Abstract
The present study assessed the effect of the lipid metabolism, fat mass and the obesity-associated gene (FTO), on energy metabolism of breast cancer cells. The human breast cancer cell lines, MCF-7 and MDA-MB-231, and HCC1937 human breast cells were studied. Real-time PCR was used to measure the levels of FTO mRNA from breast cancer cells and normal breast cells. MDA-MB-231 cells were transfected with miFTO inhibitor or inhibitor control, and cells were assessed for levels of lactic acid, ATP, pyruvate kinase activity, and hexokinase activity assay using specific kits. Western blot analysis was used to measure the levels of phosphatidylinositol 3-kinase (PI3K), p-PI3K, protein kinase B (Akt) and p-Akt in transfected breast cancer cells. The expression of FTO was significantly increased in MCF-7 and MDA-MB-231 cells compared with HCC1937 cells (P<0.01). The lactic acid content of breast cancer cells transfected with the miFTO inhibitor was significantly lower compared with cells transfected with the miFTO inhibitor control and nontransfected cells (P<0.05). The ATP content of breast cancer cells transfected with the miFTO inhibitor was significantly lower compared with the control group and inhibitor control group (P<0.05). The pyruvate kinase activity and hexokinase activity of breast cancer cells transfected with the miFTO inhibitor were significantly lower compared with the control group and inhibitor control group (P<0.01). Western blot analysis showed that after breast cancer cells were transfected with the miFTO inhibitor, the levels of PI3K, p-PI3K, Akt and p-Akt were significantly lower than in the control group and inhibitor control group. In conclusion, the FTO gene is overexpressed in breast cancer cells. Overexpression of the FTO gene can promote breast cancer cell glycolysis and the mechanism is related to the PI3K/AKT signaling pathway.
Keywords: breast cancer, energy metabolism, fat mass and obesity-associated, phosphatidylinositol 3-kinase/protein kinase B
Introduction
Breast cancer is a common malignant tumor in women. According to statistics, breast cancer accounts for 10% of malignant tumors and its incidence is second to that of uterine endometrial carcinoma (1). There are several causes of breast cancer. Early detection is difficult, women aged 40–60 years are at high risk for breast cancer, and its incidence is highest during the peri-menopausal period (2). Since breast cancer relapses and metastasizes easily, and has poor prognosis, there are great challenges in diagnosis and treatment of breast cancer.
In recent years, obesity was shown to increase the risk of a variety of diseases (3). Additional studies revealed that obesity related genes, such as fat mass and obesity-associated (FTO) are widely expressed in the human body (4). The FTO gene was found to be overexpressed in prostate cancer, pancreatic cancer, endometrial cancer and liver cancer. Its overexpression affected the energy metabolism of cancer cells, and was closely related to the occurrence and development of cancer (5). To our knowledge, there are no studies on the effect of FTO gene expression on breast cancer cell energy metabolism. In the present study, we used breast cancer cells as a model to explore the relationship between FTO gene expression and energy metabolism, and performed preliminary studies on its mechanism of action, to provide a new potential target for the treatment and diagnosis of breast cancer.
Materials and methods
Cells
The human breast cancer cell lines (MCF-7 and MDA-MB-231), and human breast cells (HCC1937) purchased from Cell Bank of Chinese Academy of Sciences (Shanghai, China) were used. Additional instruments and reagents used are shown in Table I. MCF-7, MDA-MB-231 and HCC1937 cells were removed from storage in liquid nitrogen and thawed in a water bath set to 37°C. Cells were then added to culture medium [Dulbecco's modified Eagle's medium/F12 (DMEM/F12) supplemented with 5% fetal bovine serum (FBS), 0.1 mg/ml streptomycin, 100 U/ml penicillin and 2 mmol/l glutamine]. Cells were grown in culture bottles in an incubator (37°C, 5% CO2) for 48 h. Culture medium was removed when cells reached 90% confluence. Cells were trypsinized in 0.25% trypsin, and centrifuged at 800 × g for 10 min at room temperature. Cells were washed with DMEM/F12 and then seeded again in cell culture bottles.
Table I.
