Abstract
The present study investigated the regulatory mechanism of signal-regulatory protein (SIRP)-α in the apoptosis and proliferation of prostate cancer (CaP) cells. The expression profile of SIRP-α in prostate cancer cells was analyzed using reverse transcription-quantitative polymerase chain reaction and western blotting. Then SIRP-α function in CaP cells was further analyzed with the overexpression and RNA interference of SIRP-α. The results revealed that SIRP-α expression levels were decreased in CaP tissues and cell lines, with androgen-independent CaP exhibiting a lower SIRP-α expression compared with androgen-dependent CaP. Overexpression of SIRP-α resulted in a significantly reduced number of live CaP cells by enhancing apoptosis, whereas SIRP-α silencing increased CaP cell proliferation. Mechanistically, SIRP-α decreases cyclooxygenase-2 (COX-2) expression and cytokine production by negatively regulating p38 mitogen-activated protein kinase and nuclear factor-κB pathway. Therefore, SIRP-α knockdown decreases cell apoptosis by enhancing COX-2 expression. The present results indicate that SIRP-α may function as a novel negative regulator to modulate cellular proliferation, survival and migration in CaP cells. The heightened sensitivity of cells restoring SIRP-α function could be exploited in the development of therapeutics that may potentiate the antineoplastic effects of conventional cytokines or chemotherapeutic agents.
Keywords: prostate cancer, androgen-independent prostate cancer, signal regulatory protein, apoptosis
Introduction
Prostate cancer (CaP) is a leading cause of cancer mortality in western countries, and is mainly observed as adenocarcinoma of epithelial cell-origin (1). Although CaP morbidity in China is low compared with that in western countries, it is on the rise (2). Early stage CaP requires androgens for growth, and thus responds well to androgen deprivation therapy (3). However, following the remittent stage (18–24 months in average), androgen dependent prostate cancer (ADPC) may become androgen-independent prostate cancer (AIPC). There is currently no curative therapy available for AIPC.
The mechanism underlying AIPC transformation is of importance in the CaP field. The primary mechanisms for the conversion of ADPC into AIPC are variations in gene expression, signal pathway abnormity, dysregulation of proto-oncogenes, cancer suppressor genes and growth factors (4,5). Thus far, a number of relevant genes and signal pathways in CaP have been described (6). However, due to the extremely complex biological behavior of CaP, no theory has clarified the pathogenic mechanism of AIPC (6,7). Therefore, the identification of genes involved in the transition from ADPD to AIPC is important to expand the current knowledge of AIPC (7).
Signal regulatory protein (SIRP)-α is a transmembrane regulatory protein originally identified in rat cells through its association with cytoplasmic tyrosine phosphatase Src homology region 2 domain-containing phosphatase (SHP-2). SHP-2 was later shown to be highly conserved in other mammals, including humans, mice, and cattle. The cytoplasmic region of SIRP-α contains 2 immunoreceptor tyrosine-based inhibitory motifs with 4 tyrosine residues that are phosphorylated in response to a variety of growth factors and ligand binding. This phosphorylation enables the recruitment and activation of Src homology region 1 domain-containing phosphatase (SHP-1) and SHP-2, which in turn dephosphorylate specific protein substrates involved in the mediation of various physiological effects (8,9).
Previously, decreased SIRP-α expression levels have been reported in various types of cancer, indicating its important role in oncology (9,10). The present study identified that SIRP-α expression tended to be lower in AIPC tissues compared with paired ADPC tissues. The present study also established the human prostate cancer LNCaP and LNCaP-A cell model to further explore the regulatory mechanism of SIRP-α in CaP, and demonstrated that it modulates CaP cell apoptosis and proliferation through the p38 mitogen activated protein kinase (MAPK)/nuclear factor (NF)-κB/cyclooxygenase (COX)-2 pathway.
Materials and methods
Cell culture and transfection
LNCaP, PC-3 and C4-2 cells were purchased from American Type Culture Collection (Manassas, VA, USA) and maintained in RPMI 1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS), 2 mM L-glutamine, and 25 mM 4-(2-hydroxylethyl)-1-piperazineethanesulphonic acid. The LNCaP-A cell line, an androgen independent LNCaP variant, was maintained in phenol-red free RPMI 1640 (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% charcoal stripped FBS, 300 mg/l L-glutamine, 2,000 mg/l glucose, and 2,000 mg/l NaHCO3. The normal prostate PWR-1E and RWPE-1 cell lines were cultured in keratinocyte serum free medium (Gibco; Thermo Fisher Scientific, Inc.). pcDNA3.1-myc-COX-2 was purchased from Biogot Technology Co., Ltd., (Nanjing, China). Vector-based shRNAs containing the target sequences 5′-AAGTGAAGGTGACTCAGCCTG-3′ and 5′-AATCAGTGTCTGTTGCTGCTG-3′ for SIRP-α were constructed using the pSUPER-neo vector (OligoEngine, Seattle, WA, USA) according to the manufacturer's protocol. COX-2 siRNA was obtained from Guangzhou RiboBio Co., Ltd (Guangzhou, China) and sequence was as follows: Forward, 5′-GCUGGGAAGCCUUCUCUAA-3′ and reverse, 5′-TCGACCCUUCGGAAGAGAUU-3′. The pcDNA3.1-SIRP-α plasmid was constructed according to a previous study (11). The plasmid pcDNA3.1 was used as the control vector. Transfections were performed using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.).
