Abstract
Diseases of muscle that are caused by pathological interactions between muscle and the immune system are devastating, but rare. However, muscle injuries that involve trauma and regeneration are fairly common, and inflammation is a clear feature of the regenerative process. Investigations of the inflammatory response to muscle injury have now revealed that the apparently nonspecific inflammatory response to trauma is actually a complex and coordinated interaction between muscle and the immune system that determines the success or failure of tissue regeneration.
Acute injuries to skeletal muscle occur frequently, partly because muscles comprise a major proportion of total body mass and because many are located superficially where they are prone to trauma and they serve mechanical functions that place them under damaging loads. Muscle injuries have increased worldwide over the last 20 years, partly because of increased numbers of blast injuries in armed conflicts and musculoskeletal trauma in road traffic accidents, which alone account for tens of millions of injuries every year1. In addition, recovery from medical conditions involving secondary loss of muscle mass can be greatly slowed by limited rates of muscle regeneration and growth, leading to prolonged functional impairment even after patients return to daily activities2. These medical complications will continue to increase in cost and frequency as the world population ages. Thus, identifying interventions to improve muscle growth or regeneration following injury or disuse has immediate clinical value.
Functionally important links between muscle regeneration and inflammation after acute injury or periods of disuse have been assumed for decades. However, the collaborative efforts of developmental biologists, cellular immunologists and muscle pathophysiologists have only recently provided mechanistic links between muscle inflammation and regeneration, and have revealed a surprising level of coordination between the processes. Importantly, details of how leukocyte-derived molecules exert epigenetic controls on muscle have been identified. Although myeloid cells have taken ‘centre stage’ in studies of leukocyte regulation of muscle regeneration, new findings also show unexpected roles for lymphoid cells in modulating regeneration. Perhaps most gratifying, recent advances are leading to new strategies through which manipulating the inflammatory response to muscle injury can be used to improve regeneration, not only following muscle trauma but also in chronic muscle diseases.
Key events in myogenesis and regeneration
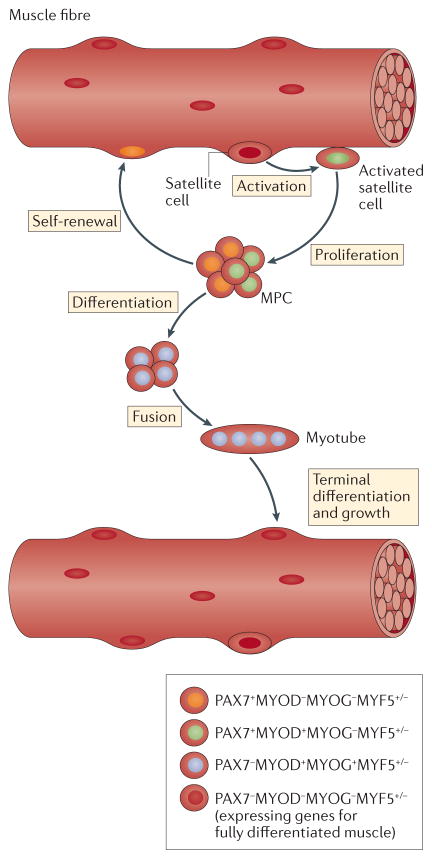
The regeneration of muscle relies on processes that mainly reuse programmes of gene expression that are necessary for the embryonic growth of muscle. Muscle regeneration begins with the activation of myogenic precursor cells (MPCs) — known as satellite cells — that reside on the surface of muscle fibres3 (BOX 1). Following muscle damage, satellite cells escape quiescence and proliferate; some daughter cells continue to differentiate, whereas others return to quiescence to replenish the reserve population of satellite cells. Postmitotic MPCs derived from activated satellite cells then form multinucleated myotubes and proceed through a stage of regeneration that is dominated by terminal differentiation and growth. Throughout this process, each stage is coupled to changes in the expression of myogenic transcription factors that are encoded by master regulatory genes3 (FIG. 1). Quiescent satellite cells express the paired-box transcription factor PAX7 but do not express the basic helix–loop–helix (bHLH) transcription factor myoblast determination protein 1 (MYOD; they are PAX7+MYOD−). Activated satellite cells are PAX7+MYOD+, and daughter MPCs that are committed to undergo differentiation express the bHLH protein myogenin. On the basis of this progression of developmental events and changes in the expression of transcription factors, regeneration is broadly divided into an early stage of MPC activation and proliferation, and a later stage of terminal differentiation and growth.