Major instruments and reagents.
| Instruments and reagents | Sources |
|---|---|
| Enzyme-labeled instrument | Nanjing Detie Laboratory Equipment Co., Ltd., Nanjing, China |
| Ultraviolet spectrophotometer | Thermo Fisher Scientific, Inc., Waltham, MA, USA |
| CO2 incubator | Sanyo, Tokyo, Japan |
| Laminar flow cabinet | Suzhou Purification Equipment Co., Ltd., Suzhou, China |
| Inverted microscope | Nikon, Tokyo, Japan |
| PCR instrument | Beckman Coulter, Inc., Brea, CA, USA |
| Centrifuge | Hunan Hengnuo Instrument Equipment Co., Ltd., Changsha, China |
| RevertAid First Strand cDNA Synthesis kit | Beyotime Institute of Biotechnology, Haimen, China |
| DMEM/F12 culture medium | Sigma-Aldrich, St. Louis, MO, USA |
| Lactic acid test kit | Sigma-Aldrich, St. Louis, MO, USA |
| ATP content test kit | Sigma-Aldrich, St. Louis, MO, USA |
| Pyruvate kinase test kit | Sigma-Aldrich, St. Louis, MO, USA |
| Hexokinase test kit | Sigma-Aldrich, St. Louis, MO, USA |
| Real-time fluorescent quantitative PCR kit | Thermo Fisher Scientific, Inc., Waltham, MA, USA |
| Agarose | Thermo Fisher Scientific, Inc., Waltham, MA, USA |
| Antibody dilution | MultiSciences (Lianke) Biotech Co., Ltd., Hangzhou, China |
| FBS | Hangzhou Sijiqing Biology Engineering Materials Co., Ltd., Hangzhou, China |
| Protein concentration test kit | Beyotime Institute of Biotechnology, Hangzhou, China |
| Mycillin | Sigma-Aldrich, St. Louis, MO, USA |
| Trypsin | Sigma-Aldrich, St. Louis, MO, USA |
| RNA isolating reagent kit | Beyotime Institute of Biotechnology, Haimen, China |
| Cell total protein extraction kit | Beyotime Institute of Biotechnology, Haimen, China |
| PBS | SinoBio Biotech Co., Ltd., Shanghai, China |
| P13K monoclonal antibody | Abcam, Cambridge, MA, USA |
| p-P13K monoclonal antibody | Abcam, Cambridge, MA, USA |
| AKT monoclonal antibody | Abcam, Cambridge, MA, USA |
| p-AKT monoclonal antibody | Abcam, Cambridge, MA, USA |
| HRP-anti-antibody | Abcam, Cambridge, MA, USA |
DMEM, Dulbecco's modified Eagle's medium; FBS, fetal bovine serum; PBS, phosphate-buffered saline; P13K, phosphatidylinositol 3-kinase; AKT, protein kinase B.
RT-PCR
TRIzol was added to cell lysis buffer for lysis of MCF-7, MDA-MB-231 and HCC1937 cells that were in the logarithmic growth phase. After 5 min of digestion, lysates were placed in new Eppendorf (EP) tubes (Corning, Inc., Corning, NY, USA), and 200 µl chloroform was added. Solutions were shaken up and down 15 times, and placed at room temperature for 5 min. Samples were then centrifuged (8,500 × g, 4°C, 10 min). RNA in the supernatant was transferred to new EP tubes. Next, 75% ethanol was added and samples were centrifuged (6,500 × g, 4°C, 5 min). The supernatant was discarded, and solutions were placed on a super-clean worktable to air dry. DEPC water (Biosharp, Hefei, China) was then added and mixed well. The concentration and purity of RNA were determined by UV spectrophotometry. According to the instructions of the reverse transcription kit, RNA was reverse transcribed into cDNA. Real-time PCR amplification of cDNA was performed to measure the expression of FTO mRNA in each group of cells.