Clinical samples
CaP tissues included in the present study were from 28 cases with adenocarcinoma of the prostate that were diagnosed by 2 pathologists at The People's Liberation Army 309th Hospital (Beijing, China). Medical history, transrectal ultrasound, computed tomography, magnetic resonance imaging and isotope scanning of the skeleton were combined to determine the clinical staging. A total of 12 patients who accepted radical prostatectomy did not exhibit metastasis and maintained very low prostate specific antigen (PSA) levels (<0.2 ng/ml) with no relapse. They were considered as androgen dependent prostate cancer (ADPC) patients according to previous studies (2). AIPC were defined as following: 1, serum testosterone <50 ng/ml; 2, high PSA level (as measured 3 times, every 2 weeks); 3, anti-androgen treatment failure. A total of 16 AIPC patients were used in the present study. The study was approved by the ethics committee of The People's Liberation Army 309th Hospital. Written informed consent was gained prior to the start of the study.
Cell viability assays
The MTT assay was used in the present study to quantify cell viability. Medium without cells served as a negative control for this experiment. Cells were incubated in 96-well culture plates (5×103 cells per well) at 37°C for 72 h. 50 µl MTT solution was added to each well and incubated at 37°C for a further 4 h. Following incubation, MTT was aspirated and 150 µl of dimethyl sulfoxide was added to each well to dissolve the formazan precipitate. Subsequently, an ELISA plate reader was used to obtain absorbance values at 570 nm.
Cell apoptosis assay
An Annexin V-Fluos staining kit (cat. no. 11988549001; Roche Applied Science, Penzberg, Germany) was used to assess early apoptosis, as represented by a phosphatidylinositol flip to the outer membrane. Cells were washed with PBS and stained with Annexin V and propidium iodide according to the manufacturer's protocol. Subsequently, slides were mounted with the Permafluor mounting medium (Immunotech; Beckman Coulter, Inc., Brea, CA, USA) and viewed under a fluorescence microscope (Axiophot, Olympus Corporation, Tokyo, Japan).
Cell cycle analysis
CaP cells were synchronized in G0 by serum starvation for 3 days followed by stimulation in DMEM supplemented with 10% FBS. Progression through the cell cycle was monitored by detection of the DNA content as previously described (12).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cells using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. Medium without cells served as a negative control for this experiment. Subsequently, RT-qPCR was carried out with the PrimeScript RT-PCR kit (Takara, Bio, Inc., Shiga, Japan), using β-actin as an internal control, in the Eppendorf Realplex4 machine (cat. no. X222687G; Hamburg, Germany). The sequences of primers used for SIRP-α and β-actin were as previously described (11). Reverse transcription reactions were performed using the following parameters: 16°C for 30 min, 42°C for 30 min and 84°C for 5 min. The 2−∆∆Cq method was used for normalization (13). All experiments were repeated three times.
Western blotting
SIRP-α (cat. no. 13379) and GAPDH (cat. no. 2118) antibodies were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). A COX-2 antibody (cat. no. sc-7951) was purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA); Bcl-2 (cat no. ab694) and Bcl-2 associated × (Bax; cat. no. ab32503) antibodies were purchased from Abcam (Cambridge, UK). Anti-rabbit (cat. no. sc-2054) and anti-mouse (cat. no. sc-358914) secondary antibodies were purchased from Santa Cruz Biotechnology, Inc.
Cells were washed with ice-cold PBS and lysed with protein lysis buffer (Pierce; Thermo Fisher Scientific, Inc.). Subsequent to centrifugation at 5,000 × g for 15 min at 4°C, the protein concentration was measured with a bicinchoninic acid protein assay kit (Pierce; Thermo Fisher Scientific, Inc.). A total of 50 µg aliquots of lysates were separated by 10% SDS-PAGE and transferred onto a polyvinylidene difluoride membrane. The membranes were blocked with 5% dried skimmed milk in Tris-buffered saline (pH 7.4) containing 0.05% Tween 20, and sequentially incubated with primary (dilution, 1:200) and horseradish peroxidase-conjugated secondary (dilution, 1:5,000) antibodies, according to the manufacturers' protocols. The proteins of interest were visualized using an enhanced chemiluminescence western blotting substrate (Pierce; Thermo Fisher Scientific, Inc.) and the Chemidoc XRS Gel Documentation System (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Cytokine assay
ELISA kits for tumor necrosis factor α (TNFα; cat. no. MTA00B), interleukin (IL)-6 (cat no. HS600B), nitric oxide (cat. no. KGE001), CC chemokine ligand (CCL) 2 (cat. no. DCP00), CCL5 (cat. no. DRN00B) and chemokine (C-X-C motif) ligand 2 (CXCL2; cat. no. DY995) were purchased from R&D Systems, Inc. (Minneapolis, MN, USA). Cytokine levels in culture supernatant or sera were determined using the ELISA kits, according to the manufacturer's protocols.
