SUMMARY
Objective
Bisphosphonates are commonly used anti-osteoporotic drugs which have controversial effects on joint diseases including osteoarthritis. Certain bisphosphonates have been shown to have anabolic effects on cartilage which could have important ramifications for their proposed effects in vivo; however, the underlying mechanisms are poorly understood. Thus, the purpose of this study was to characterize the effects of clodronate on primary articular chondrocyte metabolism and to determine the underlying signaling pathways responsible.
Design
The effects of clodronate and pamidronate on extracellular matrix (ECM) biosynthesis, accumulation and MMP-13 activity were observed in high density, 3D cultures of bovine articular chondrocytes for up to 4 weeks were evaluated. Mechanisms were delineated by measuring intracellular Ca2+ signaling and the effects of pharmacologic inhibition of the purinergic receptor pathway.
Results
Clodronate (100 μM) induced an anabolic effect (increased biosynthesis by 13–14%) which resulted in an 89–90% increase in ECM accumulation after 4 weeks of culture and without an associated effect on matrix turn-over. Stimulation by clodronate resulted in a 3.3-fold increase in Ca2+ signaling and pharmacological inhibitor experiments suggested that the anabolic effects exerted by clodronate are transduced through the purinergic receptor pathway.
Conclusions
These findings support the previous notion that certain bisphosphonates may be useful as adjunctive therapies to potentially ameliorate progression of cartilage degeneration and improve arthritis management.
Keywords: Chondrocytes, Bisphosphonates, Clodronate, Anabolism, Calcium signaling, Purinergic signaling
Introduction
Bisphosphonates are commonly used anti-osteoporotic drugs that are chemically stable analogs of inorganic pyrophosphate (PPi). They exert both physiochemical (mineralization inhibition) and biological (osteoclast apoptosis) effects that make them well suited to inhibit bone resorption1,2. As these molecules have also been proposed to possess anti-inflammatory, chondro-protective, and anti-catabolic effects, they may be promising drugs for the treatment of osteoarthritis and rheumatoid arthritis3,4. Interestingly, certain bisphosphonates (i.e., etidronate, clodronate) have been shown to have anabolic effects on cartilaginous extracellular matrix (ECM) biosynthesis5–7, which could have important ramifications for their proposed effects on arthritis in vivo. However, recent clinical studies have been relatively inconclusive8–13.
Although the physiochemical effects of bisphosphonates on mineralization are well understood, the molecular mechanisms of bisphosphonates on cellular function are more variable, and have been broadly categorized into two groups based on drug structure. After cellular uptake by fluid-phase endocytosis (pinocytosis)14,15, the nitrogen-containing bisphosphonates (e.g., pamidronate, zoledronate) interfere with the mevalonate biosynthetic pathway that leads to inhibition of bone resorption by disrupting both osteoclast function and survival16,17. Alternatively, the non-nitrogen-containing bisphosphonates (e.g., etidronate, clodronate) are metabolized into the non-hydrolyzable ATP analog adenosine 5′(β,γ-dichloromethylene) triphosphate (AppCCl2p) by aminoacyl-tRNA synthetases18–20. In osteoclasts, this metabolite is believed to be responsible for inducing apoptosis by inhibiting mitochondrial metabolism (through the disruption of ATP translocation)21 — which is believed to be the primary means by which these bisphosphonates inhibit bone resorption22. Less is known about the effects of bisphosphonate on articular cartilage. Chondrocytes are known to release ATP in response to mechanical or osmotic stimuli which is then utilized as an autocrine/paracrine signal23,24. This pathway (termed the purinergic receptor pathway) is not exclusive to chondrocytes but also functions in other cells that utilize extracellular ATP as a mechanotransduction signal25,26. Due to the structural similarity between ATP and the non-hydrolyzable bisphosphonate metabolite AppCCl2p, we propose that non-nitrogen containing bisphosphonates elicit an anabolic response in chondrocytes through extracellular transport of the metabolite AppCC2p and its interactions with components of the purinergic receptor pathway. Thus, the purpose of this study was to characterize the anabolic effects of clodronate on chondrocytes and to determine the underlying mechanism.
Materials and methods
Cell isolation and high-density 3D culture
High-density 3D chondrocyte cultures were generated from isolated chondrocytes harvested from calf (12–18 months old) metacarpal-phalangeal articular cartilage obtained from a local abattoir after slaughter (Brian Quinn’s Meats Ltd., Yarker, ON, Canada) by sequential enzymatic digestion, as described previously27. Tissue was obtained from several joints (up to four per isolation) and pooled together to collect a sufficient cell population. The cells were seeded on the surface of type II collagen-coated Millicell™ filters (Millipore, Billerica, MA, USA) in high-density 3D culture (2 × 106 cells/filter or 35,000 cells/mm2)27 and maintained in Ham’s F12 media containing 10 mM glucose supplemented with 20% fetal bovine serum (FBS), 100 μg/mL ascorbate and 20 mM HEPES (N-2-hydroxyethylpiperazine-N′-ethanesulfonic acid) (Sigma-Aldrich Ltd., Burlington, ON, Canada). The cultures were maintained in an incubator at 37°C with 95% relative humidity supplemented with 5% CO2: 95% atmospheric air. The culture medium (1 mL per filter) was changed every 2–3 days.
Bisphosphonate supplementation and assessment of ECM synthesis
To determine the short-term effect of bisphosphonates on ECM synthesis, cultures were supplemented with media containing either clodronate or pamidronate (Sigma-Aldrich Ltd.) 2 days after seeding at varying concentrations (0, 1, 10, and 100 μM) and incubated in the presence of both [35S]SO4 (5 μCi/culture) to label proteoglycans and [3H] proline (5 μCi/culture) to label collagen for a period of 24 h. Although proline can be incorporated into different proteins, in chondrocyte cultures approximately 90% of proline becomes incorporated into collagen28,29. The unincorporated isotope from the tissue cultures was removed by gently washing the samples three times in phosphate-buffered saline29. Cultures were then digested by papain (40 μg/ml in 20 mM ammonium acetate, 1 mM EDTA, and 2 mM DTT) for 48 h at 65°C. The accumulation of newly synthesized proteoglycan and collagen in the matrix was then estimated by quantifying radioisotope incorporation from aliquots of the papain digest using a β-liquid scintillation counter (Beckman Coulter LS6500, Mississauga, ON, Canada). The amounts of synthesized molecules were calculated relative to the DNA content of the tissue, determined from aliquots of the papain digest using the Hoechst dye 33258 assay30.
Long-term culture and assessment of ECM accumulation
Bisphosphonate supplemented cultures were maintained for a period of 4 weeks to determine the effect on ECM accumulation. In these experiments, only the high concentrations of bisphosphonates (100 μM of pamidronate or clodronate) were used based on the maximal effect observed during the previous short-term ECM synthesis experiments. After long-term culture, accumulated tissues were removed from the filter units and weighed (wet weight). Tissues were then digested by papain (as described earlier) and stored at −20°C until analysis. Aliquots of the digest were assayed separately for proteoglycan, collagen and DNA contents. The proteoglycan content was estimated by quantifying the amount of sulfated glycosaminoglycans using the dimethyl-methylene blue dye binding assay31. Collagen content was estimated from the determination of the hydroxyproline content. Aliquots of the papain digest were hydrolyzed in 6 N HCl at 110°C for 18 h and the hydroxyproline content of the hydrolyzate was then determined using chloramine-T/Ehrlich’s reagent assay32. Collagen content was estimated assuming hydroxyproline accounts for 10% of the total collagen mass in cartilage33. The DNA content was also determined from aliquots of the papain digest (as described earlier)30.