Transfection
After trypsinization of MDA-MB-231 cells in the logarithmic growth phase, cells were washed with DMEM/F12, cell growth medium was added, and the cell concentration was adjusted to 2×105/ml. Cells were then seeded in 6-well culture plates, and placed at 37°C, 5% CO2 for 48 h. When cells were 50% confluent, growth medium was replaced with incomplete culture medium (without FBS), and placed at 37°C, 5% CO2 for 1 h. The incomplete, serum-free medium was mixed with miFTO inhibitor or inhibitor control, and incubated at 37°C for 5 min (solution A). The serum-free medium was mixed with Lipofectamine 2000 (solution B) (KeyGen, Nanjing, China). Next, solutions A and B were mixed, and left to incubate at room temperature for 20 min. The cell culture medium was discarded, and cells were repeatedly washed with phosphate-buffered saline (PBS). The transfection reagent and miRNA were added to the cell culture plates, and cells were placed at 37°C, 5% CO2 for 6 h. The medium was replaced with complete culture medium, and cells were left in the incubator for an additional 48 h.
Measurement of lactic acid content in culture medium of breast cancer cells after transfection
MDA-MB-231 cells were transfected with miFTO inhibitor, or inhibitor control and left to incubate at 37°C, 5% CO2 for 48 h. Culture supernatant was harvested and transferred to EP tubes, and centrifuged (800 × g, 5 min). The content of lactic acid in supernatant was measured according to the instructions of the Sigma Lactic Acid Test kit (Sigma-Aldrich, St. Louis, MO, USA).
Detection of ATP content in transfected cells
Transfected MDA-MB-231 cells grown in the incubator (37°C, 5% CO2) for 48 h were trypsinized and washed with PBS. Cell culture medium was added to resuspend the cells, then cells were transferred to EP tubes and centrifuged (800 × g, 5 min). The supernatant was discarded. Cells were washed twice with PBS and the supernatant was discarded after centrifugation at 800 × g for 3 min. The cells were mixed homogeneously with ultrapure water. The cell homogenates were transferred to EP tubes and heated in a water bath (100°C, 10 min). The content of ATP was determined according to the instructions of the ATP test kit.
Detection of hexokinase and pyruvate kinase activity in breast cancer cells
MDA-MB-231 cells were transfected with miFTO inhibitor or inhibitor control for 48 h. The activity of hexokinase and pyruvate kinase were detected according to the instructions of the hexokinase and pyruvate kinase test kits.
Western blot analysis
After 48 h culture, culture medium from transfected MDA-MB-231 cells was discarded, cells were washed with PBS and lysed on ice for 30 min. Protein extracts were mixed with loading buffer, and boiled at 100°C for 5 min. A total volume of 50 µl of the denatured protein samples were loaded on gels (12% separation gel and 5% spacer gel). A voltage of 80 V was applied to samples and then adjusted to 120 V when protein reached the separation gel. When bromophenol blue entered the separation gel, electrophoresis was stopped. Protein was transferred to PVDF membranes overnight at 4°C. PVDF membranes were washed with TBST 3 times, and blocked with skim milk for 2 h at 37°C. Membranes were treated with primary antibody overnight at 4°C, washed with TBST, and incubated with secondary antibody for 1.5 h at room temperature. Membranes were washed and developed. Protein expression was analyzed with the Odyssey scanning system (LI-COR, Inc., Lincoln, NE, USA). Primary rabbit polyclonal AKT antibody (dilution, 1:500; cat. no. ab38449); rabbit monoclonal p-AKT antibody (dilution, 1:500; cat. no. ab81283), rabbit monoclonal PI3K antibody (dilution, 1:500; cat. no. ab86714), rabbit polyclonalp-PI3K antibody (dilution, 1:500; cat. no. ab182651) and secondary goat anti-rabbit (HRP) IgG antibody (dilution, 1:2,000; cat. no. ab6721) were all purchased from Abcam (Cambridge, MA, USA).
Statistical analysis
Data were analyzed with SPSS 16.0 statistical software (SPSS, Inc., Chicago, IL, USA). Comparisons between groups were by t-test. P<0.05 was considered to indicate a statistically significant difference.
Results
Measurement of FTO mRNA expression in breast cancer cells and breast cells by RT-PCR
In the logarithmic growth phase, MCF-7, MDA-MB-231 and HCC1937 cells were harvested for extraction of total RNA. The expression levels of FTO in the 3 groups of cells were detected by real-time quantitative PCR. The relative expression levels of FTO mRNA in MCF-7 cells was 26.89±2.31, 36.23±2.91 in MDA-MB-231 cells and 8.96±3.01 in HCC1937 cells. The levels of FTO mRNA in MCF-7 and MDA-MB-231 cells were significantly higher than in HCC1937 cells (P<0.01) (Fig. 1).
