0 | IIA | GCLS | UT | NED | 1,350 | + | − | 100 | 19 | 0 | 80 | 1.8 (+) |
| 3 | Male | 52 | 2 | 1 | 3 | 0 | 0 | IB | GCLS | MT | NED | 1,525 | + | − | 70 | 70 | 0 | 10 | 2.2 (+) |
| 4 | Male | 53 | 3 | 1 | 3 | 1 | 0 | IIB | GCLS | MT | NED | 1,839 | + | − | 3 | 15 | 65 | 190 | 2.2 (+) |
| 5 | Male | 74 | 1b | 1 | 0 | 1 | 0 | IB | GCLS | UT | NED | 1,512 | + | − | 5 | 15 | 0 | 40 | 1.8 (+) |
| 6 | Male | 63 | 1b | 0 | 0 | 0 | 0 | IA | GCLS | UT | NED | 845 | + | − | 80 | 80 | 0 | 70 | 2.4 (+) |
| 7 | Male | 77 | 1b | 0 | 0 | 0 | 0 | IA | GCLS | MT | NED | 700 | + | − | 90 | 90 | 35 | 225 | 2.0 (−) |
| 8 | Male | 54 | 1b | 2 | 1 | 0 | 0 | IA | GCLS | MT | NED | 675 | + | − | 30 | 75 | 60 | 110 | 1.4 (+) |
| 9 | Male | 45 | 1b | 0 | 0 | 0 | 0 | IA | GCLS | UT | NED | 657 | + | − | 90 | 85 | 0 | 160 | 1.4 (+) |
| 10 | Male | 60 | 1b | 0 | 0 | 0 | 0 | IA | GCLS | MT | NED | 391 | + | − | 30 | 24 | 0 | 60 | 0.2 (−) |
| 11 | Male | 58 | 3 | 2 | 2 | 2 | 0 | IIIA | por2>tub2 | LT | NED | 706 | + | − | 53 | 120 | 110 | 220 | 1.4 (−) |
| EBV(−) GC | |||||||||||||||||||
| 12 | Male | 77 | 2 | 1 | 1 | 0 | 0 | IB | GCLS | LT | NED | 1,115 | − | − | 60 | 8 | 140 | 70 | 1.8 (−) |
| 13 | Male | 73 | 3 | 2 | 3 | 2 | 0 | IIIA | GCLS | R | STD | 2,250 | − | − | 170 | 10 | 60 | 210 | 1.6 (−) |
| 14 | Male | 73 | 1b | 0 | 0 | 0 | 0 | IA | GCLS | MT | NED | 773 | − | − | 120 | 50 | 0 | 260 | 2.0 (+) |
| 15 | Female | 68 | 2 | 1 | 2 | 0 | 0 | IB | GCLS | LT/MT | NED | 2,161 | − | − | 10 | 55 | 105 | 125 | 2.0 (+) |
| 16 | Male | 76 | 1b | 1 | 0 | 0 | 0 | IA | GCLS | R | NED | 2,943 | − | − | 110 | 17 | 0 | 90 | 2.0 (+) |
| 17 | Male | 77 | 1b | 2 | 2 | 0 | 0 | IA | tub2>tub1 | MT | SAC | 127 | − | − | 9 | 140 | 150 | 40 | 2.0 (+) |
| 18 | Male | 71 | 2 | 1 | 1 | 0 | 0 | IB | sig>por2 | MT | NED | 2,876 | − | − | 3 | 140 | 0 | 20 | 2.0 (+) |
| 19 | Male | 69 | 2 | 2 | 1 | 0 | 0 | IB | tub1> tub2>muc | UT | NED | 187 | − | − | 135 | 240 | 100 | 16 | 2.4 (+) |
| 20 | Male | 66 | 1b | 1 | 1 | 0 | 0 | IA | tub>por2 | MT | NED | 2,610 | − | − | 30 | 295 | 120 | 80 | 1.6 (+) |
| 21 | Female | 58 | 1b | 2 | 2 | 0 | 0 | IA | tub2 | MT | NED | 2,511 | − | − | 70 | 280 | 120 | 95 | 2.2 (+) |
| 22 | Male | 57 | 2 | 1 | 1 | 0 | 0 | IB | sig | MT | NED | 2,530 | − | − | 150 | 110 | 0 | 60 | 1.2 (+) |
| 23 | Male | 49 | 1b | 1 | 1 | 0 | 0 | IA | por2>tub2 | UT | NED | 1,078 | − | − | 45 | 295 | 100 | 95 | 2.4 (+) |
| 24 | Male | 59 | 1b | 1 | 0 | 0 | 0 | IA | tub1>tub2 | MT | NED | 2,493 | − | − | 30 | 255 | 70 | 24 | 2.2 (+) |