Box 1. Satellite cells in health and disease.
Satellite cells, which are sometimes referred to as muscle stem cells, are a population of committed myogenic cells that normally reside in fully differentiated muscle. In young, perinatal mice, which experience rapid muscle growth, satellite cell nuclei comprise approximately one-third of total muscle nuclei, but their proportion declines steadily to less than 10% in healthy adults109,110. Satellite cells are activated by numerous stimuli, including mechanical stresses, oxidative stress, endocrine factors and inflammatory factors. Early studies of satellite cell activation in injured muscle showed that the injured muscle itself was the source of ‘wound hormones’ that were released to activate satellite cells111. Once activated, the satellite cells enter the cell cycle but soon encounter an important developmental watershed event at which point daughter cells either continue to differentiate or return to the quiescent pool of satellite cells to self-renew that population. In healthy, young muscle, self-renewal and differentiation are balanced, and the pool of satellite cells is maintained. However, in injury and ageing, the population of satellite cells and the regenerative capacity of muscle reduce112, which may be attributable to an age-related bias of daughter cells to proceed to differentiate rather than to return to quiescence. Undoubtedly, numerous age-related and pathology-related factors influence the symmetry of satellite cell divisions and developmental fate. However, recent findings have shown an increase in serum interleukin-6 (IL-6) levels in ageing animals113, suggesting a role for immunomodulation in determining the developmental fate of satellite cells.
Figure 1. Myogenic precursor cells proceed through a tightly regulated sequence of myogenic-regulatory gene expression during muscle regeneration.
Satellite cells are myogenic precursor cells (MPCs) that reside in a quiescent state on the surface of fully differentiated muscle fibres, and they can be identified by their location and by their expression of myogenic-regulatory genes (they express PAX7, lack expression of myoblast determination protein 1 (MYOD) and myogenin (MYOG), and may or may not express myogenic factor 5 (MYF5)). Following their activation by injury, exercise or other perturbations, they can enter the cell cycle to give rise to two daughter cells that have the same developmental destiny (symmetrical cleavage) or cells that have non-identical developmental paths (asymmetrical cleavage). Many of the proliferative daughter cells will begin to express MYOD and continue to differentiate. Other daughter cells will return to the quiescent state and remain PAX7+MYOD−MYOG−. If the cells continue to differentiate and lose the capacity to self-renew, they permanently downregulate PAX7 expression and begin to express the transcription factor MYOG, which is required for further differentiation and survival123,124. Those PAX7−MYOD+MYOG+MYF5+/− cells then have the capacity to fuse with neighbouring MPCs to form multinucleated myotubes. Myotubes then undergo rapid growth and begin to express genes required for terminal differentiation, many of which are under the control of MYOG, and become nascent muscle fibres. Eventually, nuclei derived from the originally activated satellite cell population become myonuclei that reside within the muscle fibre, in which myogenic-regulatory genes are permanently silenced (PAX7−MYOD−MYOG−MYF5−).