Signal inhibitors
NF-κB (BAY-117082; cat. no. EY1330), p38 MAPK (SB203580; cat. no. EY0411), ERK1/2 (U0126; cat. no. EY1161) and JNK (SP600125; cat. no. EY0021) inhibitors were purchased from Amquar Biological Technology Co., Ltd. (Shanghai, China). The LNCaP cells were incubated in 6-well plates (106 cells/well). The inhibitors BAY-117082 (20 µM), SB203580 (20 µM), U0126 (10 µM) and SP600125 (20 µM) were added to the appropriate well and incubated at 37°C for 45 min. The cells were subsequently incubated at 37°C for 24 h prior to being harvested.
Statistical analyses
Data are represented as the mean ± standard deviation from ≥3 separate experiments performed in triplicate. The differences between groups were determined using two-tailed Student's t-test with SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA). The differences of ELISA data between groups were determined using analysis of variance. P<0.05 was considered to indicate a statistically significant difference. The χ2 test or Fisher's exact test was used to analyze the association between SIRP-α expression and clinicopathological features.
Results
SIRP-α expression is decreased in CaP tissues
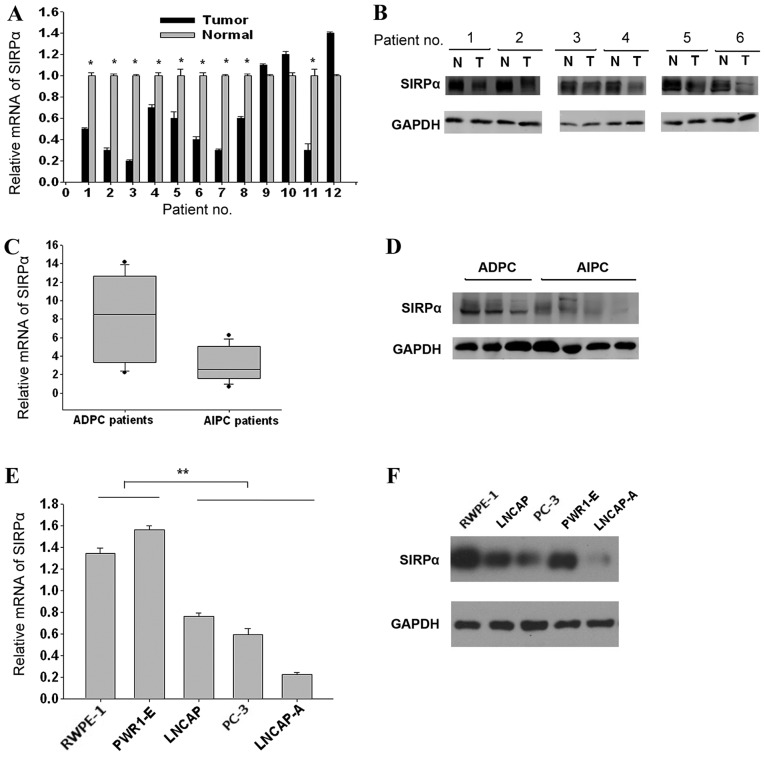
PCR results demonstrated that 9/12 (75%) CaP samples showed lower SIRP-α levels compared with matched normal tissues (Fig. 1A; P=0.044). Western blotting data confirmed this SIRP-α expression trend in the same patient groups (Fig. 1B, data of 6 patients are shown; P=0.042). The SIRP-α expression levels between the AIPC and ADPC groups were compared; it was revealed that they were significantly lower in AIPC samples compared with the ADPC groups (RT-qPCR, Fig. 1C, P=0.026; western blotting, Fig. 1D, P=0.031). To confirm these findings, SIRP-α expression levels were assessed in 3 prostate cancer LNCaP, LNCaP-A, and PC3 cell lines along with the 2 normal prostate epithelial RWPE-1 and PWR-1E cell lines. The expression of SIRP-α was lowest in LNCaP-A cells, followed by PC3 and LNCaP, whereas RWPE-1 and PWR-1E showed higher SIRP-α expression levels (RT-qPCR, Fig. 1E, P=0.006; western blotting, Fig. 1F, P=0.008). The data indicated that SIRP-α expression was decreased in CaP tissues and cell lines, with AIPC showing lower SIRP-α expression compared with ADPC.
Figure 1.
SIRP-α is decreased in CaP cells and tissues. (A) Reverse transcription-qPCR analysis of SIRP-α expression in CaP and adjacent normal tissues. (B) Western blotting for SIRP-α in paired normal and tumor tissues. (C) qPCR and (D) western blotting results comparing SIRP-α levels between ADPC and AIPC tissues. (E) mRNA and (F) protein expression levels of SIRP-α in the prostate RWPE-1, PWR1-E, LNCAP, LNCAP-A and PC-3 cell lines. SIRP, signal regulatory protein; CaP, prostate cancer; qPCR, quantitative polymerase chain reaction; ADPC, androgen-dependent prostate cancer; AIPC, androgen-independent prostate cancer.