| 25 | Male | 67 | 1b | 1 | 0 | 0 | 0 | IA | por2>sig | MT | NED | 2,435 | − | − | 105 | 140 | 10 | 75 | 1.8 (+) |
| 26 | Male | 69 | 1b | 1 | 1 | 0 | 0 | IA | tub1>tub2 | UT | NED | 2,211 | − | − | 90 | 230 | 100 | 180 | 2.0 (+) |
| 27 | Male | 77 | 1b | 1 | 3 | 0 | 0 | IA | tub2 | R | NED | 1,634 | − | − | 80 | 125 | 90 | 170 | 0.8 (−) |
| 28 | Male | 70 | 1b | 2 | 2 | 1 | 0 | IB | tub2 | UT | SAC | 403 | − | − | 70 | 180 | 60 | 170 | 1.8 (+) |
| 29 | Male | 63 | 3 | 1 | 1 | 0 | 0 | IIA | tub1 | MT | NED | 1,520 | − | − | 35 | 150 | 100 | 75 | 1.8 (+) |
| 30 | Male | 70 | 3 | 2 | 3 | 1 | 0 | IIB | tub2 | MT | NED | 898 | − | − | 145 | 40 | 0 | 5 | 2.2 (+) |
Table III.
Association between EBV infection and expression of AID and AID-regulatory factors in GC with or without LS.
| GC type | AID(+) % | P-value | NF-κB (+) (AID activator) (%) | P-value | PAX5 (+) (AID activator) (%) | P-value | c-Myb (+) (AID silencer) (%) | P-value |
|---|---|---|---|---|---|---|---|---|
| 0.025a | 0.005a | 0.01a | 0.354 | |||||
| EBV(+) GC (n=11) | 0 (0) | 1 (9) | 1 (9) | 5 (45) | ||||
| EBV(−) GC (n=19) | 7 (37) | 12 (63) | 11 (58) | 6 (32) | ||||
| NA | NA | NA | NA | |||||
| EBV(+) LS(+) GC (n=10) | 0 (0) | 1 (10) | 0 (0) | 4 (40) | ||||
| EBV(−) LS(+) GC (n=5) | 3 (60) | 0 (0) | 2 (40) | 3 (60) | ||||
| 0.500 | <0.001a | 0.003a | 0.256 | |||||
| LS(+) GC (n=15) | 3 (20) | 1 (7) | 2 (13) | 7 (47) | ||||
| LS(−) GC (n=15) | 4 (27) | 12 (80) | 10 (67) | 4 (27) |
EBV, Epstein-Barr virus; AID, activation-induced cytidine deaminase; GC, gastric carcinoma; LS, lymphoid stroma; NF-κB, nuclear factor κB; PAX5, paired box 5; c-Myb, c-Myb proto-oncogene; NA, not applicable (insufficient case numbers for statistical analysis).
Statistically significant.
Detection of EBV markers in tissue samples suspected of EBVaGC
The expression of EBER1 was only detected in 10/15 GCLS samples, the majority of which were histopathologically suspected of EBVaGC. On the other hand, 1/15 GC without LS samples tested positive for EBER1. All 30 samples were negative for LMP-1 (data not shown).
Grading of atrophic gastritis in sample tissue backgrounds
Background atrophic gastritis was evaluated and scored in sample tissues according to the USS. The mean USS scores (± SD) in EBV (+)GC (n=11) and in EBV (−)GC (n=19) were 1.67 (±0.57) and 1.88 (±0.38), respectively, and were not significantly different (P=0.328).
EBV(−) GC is more likely to express AID and AID-regulatory factors
AID H-scores of ≥105 were considered to be positive. Similarly, NF-κB, PAX5 and c-Myb H-scores of ≥125, ≥70 and ≥100, respectively, were considered to be positive.