Satellite cells and their progeny are essential for muscle regeneration. For example, depleting satellite cells abolishes the regeneration of injured muscles4–6. However, satellite cell presence alone is insufficient for muscle regeneration; the cells must have the capacity to proceed through the sequence of activation, proliferation and differentiation. When this sequence is disrupted by mutation of MYOD, regeneration is impaired (despite there being an increase in the number of satellite cells in muscle)7. Finally, activated satellite cells must have the capacity to restore the reserve population of satellite cells following injury to maintain the regenerative potential of muscle. This was shown by satellite cell-specific deletion of NUMB, a protein that regulates asymmetric cell cleavage in which one daughter cell returns to the undifferentiated reserve population, and the other daughter cell proceeds to differentiate. Ablation of NUMB in PAX7-expressing cells reduced satellite cell numbers and impaired regeneration, showing the need to restore reserve populations of satellite cells for successful muscle regeneration8.
The muscle immune system
Leukocytes are a non-obtrusive, long-overlooked component of healthy skeletal muscle. Although they appear scant in histological observations, there are 500 to 2,000 leukocytes per mm3 of adult, rodent limb muscles9–11, which is equivalent to approximately 109 leukocytes per litre of muscle. This is a surprisingly high number considering that leukocyte concentrations in the blood are 1011 leukocytes per litre for a typical adult human; total blood contains approximately 4 × 1011 leukocytes, whereas total muscle contains 4 × 1010 leukocytes. Although intramuscular leukocytes comprise various diverse cell types, including CD8+ cytotoxic T cells, regulatory T (Treg) cells, neutrophils and eosinophils, each population constitutes a small proportion of the total leukocyte population in healthy muscle. The vast majority of intramuscular leukocytes are monocytes or macrophages located primarily in either the sheath of connective tissue that surrounds entire muscles or near blood vessels12,13. Similarly to satellite cells, resident macrophages reside in a quiescent state in healthy muscle14, but increased muscle use or trauma causes their rapid activation, which is necessary for normal muscle regeneration.
Myeloid cell regulation of muscle regeneration
Muscle–myeloid cell interactions during the early, proliferative stage of muscle regeneration
Muscle damage caused by acute trauma, burns, freezing, toxins, exercise or some diseases initiates a stereotypical inflammatory response in which the number of intramuscular leukocytes can rapidly increase more than 100-fold (BOX 2). Within hours, neutrophils (that express LY6G and high levels of CD11b (also known as integrin-αM)) invade damaged muscle and reach maximum numbers at approximately 12 to 24 hours post injury, after which they rapidly return to near-normal numbers15–17. Resident macrophages (that express F4/80, LY6C and CD11b, but lack expression of CXC-chemokine receptor 1 (CXCR1)) promote this marked neutrophil influx by releasing the neutrophil chemoattractants CXC-chemokine ligand 1 (CXCL1) and CC-chemokine ligand 2 (CCL2)13. Several compelling investigations have established the importance of CCL2-mediated signalling in driving both muscle inflammation and muscle regeneration following injury, providing early evidence that muscle regeneration and growth are strongly influenced by inflammation. For example, genetic ablation of Ccl2 or its receptor CC-chemokine receptor 2 (Ccr2) in mice reduced macrophage numbers in injured muscle, and this reduction was accompanied by slowed growth of nascent muscle fibres9,18–21. Although CCL2-mediated events are mostly driven by myeloid cells, T cells have unexpected, important roles in initiating the cascade of events leading to muscle repair. Genetic deletion of Cd8a reduced CCL2 production by T cells and impaired the recruitment of macrophages into muscle following injury caused by injection of snake cardiotoxin (CTX)22; this led to defects in muscle regeneration that were similar to those caused by deletion of Ccl2 or Ccr2 (REFS 9,18–20). The most interesting implications of these findings are that resident CD8+ T cells are early responders to acute muscle injury and that signalling through the T cell receptor on CD8+ T cells has an important role in promoting the innate immune response in injured muscle, thereby affecting regeneration.
Box 2. Models of acute muscle injury and repair.
