Abstract
To date, no comprehensive prognostic or predictive marker profiling analysis has been performed in association with the age of patients with breast cancer. In the present study, 632 breast cancer tissue samples were analyzed for expression of estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2), B-cell lymphoma (Bcl)-2 protein, HER2 gene amplification, proliferation [as evaluated by proliferating cell nuclear antigen (PCNA) and Ki-67 index], tumor grade, histological type and molecular subtype. The data revealed correlations with the age of patients. A statistically significant positive correlation was identified between patient age and expression of ER (P<0.0001). There was no significant association between patient age and PR, HER2 protein expression, HER2 gene amplification or PCNA. A significant negative correlation between age and Ki-67 expression (P<0.0001) as well as grade of tumor (P=0.007) was identified. The spectrum of molecular subtypes differed according to age (P=0.0003). The highest incidence of aggressive triple-negative and HER2-positive breast cancer was present in patients aged between 20 and 39 years. Luminal A subtype was the most frequent cancer subtype in patients from age 40 onwards, where proliferation activity declined with age and expression of hormone receptors increased along with Bcl-2 expression. Aggressive forms of breast cancer were more common in younger patients. Prognostic and predictive markers have a complex age-specific distribution. The findings of less aggressive luminal A and B subtypes in older patients, and the positive correlation with ER, PR and Bcl-2 expression reveal the potential efficacy of Bcl-2 as a marker of hormone responsiveness in these patients.
Keywords: breast cancer, age-associated expression, biomarkers, B-cell lymphoma 2, molecular subtypes
Introduction
Breast cancer is a heterogeneous group of diseases that develops from the mammary gland. It varies in morphology, biological characteristics, behavior and response to therapy (1). However, the complex associations between various tumor characteristics and patient age at the time of diagnosis have not yet been elucidated. A large number of risk factors for breast cancer have now been identified. These include: Geographic variations; race and ethnicity (2,3); prolonged exposure to exogenous estrogens post-menopausally (including hormone replacement therapy used in the prevention of osteoporosis) (4–7); obesity (due to estrogens produced by the adipose tissue) (8–10); alcohol abuse (11); genetic inheritance (mutations in breast cancer 1 and 2) (12–14); lack of physical activity (15); ionizing radiation to the chest (depending on radiation dose, age and time following exposure) (16–19); and early age at menarche or late age of pregnancy and menopause (20).
It is also established that the incidence of breast cancer increases with age. The number of elderly patients with breast cancer is increasing and the majority of females who succumb to breast cancer are >65 years old (21). However, older patients are more likely to present with tumors that are estrogen receptor (ER)- and progesterone receptor (PR)-positive and human epidermal growth factor receptor 2 (HER2)-negative, and these tumors are associated with improved prognosis and clinical outcomes (22,23). By contrast, younger patients with triple-negative and HER2-positive breast cancers have an increased risk of relapse within 5 years of diagnosis (24). Breast cancer that arises in young females is associated with reduced survival and higher incidence of unfavorable prognostic and predictive tumor markers (25–28).
Using gene expression analysis, breast cancer is able to be divided into six intrinsic molecular subtypes: Luminal A (ER+ and/or PR+, HER2− and Ki-67 <14%), luminal B (ER+ and/or PR+, HER2− and Ki-67 ≥14%, or ER+ and/or PR+ and HER2+), HER2-enriched (ER−, PR− and HER2+), basal-like/triple-negative (ER−, PR− and HER2−), normal breast-like and claudin-low [cluster of differentiation (CD)44+ and CD24− or low] (29,30). Molecular subtyping using four biomarkers (ER, PR, HER2 and Ki-67) and dividing tumors into four subtypes (basal cell-like, HER2 positive luminal A and luminal B) also provide clinically useful information concerning the biology of tumors and their clinical behavior. Therefore, they have been proposed for use in determining the efficacy of therapy and surveillance strategies (31–37).
To the best of our knowledge, no comprehensive prognostic or predictive marker analysis has been performed to date in association with age in patients with breast cancer. The present study therefore aims to correlate the comprehensive basic clinicopathological data with age.
Materials and methods
Patients and diagnostic tests
The present study analyzed the age-specific presence of prognostic and predictive markers in a sample of 632 formalin-fixed, paraffin-embedded breast cancer samples obtained from core-cut biopsies or mastectomies performed at the Department of Clinical and Molecular Pathology, University Hospital, Palacky University (Olomouc, Czech Republic) between January 2010 and April 2014, using standard immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH). The present study was approved by the Ethics Committee of the University Hospital and the Faculty of Medicine and Dentistry of Palacky University. Informed consent was obtained from patients for the use of their tissues. The median patient age was 65 years (range, 26–95 years). Sections of breast cancer samples (5-µm thick) were used for examination of ER and PR expression, HER2 protein expression, markers of proliferation [including proliferating cell nuclear antigen (PCNA) and Ki-67], and B-cell lymphoma (Bcl)-2 and HER2 gene amplification. The clinicopathological data were obtained from the primary pathology reports. All findings were verified by two independent pathologists of the Department of Clinical and Molecular Pathology, University Hospital, Palacky University.