The positive H-score rates of AID and its regulatory factors were compared between EBV(+) GC (n=11) and EBV(−) GC (n=19). The rates were 0/11 vs. 7/19 (0 vs. 37%; P=0.025) for AID, 1/11 vs. 12/19 (9 vs. 63%; P=0.005) for NF-κB, 1/11 vs. 11/19 (9 vs. 58%; P=0.01) for PAX5 and 5/11 vs. 6/19 (45 vs. 32%; P=0.35) for c-Myb for EBV(+) GC and EBV(−) GC, respectively. The positive rates of AID, NF-κB and PAX5, but not c-Myb, were significantly increased in EBV(−) GC compared with in EBV(+) GC (Table III).
No association between the expression of AID and USS scores was identified (P=0.623; Table II).
Table II.
Association between expression of AID and AID-regulatory factors and clinicopathological parameters in gastric cancers (n=30) regardless of EBV infection.
| Factor | AID (+) | P-value | NF-κB (+) (AID activator) | P-value | PAX-5 (+) (AID activator) | P-value | c-Myb (+) (AID silencer) | P-value |
|---|---|---|---|---|---|---|---|---|
| T | 0.572 | 0.215 | 0.831 | 0.465 | ||||
| 1b (n=18) | 3 | 10 | 7 | 7 | ||||
| 2 (n=6) | 2 | 2 | 3 | 1 | ||||
| 3 (n=6) | 2 | 1 | 2 | 3 | ||||
| ly | 0.539 | 0.100 | 0.134 | 0.218 | ||||
| 0 (n=5) | 1 | 0 | 0 | 3 | ||||
| 1 (n=17) | 3 | 9 | 8 | 4 | ||||
| 2 (n=8) | 3 | 4 | 4 | 4 | ||||
| v | 0.456 | 0.317 | 0.022a | 0.292 | ||||
| 0 (n=10) | 3 | 3 | 1 | 3 | ||||
| 1 (n=10) | 2 | 6 | 6 | 2 | ||||
| 2 (n=5) | 0 | 3 | 4 | 3 | ||||
| 3 (n=5) | 2 | 1 | 1 | 3 | ||||
| N | 0.642 | 0.285 | 0.213 | 0.114 | ||||
| 0 (n=24) | 5 | 12 | 11 | 7 | ||||
| 1 (n=4) | 1 | 1 | 0 | 2 | ||||
| 2 (n=2) | 1 | 0 | 1 | 2 | ||||
| Stage | 0.663 | 0.368 | 0.806 | 0.264 | ||||
| IA (n=16) | 3 | 9 | 7 | 6 | ||||
| IB (n=8) | 2 | 3 | 3 | 2 | ||||
| IIA (n=2) | 0 | 1 | 1 | 0 | ||||
| IIB (n=2) | 1 | 0 | 0 | 1 | ||||
| IIIA (n=2) | 1 | 0 | 1 | 2 | ||||
| Location | 0.179 | 0.387 | 0.172 | 0.389 | ||||
| Upper third (n=9) | 1 | 5 | 3 | 3 | ||||
| Middle third (n=15) | 4 | 7 | 5 | 4 | ||||
| Lower third (n=3) | 0 | 0 | 3 | 2 | ||||
| Remnant GC (n=3) | 2 | 1 | 1 | 2 | ||||
| Age, years | 0.089 | 0.462 | 0.590 | 0.421 | ||||
| <65 (n=13) | 1 | 5 | 5 | 4 | ||||
| ≥65 (n=17) | 6 | 8 | 7 | 7 | ||||
| Mean USS | 0.623 | 0.515 | 0.073 | 0.310 | ||||
| ≥2.2 (n=22) | 5 | 9 | 11 | 7 | ||||
| <2.2 (n=8) | 2 | 4 | 1 | 4 |
AID, activation-induced cytidine deaminase; NF-κB, nuclear factor κB; PAX5, paired box 5; c-Myb, c-Myb proto-oncogene; T, tumor; ly, lymphatic; v, venous; N, node; GC, gastric carcinoma; USS, updated Sydney system.
Statistically significant.