Acute muscle injuries offer an attractive system for exploring interactions between the immune system and tissue regeneration because the onset of tissue damage is well defined and the time courses of inflammation and regeneration are predictable. Across the range of damage models, the regenerative processes appear similar, at least on first approximation. Models include those in which muscles undergo a period of unloading following a return to normal weight-bearing; in these models damage is minor and causes necrosis of only a small percentage of muscle fibres14,52,56. Intense exercise can produce structural damage in a large proportion of fibres114, whereas muscle freezing18,66, crushing or injection of snake toxins9,21,22,26,37,57,62,97,115,116 can cause a huge amount of damage that destroys nearly all fibres at the injury site. However, recent direct comparisons of the inflammatory response in different injury models have revealed differences. For example, muscle neutrophilia persists for several days in muscle damaged by injection of cardiotoxin (CTX), the most common muscle injury model, whereas muscle neutrophilia is much more rapidly resolved in muscle injured by injection of notexin (NTX) or barium chloride (BaCl2)117, or in muscle injured by exercise or modified loading14,42,52,56. Although differences in the magnitude of injury may contribute to the differences between models, inflammation resolves more quickly in CTX-injured muscles than in BaCl2-injured or NTX-injured muscles117, indicating qualitative differences between models in the inflammatory response to acute injury. Part of the explanation may lie in unexamined differences in the response of the immune system to the toxins themselves, which not only injure the muscle but might also affect inflammatory cells. For example, many snake venoms including CTX and NTX contain phospholipase A2 (PLA2), which not only lyses the muscle cell membrane but also stimulates neutrophils, acts as an anticoagulant, is haemolytic, causes mast cell degranulation and activates macrophages118–121. Snake venoms can also interact with endogenous PLA2 in the injured muscle to produce the same perturbations to the inflammatory response to injury122.
Early invasion of injured muscle by neutrophils is a generic and indispensable response to acute muscle damage. As in other injuries, the early arrival of neutrophils in injured muscle enables them to condition the inflammatory environment to influence the activation state of subsequent immune cell populations. Following the onset of neutrophil invasion, circulating monocytes and macrophages extravasate and enter a muscle environment that is enriched with pro-inflammatory cytokines, including interferon-γ (IFNγ) and tumour necrosis factor (TNF)23–26. Together, these cytokines can activate macrophages to a pro-inflammatory phenotype, and these macrophages are named M1 macrophages to reflect their activation by pro-inflammatory T helper 1 (TH1)-type cytokines27–29. This distinguishes them from M2 macrophages, which are activated by TH2-type cytokines and are associated with the resolution of inflammation and with tissue repair. However, the M1 versus M2 macrophage phenotype dichotomy represents polarized extremes of a range of phenotypes that does not reflect the true diversity or plasticity of macrophage populations30. Even in individual macrophages isolated from injured muscle, transcripts associated with both the M1 and M2 phenotypes can be expressed simultaneously, which provides these macrophages with functions that differ from either polarized phenotype31. Furthermore, at any time point following injury, the inflammatory infiltrate will consist of a mix of macrophage phenotypes and diverse states of activation, with some macrophages expressing M1-associated activation markers and neighbouring macrophages expressing M2-associated activation markers32. Nevertheless, the M1–M2 nomenclature is used in this Review to indicate the predominant bias of macrophage populations towards a pro-inflammatory (M1) or pro-regenerative (M2) phenotype, while recognizing the phenotypic complexity of macrophages that reside between the extremes.