Figure 1.
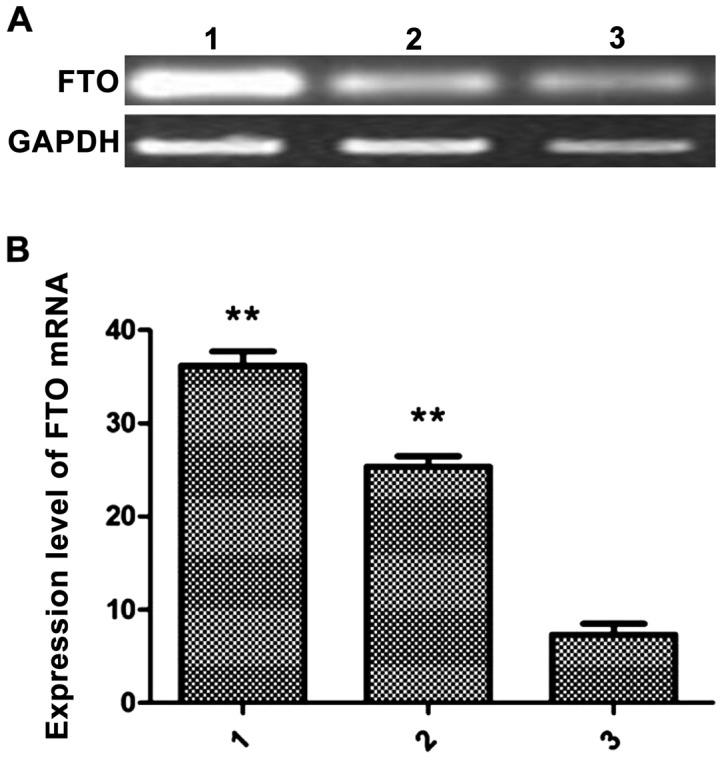
Relative FTO mRNA expression levels in MCF-7, MDA-MB-231 and HCC1937 cells by RT-PCR. (A) FTO mRNA expression level detected by agarose gel electrophoresis. (B) FTO mRNA expression level. Lane 1, MDA-MB-231; lane 2, MCF-7; and lane 3, HCC1937; **P<0.01 vs. HCC1937. FTO, fat mass and obesity-associated.
Measurement of lactic acid and ATP content
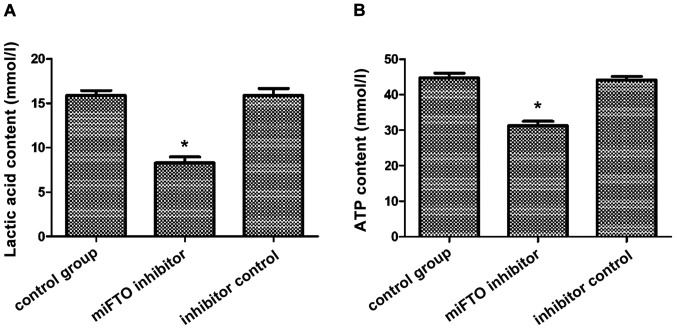
The content of lactic acid and ATP in MDA-MB-231 cells transfected with the miFTO inhibitor or inhibitor control were detected according to the lactic acid test kit and ATP test kit. After 48 h culture, the lactic acid content of the miFTO inhibitor group was 8.97±0.25 mmol/l, the lactic acid content of the inhibitor control group was 17.11±1.02 mmol/l, and the lactic acid content of the blank control group was 17.08±1.32 mmol/l. The lactic acid content of breast cancer cells transfected with miFTO inhibitor was significantly lower compared with the control group and inhibitor control group. FTO mRNA inhibitors can inhibit the production of lactic acid in breast cancer cells (Fig. 2A). After 48 h culture, the ATP content of the miFTO inhibitor group was 31.45±1.58 mmol/l, the ATP content of the inhibitor control group was 44.12±3.12 mmol/l, and the ATP content of the blank control group was 44.56±2.45 mmol/l. The ATP content of breast cancer cells transfected with the miFTO inhibitor was significantly lower compared with the control group and inhibitor control group (P<0.05). FTO mRNA inhibitors can inhibit the production of ATP in breast cancer cells (Fig. 2B).