SIRP-α negatively regulates CaP cell proliferation by enhancing cell apoptosis
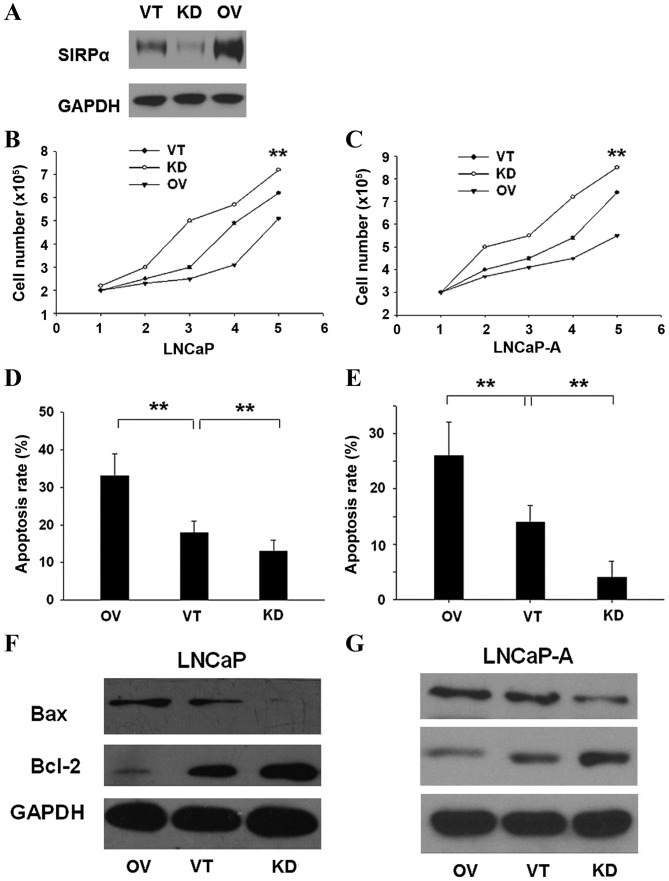
To investigate the biological function of SIRP-α in CaP cell lines, SIRP-α was overexpressed by transfecting pcDNA3.1-SIRP-α (OV), and suppressed by shRNA-SIRP-α (KD) in 2 cell lines: LNCaP and LNCaP-A. Control vector (VT) transfection was also performed (Fig. 2A). As shown in Fig. 2B, OV transfection of LNCaP cells resulted in significantly reduced number of live cells, whereas KD transfection increased CaP cellular viability (P=0.003). The same trend was observed in LNCaP-A cells (Fig. 2C; P=0.005). Cell apoptosis rates were determined using the Annexin V-Fluos staining kit. OV significantly enhanced the apoptosis rate (Fig. 2D; P=0.005), whereas KD had the opposite effect (Fig. 2E; P=0.004). Additionally, OV resulted in overtly higher Bax (pro-apoptotic) and lower Bcl-2 (anti-apoptotic) mRNA and protein expression levels 24 h after transfection (LNCaP cells, Fig. 2F, P=0.004; LNCaP-A cells, Fig. 2G, P=0.006).
Figure 2.
SIRP-α negatively regulates CaP cellular proliferation by enhancing cell apoptosis. Cells were transfected with VT, KD and OV. (A) Efficiency of transfection was examined in LNCaP and LNCaP-A. Growth curves for (B) LNCaP and (C) LNCaP-A were determined, and apoptosis rates for (D) LNCaP and (E) LNCaP-A were determined. Apoptosis marker expression for (F) LNCaP and (G) LNCaP-A were determined. Error bars represent the standard deviation. **P<0.01. SIRP, signal regulatory protein; OV, pcDNA3.1-SIRP-α; VT, control vector; KD, shRNA for SIRP-α; Bax, Bcl associated X.
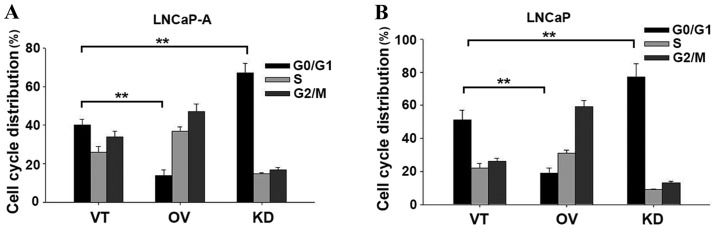
Based on the growth inhibitory effects of SIRP-α in CaP cells, the present study then examined its effect on cell cycle progression. As demonstrated in LNCaP-A cells in Fig. 3A (P=0.008) and LNCaP cells in Fig. 3B (P=0.006), downregulation of SIRP-α increased the number of G0/G1 cell numbers. This data indicated an important role for SIRP-α in apoptosis.
Figure 3.
Cell cycle distribution. Cells were transfected with VT, KD and OV, and then cell cycle distribution of (A) LNCaP-A and (B) LNCaP cells were determined. Error bars represent the standard deviation. **P<0.01. VT, control vector; KD, shRNA for SIRP-α; OV, pcDNA3.1-SIRP-α; SIRP, signal regulatory protein.