Histological and immunohistochemical evaluation
Representative cultures from the long-term experiments were harvested and fixed overnight in 4% paraformaldehyde (in 0.1 M phosphate buffer, pH 7.2), dehydrated in graded ethanol solutions and embedded in paraffin at 65°C. Thin (5 μm thick) sections were cut and mounted on Superfrost slides (Fisher Scientific, Mississauga, ON, Canada) and dried for 24 h at 37°C. Sections were stained with hematoxylin & eosin (H&E) or assessed for localization of collagen types I and II by immunohistochemistry. Briefly, after deparaffinization and dehydration, sections were enzymatically treated with 0.05% of trypsin (pH 7.8) for 30 min at 37°C to facilitate antibody binding. Endogenous peroxidase activity was blocked with 1% H2O2 and 1% bovine serum albumin (BSA) in phosphate buffer for 30 min. Sections were then incubated with mouse monoclonal antibodies against collagen type I (I-8H5 at 40 μg/mL; Daiichi Fine Chemicals Co Ltd, Takaoka, Japan) or collagen type II (II-II6B3 at 187 μg/mL; Developmental Studies Hybridoma Bank, Iowa City, IA, USA) all diluted in phosphate buffer (pH 7.4) containing 1% BSA overnight at 4°C. Following primary antibody incubation, sections were rinsed in PBS (pH 7.4), and incubated with biotinylated anti-mouse secondary antibodies (Vector Laboratories Inc., Burlingame, CA, USA) using the Vectastain Elite ABC kit (Vector Laboratories) for 2 h at room temperature, followed by incubation with diaminobenzidine (DAB) for 6 min at room temperature. The sections were counter-stained with Harris’ hematoxylin and mounted in permanent mounting medium. Non-specific staining was assessed by replacement of the primary antibody with non-immune mouse serum. Stained sections were examined by light microscopy using a Zeiss Axio-Image M1 microscope (Göttingen, Germany). All the experiments were completed at least three times with no positive staining detected in the negative controls.
Assessment of MMP-13 activity
Additional long-term cultures were used to determine the effect of bisphosphonates on MMP-13 activity. MMP-13 mediates ECM catabolism, reflects altered chondrocyte phenotype34 and is a major factor in the matrix degradation that occurs in osteoarthritis35. In these experiments, the serum concentration was decreased to 5% at the last feeding prior to harvest to reduce potential interference with the MMP-13 activity assay. After harvest, protein was extracted from the tissue cultures following a protocol described by Nielson et al.36 Samples were snap frozen in liquid N2, pulverized and then homogenized (2 × 30 s) (Power Gen 125, Fisher Scientific, Hampton, NH, USA) in the presence of extraction buffer (Tris–HCl 0.05 M, NaCl 0.1 M, Triton X-100 0.1% (v/v), pH 7.4) and one complete mini EDTA-free protease inhibitor cocktail tablet (Roche Diagnostics, Laval, QC, Canada) per 10 mL of buffer. Samples were centrifuged for 10 min at 7500 g and 4°C. The supernatant was collected and further centrifuged at 10,000 g for 10 min at 4°C and then stored at −20°C until analysis. To determine the amount of active MMP-13, a FRET based assay using the Mca MMP-13 FRET substrate (MCA-Pro-Cha-Gly-Nva-His-Ala-Dpa-NH2) (AnaSpec Inc., Fremont, CA, USA) which fluoresces when cleaved by MMP-1337. Equal volumes of the Mca MMP-13 FRET substrate and protein extracts were combined in 20% DMSO (v/v) and mixed with assay buffer (200 mM NaCl, 50 mM Tris base CaCl2, 20 μM ZnSO4, and 0.05% BRIJ 35 (w/v), pH 7.5) then incubated at 37°C with 95% humidity for 18 h in the dark. After incubation, fluorescence was measured at 325 nm excitation and 393 nm emission with Mca MMP FRET fluorescence peptides (MCA-Pro-Leu-OH) reconstituted in 20% DMSO (v/v) used as assay standards. MMP-13 activity was calculated relative to the DNA content of the tissue culture (as described earlier)30.
Assessment of intracellular calcium signaling and the mechanisms of drug import/export and involvement of the purinergic receptor pathway
As many of the cellular responses to extracellular ATP are mediated through intracellular calcium signaling38–40, the mechanism by which clodronate affected chondrocyte biosynthesis was investigated by assessing intracellular calcium transients. Isolated chondrocytes were resuspended at a concentration of 20 ×106 cells/mL in Ham’s F-12 with 25 mM HEPES. A 4% w/v solution of type VII low melt agarose (Sigma-Aldrich) was prepared in PBS (pH 7.4) and mixed in equal parts with the cell suspension to form 2% agarose gels with a concentration of 10 ×106 cells/mL. The suspension was poured into petri dishes and allowed to gel at room temperature. Individual cell-seeded hydrogel constructs (4 mm dia. × 4 mm height) were then created using a 4 mm biopsy punch and cultured in complete media for 2 days. Cell-seeded constructs were then incubated in the intracellular calcium ion dye Fluo-4 AM (3 μM; Life Technologies, Carlsbad, CA, USA) in complete media for 90 min in the dark, followed by washing for 10 min in complete media. In the first set of experiments, the time course of Ca2+ signaling as a result of bisphosphonate stimulation was assessed by transferring the labeled constructs to media containing either clodronate or pamidronate (100 μM) and measuring resultant Ca2+ transients at 30 min intervals for a period of up to 180 min. At each time point, the mid-section of the construct was imaged by confocal microscopy (IX81, Olympus Canada, Richmond Hill, ON, Canada) with a 10× objective (ex: 488 nm; em: 516 nm). Images (640 ×640 pixels) were captured every 5 s over a 5 min imaging period. Captured images were then analyzed using ImageJ software (US National Institutes of Health, Bethesda, MD, USA) by selecting circular regions of interest around individual cells experiencing Ca2+ transients and measuring the change in intensity over the series of collected images. Fluorescent intensity traces (as a function of time) were plotted and the number cells experiencing multiple transients (more than one) were counted. Once the pattern of calcium transients elicited by clodronate was established, we set out to delineate the mechanisms involved. In these studies, we used pharmacologic inhibitors of the purinergic receptor pathway as well as inhibitors of drug import or efflux mechanisms. Cell-seeded gel constructs were pre-incubated in the presence of pharmacological inhibitors to block discrete elements of the ATP-purinergic receptor pathway (Table I) prior to clodronate stimulation (100 μM). It was also determined whether the blockade of clodronate import (dansylcadaverine) and AppCCl2p efflux (flufenamic acid (FFA)) could be rescued by direct stimulation of the purinergic receptors by ATP (100 μM). In the inhibitor and rescue experiments, confocal imaging to assess resultant changes in Ca2+ transients was assessed at the optimal time identified in the previous study.
Table I.