Certain cases of EBV(−) GCLS express AID and PAX5 (AID activator)
H-scores of AID and its regulatory factors were compared between EBV(+) GCLS (n=10) and EBV(−) GCLS (n=5). The positive H-score rate of AID and PAX5 was increased in EBV(−) GCLS (3/5, 60% for AID and 2/5, 40% for PAX5) compared with in EBV(+) GCLS (both 0/10, 0%). However, no significant differences were identified for AID or AID-regulatory factors between EBV(+) GCLS and EBV(−) GCLS, owing to an insufficient number of cases examined (Table III).
Expression of NF-κB and PAX5 is decreased in GCLS compared with in GC without LS
The aberrant expression of NF-κB and PAX5 was significantly decreased in GCLS [1/15 (7%) and 2/15 (13%); P<0.001] compared with in GC without LS [12/15 (80%) and 10/15 (67%); P=0.003] (Table III).
AID and AID-regulatory factors except PAX5 are not associated with clinicopathological parameters
The relationships of the expression of AID and its regulatory factors with clinicopathological parameters including tumor category, lymphatic invasion, venous invasion, node category, stage, location of GC, age and USS were investigated. No association between AID and its regulatory factors except PAX5 and the clinical parameters examined in EBV(+) and EBV(−) GCs was identified (n=30), whereas PAX5 expression was significantly associated with venous invasion (P=0.022; Table II).
Association between AID and AID-regulatory factors and survival rates
Among 30 patients who participated in the present study, only one succumbed to GC (Table I, case 13). Therefore, in the survival analysis, it was not possible to conclude whether AID and AID-regulatory factors affected the survival rate.
Discussion
Previous comprehensive genome analyses using next-generation sequencing of various cancer tissues have revealed that cancer cells have numerous nucleotide alterations (14). Cancer cells are considered to be generated from the stepwise accumulation of genetic alterations in various genes in inflammation-associated carcinogenesis (7). AID, a nucleotide-editing enzyme that is essential for somatic hypermutation and class-switch recombination of the immunoglobulin gene, is also known to serve a role as a genomic mutator in carcinogenesis (9). Shimizu et al (7) demonstrated that pathogenic bacterial or viral factors and subsequent inflammatory reactions in H. pylori-related gastritis, chronic viral hepatitis, Barrett's esophagus and inflammatory bowel disease lead to the aberrant expression of AID in various epithelial cells via NF-κB activation, which causes the accumulation of genetic alterations in tumor-related genes. The aberrant expression of AID was observed in the gastric epithelium and gastric cancer cells with an H. pylori-infected background (10,15,16). EBVaGC, a distinct type of GC, the majority of cases of which have lymphoid stroma, is an EBV-infected and inflammation-associated cancer. The results of the present study identified that the aberrant expression of AID and NF-κB was significantly decreased in EBVaGC compared with in EBV-non-associated GC, and also that EBV was not likely to be involved in pathogen-induced AID expression via NF-κB activation in gastric cancers. These results suggested that the genomic modulation of tumor-related genes by EBV-induced AID expression is not a primary causative mechanism in the carcinogenesis of EBVaGC. Unexpectedly, the aberrant expression of NF-κB was less frequent in EBVaGC with LS or GCLS compared with in GC without LS. A potential reason for the decreased expression of NF-κB is that EBV-LMP-1, a strong activator of NF-κB, was not expressed in EBVaGC with latency type I or II EBV infection. However, this does not explain the decreased expression of NF-κB in GCLS compared with in GC without LS regardless of EBV infection because LS is a hallmark of chronic inflammation. Furthermore, no significant differences were identified in the expression of AID between GCLS and GC without LS. These results suggest that LS, a histological characteristic of chronic inflammation, does not necessarily reflect NF-κB-inducing conditions, and that NF-κB and homeobox protein C4 may promote the transcription of AID (8). The activation of NF-κB is a complex process induced by various stimuli, including microbial and viral products, cytokines, DNA damage, oxidative damage and radiation (17). Aberrant NF-κB activity has been demonstrated to contribute to a number of human diseases that possess an immune or inflammatory component, including tumorigenesis and H. pylori infection (17). Other stimuli, including H. pylori infection, appear to be more important in the induction of aberrant AID expression via NF-κB in the stomach compared with EBV infection (15). In the present study, no significant differences were observed in mean USS scores or H. pylori-infected rates between EBVaGC and EBV-non-associated GC. No association was identified between EBV infection and H. pylori infection in gastric cancer patients from northern Brazil (18). Furthermore, no association between the expression of AID and USS scores was identified; however, H. pylori infection is generally considered to induce atrophic gastritis and intestinal metaplasia with the aberrant expression of AID, which subsequently leads to gastric cancers through the accumulation of genetic mutations (19).