The inflammatory response during the early stages of muscle regeneration is coupled temporally and spatially to the initial stages of myogenesis, when satellite cells are first activated and begin to proliferate and differentiate (FIG. 2). However, exactly how the initial inflammatory response and early myogenesis are co-regulated has only recently emerged. Among several candidate molecules, IFNγ stands out as potentially important for coordinating the initial inflammatory response with the early stages of regeneration. Within the first 24 hours following acute muscle injury by CTX injection, IFNγ expression levels in the injured tissue increase, coinciding with increased numbers of neutrophils, macrophages and activated satellite cells expressing MYOD25. Furthermore, blockade of IFNγ signalling in injured muscle reduces the expression in macrophages of transcripts that indicate M1 activation (such as those encoding interferon-regulatory factor 1 (IRF1) and inducible nitric oxide synthase (iNOS))25. As IFNγ-mediated induction of IRF1 and iNOS expression occurs through a signal transducer and activator of transcription 1 (STAT1)-mediated pathway33,34, these findings indicate that STAT1 signalling may have a role in IFNγ-mediated activation of macrophages in regenerating muscles. IFNγ also regulates macrophage phenotype in muscle from dystrophic mice (mdx mice) during the period of acute muscle damage and the following regenerative stage. Ablation of IFNγ expression in mdx mice reduces iNOS protein levels in muscle macrophages, increases the expression in vivo of transcripts that indicate M2 activation of macrophages (such as those encoding interleukin-4 (IL-4), arginase 1 (ARG1), monoglyceride lipase 2 (MGL2) and FIZZ1 (also known as resistin-like-α)) and shifts macrophages to a CD206+ M2-biased phenotype in vivo35. Despite these findings showing a role for IFNγ in regulating macrophage phenotype in injured muscle in vivo, one recent investigation did not find signs of macrophage activation to an M1-biased phenotype for muscle macrophages ex vivo36. Following the sorting of macrophages into LY6C+ and LY6C− groups, which were expected to represent M1-biased and M2-biased populations37, the investigators did not find significant differences in the expression of some M1-associated or M2-associated transcripts between the groups at any time point post injury. They also did not see elevations in the levels of transcripts associated with IFNγ-mediated induction of STAT1 signalling in sorted macrophage samples. Although numerous differences could underlie incongruous outcomes when assessing macrophage activation states in vivo versus ex vivo, a potential biological explanation may be that the earliest time point examined in the ex vivo investigation used macrophages isolated from muscle 24 hours post injury or later, without a baseline comparison group36. At 24 hours post injury, IFNγ expression has already been increased25, and macrophages have already been activated to an M1-biased phenotype. Although the investigators also showed no significant difference in the levels of expression of some M1-associated transcripts in LY6C+ and LY6C− macrophages collected 24 hours post injury, which could indicate no activation to an M1-biased phenotype at this stage, they also detected no significant difference in the expression of LY6C between the LY6C+ and LY6C− groups at that time.
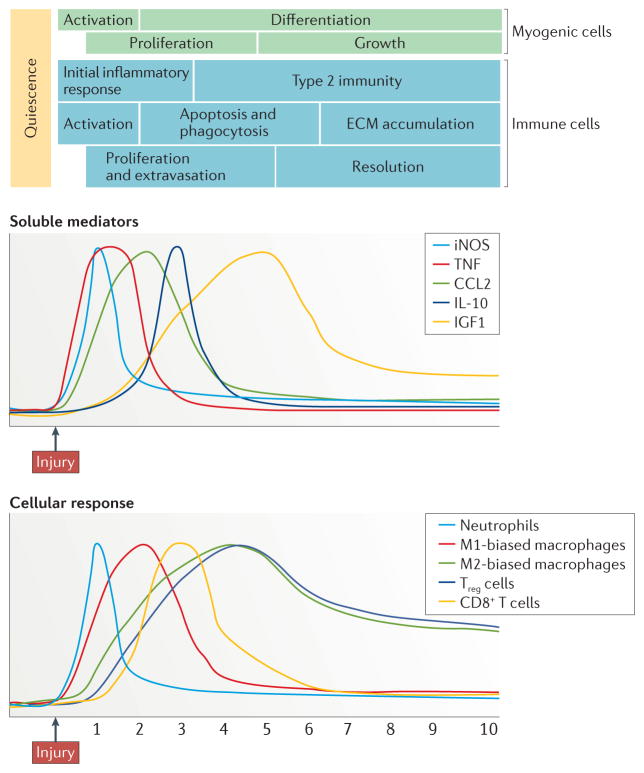
Figure 2. Myogenesis during muscle regeneration is temporally linked with a transition from the initial inflammatory response to a type 2 immune response.