Pharmacological inhibitors (all previously used on chondrocytes) used to block elements of the ATP-purinergic receptor pathway
| Inhibitor | Carrier | Target | Concentration | Pre-incubation time |
|---|---|---|---|---|
| Suramin | Media | Broad spectrum P2 receptor inhibitor41 | 100 μM | 30 min |
| Reactive blue 2 (RB2) | Media | P2Y receptor inhibitor42 | 100 μM | 30 min |
| Brilliant blue G (BBG) | Media | P2X receptor inhibitor43 | 50 μM | 30 min |
| Apyrase | Media | Deplete extracellular ATP (ATPase)41 | 10 units/mL | 30 min |
| FFA | DMSO | Connexin 43 hemi-channel blocker41 | 25 μM | 30 min |
| U73122 | Chloroform | PLC inhibitor40 | 10 μM | 30 min |
| Dansylcadaverine | Methanol | Pinocytosis inhibitor15 | 500 μM | 240 min |
Statistical analyses
Chondrocytes isolated from a single animal (from up to four legs of the same animal) were used for each experiment. Each experiment was repeated three times with chondrocytes from three different animals (N = 3 animal donors). Within each experiment, replicates of 2–3 samples per group were generated and all quantitative data was normalized to the control of the same animal donor. Statistical analyses were then performed on the composite data obtained from all three experiments (total sample size of n = 6–9 samples/group). Note that due to the relatively small number of samples created from each animal, the effect of animal donor was not incorporated into the statistical models. All results were expressed as the mean (lower 95% confidence interval limit, upper 95% confidence interval limit). Collected data was analyzed statistically (SPSS version 16, SPSS Inc., Chicago, IL, USA) using a one-way or two-way ANOVA and the Tukey’s post-hoc test (where appropriate) depending on the experimental design. One-way ANOVA testing was used to determine the effect of bisphosphonate dose and the time course of Ca2+ signaling; whereas two-way ANOVA testing was used to determine the effect of each specific inhibitor used to block various elements of the ATP-purinergic receptor pathway. All data were checked prior to performing statistical tests for both normality and equal-variance. Significance was defined by P-values less than 0.05 and trends associated with P-values between 0.05 and 0.1.
Results
Effect of bisphosphonates on cartilaginous ECM synthesis, ECM accumulation, and MMP-13 activity
To determine the short-term effect of bisphosphonate stimulation on the synthesis of cartilaginous ECM macromolecules, isolated articular chondrocytes were seeded in high-density 3D culture supplemented with varying doses of clodronate or pamidronate (0–100 μM) 2 days after seeding. As determined by radiolabel incorporation, only stimulation by 100 μM clodronate significantly upregulated ECM synthesis by 13–14% (collagen: P = 0.018, proteoglycans: P = 0.036; Fig. 1). In contrast, pamidronate had no effect on ECM synthesis under any of the doses investigated (Fig. 1). Bisphosphonate stimulation (clodronate or pamidronate) also had no effect on tissue cellularity (data not shown).
Fig. 1.
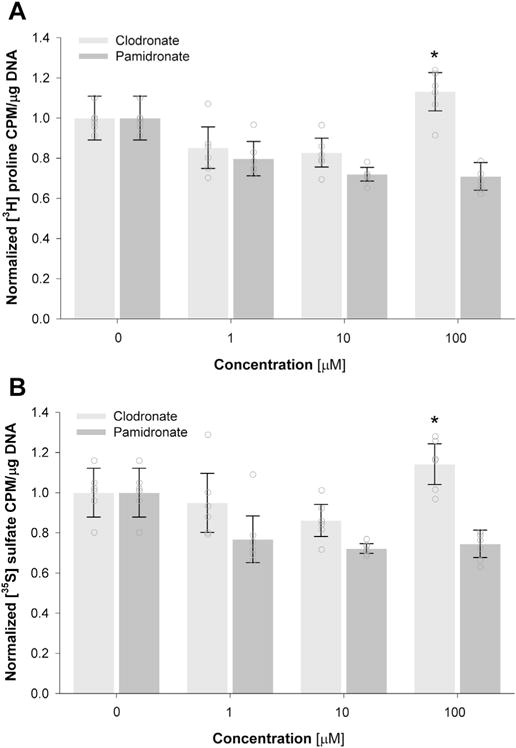
The effect of short-term clodronate and pamidronate exposure on collagen and proteoglycan synthesis by articular chondrocytes. The effect of clodronate and pamidronate on collagen (A) and proteoglycan (B) synthesis by chondrocytes was determined 24 h after exposure to the bisphosphonate. Data was normalized to control (as described in the Methods) and expressed as the mean with errors bars representing the 95% confidence interval (N = 3 donors; total n = 6 samples/group) and open circles representing individual data points. *denotes a significance difference between all other groups (panel A: P = 0.018; panel B: P = 0.036).
To determine the long-term effect of bisphosphonate stimulation on the growth of cartilaginous tissue formed in vitro, high-density 3D cultures were cultured in the presence of clodronate or pamidronate (at 100 μM) for a period of 4 weeks. Clodronate induced a sustained anabolic effect as observed by an increase in tissue wet weight by 66% (P = 0.001). Further analysis of the accumulated ECM revealed that stimulation by clodronate resulted in a significant increase of 89–90%, in collagen (P < 0.001) and proteoglycans (P = 0.007) compared to control (Table II). In contrast to the short-term studies, long-term culture in the presence of clodronate appeared to elicit a modest proliferative response with the stimulated cultures having an approximate 25% higher DNA content (P = 0.022) compared to control (Table II). Similar to the results of short-term stimulation, long-term culture with pamidronate induced no apparent effect on ECM accumulation (wet weight: P = 0.655, collagen: P = 0.196, proteoglycans: P = 0.876, DNA: P = 0.718). Histological assessment of the developed tissues displayed the same trends in terms of tissue thickness as a result of long-term bisphosphonate stimulation with only clodronate inducing an anabolic effect (Fig. 2). Immunohistochemical evaluation confirmed that the increased collagen accumulation as a result of long-term culture with clodronate was primarily type II with no detectable presence of type I collagen synthesized by the cells (Fig. 2). Maintenance of a healthy chondrocyte phenotype and stable ECM catabolism were confirmed by the lack of altered MMP-13 activity after bisphosphonate treatment (P = 0.560; Table II).
Table II.
Effect of the bisphosphonates on cartilaginous tissue formation and MMP-13 activity. Data was normalized to control (as described in the Methods) and expressed as the mean (lower 95% confidence interval limit, upper 95% confidence interval limit) (N = 3 donors; total n = 8 samples/group)
| Control | 100 μM pamidronate | 100 μM clodronate | |
|---|---|---|---|
| Wet weight [mg/culture] | 1.0 (0.8, 1.2) | 1.1 (0.8, 1.4) | 1.66 (1.60, 1.72)a |
| Proteoglycans [μg/culture] | 1.0 (0.8, 1.2) | 1.1 (0.8, 1.4) | 1.9 (1.5, 2.3)b |
| Collagen [μg/culture] | 1.0 (0.8, 1.2) | 0.8 (0.6, 1.0) | 1.89 (1.83, 1.95)c |
| DNA [μg/culture] | 1.0 (0.8, 1.2) | 0.9 (0.8, 1.0) | 1.2 (1.0, 1.4)d |
| Proteoglycans/Wet weight [μg/mg] | 1.0 (0.9, 1.1) | 1.0 (0.9, 1.1) | 1.1 (0.9, 1.3) |
| Proteoglycans/DNA [μg/μg] | 1.00 (0.92, 1.08) | 1.2 (0.9, 1.5) | 1.5 (1.2, 1.8)e |
| Collagen/Wet weight [μg/mg] | 1.0 (0.7, 1.3) | 0.9 (0.6, 1.2) | 1.2 (1.1, 1.3) |
| Collagen/DNA [μg/μg] | 1.0 (0.7, 1.3) | 0.8 (0.6, 1.0) | 1.6 (1.3, 1.9)f |
| MMP-13 activity [ng substrate hydrolyzed/μg DNA] | 1.0 (0.6, 1.4) | 1.0 (0.8, 1.2) | 1.3 (0.8, 1.8) |
Significantly different from all other groups (P = 0.001).