Figure 2.
Lactic acid content and ATP content after transfection of MDA-MB-231 cells. (A) Lactic acid content and (B) ATP content; *P<0.05 vs. control group.
Detection of pyruvate kinase and hexokinase activity
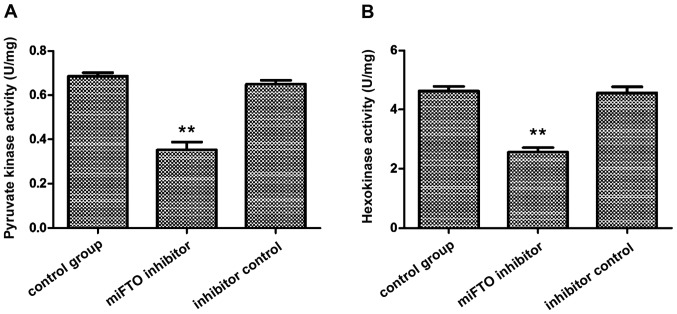
The activity of hexokinase and pyruvate kinase in MDA-MB-231 cells transfected with miFTO inhibitor or inhibitor control were detected according to the instructions of the hexokinase and pyruvate kinase test kits. The results showed that pyruvate kinase activity in breast cancer cells transfected with miFTO inhibitor and inhibitor control were 0.39±0.01 and 0.68±0.02, respectively, and 0.71±0.03 in the nontransfected cells. Pyruvate kinase activity of breast cancer cells transfected with miFTO inhibitor was significantly lower compared with the control group and inhibitor control group (P<0.01). Hexokinase activity in breast cancer cells transfected with the miFTO inhibitor and inhibitor control were 2.54±0.21 and 4.86±0.25, respectively, and 4.84±0.20 in the nontransfected cells. Hexokinase activity of breast cancer cells transfected with the miFTO inhibitor was significantly lower compared with the control group and inhibitor control group (P<0.01). Therefore, miFTO inhibitors can reduce the activity of hexokinase and pyruvate kinase in breast cancer cells (Fig. 3).
Figure 3.
Pyruvate kinase and hexokinase activity in breast cancer cells. (A) Pyruvate kinase activity and (B) hexokinase activity; **P<0.01 vs. control group.
Western blot analysis to detect the expression levels of related proteins in cells
MDA-MB-231 cells transfected with the miFTO inhibitor or inhibitor control for 48 h were harvested, and lysates were used to analyze the expressions of phosphatidylinositol 3-kinase (PI3K), p-PI3K, protein kinase B (Akt), and p-AKT by western blot analysis. After transfection with the miFTO inhibitor, the expressions of PI3K, p-PI3K, AKT and p-AKT were significantly lower compared with the control group and inhibitor control group. The phosphorylated forms of PI3K and AKT decreased significantly (Fig. 4).
Figure 4.