PAX5, known as a B cell-specific activator protein, serves a role as an activator for AID (9). In the present study, the aberrant expression of PAX5 and AID was more frequently observed in EBV-non-associated GC compared with in EBVaGC (P=0.01 and P=0.025, respectively). The expression of PAX5 was also increased in GC without LS compared with in GCLS (P=0.003). This was attributed to the aberrant expression of PAX5 contributing to the induction of AID expression in EBV-non-associated GC, the majority of cases of which exhibited no LS background. PAX5 has been identified as a tumor suppressor gene and the epigenetic inactivation of PAX5 through the direct up-regulation of p53 is associated with a poor prognosis in gastric cancer patients (20). However, it is noteworthy that PAX5 expression was significantly associated with venous invasion of GC in that study.
The cellular oncogene c-Myb, a v-Myb avian myeloblastosis viral oncogene homolog, has been implicated in leukemogenesis, and c-Myb was demonstrated to be increased in acute myeloid leukemia and acute lymphoblastic leukemia samples (21). c-Myb also serves a role as a repressor or silencer of the expression of AID (8). The aberrant expression of c-Myb in the present study was not significantly different between EBVaGC and EBV-non-associated GC, or between GCLS and GC without LS. The expression of c-Myb as a silencer for the induction of AID appears to serve certain roles in the expression of AID in gastric cancers, regardless of the EBV status.
The Cancer Genome Atlas Research Network recently provided a comprehensive molecular characterization of gastric adenocarcinoma and defined EBVaGC as a specific subtype, which displays recurrent mutations in phosphoinositide 3-kinase catalytic α polypeptide (PIK3CA) and AT-rich-interaction domain 1A (ARID1A), extreme DNA hypermethylation, and the amplification of genes encoding c-Jun-associated kinase 2, cluster of differentiation 274 (also known as programmed death ligand 1) and programmed cell death 1 ligand 2 (also known as programmed death ligand 2) with the extremely rare tumor protein 53 gene mutation (22). Furthermore, mutations in PIK3CA and ARID1A may precede EBV infection because mutation events have been detected in chronically inflamed and H. pylori-infected gastric mucosa (23). EBVaGC is characterized by extreme DNA hypermethylation and relatively rare somatic gene mutations. The decreased aberrant expression of AID observed in EBVaGCs in the present study is consistent with the relatively small number of somatic gene mutations detected using the comprehensive molecular analysis in this specific subtype of GC.
The prognostic effects of EBV positivity on gastric cancer are controversial (23). However, a recent meta-analysis of 4599 GC cases identified a decreased mortality rate in EBVaGC following adjustment for the cancer stage and other factors (24). The 10-year disease-specific rate of patients with EBVaGCLS (89.1%) was previously demonstrated to be significantly increased compared with that of EBV-non-associated GCLS (66.9%) (P=0.009) (4). These results suggest that the EBV infection status in GC is an important prognostic factor. The aberrant expression of AID and AID-regulatory factors (NF-κB and c-Myb) in the present study was not identified to be associated with clinicopathological parameters, whereas only PAX5 expression was significantly associated with venous invasion. The relevance of AID and its regulatory factors for the survival rate of patients with GC was not demonstrated in the present study because only 1/30 patients succumbed to GC during the observation period.