Immunohistochemistry (IHC)
The protocol for IHC was as follows: Slides were deparaffinized, exposed to heat-induced antigen retrieval in a microwave oven for 15 min at 121°C in a 10 mM sodium citrate buffer (pH 6.0; cat. no. C8532; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), and endogenous peroxidase activity was blocked by incubation with a 5% hydrogen peroxide blocking solution (0.01 M PBS, pH 7.4, containing 0.01% thimerosal) for 10 min. The sections were incubated with diluted primary antibodies for 60 min at room temperature (RT) and subsequently with the secondary antibody Dako EnVision+ Dual Link System-HRP (cat. no. K4061; Agilent Technologies, Inc., Santa Clara, CA, USA) for 60 min at room temperature. The Dako Liquid DAB+ Substrate Chromogen System (cat. no. K3468; Agilent Technologies, Inc., Santa Clara, CA, USA) was used for the visualization according the manufacture's protocol. Sections were then counterstained with hematoxylin, dehydrated, cleared, mounted and covered. IHC evaluation of ER expression was performed using monoclonal mouse anti-human primary antibody, clone 1D5 (cat. no. M7047; dilution, 1:20; Dako; Agilent Technologies, Inc., Santa Clara, CA, USA). PR expression was determined by monoclonal mouse anti-human antibody, clone PgR 636 (cat. no. M3569; dilution, 1:100; Dako; Agilent Technologies, Inc., Santa Clara, CA, USA). The proliferative markers PCNA and Ki-67 were detected using monoclonal mouse anti-PCNA, clone PC10 (cat. no. M0879; dilution, 1:1,500; Dako; Agilent Technologies, Inc.) for PCNA and monoclonal mouse anti-human Ki-67 antigen, clone MIB-1 (cat. no. M7240; dilution, 1:200; Dako; Agilent Technologies, Inc.) for Ki-67. Bcl-2 was determined by anti-Bcl-2 oncoprotein, clone 100, which reacts with Bcl-2 alpha oncoprotein (Cat. no. AM287-10M; dilution, 1:10; BioGenex; Fremont, CA, USA). Hormone receptors (ER and PR), PCNA, Ki-67 and Bcl-2 were evaluated using the histological score (H-score) as follows: Percentage of positive cells × intensity of staining (1, 2 or 3). The age distribution of the analyzed markers was evaluated. Due to the small number of patients aged 20–29 and 90–99 years, the 20–29 years group was combined with the 30–39 years group and the 80–89 years group was combined with the 90–99 years group for statistical evaluation. HER2 protein expression was determined according manufacturer's protocol using the in vitro diagnostic certified kit HercepTest™ (Dako; Agilent Technologies, Inc., Catalogue No. K5204). The expression of HER2 was scored on a qualitative scale from 0 to 3+ according to the Dako manual (Agilent Technologies, Inc.) and the guidelines for HER2 testing in breast cancer from the American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP) (38). A score of 0 or 1+ was assessed as negative, 2+ as moderately positive and 3+ as positive (uniform intense staining of >30% of invasive tumor cells). IHC with HercepTest™ and anti-hormone receptor primary antibodies were performed on the invasive breast cancer tissue samples.
Fluorescence in situ hybridization
The HER2 gene status was assessed using FISH analysis, which was performed according to the manufacturer's protocol on formalin-fixed, paraffin-embedded tissues. Locus-specific identifier HER2/neu (Spectrum Orange™) and chromosome 17 centromere (CEP17; Spectrum Green™) probes (cat. no. IM_001; IntellMed, Ltd., Olomouc, Czech Republic) were used for gene/chromosome copy number enumeration. The signals were observed and counted using fluorescence microscopy. At least 100 non-overlapping nuclei were selected in each sample. Cut-off levels were determined according to the ASCO/CAP recommendations. A HER2/CEP17 ratio of >2.2 was considered as positive (38,39).
Statistical analysis
The data were evaluated using IBM SPSS version 22.0 (IBM SPSS, Armonk, NY, USA). The correlation analysis for ER, PR, PCNA, Ki-67, Bcl-2, HER2 protein (using IHC) and HER2 gene (using FISH) expression with age and tumor grade was performed using the Spearman's rank correlation coefficient. The associations between ER, PR, PCNA, Bcl-2, Ki-67, grade, HER2 protein and HER2 gene with histological type and molecular subtype were evaluated using the Kruskal-Wallis test. Mann-Whitney U-tests with Bonferroni correction were used for pairwise comparisons. The data distribution was presented using box graphs. To examine the correlations between HER2 protein expression and molecular subtype or between histological type and molecular subtype, Fisher's exact test was used. P<0.05 was considered to indicate a statistically significant difference.