Within hours of trauma, muscles experience rapid activation of resident myeloid cells, and invasion by neutrophils and by macrophages that are biased towards an M1 phenotype. During the first 24 hours, the immune response is characterized by high levels of expression of tumour necrosis factor (TNF), which promotes the initial inflammatory response, and CC-chemokine ligand 2 (CCL2), which drives the recruitment of both myeloid and lymphoid populations. However, elevated expression of interleukin-10 (IL-10) coincides with the attenuation of the initial inflammatory response and with the reduced proliferation of myogenic precursor cells (MPCs) as they begin to exit the cell cycle and differentiate, which corresponds to a change in the macrophage population from an M1-biased phenotype to an M2-biased phenotype. The shift towards a pro-regenerative environment is also coupled with an increase in regulatory T (Treg) cell numbers in the regenerative tissue. The time courses are compiled from aggregate data from numerous publications (REFS 20,24,25,37,42,54,56,125). ECM, extracellular matrix; IGF1, insulin-like growth factor 1; iNOS, inducible nitric oxide synthase.
Within the post-injury debris and inflammatory lesions that are dominated by neutrophils and by macrophages biased towards the M1 phenotype, the earliest stages of regeneration begin, and they are influenced by IFNγ25,35. IFNγ regulates gene expression in muscle cells through a pathway mediated by MHC class II transactivator (CIITA)38,39. These dual roles of IFNγ, whereby it functions both to activate macrophages and to directly regulate myogenic cells in the process of differentiation, is reflected in the extended period of time during which its expression is increased following muscle injury; although it is first markedly increased at day 1 post injury, expression continues to increase until day 5 (REF. 25). Similarly to the pathway through which IFNγ activates macrophages to the M1-biased phenotype, IFNγ receptor binding on MPCs activates the Janus kinase (JAK)–STAT1 signalling pathway, leading to the expression of target genes, including CIITA40 (FIG. 3a). In MPCs, IFNγ-mediated induction of CIITA expression is essential for inhibiting MPC differentiation. Whereas IFNγ normally suppresses the expression of myogenin, an essential transcription factor for muscle terminal differentiation, myogenin suppression did not occur if CIITA expression was knocked down38. Thus, IFNγ-mediated mechanisms are well suited for coordinating the immune response to muscle injury with the early stage of muscle regeneration. Although phagocytic macrophages biased towards the M1 phenotype remove debris resulting from muscle injury23,41,42, they also express IFNγ25, which may reinforce the macrophage phenotype and retain MPCs in a proliferative, non-differentiated state so that their populations can expand and support tissue repair.
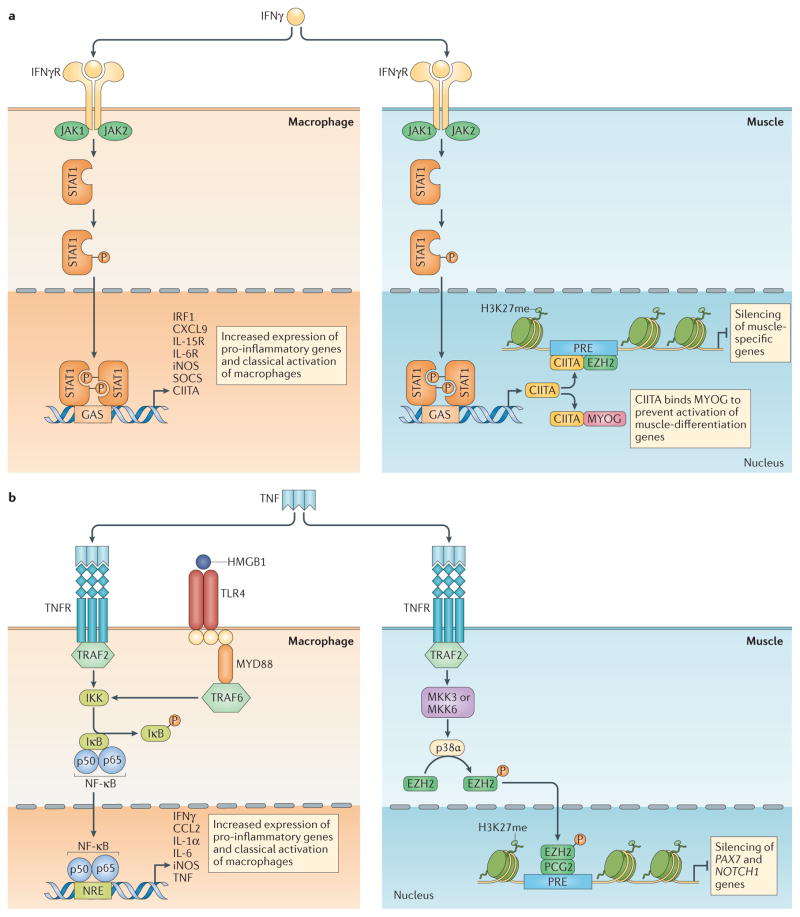
Figure 3. Pro-inflammatory cytokines link inflammation with muscle regeneration.