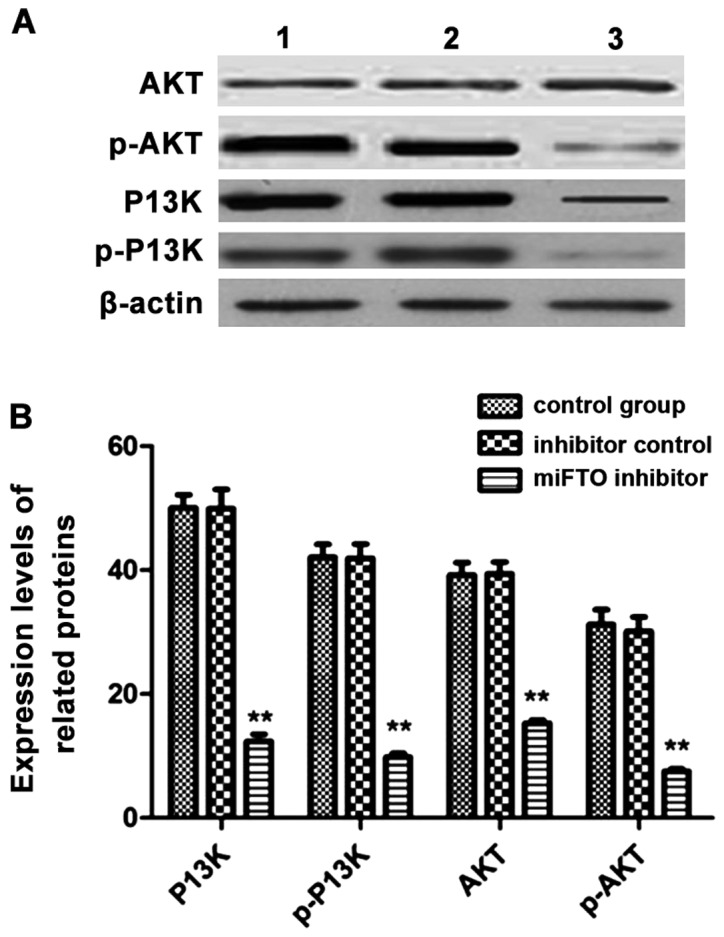
The expression levels of PI3K, p-PI3K, AKT and p-AKT in breast cancer cells by western blot analysis. (A) The expression levels of PI3K/AKT signaling proteins by western blot analysis. (B) The relative protein expression levels. Lane 1, control group; lane 2, inhibitor control group; and lane 3, miFTO inhibitor group; **P<0.01 vs. control group. PI3K, phosphatidylinositol 3-kinase; AKT, protein kinase B.
Discussion
Breast cancer is a common malignant tumor in women. According to statistics, the incidence of breast cancer accounts for 10% of all malignant tumors. There are 1.3 million newly diagnosed cases of breast cancer worldwide every year, and ~50 million people die from breast cancer each year. Therefore, breast cancer is a serious threat to the health of women. The occurrence of breast cancer has regional differences. The morbidity of breast cancer in developed countries is higher than that in developing countries (6). There are several causes of breast cancer and it is difficult to detect in the early stage. Research on the pathogenesis of breast cancer is important for treatment and diagnosis.
In recent years, studies have shown that obesity can increase the risk of breast cancer. Obese women were 3 times more likely to have breast cancer than nonobese women (3). The lipid metabolism gene FTO, has been found to be closely related to obesity. The FTO gene contains nine exons and is located on chromosome 16. It is widely expressed in adults and in the fetus. FTO is most highly expressed in the pituitary, pancreatic islets, hypothalamus and adrenal glands (7,8). FTO is overexpressed in prostate cancer, pancreatic cancer, hepatocellular carcinoma and endometrial carcinoma. It therefore has a close relationship with the occurrence and development of cancer. In this study, we used human breast cancer cells (MCF-7 and MDA-MB-231) and human breast cells (HCC1937) to determine the levels of FTO mRNA by real-time fluorescence quantitative PCR. The results showed that the FTO mRNA levels in breast cancer cells were significantly higher than in normal breast cells, suggesting that FTO is an oncogene, which represents a potential new marker for the early diagnosis of breast cancer.
Cell energy metabolism is the process of transforming organic matter into energy. In normal cells, ATP is produced by oxidative decomposition of glucose, which can be divided into aerobic oxidation and glycolysis (9,10). Glucose can be oxidized to produce ATP under aerobic conditions, while under anoxic conditions, ATP can be generated by glycolysis (11). The energy metabolism of tumor cells is different from that of normal cells. In cases where tumor cells receive sufficient oxygen, energy is also produced by glycolysis which converts pyruvate to lactic acid (the ‘Warburg effect’) (12). Studies have shown that aerobic glycolysis can be found in lung cancer, breast cancer, colon cancer and renal cancer cells (13). Pyruvate kinase and hexokinase play a key role in glycolysis. In tumor cells, hexokinase exists as isozymes. The expression of hexokinase is related to the occurrence and development of colon cancer and renal cell carcinoma. Downregulated expression of pyruvate kinase can inhibit the production of lactic acid by glycolysis (14). In this study, after cells were transfected with the FTO mRNA inhibitor, the ATP levels in breast cancer cells decreased, pyruvate kinase and hexokinase activity decreased significantly, and the content of lactic acid in the medium decreased significantly. These results demonstrate that overexpression of the FTO gene could promote glycolysis in breast cancer cells.