Results
Age-specific associations with hormone receptors
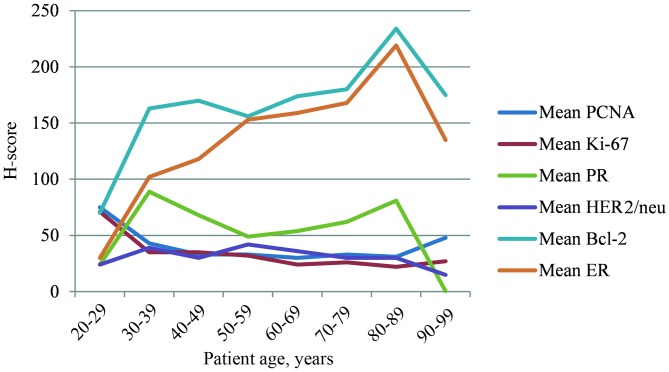
The present study identified a significant positive correlation between age and ER expression, between ER and PR expression and between Bcl-2 expression and molecular subtypes (all P<0.0001). By contrast, an inverse association between ER expression and the grade of tumor (P<0.0001), amplification of the HER2 gene [all P<0.0001; odds ratio (OR), 1.003; 95% confidence interval (CI), 1.000–1.005] and the markers of proliferation PCNA and Ki-67 (P<0.0001) was detected. No statistically significant correlation between age and PR expression was identified; however, there were positive associations between PR expression and the expression of HER2 protein (P=0.001), Bcl-2 protein (P<0.0001) and molecular subtypes (P<0.0001). By contrast, there were inverse associations with tumor grade (P<0.0001), PCNA (P=0.004) and Ki-67 (P<0.0001). The highest levels of ER and Bcl-2 expression were observed in patients aged 70–79 years old, whereas PR expression was highest in patients aged 30–39 years old (Fig. 1).
Figure 1.
Mean histological score (H-score) of PCNA, Ki-67, PR, HER2, Bcl-2 and ER in age decades. PCNA, proliferating cell nuclear antigen; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; Bcl, B-cell lymphoma; ER, estrogen receptor.
Age-specific associations with HER2 protein expression
A statistically significant positive correlation between HER2 protein expression and PR expression (P=0.001), amplification of the HER2 gene (P<0.0001; OR, 1,290; 95% CI, 1.000–1.665) and tumor grade (P=0.0002), and a negative correlation between HER2 expression and Bcl-2 expression (P=0.003) were identified. No statistically significant negative correlation was identified between age and HER2 protein expression (P=0.159).
Age-specific associations with proliferative markers
A significant negative correlation was identified between age and Ki-67 expression (P<0.0001). Ki-67 also exhibited an inverse association with Bcl-2 (P<0.0001), and was associated with tumor grade (P<0.0001), HER2 protein expression (P=0.032), HER2 gene amplification (P=0.007), PCNA expression (P<0.0001), histological type (P<0.0001) and molecular subtype (P<0.0001). No significant associations between age and PCNA expression were identified.
Age-specific associations with Bcl-2 expression
A statistically significant positive correlation between Bcl-2 and hormone receptor expression and molecular subtype was identified. High levels of Bcl-2 expression in luminal A and luminal B subtypes were observed in comparison with the HER2+ and triple-negative breast cancer (TNBC) molecular subtypes. The significant correlation between Bcl-2 expression levels and hormone receptor expression, demonstrates that Bcl-2 is a potential effective marker of breast cancer hormonal responsiveness. By contrast, an inverse association was identified between Bcl-2 and HER2 protein expression levels and proliferative markers.
Age-specific associations between tumor grade and histological type
A statistically significant positive correlation between tumor grade and HER2 protein expression (P=0.0002), Ki-67 (P<0.0001) and molecular subtype (P<0.0001) was identified. Conversely an inverse association was detected between tumor grade and hormone receptor (ER and PR) expression levels. In the present study of 632 breast cancer tissue samples, the following distribution of histological types was observed: Invasive ductal breast cancer [invasive cancer of no special type according to the WHO Classification of Tumors of the Breast (1)], 82.0%; in situ ductal breast cancer, 9.7%; invasive lobular breast cancer, 5.8%; breast cancers with poor prognosis, including metaplastic and micropapillary breast cancer, 0.5%; medullary breast cancer, 0.3%; and other types of breast cancer with improved prognosis (tubular, mucinous, cribriform and papillary; 1.7%) (Table I). The highest incidence of invasive and non-invasive ductal breast cancer cases was observed in patients aged 60–69 years (mean age, 65 years). The occurrence of these types of breast cancer was predominant also in younger patients (<50 years old), and these two histological types exhibited the highest levels of HER2 expression. The incidence of invasive lobular breast cancer increased between the ages of 50 and 70 years.
Table I.
Age-associated distribution of tumor histological types.
| Histological typea | ||||||||
|---|---|---|---|---|---|---|---|---|
| Age, years | Number of patients | Mean age, years | 1 | 2 | 3 | 4 | 5 | 6 |
| 20–29 | 4 | 29 | 4 | 0 | 0 | 0 | 0 | 0 |
| 30–39 | 30 | 36 | 25 | 4 | 1 | 0 | 0 | 0 |
| 40–49 | 79 | 46 | 66 | 8 | 4 | 0 | 0 | 1 |
| 50–59 | 158 | 56 | 130 | 16 | 8 | 0 | 1 | 3 |
| 60–69 | 203 | 65 | 163 | 25 | 10 | 2 | 0 | 3 |
| 70–79 | 111 | 75 | 88 | 7 | 11 | 1 | 1 | 3 |
| 80–89 | 41 | 84 | 36 | 1 | 3 | 0 | 0 | 1 |
| 90–99 | 6 | 93 | 6 | 0 | 0 | 0 | 0 | 0 |
| Total | 632 | 61 | 518 | 61 | 37 | 3 | 2 | 11 |
1, Invasive breast cancer of no special type according to the WHO classification (formerly termed invasive ductal cancer); 2, in situ ductal breast cancer; 3, invasive lobular breast cancer; 4, breast cancer with a poor prognosis (metaplastic and micropapillary); 5, medullary breast cancer; 6, other types of breast cancer with a good prognosis (tubular, mucinous, cribriform and papillary).