a | Interferon-γ (IFNγ)-mediated induction of macrophage activation coordinates the initial inflammatory response and the regulation of muscle differentiation. In the well-characterized signalling pathway through which IFNγ activates the M1 phenotype, the cytokine binds its receptor, leading to the recruitment of the tyrosine kinases Janus kinase 1 (JAK1) and JAK2, which in turn phosphorylate and activate signal transducer and activator of transcription 1 (STAT1) and IFN-regulatory factor 1 (IRF1)126. Nuclear targeting of dimerized, phosphorylated STAT1 enables it to bind γ-activated sequences (GASs) of target genes, leading to the activation of a range of genes that can promote the pro-inflammatory M1 phenotype. Simultaneously, IFNγ-activated signalling in muscle cells through the JAK–STAT1 pathway increases the expression of MHC class II transactivator (CIITA). During early stages of myogenesis, CIITA binds directly to myogenin (MYOG) and inactivates it, preventing MYOG-mediated induction of myogenic genes that are required for muscle differentiation and function (for example, TNNI2 (which encodes fast skeletal muscle troponin I2 and LMOD2 encoding leiomodin 2)). This helps to retain myogenic precursor cells (MPCs) in an undifferentiated, proliferative state. In later stages of differentiation, increased levels of CIITA lead to its recruitment of EZH2 to the Polycomb-response element (PRE) of muscle-specific genes, which leads to their silencing and prevents the expression of genes that are required to reach the fully mature muscle phenotype. b | Increased release of tumour necrosis factor (TNF) coordinates early myogenic events with the initial inflammatory response during early stages of muscle injury and repair. Although numerous cells, including those of muscle itself, can express TNF, inflammatory cells are the primary sources at early stages post injury24. TNF binding to its receptor (TNFR) on macrophages activates signalling through TNFR-associated factor 2 (TRAF2), leading to activation of the inhibitor of nuclear factor-κB kinase (IKK), and the subsequent phosphorylation of inhibitor of nuclear factor-κB (IκB) and activation of nuclear factor-κB (NF-κB). Activated NF-κB then translocates to the cell nucleus to bind to the NF-κB-response element (NRE) of target genes to activate their expression. Expression of those target genes is essential to maintain the initial inflammatory response. Alternatively, the binding of molecules containing damage-associated molecular patterns (DAMPs), especially high mobility group protein B1 (HMGB1) can also activate NF-κB127. HMGB1 binds to Toll-like receptor 4 (TLR4) on macrophages to activate NF-κB via myeloid differentiation primary response gene 88 (MYD88) and TRAF6. At the same time, TNF can bind its receptor on the MPC surface to activate mitogen-activated protein kinase (MAPK) kinase 3 (MKK3) or MKK6, leading to the activation of the MAPK p38α. Phosphorylation of EZH2 by p38α causes EZH2 recruitment to the Polycomb repressive complex 2 (PRC2), which leads to binding at the PRE of target genes. Activated EZH2 then trimethylates histone 3 lysine 27 (H3K27) at the target genes, leading to their repression. Two functionally important target genes in activated satellite cells are PAX7 and NOTCH1, and their silencing prepares the satellite cells to transition to the early stages of differentiation. CCL2, CC-chemokine ligand 2; CXCL9, CXC-chemokine ligand 9; IFNγR, IFNγ receptor; IL, interleukin; IL-6R, IL-6 receptor; IL-15R, IL-15 receptor; iNOS, inducible nitric oxide synthase; PCG2, Polycomb group 2; SOCS, suppressor of cytokine signalling.