SIRP-α alters the production pattern of various cytokines in CaP cells
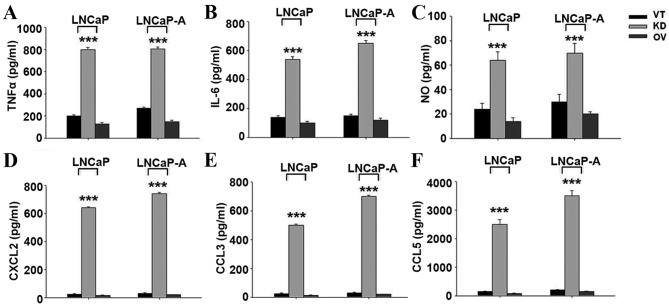
It is known that SIRP-α regulates the production of various cytokines. In LNCaP and LNCaP-A cells, transfection with KD produced significantly more TNFα (Fig. 4A), IL-6 (Fig. 4B), and nitric oxide (Fig. 4C) compared with control and SIRP-α overexpression groups (P<0.001). The chemoattractants CXCL2 (Fig. 4D), CCL3 (Fig. 4E) and CCL5 (Fig. 4F) were also upregulated following SIRP-α knockdown (P<0.001).
Figure 4.
Increased cytokine production of SIRP-α knockdown in CaP cells. Cells were transfected with VT, KD and OV, and cytokine production was determined using ELISA kits. (A) TNFα, (B) IL-6, (C) NO, (D) CXCL2, (E) CCL3, (F) CCL5. SIRP, signal regulatory protein; CaP, prostate cancer; VT, control vector; KD, shRNA for SIRP-α; OV, pcDNA3.1-SIRP-α; TNF, tumor necrosis factor; IL-6, interleukin-6; NO, nitric oxide; CXCL2, chemokine (C-X-C motif) ligand 2; CCL, CC chemokine ligand.
SIRP-α knockdown decreases cell apoptosis by enhancing COX-2 expression
Using LNCaP and LNCaP-A cells as models, the present study identified that SIRP-α silencing significantly upregulated COX-2 expression (Fig. 5A, P<0.001). Considering that COX-2 is positively associated with cancer cell apoptosis, it was then explored whether silencing of SIRP-α could suppress apoptosis by increasing COX-2 expression. Cells were transiently co-transfected with COX-2 siRNA (si-COX-2) and si-SIRP-α for 24 h. As shown in Fig. 5B, co-transfection of si-COX-2 blocked COX-2 expression enhancement (P<0.001). Bax was also upregulated in the si-COX-2 group, and Bcl-2 was downregulated (P<0.001). The apoptosis rate was sharply reduced in the si-SIRP-α group, and si-COX-2 co-transfection blocked this apoptosis inhibition (P=0.002). The COX-2 independent inhibitor NS-398 did not downregulate COX-2 expression, but also caused elevated apoptosis rates (data not shown). Taken together, these findings suggested that SIRP-α knockdown decreased cell apoptosis by enhancing COX-2 expression.