Significantly different from all other groups (P = 0.007).
Significantly different from all other groups (P < 0.001).
Significantly different from all other groups (P = 0.022).
Significantly different from all other groups (P = 0.048).
Significantly different from all other groups (P = 0.010).
Fig. 2.
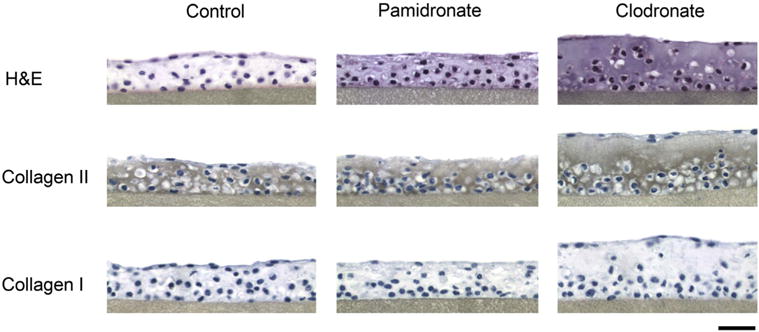
The effect of long-term exposure to clodronate and pamidronate on chondrocyte ECM. Histological and immunohistochemical assessment of cartilaginous tissue constructs after 4 weeks of exposure to clodronate (100 μM) or pamidronate (100 μM). Tissue sections were stained with hematoxylin and eosin (H&E) as well as collagen II and collagen I assessed by immunohistochemical methods (positive protein staining appears in brown). Original magnification of 40 × and the scale bar represents 100 μm.
Effect of bisphosphonates on intracellular calcium signaling and the delineation of membrane transport and signaling mechanisms
Additional experiments were conducted to determine whether, like ATP, clodronate signals by inducing intracellular Ca2+ transients. Fluo-4 AM loaded chondrocytes in suspended in agarose hydrogels were stimulated with clodronate (100 μM) and imaged by confocal microscopy. Measurement of fluorescent intensity over the 5 min imaging window showed that the cells (control and bisphosphonate stimulated) experienced Ca2+ transients of similar magnitude and duration (Fig. 3). However, after 30 min of incubation, the number of Ca2+ transients experienced significantly increased upon stimulation with clodronate as opposed to pamidronate (fold changes in Ca2+ signaling; control: 1.0 (0.9, 1,1), clodronate: 3.3 (3.1, 3.5), pamidronate: 1.1 (0.8, 1.4); P < 0.001, n = 8). This delay in signaling supports the concept that a drug metabolite is responsible for the observed calcium transients. The induced Ca2+ signaling response as a result of clodronate stimulation was relatively unchanged 120 min after stimulation which then started to decline upon longer durations (data not shown). For this reason, all subsequent experiments were conducted with Ca2+ signaling measured 30 min after stimulation. In the second series of experiments, cultures were pre-incubated in the presence of different inhibitors to block discrete elements of the purinergic receptor pathway prior to stimulation by clodronate. Broad spectrum purinergic P2 receptor inhibitors (suramin, reactive blue 2, brilliant blue G) each appeared to inhibit clodronate induced Ca2+ signaling by varying degrees [Fig. 4(a)]. Both suramin (a general P2 receptor antagonist) and reactive blue 2 (P2Y receptor antagonist) induced complete inhibition (P < 0.001) whereas brilliant blue G (P2X receptor antagonist) only partially inhibited the response (by ~70%; P = 0.030). Downstream signaling through the purinergic receptor pathway was confirmed as the phospholipase C (PLC) inhibitor U73122 also completely inhibited clodronate induced Ca2+ signaling [P = 0.006, Fig. 4(a)]. Similarly, while the hemi-channel blocker FFA also completely inhibit clodronate induced Ca2+ signaling [P < 0.001, Fig. 4(a)]; however, the release of extracellular ATP appeared not to be involved as depletion by apyrase had no measurable effect on Ca2+ signaling [Fig. 4(a)]. There was also a trend towards complete inhibition of the response with dansylcadaverine, a pinocytosis (fluid-phase endocytosis) inhibitor [P = 0.093, Fig. 4(a)]. In the last series of experiments, it was determined whether the inhibitory effects of FFA and dansylcadaverine could be rescued by direct stimulation of the purinergic receptors. Direct stimulation of the purinergic receptor pathway by ATP (100 μM) rescued the inhibited Ca2+ signaling response to similar levels observed in the previous experiments [P < 0.001, Fig. 4(b)].
Fig. 3.
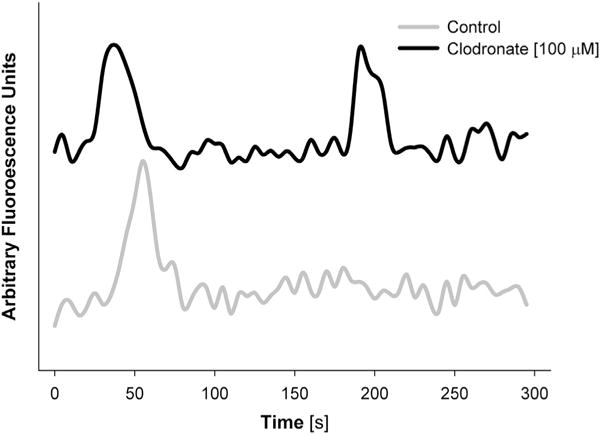
The effect of clodronate on calcium transients in chondrocytes. Overall cell fluorescent intensity (arbitrary units) of individual chondrocytes pre-incubated with Fluo-4 using confocal microscopy is shown here for cultures supplemented with (top trace) or without (bottom trace) clodronate (100 μM). Fluorescent intensity was determined from images captured every 5 s over a 300 s imaging period.
Fig. 4.