Age-specific associations with molecular subtypes
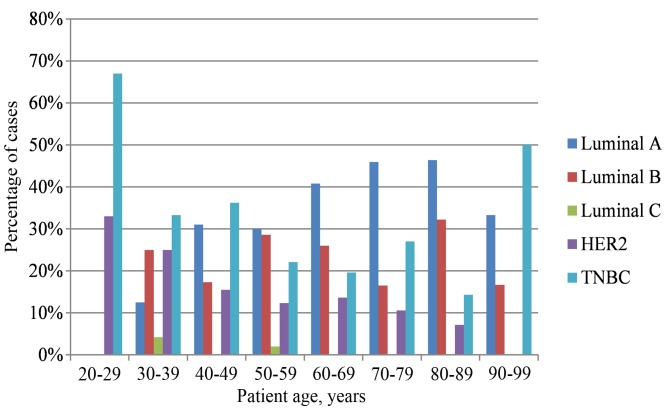
The distribution of molecular subtypes was as follows: 35% luminal A, 25% luminal B, 1% luminal C, 14% HER2+ and 25% TNBC. The highest incidence of TNBC and HER2-positive breast cancer was detected in the youngest patient groups (20–39 years old). However, in patients aged ≥40 years old, the luminal A molecular subtype was most commonly observed (Table II and Fig. 2). The age of patients with luminal A breast cancer was statistically significantly higher compared with that of patients with the HER2+ and TNBC molecular subtypes (P<0.0001). Statistically significant positive correlations between molecular subtypes and ER expression (P<0.0001), PR expression (P<0.0001), Ki-67 expression (P<0.0001), PCNA expression (P<0.0001), tumor grade (P<0.0001), histological type (P<0.0001), expression of HER2 protein (P<0.0001), and amplification of the HER2 gene (P<0.0001) and the Bcl-2 proto-oncogene (P<0.0001) were also observed. Those breast cancer cases that were identified as luminal A molecular subtype were exclusively composed of the most favorable histological types in terms of prognosis, including mucinous, tubular and papillary cribriform breast cancer. Luminal B molecular subtype cancer cases had a significantly higher grade than luminal A cancer cases (P=0.001) and a significantly lower grade than HER2+ cases (P<0.0001). The distribution of invasive and non-invasive ductal breast cancer [invasive ductal carcinoma (IDC) or ductal carcinoma in situ (DCIS)] between molecular subtypes was more heterogeneous (IDC: 35.8% luminal A, 24.7% luminal B, 10.9% HER2+ and 27.9% TNBC; and DCIS: 26.7% luminal A, 26.7% luminal B, 33.3% HER2+ and 11.7% TNBC). In the IDC and DCIS groups, the highest incidence of HER2 positivity was identified. The overexpression and amplification of HER2 were significantly higher in HER2+ and luminal B molecular subtypes compared with the other molecular subtypes. The distribution of invasive and non-invasive lobular breast cancer was divided between the luminal A (53.3%), luminal B (33.3%) and HER2+ (13.3%) subtypes. The expression levels of Ki-67 and PCNA were significantly higher in the HER2+ and TNBC molecular subtypes than in the two luminal subtypes.
Table II.
Age-associated distribution of molecular subtypes.
| Age, years | Luminal A, % | Luminal B, % | Luminal C, % | HER2, % | TNBC, % |
|---|---|---|---|---|---|
| 20–29 | 0.00 | 0.00 | 0.00 | 33.00 | 67.00 |
| 30–39 | 12.50 | 25.00 | 4.20 | 25.00 | 33.30 |
| 40–49 | 31.00 | 17.30 | 0.00 | 15.50 | 36.20 |
| 50–59 | 30.00 | 28.60 | 2.00 | 12.30 | 22.10 |
| 60–69 | 40.80 | 26.00 | 0.00 | 13.60 | 19.60 |
| 70–79 | 45.90 | 16.50 | 0.00 | 10.60 | 27.00 |
| 80–89 | 46.40 | 32.20 | 0.00 | 7.10 | 14.30 |
| 90–99 | 33.30 | 16.70 | 0.00 | 0.00 | 50.00 |
HER2, human epidermal growth factor receptor 2; TNBC, triple-negative breast cancer.
Figure 2.
Graphical presentation of age-associated distribution of molecular subtypes. HER2, human epidermal growth factor receptor 2; TNBC, triple-negative breast cancer.
Discussion
The present study aimed to elucidate the associations between common clinicopathological characteristics of breast cancer and the patient age distribution. The results revealed that these prognostic and predictive markers have an age-specific distribution. More aggressive breast cancers were observed primarily in younger patients (20–39 years old), whereas the breast cancer types with improved prognosis were associated with older patients (≥40 years old). Proliferative activity declined with age, and the expression of hormone receptors and Bcl-2 increased with age. Young females exhibited tumors with a higher grade and HER2+ and TNBC molecular subtypes. To the best of our knowledge, no comprehensive analysis of all these characteristics has been performed to date, and there are limited previous studies concerning this subject. Diab et al (22) described the association between increasing age at the time of diagnosis and HER2 protein expression in patients with breast cancer who were ≥55 years old. Benz (25) compared the biology of early-onset with late-onset breast cancer, and demonstrated that late-onset tumors develop more slowly and are biologically less aggressive than early-onset tumors. Anders et al (40) revealed that young females have lower ER positivity, larger tumors, higher levels of human epidermal growth factor receptor 2 expression, higher-grade tumors, lymph node positivity and a tendency towards reduced disease-free survival.