The role of the PI3K/AKT signaling pathway in tumor cells is an area of intense study (15,16). The PI3K/AKT signaling pathway is related to the proliferation and apoptosis of cancer cells, and can regulate the activity of caspase-9 (17), p53 (18), Bad (19), and other proteins, and inhibit apoptosis. The PI3K/AKT signaling pathway is active in several types of cells, and it plays an important role when cells are under hypoxic conditions. Under hypoxic conditions, the PI3K/AKT signaling pathway can upregulate insulin, epidermal growth factor and cytokine expression. It can deliver messages to protein tyrosine kinases via transmembrane receptors, activate PI3K, and catalyze the generation of PIP3, which then delivers messages to Akt, and activates the Ras-MAPK signaling pathway, which consequently causes a series of complex reactions in the body (20,21). In this study, through transcriptional inhibition of the FTO gene, the protein expression of PI3K, p-PI3K, Akt and p-Akt in cells increased significantly according to western blot analysis, demonstrating that expression of the FTO gene affected the energy metabolism of breast cancer cells through the PI3K/AKT signaling pathway.
In conclusion, overexpression of FTO in breast cancer cells can result in upregulation of pyruvate kinase and hexokinase activity, increase the amount of ATP generation in cells, and promote glycolysis and lactic acid production. FTO overexpression affects the energy metabolism of breast cancer cells, and the mechanism is related to the PI3K/AKT signaling pathway. Our results represent a potential new therapeutic option for the treatment and diagnosis of breast cancer.
References
- 1.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, et al. Herceptin Adjuvant (HERA) Trial Study Team: Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 2.Dowsett M, Forbes JF, Bradley R, Ingle J, Aihara T, Bliss J, Boccardo F, Coates A, Coombes RC, Cuzick J, et al. Early Breast Cancer Trialists' Collaborative Group (EBCTCG): Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015;386:1341–1352. doi: 10.1016/S0140-6736(15)61074-1. [DOI] [PubMed] [Google Scholar]
- 3.Gallagher EJ, LeRoith D. Obesity and diabetes: the increased risk of cancer and cancer-related mortality. Physiol Rev. 2015;95:727–748. doi: 10.1152/physrev.00030.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sevgi M, Rigoux L, Kühn AB, Mauer J, Schilbach L, Hess ME, Gruendler TO, Ullsperger M, Stephan KE, Brüning JC, et al. An obesity-predisposing variant of the FTO gene regulates D2R-dependent reward learning. J Neurosci. 2015;35:12584–12592. doi: 10.1523/JNEUROSCI.1589-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Milagro FI, Moreno-Aliaga MJ, Martinez JA. FTO obesity variant and adipocyte browning in humans. N Engl J Med. 2016;374:190–191. doi: 10.1056/NEJMc1513316. [DOI] [PubMed] [Google Scholar]
- 6.Kuchenbaecker KB, Ramus SJ, Tyrer J, Lee A, Shen HC, Beesley J, Lawrenson K, McGuffog L, Healey S, Lee JM, et al. EMBRACE; GEMO Study Collaborators; Breast Cancer Family Registry; HEBON; KConFab Investigators; Australian Cancer Study (Ovarian Cancer Investigators); Australian Ovarian Cancer Study Group; Consortium of Investigators of Modifiers of BRCA1 and BRCA2: Identification of six new susceptibility loci for invasive epithelial ovarian cancer. Nat Genet. 2015;47:164–171. doi: 10.1038/ng.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hasstedt SJ, Coon H, Xin Y, Adams TD, Hunt SC. APOH interacts with FTO to predispose to healthy thinness. Hum Genet. 2016;135:201–207. doi: 10.1007/s00439-015-1629-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salgado-Montilla J, Rodríguez-Caban J, Gonzalez-Sepulveda L, Sanchez-Ortiz R, Irizarry-Ramirez M. Presence of FTO rs9939609 and rs9930506 and severity of prostate cancer in Puerto Ricans; Cancer Res (106th Annual Meeting Abstracts); 2015.p. 4836.