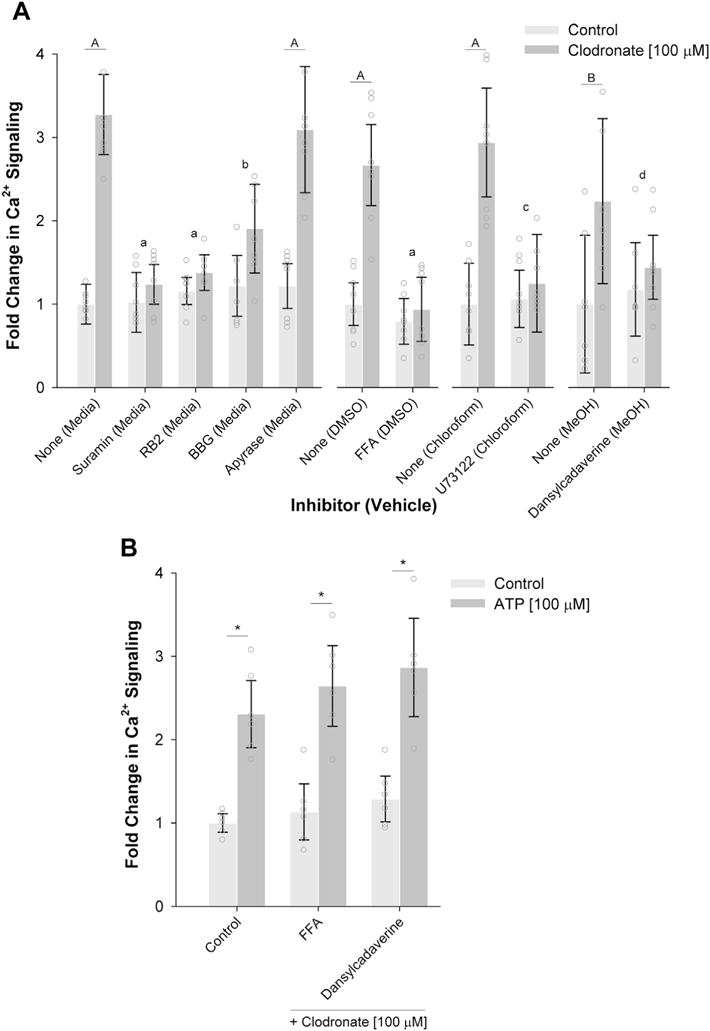
The effect of purinergic receptor pathway inhibitors on calcium transients induced by clodronate. (A) Fold changes in intracellular Ca2+ signaling of Fluo-4 labeled chondrocytes stimulated by clodronate (100 μM) after pre-incubation with pharmacological inhibitors to block discrete elements of the purinergic receptor pathway (Table I). Confocal images (640 × 640 pixels) were captured every 5 s over a 300 s imaging period and the number of cells experiencing multiple transients were counted. For each inhibitor, the number of cells experiencing multiple transients was normalized to vehicle-only control (shown in parentheses) and expressed as the mean with errors representing the 95% confidence interval (N = 3 donors; total n = 8–9 samples/group) and open circles representing individual data points. (B) Fold changes in intracellular Ca2+ signaling of Fluo-4 labeled chondrocytes pre-incubated with FFA or dansylcadaverine (Table I) and stimulated by clodronate (100 μM) with or without additional ATP stimulation (100 μM). The number of cells experiencing multiple transients was normalized to vehicle-only control and expressed as the mean with errors representing the 95% confidence interval (N = 3 donors; total n = 6–8 samples/group) and open circles representing individual data points. A,Bdenotes a significant effect of clodronate (P < 0.001 and P = 0.045, respectively), a,b,cdenotes a significant effect of the inhibitor (P < 0.001, P = 0.030 and P = 0.006, respectively), and ddenotes a trend of the inhibitor (P = 0.093). *denotes a significant effect of ATP (P < 0.001).
Discussion
This study demonstrates that clodronate induces an anabolic effect in chondrocyte cultures and that the response appears to be mediated by export of an intracellular ATP-analog which then signals through the purinergic receptor pathway. Upon stimulation by clodronate, short-term matrix synthesis was upregulated by a relatively small degree (13–14%); however, there was a sustained effect with 89–90% increase in proteoglycan and collagen content in clodronate-treated cultures observed over the long-term. Interestingly, this response occurred without alterations in chondrocyte phenotype, or increased catabolism as determined by MMP-13 activity. These results were similar to several other studies that reported an anabolic effect of clodronate in chondrocytes5,6. Alternatively, pamidronate had no apparent effect on ECM synthesis, accumulation or matrix turn-over. The difference in these responses was most likely attributed to the differences in bisphosphate structure. Pamidronate is a nitrogen-containing bisphosphonate that has been proposed to disrupt osteoclast function through interference with the mevalonate biosynthetic pathway16,17. Clodronate, a non-nitrogen containing bisphosphonate, has been shown to become metabolized into the non-hydrolyzable ATP analog adenosine 5′(β,γ-dichloromethylene) triphosphate (AppCCl2p) by aminoacyl-tRNA synthetases18–20.
Due to the structural similarities between ATP and AppCCl2p, we hypothesized that this metabolite could then act via the ATP-purinergic receptor pathway leading to an anabolic response. Chondrocytes utilize the purinergic receptor pathway as part of the mechanotransduction cascade in which ATP is released through connexin 4344, pannexin 1 hemi-channels45 and/or ANK46 into the extracellular space where it can bind and signal through a variety of purinergic P2 receptors. Both P2X and P2Y receptors have been identified on articular chondrocytes23,24. P2Y receptors are G-protein coupled receptors which utilize the IP3 – Ca2+ second messenger system, leading to the stimulation of ECM gene expression and protein synthesis24, while P2X receptors may have both catabolic functions and regulate hemichannel activity, through their interactions with pannexins45 In the present study, further examination of the underlying signaling pathways revealed that stimulation by clodronate, and not pamidronate, resulted in a 3-fold increase in Ca2+ signaling, which could be inhibited through pharmacological inhibition of discrete elements of the purinergic receptor pathway (Fig. 5). Involvement of the purinergic receptor pathway was also confirmed by additional experiments showing that this response could be rescued by direct stimulation of the purinergic receptors (P2Y) by ATP; which have been shown to elicit an anabolic response in both bovine and human chondrocytes47–50. After uptake by fluid-phase endocytosis (pinocytosis), clodronate is metabolized into AppCCl2p which is then released into the extracellular space likely through hemi-channels. As approximately 70% of clodronate induced Ca2+ signaling was abolished by inhibiting P2X receptors, this suggests that the released AppCCl2p may bind to the P2X7 or P2X4 receptor leading to additional release through its known association with pannexin 1, a channel forming glycoprotein that primarily responsible for ATP release45. Extracellular AppCCl2p then binds to P2Y receptors (most likely P2Y2) leading to the release of intracellular calcium from intracellular stores through the IP3 second messenger system. This proposed pathway is also supported by the previous observation that clodronate can induce Ca2+ signaling in carcinoma cells51.
Fig. 5.
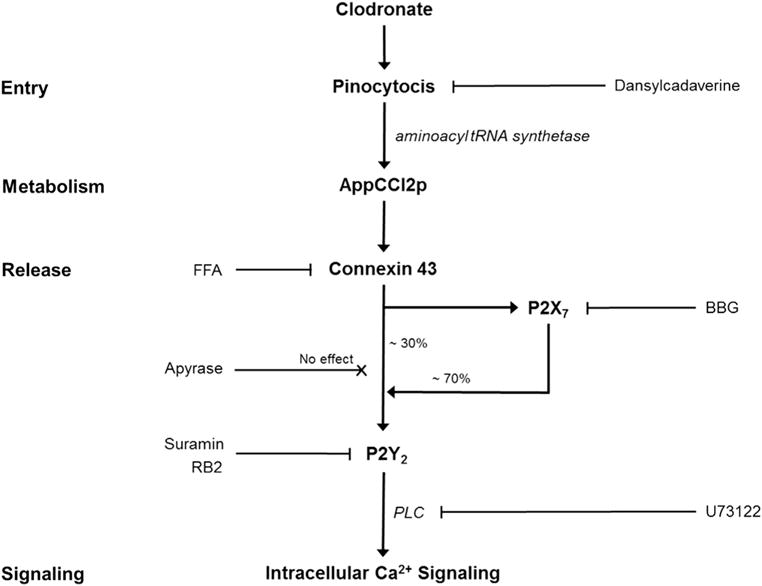
Schema of proposed clodronate signaling pathway. Proposed clodronate signaling pathway. Pharmacological inhibitors used to block discrete elements of the signaling pathway (irrespective of their effect) are shown.