In inflammation-associated cancer development, in addition to infectious agents including H. pylori, a number of intrinsic mediators of inflammation, including proinflammatory cytokines, growth factors, and reactive oxygen and nitrogen species, have the ability to induce the genetic and epigenetic modulation of tumor-related genes through various mechanisms, including enhancements in cell growth, mobility and angiogenesis, the inhibition of apoptosis and somatic gene mutations or rearrangements, and DNA methylation (6,7). Furthermore, inflammation modulates the expression of microRNAs that influence the production of numerous tumor-related mRNAs or proteins (5). On the other hand, in the development of EBVaGC, mechanisms other than the AID-induced genetic mutations described above, particularly DNA methylation, may have more important functions as the mechanisms underlying somatic gene mutations through EBV-induced aberrant AID expression were not demonstrated in EBVaGC in the present study.
In the present study, significant aberrant expression of AID was not observed in EBVaGC and is considered irrelevant to the mechanisms underlying carcinogenesis in this specific subtype of GC, whereas aberrant AID expression contributes to carcinogenesis in certain cases of EBV-non-associated GC.
Acknowledgements
The authors thank former Professor Masahide Ikeguchi of the First Surgery Department, Tottori University Hospital (Yonago, Japan) for providing the clinical samples.
References
- 1.Jha HC, Banerjee S, Robertson ES. The role of Gammaherpesviruses in cancer pathogenesis. Pathogens. 2016;5(pii):E18. doi: 10.3390/pathogens5010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Epstein MA, Achong BG, Barr YM. Virus particle in cultured lymphoblasts from Burkitt's Lymphoma. Lancet. 1964;1:702–703. doi: 10.1016/S0140-6736(64)91524-7. [DOI] [PubMed] [Google Scholar]
- 3.Shinozaki-Ushiku A, Kunita A, Fukayama M. Update on Epstein-Barr virus and gastric cancer (review) Int J Oncol. 2015;46:1421–1434. doi: 10.3892/ijo.2015.2856. [DOI] [PubMed] [Google Scholar]
- 4.Lim H, Park YS, Lee JH, Son da H, Ahn JY, Choi KS, Kim do H, Choi KD, Song HJ, Lee GH, et al. Features of gastric carcinoma with lymphoid stroma associated with Epstein-Barr virus. Clin Gastroenterol Hepatol. 2015;13:1738–1744.e2. doi: 10.1016/j.cgh.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 5.Chiba T, Marusawa H, Ushijima T. Inflammation-associated cancer development in digestive organs: Mechanisms and roles for genetic and epigenetic modulation. Gastroenterology. 2012;143:550–563. doi: 10.1053/j.gastro.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 6.Marusawa H, Takai A, Chiba T. Role of activation-induced cytidine deaminase in inflammation-associated cancer development. Adv Immunol. 2011;111:109–141. doi: 10.1016/B978-0-12-385991-4.00003-9. [DOI] [PubMed] [Google Scholar]
- 7.Shimizu T, Marusawa H, Endo Y, Chiba T. Inflammation-mediated genomic instability: Roles of activation-induced cytidine deaminase in carcinogenesis. Cancer Sci. 2012;103:1201–1206. doi: 10.1111/j.1349-7006.2012.02293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zan H, Casali P. Regulation of Aicda expression and AID activity. Autoimmunity. 2013;46:83–101. doi: 10.3109/08916934.2012.749244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Honjo T, Kobayashi M, Begum N, Kotani A, Sabouri S, Nagaoka H. The AID dilemma: Infection, or cancer? Adv Cancer Res. 2012;113:1–44. doi: 10.1016/B978-0-12-394280-7.00001-4. [DOI] [PubMed] [Google Scholar]