Figure 5.
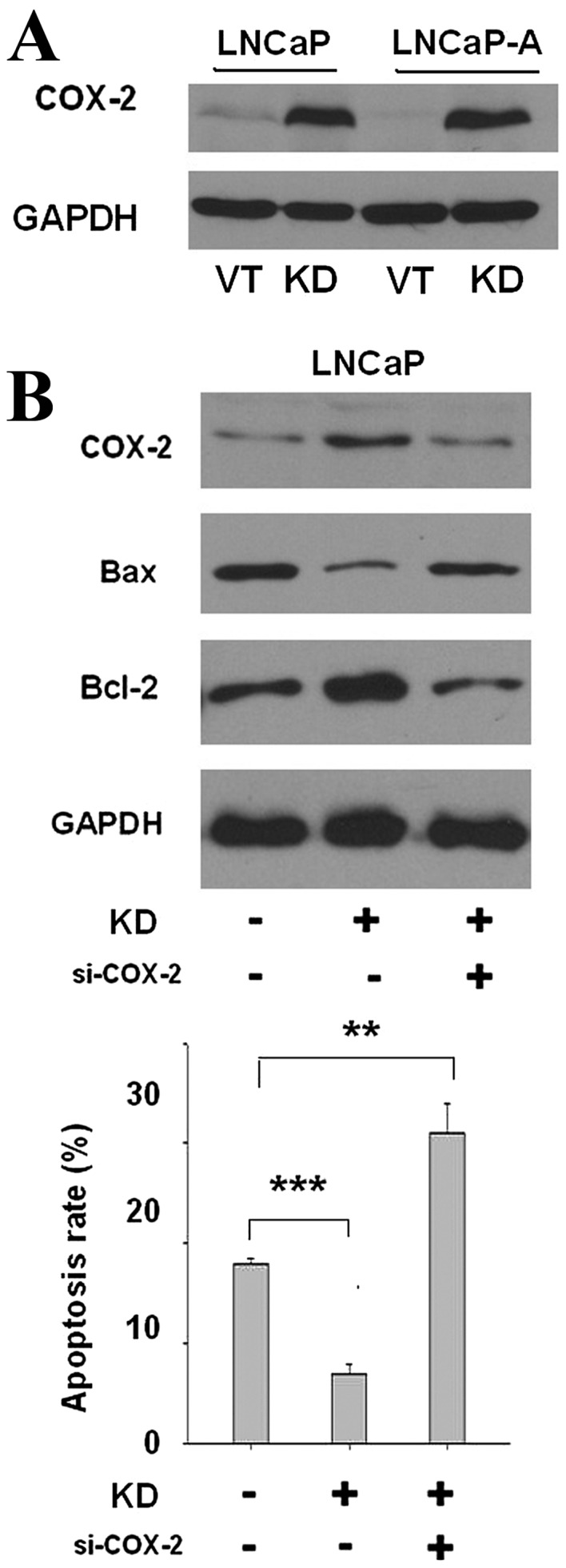
SIRP-α knockdown decreases cell apoptosis by enhancing COX-2 expression. (A) Cells were transfected with KD, and COX-2 expression were determined by western blotting. VT and GADPH were used as controls. (B) Cells were co-transfected with si-COX-2 and KD and then the apoptosis rate and apoptosis marker were determined. Error bars represent the standard deviation. **P<0.01; ***P<0.001. SIRP, signal regulatory protein; COX-2, cyclooxygenase-2; KD, shRNA-SIRP-α; VT, vector control; si-, small interfering RNA; Bax, Bcl associated X.
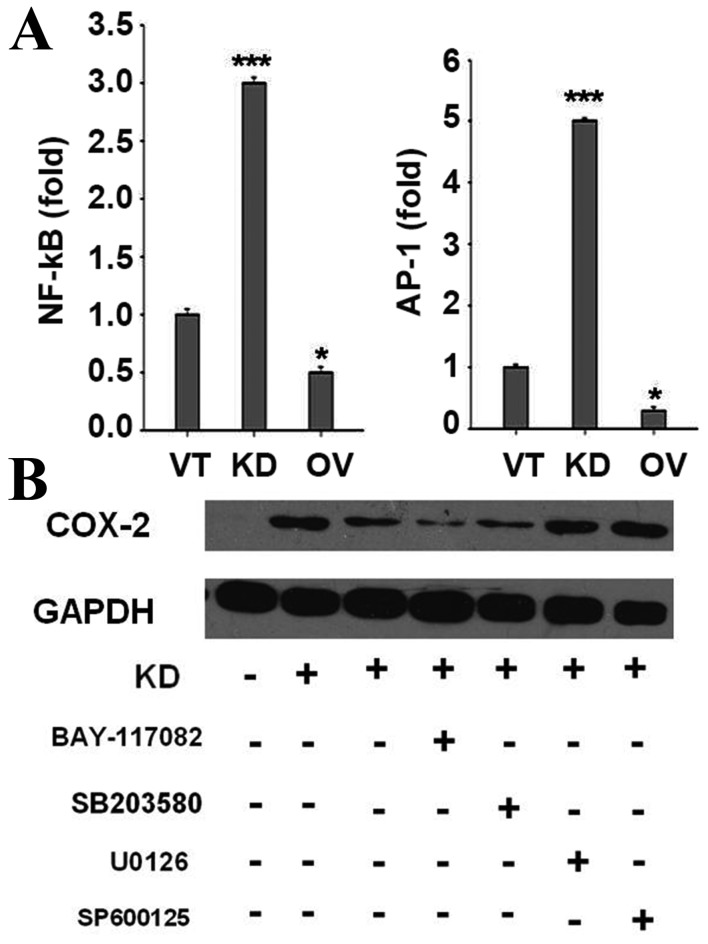
SIRP-α decreases COX-2 expression and cytokine production by negatively regulating p38 MAPK and NF-κB
In the present study, NF-κB and AP-1, targets of TLR-activated MAPKs and inhibitors of κB kinase, were enhanced by SIRP-α knockdown, as assessed by a luciferase reporter assay (Fig. 6A). LNCaP cells were pre-treated with NF-κB, p38 MAPK, ERK1/2 or JNK inhibitors, followed by SIRP-α knockdown. Notably, NF-κB and p38MAPK inhibitors could block SIRP-α knockdown induced COX-2 expression (Fig. 6B, P<0.001), indicating that SIRP-α decreased COX-2 expression by negatively regulating p38 MAPK and NF-κB.
Figure 6.
SIRP-α decreases COX-2 expression and cytokine production by negatively regulating p38 MAPK and NF-κB. (A) Cells were transfected with VT, KD and OV, together with the NF-κB or AP-1 reporter plasmids (0.2 µg), and the control plasmid pRL-TK (0.02 µg), and then luciferase activities were detected. (B) Cells were transfected with VT, KD, together with various key protein inhibitors, and then the expression of COX-2 were determined. SIRP, signal regulatory protein; COX-2, cyclooxygenase-2; MAPK, mitogen activated protein kinase; NF, nuclear factor; VT, control vector; KD, shRNA for SIRP-α; OV, pcDNA3.1-SIRP-α; AP-, activator protein-; pRL-TK, thymidine kinase promoter-Renilla luciferase reporter plasmid.