There were a few limitations of the present study. The most notable was that we were unable to measure the generation of the clodronate metabolite AppCCl2p. Similarly, as it is presently not known which specific aminoacryl transfer RNAases are involved in the generation of AppCCl2p, it was not possible to perform any specific inhibitor studies to block the generation of the metabolite. Aminoacryl transfer RNAases are a large family of enzymes with numerous isoforms and there are no broad spectrum inhibitors currently available. In spite of these limitations, the underlying mechanism(s) responsible for the anabolic effect of clodronate on chondrocytes appears to involve the generation of a non-hydrolyzable ATP analog. Studies conducted in the presence of apyrase (to degrade extracellular ATP) had no apparent effect and the response was inhibited by blocking either P2X or P2Y receptors – both of which have high affinity for ATP and ATP analogs. Similarly, the pharmacologic inhibitors used in this study have potential off-target effects which do not allow for the direct inhibition of this pathway during long-term culture. However, previous studies have demonstrated that purinergic receptors agonists (e.g., ATP) elicit an anabolic response in both bovine and human chondrocytes under similar concentrations to the bisphosphonate concentrations used in the present study47–50. There is also uncertainty related to the levels of clodronate in cartilage achieved in vivo. However, previous studies involving intra-articular injections of clodronate showed positive effects of clodronate with doses in the range of 10−4 to 10−3 M11 which were comparable to levels in which anabolic effects on chondrocytes were observed. Lastly, as these studies were conducted using healthy bovine chondrocytes, future studies will need to be conducted with mature human chondrocytes as the choice of the chondrocyte species, age, and (lack of) disease state may have each contributed to the observed response. However, several studies using bovine chondrocytes have elucidated the mechanism of release41,44 as well as the subsequent anabolic effect47,48 of purinergic receptor agonists indicating their utility for understanding the role of the purinergic receptor pathway in chondrocyte metabolism.
As bisphosphonates have been ascribed to possess anti-inflammatory, chondro-protective, and anti-catabolic effects they have been suggested to be promising drugs for the treatment of arthritis. While recent reports have concluded that bisphosphonates do not have any significant clinical effects on either disease activity or pain in osteoarthritis8 and rheumatoid arthritis9, there has been little attempt to distinguish clinical efficacy based on bisphosphonate type. Interestingly, while the nitrogen-containing bisphosphonates risedronate, alendronate and zoledronate are generally ineffective8,9, the non-nitrogen containing bisphosphonate clodronate appears to be have some limited effects in reducing pain associated with both osteoarthritis10,11 and rheumatoid arthritis12,13. Only a single study involving clodronate showed no effect of the treatment, which the authors attributed to the method of delivery (iv. infusion as opposed to liposomal encapsulation)52. The observed anabolic effect of clodronate may serve to limit or restrict cartilage degradation as a result of osteoarthritis and rheumatoid arthritis. However, the significance of this effect and whether it can be extended to all non-nitrogen containing bisphosphonates, including etidronate, and tiludronate is currently unclear53,54. This proposed mechanism of action may be also one of several through which bisphosphonates might ameliorate cartilage damage. For example, bisphosphonates also bind and inactivate basic calcium phosphate crystals, which are common mediators of articular destruction in damaged joints55,56.
The results of this in vitro study demonstrate that clodronate has an anabolic effect in articular cartilage and this response appears to be transduced through the purinergic receptor pathway. After cellular uptake by fluid-phase endocytosis, clodronate is metabolized into the non-hydrolyzable ATP analog AppCCl2p and released extracellularly where it can bind to purinergic receptors resulting in increased matrix synthesis. These findings support the previous notion that certain bisphosphonates may be useful as adjunctive therapies10–13 to potentially ameliorate progression of cartilage degeneration and improve arthritis management.
Acknowledgments
Funding for this work was provided by the Natural Sciences and Engineering Research Council of Canada (NSERC).
Footnotes
Author contributions
Conception and design: RGR AKR SDW. Acquisition of data: RGR KC AL ED JFW. Analysis and interpretation of the data: RGR KC AL AKR SDW. Obtaining of funding: SDW. Drafting of the article: RGR SDW. Critical revision of the article for important intellectual content: KC AL ED JFW AKR. Final approval of the article: RGR KC AL ED JFW AKR SDW.
Conflict of interest
None.
References
- 1.Fleisch H, Russell RG, Francis MD. Diphosphonates inhibit hydroxyapatite dissolution in vitro and bone resorption in tissue culture and in vivo. Science. 1969;165(3899):1262–4. doi: 10.1126/science.165.3899.1262. [DOI] [PubMed] [Google Scholar]
- 2.Fleisch H, Russell RG, Straumann F. Effect of pyrophosphate on hydroxyapatite and its implications in calcium homeostasis. Nature. 1966;212(5065):901–3. doi: 10.1038/212901a0. [DOI] [PubMed] [Google Scholar]
- 3.Dombrecht EJ, Schuerwegh AJ, Bridts CH, Ebo DG, Van Offel JF, Stevens WJ, et al. Effect of bisphosphonates on nitric oxide production by inflammatory activated chondrocytes. Clin Exp Rheumatol. 2007;25(6):817–22. [PubMed] [Google Scholar]
- 4.van Offel JF, Dombrecht EJ, Bridts CH, Schuerwegh AJ, Ebo DG, Stevens WJ, et al. Influence of bisphosphonates on the production of pro-inflammatory cytokines by activated human articular chondrocytes. Cytokine. 2005;31(4):298–304. doi: 10.1016/j.cyto.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 5.Guenther HL, Guenther HE, Fleisch H. The effects of 1-hydroxyethane-1,1-diphosphonate and dichloromethanediphosphonate on collagen synthesis by rabbit articular chondrocytes and rat bone cells. Biochem J. 1981;196(1):293–301. doi: 10.1042/bj1960293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guenther HL, Guenther HE, Fleisch H. Effects of 1-hydroxyethane-1,1-diphosphonate and dichloromethanediphosphonate on rabbit articular chondrocytes in culture. Biochem J. 1979;184(2):203–14. doi: 10.1042/bj1840203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larsson A, Larsson SE. The effects of ethylene-1-hydroxy-1, 1-diphosphonate on cellular transformation and organic matrix of the epiphyseal growth plate of the rat–a light microscopic and ultrastructural study. Acta Pathol Microbiol Scand A. 1978;86(3):211–23. doi: 10.1111/j.1699-0463.1978.tb02035.x. [DOI] [PubMed] [Google Scholar]