In the present study, the age of patients with luminal A breast cancer was significantly higher compared with that of patients with the HER2+ and TNBC molecular subtypes. Furthermore, de Kruijf et al (41) also identified a statistically significant association (P=0.02) between patient age and tumor molecular subtype with luminal tumors being more frequently identified in elderly patients, whereas HER2+, basal-like and unclassified subtypes were more commonly detected in younger patients. A similar trend was described in the study by Park et al (33), in which poor patient outcomes were associated with an increased frequency of triple-negative/HER2 subtypes and more aggressive clinical behavior in young patients, in contrast to ER-positive tumors in older patients. Jenkins et al (42) examined the association between age and subtype, and recurrence-free, disease-specific and overall survival in older females. In this previous study, the incidence of luminal subtypes increased with age and had improved outcomes compared with those of basal-cell like and HER2-enriched subtypes. Prat et al (43) reported that HER2-positive breast cancer cases had a higher frequency compared with the HER-negative types of breast cancer of the HER2-enriched subtype (47.0 vs. 7.1%) and a lower frequency of the basal-like (14.1 vs. 23.4%) and luminal A (10.7 vs. 39.0%) subtypes. In this previous study, the HER2 gene and protein expression levels were statistically significantly higher in the HER2-enriched and basal-like subtypes compared with those in the luminal subtype.
In the present cohort of patients, the distribution of particular molecular subtypes was 35% luminal A, 25% luminal B, 1% luminal C, 14% HER2+ and 25% TNBC. The expression of Ki-67 and PCNA was significantly higher in HER2+ and TNBC subtypes compared with that in either luminal subtype. By contrast, Ihemelandu et al (26) classified breast cancer into four molecular subtypes (basal cell-like, HER2/neu, luminal A and luminal B) and analyzed the prevalence and clinicopathological associations for these molecular subtypes in pre- and post-menopausal African-American females. The luminal A type was the most prevalent (55.4%), whereas the basal cell-like form was the most prevalent in the age group <35 years old, and also exhibited an age-specific bimodal distribution, with a peak in patients aged <35 and 51–65 years old (26). The basal cell-like and HER2+ subtypes had a stronger association with a more aggressive clinical course than the luminal A subtype (26). Park et al (33,44) revealed that luminal A tumors were well differentiated and more frequently co-expressed hormone receptors than the luminal B type. Patients with TNBC tumors were younger at the time of diagnosis and had larger, more undifferentiated tumors with a higher proliferation rate and frequent visceral metastases (33).
A previous study made notable progress in aiding the understanding of the role of the pro-survival protein Bcl-2, which has an important role in regulating the pro-apoptotic effector proteins Bcl-2 homologous antagonist/killer and Bcl-2-associated X protein, and also neutralizes a group of sensor proteins, including Bcl-2-like protein 11, which are triggered by cytotoxic stimuli such as chemotherapy (45). The correlation between Bcl-2 expression and patient outcome has been the focus of a number of studies on primary breast cancer and, paradoxically, Bcl-2 was identified to be a marker of improved prognosis (45) across molecular subtypes (46–49). The explanation for this paradox may be that Bcl-2 is an estrogen-responsive gene (50) or that high levels of pro-apoptotic Bcl-2 trigger mitochondrial priming (51). However, a previous study reported that Bcl-2 expression is an independent factor predicting poor prognosis in patients with hormone receptor-negative breast cancer or TNBC who did not undergo adjuvant therapy, particularly in post-menopausal females (52). The present study reveals high expression levels of Bcl-2 in the luminal A and luminal B subtypes of breast cancer in comparison with that in the HER2+ and triple-negative molecular subtypes. These results are concordant with those from Seong et al (53), which also described a significant association between Bcl-2-positive tumors, and a younger patient age, early stage, lower grade, positive expression of ER and PR, and negative expression of HER2. Patients with Bcl-2/ER/PR-positive and HER2-negative tumors in this previous study also exhibited an improved prognosis (53). A significant correlation between Bcl-2 expression levels and hormone receptor status demonstrates that Bcl-2 is a potential effective marker of hormonal responsiveness in patients with ER/PR positive breast cancer.
The present study provides a comprehensive look at natural relations between levels of the most important breast cancer prognostic and predictive biomarkers and the age of the patients. It was demonstrated that there was an age-specific distribution in the breast cancer patient population, and therefore suggested the significance of age as an additional factor for an increase in the reliability of estimation of disease progression. The present study seeks to encourage oncologists to recognize Bcl-2 expression in estrogen receptor positive breast cancer samples as a reliable indicator of the functional estrogen driven axis for patients being considered for hormonal treatment.
Acknowledgements
The authors thank Dr Kateřina Langová, Department of Medical Biophysics, Faculty of Medicine and Dentistry, Palacky University, Olomouc, Czech Republic, for statistical analysis. The present study was funded by the Czech Agency for Health Research (grant no. 16-31997A).