Discussion
SIRP-α levels were shown to be decreased in human CaP tissues. Specifically, SIRP-α expression was significantly lower in the AIPC group compared with ADPC cases. The abnormity of pro-apoptotic regulatory genes is considered a main factor in the AIPC transformation mechanism. Previous studies have demonstrated that polyinosinic-polycytidylic acid [poly (I:C)] induces apoptosis in the ADPC cell line LNCaP in a TLR3-dependent manner, but not in AIPC cell lines (14,15). Considering that suppression of SIRP-α expression by RNA interference results in enhanced apoptosis following poly (I:C) treatment (14), it was deduced that SIRP-α may be involved in AIPC transformation by regulating apoptosis.
SIRP-α has been shown to promote cell apoptosis in liver cancer (10) and breast carcinoma (9), but the molecular mechanism remains unclear. The present study confirmed that SIRP-α is involved in the regulation of cellular proliferation and apoptosis in ADPC and AIPC cell lines. Notably, the present study elucidated the negative regulatory effect of SIRP-α on COX-2 as the main cause of apoptosis promotion. COX-2 is an oncogenic protein, which is involved in numerous signaling pathways of apoptosis. Inhibition of COX-2 leads to reduced proliferation and induction of apoptosis, in connection with Bcl-2 downregulation and Bax upregulation (16). It has been previously demonstrated that COX-2 inhibits apoptosis in cancer cells by inducing a P53 mutation (17). Other studies indicated that COX-2 weakens the apoptotic signal mediated by the Fas protein (18,19). The above findings indicate that COX-2 is a negative regulator of apoptosis. In the present study, apoptosis was sharply reduced in the si-SIRP-α group, and co-transfection with si-COX-2 could block this apoptosis reduction, suggesting that SIRP-α regulated cell apoptosis was dependent on COX-2.
A number of past studies have demonstrated that chemokines, including CXCL2, CCL2, CCL3, and CCL5, are produced at the tumor site by CaP cells that also express their receptors, as well as the supporting tissues. These chemokines are likely to promote tumor development and angiogenesis (20,21). As demonstrated in the present study, CXCL2, CCL2 and CCL5 are overtly upregulated by SIRP-α knockdown. Lu et al (21) showed in 2006 that CCL2 expression correlates with pathology in human CaP. In addition, Izhak et al (20) demonstrated that effective neutralization of CCL2 could slow tumor development. Therefore, the present results provide another possible pathway for tumor inhibition by SIRP-α.
Previous studies have shown that all 3 JNK, ERK, and p38MAPK pathways can regulate COX-2 and cytokine expression. The present study, identified that JNK and ERK were upregulated along with p38MAPK and NF-κB, however, only p38MAPK and NF-κB inhibitors blocked COX-2 and cytokine expression change, indicating that SIRP-α affects CaP mainly through the p38MAPK/NF-κB pathway.
In conclusion, this is, to the best of our knowledge, the first study assessing the role of SIRP-α in CaP. SIRP-α may function as a novel negative regulator to modulate cellular proliferation, survival and migration in CaP cells. The heightened sensitivity of cells restoring SIRP-α function could be exploited in the development of therapeutics that may potentiate the antineoplastic effects of conventional cytokines or chemotherapeutic agents.
Acknowledgements
The present study was supported by the China Postdoctoral Science Foundation (grant no. 2015M572724) and the People's Liberation Army 309th Foundation (grant no. 2013MS-021).