- 8.Davis AJ, Smith TO, Hing CB, Sofat N. Are bisphosphonates effective in the treatment of osteoarthritis pain? A meta-analysis and systematic review. PLoS One. 2013;8(9):e72714. doi: 10.1371/journal.pone.0072714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le Goff B, Heymann D. Pharmacodynamics of bisphosphonates in arthritis. Expert Rev Clin Pharmacol. 2011;4(5):633–41. doi: 10.1586/ecp.11.40. [DOI] [PubMed] [Google Scholar]
- 10.Saviola G, Abdi-Ali L, Campostrini L, Sacco S, Baiardi P, Manfredi M, et al. Clodronate and hydroxychloroquine in erosive osteoarthritis: a 24-month open randomized pilot study. Mod Rheumatol. 2012;22(2):256–63. doi: 10.1007/s10165-011-0506-8. [DOI] [PubMed] [Google Scholar]
- 11.Rossini M, Viapiana O, Ramonda R, Bianchi G, Olivieri I, Lapadula G, et al. Intra-articular clodronate for the treatment of knee osteoarthritis: dose ranging study vs hyaluronic acid. Rheumatology (Oxford) 2009;48(7):773–8. doi: 10.1093/rheumatology/kep084. [DOI] [PubMed] [Google Scholar]
- 12.Rovetta G, Monteforte P. Efficacy of disodium-clodronate in the management of joint pain in rheumatoid arthritis. Six months open study. Minerva Med. 2003;94(5):353–7. [PubMed] [Google Scholar]
- 13.Cocco R, Tofi C, Fioravanti A, Nerucci F, Nannipieri F, Zampieri A, et al. Effects of clodronate on synovial fluid levels of some inflammatory mediators, after intra-articular administration to patients with synovitis secondary to knee osteoarthritis. Boll Soc Ital Biol Sper. 1999;75(11–12):71–6. [PubMed] [Google Scholar]
- 14.Coxon FP, Thompson K, Roelofs AJ, Ebetino FH, Rogers MJ. Visualizing mineral binding and uptake of bisphosphonate by osteoclasts and non-resorbing cells. Bone. 2008;42(5):848–60. doi: 10.1016/j.bone.2007.12.225. [DOI] [PubMed] [Google Scholar]
- 15.Thompson K, Rogers MJ, Coxon FP, Crockett JC. Cytosolic entry of bisphosphonate drugs requires acidification of vesicles after fluid-phase endocytosis. Mol Pharmacol. 2006;69(5):1624–32. doi: 10.1124/mol.105.020776. [DOI] [PubMed] [Google Scholar]
- 16.Luckman SP, Coxon FP, Ebetino FH, Russell RG, Rogers MJ. Heterocycle containing bisphosphonates cause apoptosis and inhibit bone resorption by preventing protein prenylation: evidence from structure–activity relationships in J774 macrophages. J Bone Miner Res. 1998;13(11):1668–78. doi: 10.1359/jbmr.1998.13.11.1668. [DOI] [PubMed] [Google Scholar]
- 17.Kavanagh KL, Guo K, Dunford JE, Wu X, Knapp S, Ebetino FH, et al. The molecular mechanism of nitrogen-containing bisphosphonates as antiosteoporosis drugs. Proc Natl Acad Sci USA. 2006;103(20):7829–34. doi: 10.1073/pnas.0601643103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rogers MJ, Brown RJ, Hodkin V, Russell RG, Watts DJ. Bisphosphonates are incorporated into adenine nucleotides by human aminoacyl-tRNA synthetase enzymes. Biochem Biophys Res Commun. 1996;224(3):863–9. doi: 10.1006/bbrc.1996.1113. [DOI] [PubMed] [Google Scholar]
- 19.Frith JC, Mönkkönen J, Blackburn GM, Russell RG, Rogers MJ. Clodronate and liposome-encapsulated clodronate are metabolized to a toxic ATP analog, adenosine 5′-(beta, gamma-dichloromethylene) triphosphate, by mammalian cells in vitro. J Bone Miner Res. 1996;12(9):1358–67. doi: 10.1359/jbmr.1997.12.9.1358. [DOI] [PubMed] [Google Scholar]
- 20.Auriola S, Frith J, Rogers MJ, Koivuniemi A, Mönkkönen J. Identification of adenine nucleotide-containing metabolites of bisphosphonate drugs using ion pair liquid chromatography–electrospray mass spectrometry. J Chromatogr B. 1997;704(1–2):187–95. doi: 10.1016/s0378-4347(97)00490-8. [DOI] [PubMed] [Google Scholar]
- 21.Lehenkari PP, Kellinsalmi M, Näpänkangas JP, Ylitalo KV, Mönkkönen J, Rogers MJ, et al. Further insight into mechanism of action of clodronate: inhibition of mitochondrial ADP/ATP translocase by a nonhydrolyzable, adenine-containing metabolite. Mol Pharmacol. 2002;61(5):1255–62. doi: 10.1124/mol.61.5.1255. [DOI] [PubMed] [Google Scholar]
- 22.Rogers MJ, Crockett JC, Coxon FP, Mönkkönen J. Biochemical and molecular mechanisms of action of bisphosphonates. Bone. 2011;49(1):34–41. doi: 10.1016/j.bone.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 23.Graff RD, Lazarowski ER, Banes AJ, Lee GM. ATP release by mechanically loaded porcine chondrons in pellet culture. Arthritis Rheum. 2000;43(7):1571–9. doi: 10.1002/1529-0131(200007)43:7<1571::AID-ANR22>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 24.Pingguan-Murphy B, El-Azzeh M, Bader DL, Knight MM. Cyclic compression of chondrocytes modulates a purinergic calcium signalling pathway in a strain rate- and frequency-dependent manner. J Cell Physiol. 2006;209(2):389–97. doi: 10.1002/jcp.20747. [DOI] [PubMed] [Google Scholar]
- 25.Tsuzaki M, Bynum D, Almekinders L, Faber J, Banes AJ. Mechanical loading stimulates ecto-ATPase activity in human tendon cells. J Cell Biochem. 2005;96(1):117–25. doi: 10.1002/jcb.20491. [DOI] [PubMed] [Google Scholar]
- 26.Yamazaki S, Weinhold PS, Graff RD, Tsuzaki M, Kawakami M, Minchew JT, et al. Annulus cells release ATP in response to vibratory loading in vitro. J Cell Biochem. 2003;90(4):812–8. doi: 10.1002/jcb.10681. [DOI] [PubMed] [Google Scholar]
- 27.Waldman SD, Grynpas MD, Pilliar RM, Kandel RA. Characterization of cartilagenous tissue formed on calcium polyphosphate substrates in vitro. J Biomed Mater Res. 2002;62(3):323–30. doi: 10.1002/jbm.10235. [DOI] [PubMed] [Google Scholar]
- 28.Peterkofsky B, Diegelmann R. Use of a mixture of protease-free collagenases for specific assay of radioactive collagen in the presence of other proteins. Biochemistry. 1971;10(6):988–94. doi: 10.1021/bi00782a009. [DOI] [PubMed] [Google Scholar]
- 29.Sun YL, Kandel RA. Deep zone articular chondrocytes in vitro express genes that show specific changes with mineralization. J Bone Miner Res. 1999;14(11):1916–25. doi: 10.1359/jbmr.1999.14.11.1916. [DOI] [PubMed] [Google Scholar]
- 30.Kim YJ, Sah RL, Doong JY, Grodzinsky AJ. Fluorometric assay of DNA in cartilage explants using Hoechst 33258. Anal Biochem. 1988;174(1):168–76. doi: 10.1016/0003-2697(88)90532-5. [DOI] [PubMed] [Google Scholar]
- 31.Goldberg RL, Kolibas LM. An improved method for determining proteoglycans synthesized by chondrocytes in culture. Connect Tissue Res. 1990;24(2–3):265–75. doi: 10.3109/03008209009152154. [DOI] [PubMed] [Google Scholar]