References
- 1.Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, van de Vijver MJ. International Agency for Research on Cancer. Lyon: 2012. WHO Classification of Tumours of the Breast. [DOI] [Google Scholar]
- 2.Clarke CA, Keegan TH, Yang J, Press DJ, Kurian AW, Patel AH, Lacey JV., Jr Age-specific incidence of breast cancer subtypes: Understanding the black-white crossover. J Natl Cancer Inst. 2012;104:1094–1101. doi: 10.1093/jnci/djs264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurian AW, Fish K, Shema SJ, Clarke CA. Lifetime risks of specific breast cancer subtypes among women in four racial/ethnic groups. Breast Cancer Res. 2010;12:R99. doi: 10.1186/bcr2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olsson HL, Ingvar C, Bladström A. Hormone replacement therapy containing progestins and given continuously increases breast carcinoma risk in Sweden. Cancer. 2003;97:1387–1392. doi: 10.1002/cncr.11205. [DOI] [PubMed] [Google Scholar]
- 5.Clavel-Chapelon F, Hill C. Hormone replacement therapy in menopause and risk of breast cancer. Presse Med. 2000;29:1688–1693. (In French) [PubMed] [Google Scholar]
- 6.Bae JM, Kim EH. Hormone replacement therapy and risk of breast cancer in Korean women: A quantitative systematic review. J Prev Med Public Health. 2015;48:225–230. doi: 10.3961/jpmph.15.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Predná L, Habánová M, Sláviková E, Wyka J. Hormonal contraceptives and hormone replacement therapy as a possible factor of breast cancer. Rocz Panstw Zakl Hig. 2015;66:269–274. [PubMed] [Google Scholar]
- 8.Orecchioni S, Reggiani F, Talarico G, Bertolini F. Mechanisms of obesity in the development of breast cancer. Discov Med. 2015;20:121–128. [PubMed] [Google Scholar]
- 9.Bertolini F. Adipose tissue and breast cancer progression: A link between metabolism and cancer. Breast. 2013;22:S48–S49. doi: 10.1016/j.breast.2013.07.009. (Suppl 2) [DOI] [PubMed] [Google Scholar]
- 10.Bertolini F, Petit JY, Kolonin MG. Stem cells from adipose tissue and breast cancer: Hype, risks and hope. Br J Cancer. 2015;112:419–423. doi: 10.1038/bjc.2014.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jayasekara H, MacInnis RJ, Room R, English DR. Long-term alcohol consumption and breast, upper aero-digestive tract and colorectal cancer risk: A systematic review and meta-Analysis. Alcohol Alcohol. 2016;51:315–330. doi: 10.1093/alcalc/agv110. [DOI] [PubMed] [Google Scholar]
- 12.van den Broek AJ, Schmidt MK, van't Veer LJ, Tollenaar RA, van Leeuwen FE. Worse breast cancer prognosis of BRCA1/BRCA2 mutation carriers: What's the evidence? A systematic review with meta-analysis. PLoS One. 2015;10:e0120189. doi: 10.1371/journal.pone.0120189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Llort G, Peris M, Blanco I. Hereditary breast and ovarian cancer: Primary and secondary prevention for BRCA1 and BRCA2 mutation carriers. Med Clin (Barc) 2007;128:468–476. doi: 10.1157/13100569. (In Spanish) [DOI] [PubMed] [Google Scholar]
- 14.Calderon-Margalit R, Paltiel O. Prevention of breast cancer in women who carry BRCA1 or BRCA2 mutations: A critical review of the literature. Int J Cancer. 2004;112:357–364. doi: 10.1002/ijc.20429. [DOI] [PubMed] [Google Scholar]
- 15.Olsen CM, Wilson LF, Nagle CM, Kendall BJ, Bain CJ, Pandeya N, Webb PM, Whiteman DC. Cancers in Australia in 2010 attributable to insufficient physical activity. Aust N Z J Public Health. 2015;39:458–463. doi: 10.1111/1753-6405.12469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drooger JC, Hooning MJ, Seynaeve CM, Baaijens MH, Obdeijn IM, Sleijfer S, Jager A. Diagnostic and therapeutic ionizing radiation and the risk of a first and second primary breast cancer, with special attention for BRCA1 and BRCA2 mutation carriers: A critical review of the literature. Cancer Treat Rev. 2015;41:187–196. doi: 10.1016/j.ctrv.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Tang J, Fernandez-Garcia I, Vijayakumar S, Martinez-Ruis H, Illa-Bochaca I, Nguyen DH, Mao JH, Costes SV, Barcellos-Hoff MH. Irradiation of juvenile, but not adult, mammary gland increases stem cell self-renewal and estrogen receptor negative tumors. Stem Cells. 2014;32:649–661. doi: 10.1002/stem.1533. [DOI] [PubMed] [Google Scholar]
- 18.Clemons M, Loijens L, Goss P. Breast cancer risk following irradiation for Hodgkin's disease. Cancer Treat Rev. 2000;26:291–302. doi: 10.1053/ctrv.2000.0174. [DOI] [PubMed] [Google Scholar]