- 10.Kawata S, Yashima K, Yamamoto S, Sasaki S, Takeda Y, Hayashi A, Matsumoto K, Kawaguchi K, Harada K, Murawaki Y. AID, p53 and MLH1 expression in early gastric neoplasms and the correlation with the background mucosa. Oncol Lett. 2015;10:737–743. doi: 10.3892/ol.2015.3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Iwasaki T, Matsushita M, Nonaka D, Nagata K, Kato M, Kuwamoto S, Murakami I, Hayashi K. Lower expression of CADM1 and higher expression of MAL in Merkel cell carcinomas are associated with Merkel cell polyomavirus infection and better prognosis. Hum Pathol. 2016;48:1–8. doi: 10.1016/j.humpath.2015.09.030. [DOI] [PubMed] [Google Scholar]
- 13.Takashima K, Ohashi M, Kitamura Y, Ando K, Nagashima K, Sugihara H, Okuno K, Sairenji T, Hayashi K. A new animal model for primary and persistent Epstein-Barr virus infection: Human EBV-infected rabbit characteristics determined using sequential imaging and pathological analysis. J Med Virol. 2008;80:455–466. doi: 10.1002/jmv.21102. [DOI] [PubMed] [Google Scholar]
- 14.Meyerson M, Gabriel S, Getz G. Advances in understanding cancer genomes through second-generation sequencing. Nat Rev Genet. 2010;11:685–696. doi: 10.1038/nrg2841. [DOI] [PubMed] [Google Scholar]
- 15.Matsumoto Y, Marusawa H, Kinoshita K, Endo Y, Kou T, Morisawa T, Azuma T, Okazaki IM, Honjo T, Chiba T. Helicobacter pylori infection triggers aberrant expression of activation-induced cytidine deaminase in gastric epithelium. Nat Med. 2007;13:470–476. doi: 10.1038/nm1566. [DOI] [PubMed] [Google Scholar]
- 16.Goto A, Hirahashi M, Osada M, Nakamura K, Yao T, Tsuneyoshi M, Takayanagi R, Oda Y. Aberrant activation-induced cytidine deaminase expression is associated with mucosal intestinalization in the early stage of gastric cancer. Virchows Arch. 2011;458:717–724. doi: 10.1007/s00428-011-1086-x. [DOI] [PubMed] [Google Scholar]
- 17.Le Negrate G. Viral interference with innate immunity by preventing NF-κB activity. Cell Microbiol. 2012;14:168–181. doi: 10.1111/j.1462-5822.2011.01720.x. [DOI] [PubMed] [Google Scholar]
- 18.de Souza CR, de Oliveira KS, Ferraz JJ, Leal MF, Calcagno DQ, Seabra AD, Khayat AS, Montenegro RC, Alves AP, Assumpção PP, et al. Occurrence of Helicobacter pylori and Epstein-Barr virus infection in endoscopic and gastric cancer patients from Northern Brazil. BMC Gastroenterol. 2014;14:179. doi: 10.1186/1471-230X-14-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimizu T, Marusawa H, Matsumoto Y, Inuzuka T, Ikeda A, Fujii Y, Minamiguchi S, Miyamoto S, Kou T, Sakai Y, et al. Accumulation of somatic mutations in TP53 in gastric epithelium with Helicobacter pylori infection. Gastroenterology. 2014;147:407–417.e3. doi: 10.1053/j.gastro.2014.04.036. [DOI] [PubMed] [Google Scholar]
- 20.Li X, Cheung KF, Ma X, Tian L, Zhao J, Go MY, Shen B, Cheng AS, Ying J, Tao Q, et al. Epigenetic inactivation of paired box gene 5, a novel tumor suppressor gene, through direct upregulation of p53 is associated with prognosis in gastric cancer patients. Oncogene. 2012;31:3419–3430. doi: 10.1038/onc.2011.511. [DOI] [PubMed] [Google Scholar]
- 21.Shetzline SE, Rallapalli R, Dowd KJ, Zou S, Nakata Y, Swider CR, Kalota A, Choi JK, Gewirtz AM. Neuromedin U: A Myb-regulated autocrine growth factor for human myeloid leukemias. Blood. 2004;104:1833–1840. doi: 10.1182/blood-2003-10-3577. [DOI] [PubMed] [Google Scholar]
- 22.Cancer Genome Atlas Research Network, corp-author. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–209. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abe H, Kaneda A, Fukayama M. Epstein-barr virus-associated gastric carcinoma: Use of host cell machineries and somatic gene mutations. Pathobiology. 2015;82:212–223. doi: 10.1159/000434683. [DOI] [PubMed] [Google Scholar]
- 24.Camargo MC, Kim WH, Chiaravalli AM, Kim KM, Corvalan AH, Matsuo K, Yu J, Sung JJ, Herrera-Goepfert R, Meneses-Gonzalez F, et al. Improved survival of gastric cancer with tumour Epstein-Barr virus positivity: An international pooled analysis. Gut. 2014;63:236–243. doi: 10.1136/gutjnl-2013-304531. [DOI] [PMC free article] [PubMed] [Google Scholar]