- 32.Woessner JF. The determination of hydroxyproline in tissue and protein samples containing small proportions of this amino acid. Arch Biochem Biophys. 1961;93(2):440–7. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 33.Heinegard D, Bayliss M, Lorenzo P. Biochemistry and metabolism of normal and osteoarthritic cartilage. In: Brandt KD, Doherty M, Lohmander LS, editors. Osteoarthritis. New York: Oxford University Press; 1998. pp. 74–84. [Google Scholar]
- 34.van der Kraan PM, van den Berg WB. Chondrocyte hypertrophy and osteoarthritis: role in initiation and progression of cartilage degeneration? Osteoarthritis Cartilage. 2012;20(3):223–32. doi: 10.1016/j.joca.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 35.Neuhold LA, Killar L, Zhao W, Sung ML, Warner L, Kulik J, et al. Postnatal expression in hyaline cartilage of constitutively active human collagenase-3 (MMP-13) induces osteoarthritis in mice. J Clin Invest. 2001;107(1):35–44. doi: 10.1172/JCI10564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nielsen RH, Stoop R, Leeming DJ, Stolina M, Qvist P, Christiansen C, et al. Evaluation of cartilage damage by measuring collagen degradation products in joint extracts in a traumatic model of osteoarthritis. Biomarkers. 2008;13(1):79–87. doi: 10.1080/13547500701615108. [DOI] [PubMed] [Google Scholar]
- 37.Knäuper V, Will H, López-Otin C, Smith B, Atkinson SJ, Stanton H, et al. Cellular mechanisms for human procollagenase-3 (MMP-13) activation. J Biol Chem. 1996;271(29):17124. doi: 10.1074/jbc.271.29.17124. [DOI] [PubMed] [Google Scholar]
- 38.Wann AK, Zuo N, Haycraft CJ, Jensen CG, Poole CA, McGlashan SR, et al. Primary cilia mediate mechano-transduction through control of ATP-induced Ca2+ signaling in compressed chondrocytes. FASEB J. 2012;26(4):1663–71. doi: 10.1096/fj.11-193649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elfervig MK, Graff RD, Lee GM, Kelley SS, Sood A, Banes AJ. ATP induces Ca(2+) signaling in human chondrons cultured in three-dimensional agarose films. Osteoarthritis Cartilage. 2001;9(6):518–26. doi: 10.1053/joca.2000.0435. [DOI] [PubMed] [Google Scholar]
- 40.D’Andrea P, Vittur F. Propagation of intercellular Ca2+ waves in mechanically stimulated articular chondrocytes. FEBS Lett. 1997;400(1):58–64. doi: 10.1016/s0014-5793(96)01356-7. [DOI] [PubMed] [Google Scholar]
- 41.Garcia M, Knight MM. Cyclic loading opens hemichannels to release ATP as part of a chondrocyte mechanotransduction pathway. J Orthop Res. 2010;28(4):510–5. doi: 10.1002/jor.21025. [DOI] [PubMed] [Google Scholar]
- 42.Inoue K, Nakazawa K, Ohara-Imaizumi M, Obama T, Fujimori K, Takanaka A. Antagonism by reactive blue 2 but not by brilliant blue G of extracellular ATP-evoked responses in PC12 phaeochromocytoma cells. Br J Pharmacol. 1991;102(4):851–4. doi: 10.1111/j.1476-5381.1991.tb12265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82(4):1013–67. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 44.Knight MM, McGlashan SR, Garcia M, Jensen CG, Poole CA. Articular chondrocytes express connexin 43 hemichannels and P2 receptors – a putative mechanoreceptor complex involving the primary cilium? J Anat. 2009;214(2):275–83. doi: 10.1111/j.1469-7580.2008.01021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Penuela S, Gehi R, Laird DW. The biochemistry and function of pannexin channels. Biochim Biophys Acta. 2013;1828(1):15–22. doi: 10.1016/j.bbamem.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 46.Rosenthal AK, Gohr CM, Mitton-Fitzgerald E, Lutz MK, Dubyak GR, Ryan LM. The progressive ankylosis gene product ANK regulates extracellular ATP levels in primary articular chondrocytes. Arthritis Res Ther. 2013;15(5):R154. doi: 10.1186/ar4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Croucher LJ, Crawford A, Hatton PV, Russell RG, Buttle DJ. Extracellular ATP and UTP stimulate cartilage proteoglycan and collagen accumulation in bovine articular chondrocyte pellet cultures. Biochim Biophys Acta. 2000;1502(2):297–306. doi: 10.1016/s0925-4439(00)00055-7. [DOI] [PubMed] [Google Scholar]
- 48.Waldman SD, Usprech J, Flynn LE, Khan AA. Harnessing the purinergic receptor pathway to develop functional engineered cartilage constructs. Osteoarthritis Cartilage. 2010;18(6):864–72. doi: 10.1016/j.joca.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 49.Bow JF, Waldman SD. The use of exogenous adenosine triphosphate (ATP) to stimulate the growth of human tissue engineered cartilage. In: Kuester E, Traugott G, editors. Adenosine Triphosphate: Chemical Properties, Biosynthesis and Functions in Cells. Hauppauge: Nova Science Publishers Inc; 2013. pp. 99–122. [Google Scholar]
- 50.Gadjanski I, Yodmuang S, Spiller K, Bhumiratana S, Vunjak-Novakovic G. Supplementation of exogenous adenosine 5′-triphosphate enhances mechanical properties of 3D cell-agarose constructs for cartilage tissue engineering. Tissue Eng Part A. 2013;19(19–20):2188–200. doi: 10.1089/ten.tea.2012.0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang DM, Teng HC, Chen KH, Tsai ML, Lee TK, Chou YC, et al. Clodronate-induced cell apoptosis in human thyroid carcinoma is mediated via the P2 receptor signaling pathway. J Pharmacol Exp Ther. 2009;330(2):613–23. doi: 10.1124/jpet.109.152447. [DOI] [PubMed] [Google Scholar]
- 52.Valleala H, Laitinen K, Pylkkänen L, Konttinen YT, Friman C. Clinical and biochemical response to single infusion of clodronate in active rheumatoid arthritis–a double blind placebo controlled study. Inflamm Res. 2001;50(12):598–601. doi: 10.1007/PL00000240. [DOI] [PubMed] [Google Scholar]
- 53.Bird HA, Hill J, Sitton NG, Dixon JS, Wright V. A clinical and biochemical assessment of etidronate disodium in patients with active rheumatoid arthritis. Clin Rheumatol. 1988;7(1):91–4. doi: 10.1007/BF02284063. [DOI] [PubMed] [Google Scholar]
- 54.Pelletier JP, Troncy E, Bertaim T, Thibaud D, Goulet AC, Abram F, et al. Treatment with tiludronic acid helps reduce the development of experimental osteoarthritis lesions in dogs with anterior cruciate ligament transection followed by reconstructive surgery: a 1-year study with quantitative magnetic resonance imaging. J Rheumatol. 2011;38(1):118–28. doi: 10.3899/jrheum.100642. [DOI] [PubMed] [Google Scholar]
- 55.Fuerst M, Bertrand J, Lammers L, Dreier R, Echtermeyer F, Nitschke Y, et al. Calcification of articular cartilage in human osteoarthritis. Arthritis Rheum. 2009;60(9):2694–703. doi: 10.1002/art.24774. [DOI] [PubMed] [Google Scholar]
- 56.McCarthy GM, Westfall PR, Masuda I, Christopherson PA, Cheung HS, Mitchell PG. Basic calcium phosphate crystals activate human osteoarthritic synovial fibroblasts and induce matrix metalloproteinase-13 (collagenase-3) in adult porcine articular chondrocytes. Ann Rheum Dis. 2001;60(4):399–406. doi: 10.1136/ard.60.4.399. [DOI] [PMC free article] [PubMed] [Google Scholar]