- 9.Barbier-Torres L, Delgado TC, García-Rodríguez JL, Zubiete-Franco I, Fernández-Ramos D, Buqué X, Cano A, Gutiérrez-de Juan V, Fernández-Domínguez I, Lopitz-Otsoa F, et al. Stabilization of LKB1 and Akt by neddylation regulates energy metabolism in liver cancer. Oncotarget. 2015;6:2509–2523. doi: 10.18632/oncotarget.3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roberts DJ, Miyamoto S. Hexokinase II integrates energy metabolism and cellular protection: akting on mitochondria and TORCing to autophagy. Cell Death Differ. 2015;22:248–257. doi: 10.1038/cdd.2014.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giménez-Cassina A, Danial NN. Regulation of mitochondrial nutrient and energy metabolism by BCL-2 family proteins. Trends Endocrinol Metab. 2015;26:165–175. doi: 10.1016/j.tem.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valenti D, Vacca RA, de Bari L. 3-Bromopyruvate induces rapid human prostate cancer cell death by affecting cell energy metabolism, GSH pool and the glyoxalase system. J Bioenerg Biomembr. 2015;47:493–506. doi: 10.1007/s10863-015-9631-y. [DOI] [PubMed] [Google Scholar]
- 13.Gatenby RA, Gillies RJ. Why do cancers have highaerobic glycolysis? Nat Rev Cancer. 2004;4:891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 14.Vincent EE, Sergushichev A, Griss T, Gingras MC, Samborska B, Ntimbane T, Coelho PP, Blagih J, Raissi TC, Choinière L, et al. Mitochondrial phosphoenolpyruvate carboxykinase regulates metabolic adaptation and enables glucose-independent tumor growth. Mol Cell. 2015;60:195–207. doi: 10.1016/j.molcel.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 15.Liang M, Liu J, Ji H, Chen M, Zhao Y, Li S, Zhang X, Li J. A Aconitum coreanum polysaccharide fraction induces apoptosis of hepatocellular carcinoma (HCC) cells via pituitary tumor transforming gene 1 (PTTG1)-mediated suppression of the P13K/Akt and activation of p38 MAPK signaling pathway and displays antitumor activity in vivo. Tumour Biol. 2015;36:7085–7091. doi: 10.1007/s13277-015-3420-4. [DOI] [PubMed] [Google Scholar]
- 16.Zhang CZ, Wang XD, Wang HW, Cai Y, Chao LQ. Sorafenib inhibits liver cancer growth by decreasing mTOR, AKT, and PI3K expression. J BUON. 2015;20:218–222. [PubMed] [Google Scholar]
- 17.Zhu JJ, Cui Y, Cui K, Li X, Zhang ZY. Distinct roles of parafibromin in the extracellular environment, cytoplasm and nucleus of osteosarcoma cells. Am J Transl Res. 2016;8:2426–2431. [PMC free article] [PubMed] [Google Scholar]
- 18.Mock CD, Jordan BC, Selvam C. Recent advances of curcumin and its analogues in breast cancer prevention and treatment. RSC Advances. 2015;5:75575–75588. doi: 10.1039/C5RA14925H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Z, Xie C, Xu W, Liu G, Cao X, Li W, Chen J, Zhu Y, Luo S, Luo Z, et al. Phosphorylation and inactivation of PTEN at residues Ser380/Thr382/383 induced by Helicobacter pylori promotes gastric epithelial cell survival through PI3K/Akt pathway. Oncotarget. 2015;6:31916–31926. doi: 10.18632/oncotarget.5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pande M, Bondy ML, Do KA, Sahin AA, Ying J, Mills GB, Thompson PA, Brewster AM. Association between germline single nucleotide polymorphisms in the PI3K-AKT-mTOR pathway, obesity, and breast cancer disease-free survival. Breast Cancer Res Treat. 2014;147:381–387. doi: 10.1007/s10549-014-3081-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bains M, Roberts JL. Estrogen protects against dopamine neuron toxicity in primary mesencephalic cultures through an indirect P13K/Akt mediated astrocyte pathway. Neurosci Lett. 2016;610:79–85. doi: 10.1016/j.neulet.2015.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]