- 19.Haffty BG. Radiation therapy and the risk of contralateral breast cancer. Int J Radiat Oncol Biol Phys. 2003;56:920–921. doi: 10.1016/S0360-3016(03)00204-9. [DOI] [PubMed] [Google Scholar]
- 20.Kato I, Tominaga S, Suzuki T. Factors related to late menopause and early menarche as risk factors for breast cancer. Jpn J Cancer Res. 1988;79:165–172. doi: 10.1111/j.1349-7006.1988.tb01573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Altekruse SF, Kosary CL, Krapcho M, Neyman N, Aminou R, Waldron W, Ruhl J, Howlader N, Tatalovich Z, Cho H, et al. SEER Cancer Statistics Review, 1975–2007. Bethesda, MD: National Cancer Institute; 2010. [Google Scholar]
- 22.Diab SG, Elledge RM, Clark GM. Tumor characteristics and clinical outcome of elderly women with breast cancer. J Natl Cancer Inst. 2000;92:550–556. doi: 10.1093/jnci/92.7.550. [DOI] [PubMed] [Google Scholar]
- 23.Jenkins EO, Deal AM, Anders CK, Prat A, Perou CM, Carey LA, Muss HB. Age-specific changes in intrinsic breast cancer subtypes: A focus on older women. Oncologist. 2014;19:1076–1083. doi: 10.1634/theoncologist.2014-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walter LC, Covinsky KE. Cancer screening in elderly patients: A framework for individualized decision making. JAMA. 2001;285:2750–2756. doi: 10.1001/jama.285.21.2750. [DOI] [PubMed] [Google Scholar]
- 25.Benz CC. Impact of aging on the biology of breast cancer. Crit Rev Oncol Hematol. 2008;66:65–74. doi: 10.1016/j.critrevonc.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ihemelandu CU, Leffall LD, Jr, Dewitty RL, Naab TJ, Mezghebe HM, Makambi KH, Adams-Campbell L, Frederick WA. Molecular breast cancer subtypes in premenopausal and postmenopausal African-American women: Age-specific prevalence and survival. J Surg Res. 2007;143:109–118. doi: 10.1016/j.jss.2007.03.085. [DOI] [PubMed] [Google Scholar]
- 27.Eppenberger-Castori S, Moore DH, Jr, Thor AD, Edgerton SM, Kueng W, Eppenberger U, Benz CC. Age-associated biomarker profiles of human breast cancer. Int J Biochem Cell Biol. 2002;34:1318–1330. doi: 10.1016/S1357-2725(02)00052-3. [DOI] [PubMed] [Google Scholar]
- 28.Quong J, Eppenberger-Castori S, Moore D, III, Scott GK, Birrer MJ, Kueng W, Eppenberger U, Benz CC. Age-dependent changes in breast cancer hormone receptors and oxidant stress markers. Breast Cancer Res Treat. 2002;76:221–236. doi: 10.1023/A:1020886801674. [DOI] [PubMed] [Google Scholar]
- 29.Camerlingo R, Ferraro GA, De Francesco F, Romano M, Nicoletti G, Di Bonito M, Rinaldo M, D'Andrea F, Pirozzi G. The role of CD44+/CD24-/low biomarker for screening, diagnosis and monitoring of breast cancer. Oncol Rep. 2014;31:1127–1132. doi: 10.3892/or.2013.2943. [DOI] [PubMed] [Google Scholar]
- 30.Gudadze M, Kankava Q, Mariamidze A, Burkadze G. Features of CD44+/CD24-low phenotypic cell distribution in relation to predictive markers and molecular subtypes of invasive ductal carcinoma of the breast. Georgian Med News. 2014;228:81–87. [PubMed] [Google Scholar]
- 31.Eroles P, Bosch A, Pérez-Fidalgo JA, Lluch A. Molecular biology in breast cancer: Intrinsic subtypes and signaling pathways. Cancer Treat Rev. 2012;38:698–707. doi: 10.1016/j.ctrv.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 32.Morrison DH, Rahardja D, King E, Peng Y, Sarode VR. Tumour biomarker expression relative to age and molecular subtypes of invasive breast cancer. Br J Cancer. 2012;107:382–387. doi: 10.1038/bjc.2012.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park S, Koo JS, Kim MS, Park HS, Lee JS, Lee JS, Kim SI, Park BW. Characteristics and outcomes according to molecular subtypes of breast cancer as classified by a panel of four biomarkers using immunohistochemistry. Breast. 2012;21:50–57. doi: 10.1016/j.breast.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 34.Reis-Filho JS, Tutt AN. Triple negative tumours: A critical review. Histopathology. 2008;52:108–118. doi: 10.1111/j.1365-2559.2007.02889.x. [DOI] [PubMed] [Google Scholar]
- 35.Rakha EA, Ellis IO. Triple-negative/basal-like breast cancer: Review. Pathology. 2009;41:40–47. doi: 10.1080/00313020802563510. [DOI] [PubMed] [Google Scholar]
- 36.Prat A, Parker JS, Karginova O, Fan C, Livasy C, Herschkowitz JI, He X, Perou CM. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res. 2010;12:R68. doi: 10.1186/bcr2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Santos C, Sanz-Pamplona R, Nadal E, Grasselli J, Pernas S, Dienstmann R, Moreno V, Tabernero J, Salazar R. Intrinsic cancer subtypes-next steps into personalized medicine. Cell Oncol (Dordr) 2015;38:3–16. doi: 10.1007/s13402-014-0203-7. [DOI] [PubMed] [Google Scholar]