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Ren SC, Chen R, Sun YH. Prostate cancer research in China. Asian J Androl. 2013;15:350–353. doi: 10.1038/aja.2013.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sooriakumaran P, Nyberg T, Akre O, Haendler L, Heus I, Olsson M, Carlsson S, Roobol MJ, Steineck G, Wiklund P. Comparative effectiveness of radical prostatectomy and radiotherapy in prostate cancer: Observational study of mortality outcomes. BMJ. 2014;348:g1502. doi: 10.1136/bmj.g1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kahn B, Collazo J, Kyprianou N. Androgen receptor as a driver of therapeutic resistance in advanced prostate cancer. Int J Biol Sci. 2014;10:588–595. doi: 10.7150/ijbs.8671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi X, Gipp J, Dries M, Bushman W. Prostate progenitor cells proliferate in response to castration. Stem Cell Res. 2014;13:154–163. doi: 10.1016/j.scr.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ianni M, Porcellini E, Carbone I, Potenzoni M, Pieri AM, Pastizzaro CD, Benecchi L, Licastro F. Genetic factors regulating inflammation and DNA methylation associated with prostate cancer. Prostate Cancer Prostatic Dis. 2013;16:56–61. doi: 10.1038/pcan.2012.30. [DOI] [PubMed] [Google Scholar]
- 7.Liu JB, Dai CM, Su XY, Cao L, Qin R, Kong QB. Gene microarray assessment of multiple genes and signal pathways involved in androgen-dependent prostate cancer becoming androgen independent. Asian Pac J Cancer Prev. 2014;15:9791–9795. doi: 10.7314/APJCP.2014.15.22.9791. [DOI] [PubMed] [Google Scholar]
- 8.Barclay AN, Brown MH. The SIRP family of receptors and immune regulation. Nat Rev Immunol. 2006;6:457–464. doi: 10.1038/nri1859. [DOI] [PubMed] [Google Scholar]
- 9.Hara K, Senga T, Biswas MH, Hasegawa H, Ito S, Hyodo T, Hirooka Y, Niwa Y, Goto H, Hamaguchi M. Recovery of anoikis in Src-transformed cells and human breast carcinoma cells by restoration of the SIRP α1/SHP-2 signaling system. Cancer Res. 2011;71:1229–1234. doi: 10.1158/0008-5472.CAN-10-3431. [DOI] [PubMed] [Google Scholar]
- 10.Yan HX, Wang HY, Zhang R, Chen L, Li BA, Liu SQ, Cao HF, Qiu XH, Shan YF, Yan ZH, et al. Negative regulation of hepatocellular carcinoma cell growth by signal regulatory protein alpha1. Hepatology. 2004;40:618–628. doi: 10.1002/hep.20360. [DOI] [PubMed] [Google Scholar]
- 11.Kong XN, Yan HX, Chen L, Dong LW, Yang W, Liu Q, Yu LX, Huang DD, Liu SQ, Liu H, et al. LPS-induced down-regulation of signal regulatory protein {alpha} contributes to innate immune activation in macrophages. J Exp Med. 2007;204:2719–2731. doi: 10.1084/jem.20062611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hinz M, Krappmann D, Eichten A, Heder A, Scheidereit C, Strauss M. NF-kappaB function in growth control: Regulation of cyclin D1 expression and G0/G1-to-S-phase transition. Mol Cell Biol. 1999;19:2690–2698. doi: 10.1128/MCB.19.4.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 14.Palchetti S, Starace D, de Cesaris P, Filippini A, Ziparo E, Riccioli A. Transfected poly((I:C)) activates different dsRNA receptors, leading to apoptosis or immunoadjuvant response in androgen-independent prostate cancer cells. J Biol Chem. 2015;290:5470–5483. doi: 10.1074/jbc.M114.601625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paone A, Starace D, Galli R, Padula F, de Cesaris P, Filippini A, Ziparo E, Riccioli A. Toll-like receptor 3 triggers apoptosis of human prostate cancer cells through a PKC-alpha-dependent mechanism. Carcinogenesis. 2008;29:1334–1342. doi: 10.1093/carcin/bgn149. [DOI] [PubMed] [Google Scholar]
- 16.Sun WH, Zhu F, Chen GS, Su H, Luo C, Zhao QS, Zhang Y, Shao Y, Sun J, Zhou SM, et al. Blockade of cholecystokinin-2 receptor and cyclooxygenase-2 synergistically induces cell apoptosis, and inhibits the proliferation of human gastric cancer cells in vitro. Cancer Lett. 2008;263:302–311. doi: 10.1016/j.canlet.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 17.Han JA, Kim JI, Ongusaha PP, Hwang DH, Ballou LR, Mahale A, Aaronson SA, Lee SW. P53-mediated induction of Cox-2 counteracts p53- or genotoxic stress-induced apoptosis. EMBO J. 2002;21:5635–5644. doi: 10.1093/emboj/cdf591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Casado M, Mollá B, Roy R, Fernández-Martinez A, Cucarella C, Mayoral R, Boscá L, Martin-Sanz P. Protection against Fas-induced liver apoptosis in transgenic mice expressing cyclooxygenase 2 in hepatocytes. Hepatology. 2007;45:631–638. doi: 10.1002/hep.21556. [DOI] [PubMed] [Google Scholar]
- 19.Nzeako UC, Guicciardi ME, Yoon JH, Bronk SF, Gores GJ. COX-2 inhibits Fas-mediated apoptosis in cholangiocarcinoma cells. Hepatology. 2002;35:552–559. doi: 10.1053/jhep.2002.31774. [DOI] [PubMed] [Google Scholar]
- 20.Izhak L, Wildbaum G, Weinberg U, Shaked Y, Alami J, Dumont D, Friedman B, Stein A, Karin N. Predominant expression of CCL2 at the tumor site of prostate cancer patients directs a selective loss of immunological tolerance to CCL2 that could be amplified in a beneficial manner. J Immunol. 2010;184:1092–1101. doi: 10.4049/jimmunol.0902725. [DOI] [PubMed] [Google Scholar]
- 21.Lu Y, Cai Z, Galson DL, Xiao G, Liu Y, George DE, Melhem MF, Yao Z, Zhang J. Monocyte chemotactic protein-1 (MCP-1) acts as a paracrine and autocrine factor for prostate cancer growth and invasion. Prostate. 2006;66:1311–1318. doi: 10.1002/pros.20464. [DOI] [PubMed] [Google Scholar]