- 38.Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. Arch Pathol Lab Med. 2014;138:241–256. doi: 10.5858/arpa.2013-0953-SA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.LSI Her-2/neu (Orange)/CEP 17 (Green) Users guide. Olomouc; Czech Republic: 2006. Intellmed s.r.o. [Google Scholar]
- 40.Anders CK, Hsu DS, Broadwater G, Acharya CR, Foekens JA, Zhang Y, Wang Y, Marcom PK, Marks JR, Febbo PG, et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol. 2008;26:3324–3330. doi: 10.1200/JCO.2007.14.2471. [DOI] [PubMed] [Google Scholar]
- 41.de Kruijf EM, Bastiaannet E, Rubertá F, de Craen AJ, Kuppen PJ, Smit VT, van de Velde CJ, Liefers GJ. Comparison of frequencies and prognostic effect of molecular subtypes between young and elderly breast cancer patients. Mol Oncol. 2014;8:1014–1025. doi: 10.1016/j.molonc.2014.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jenkins EO, Deal AM, Anders CK, Prat A, Perou CM, Carey LA, Muss HB. Age-specific changes in intrinsic breast cancer subtypes: A focus on older women. Oncologist. 2014;19:1076–1083. doi: 10.1634/theoncologist.2014-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prat A, Carey LA, Adamo B, Vidal M, Tabernero J, Cortés J, Parker JS, Perou CM, Baselga J. Molecular features and survival outcomes of the intrinsic subtypes within HER2-positive breast cancer. J Natl Cancer Inst. 2014;106:dju152. doi: 10.1093/jnci/dju152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park YH, Lee SJ, Jung HA, Kim SM, Kim MJ, Kil WH, Lee JE, Nam SJ, Ahn JS, Im YH. Prevalence and clinical outcomes of young breast cancer (YBC) patients according to intrinsic breast cancer subtypes: Single institutional experience in Korea. Breast. 2015;24:213–217. doi: 10.1016/j.breast.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 45.Merino D, Lok SW, Visvader JE, Lindeman GJ. Targeting BCL-2 to enhance vulnerability to therapy in estrogen receptor-positive breast cancer. Oncogene. 2016;35:1877–1887. doi: 10.1038/onc.2015.287. [DOI] [PubMed] [Google Scholar]
- 46.Dawson SJ, Makretsov N, Blows FM, Driver KE, Provenzano E, Le Quesne J, Baglietto L, Severi G, Giles GG, McLean CA, et al. BCL2 in breast cancer: A favourable prognostic marker across molecular subtypes and independent of adjuvant therapy received. Br J Cancer. 2010;103:668–675. doi: 10.1038/sj.bjc.6605736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choi JE, Kang SH, Lee SJ, Bae YK. Prognostic significance of Bcl-2 expression in non-basal triple negative breast cancer patients treated with anthracycline-based chemotherapy. Tumour Biol. 2014;35:12255–12263. doi: 10.1007/s13277-014-2534-4. [DOI] [PubMed] [Google Scholar]
- 48.Bouchalova K, Kharaishvili G, Bouchal J, Vrbkova J, Megova M, Hlobilkova A. Triple negative breast cancer-BCL2 in prognosis and prediction. Review. Curr Drug Targets. 2014;15:1166–1175. doi: 10.2174/1389450115666141106151143. [DOI] [PubMed] [Google Scholar]
- 49.Bouchalova K, Svoboda K, Kharaishvili G, Vrbkova J, Bouchal J, Trojanec R, Koudelakova V, Radova L, Cwiertka K, Hajduch M, Kolar Z. BCL2 is an independent predictor of outcome in basal-like triple-negative breast cancers treated with adjuvant anthracycline-based chemotherapy. Tumour Biol. 2015;36:4243–4252. doi: 10.1007/s13277-015-3061-7. [DOI] [PubMed] [Google Scholar]
- 50.Perillo B, Sasso A, Abbondanza C, Palumbo G. 17beta-estradiol inhibits apoptosis in MMCF-7 cells, including bcl-2 expression via two estrogen-responsive elements present in the coding sequence. Mol Cell Biol. 2000;20:2890–2901. doi: 10.1128/MCB.20.8.2890-2901.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Certo M, Del Gaizo Moore V, Nishino M, Wei G, Korsmeyer S, Armstrong SA, Letai A. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2015;9:351–365. doi: 10.1016/j.ccr.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 52.Honma N, Horii R, Ito Y, Saji S, Younes M, Iwase T, Akiyama F. Differences in clinical importance of Bcl-2 in breast cancer according to hormone receptor status or adjuvant endocrine therapy. BMC Cancer. 2015;15:698. doi: 10.1186/s12885-015-1686-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seong MK, Lee JY, Byeon J, Sohn YJ, Seol H, Lee JK, Kim EK, Kim HA, Noh WC. Bcl-2 is a highly significant prognostic marker of hormone-receptor-positive, human epidermal growth factor receptor-2-negative breast cancer. Breast Cancer Res Treat. 2015;150:141–148. doi: 10.1007/s10549-015-3305-7. [DOI] [PubMed] [Google Scholar]