Despite the apparently effective mechanisms through which IFNγ coordinates the early stages of inflammation with the early stages of regeneration, IFNγ signalling through CIITA at later stages could have negative consequences (FIG. 3a). For example, if MPCs undergoing terminal differentiation are stimulated with IFNγ, genes encoding proteins required for the function of fully differentiated muscle are suppressed39. This gene silencing is attributable to CIITA-mediated increases in the expression and activity of the histone methyltransferase EZH2. EZH2 is the catalytic subunit of the Polycomb group 2 (PCG2) complex that represses gene transcription by trimethylating lysine 27 on histone 3 (H3K27me3) at the Polycomb-response element (PRE) near the promoters of target genes. Increased levels of IFNγ and CIITA increase the recruitment of PCG2 to the promoter region of some muscle-specific genes, and this was associated with silencing of those genes in the late stages of differentiation but not in the early stages38,39. Although it is unknown whether these epigenetic effects occur in vivo, the observation suggests that increased levels of IFNγ at early stages of regeneration benefit repair, but persistent increases in inflammatory cells expressing IFNγ at later stages could impair recovery.
Investigation of the links between the pro-inflammatory cytokine TNF and the epigenetic control of muscle regeneration has also provided insights into the mechanisms that coordinate muscle regeneration and the early stages of the inflammatory response (FIG. 3b). Neutrophils and macrophages express most or all of the TNF in muscle following an acute injury23,24. Although TNF-induced activation of nuclear factor-κB (NF-κB)-mediated signalling in myeloid cells can influence their activation and their interactions with injured muscle, TNF can also act directly on muscle cells to affect muscle regeneration. Pioneering work established that loss of TNF signalling in acutely injured muscle abrogated signalling through p38 mitogen-activated protein kinase 3 days post injury, when MPCs transition from the proliferative to the early differentiation stages of myogenesis43. In addition, the expression of myogenin was eliminated and muscle regeneration was greatly impaired. Delving into the mechanism underlying this effect showed that p38α activation in injured muscle led to the phosphorylation of EZH2, causing its recruitment to PCG2 and an associated increase in trimethylation of H3K27 at the PRE of PAX7 (REF. 44). These observations, combined with the finding that blockade of EZH2 resulted in increased expression of PAX7, support the model that TNF-induced signalling through p38α represses PAX7 expression through EZH2 activity, thereby suppressing the proliferation and promoting the differentiation of MPCs. However, there are additional layers of complexity that determine the outcome of changes in H3K27 methylation. For example, mice that have a satellite cell-specific deletion of Ezh2 (Ezh2mKO mice) have fewer satellite cells in non-injured muscle compared with non-injured muscle from wild-type mice45,46; this is the opposite to what would be predicted to occur on the basis of blocking TNF-induced signalling in injured muscle44. Furthermore, Ezh2mKO mice show reduced expansion of satellite cell populations in injured muscle45, despite the finding that decreased EZH2 activity caused by blocking TNF and p38α signalling in injured muscle increases MPC numbers44. Although there are several possible explanations for the different outcomes, part of the explanation may lie in the stage of myogenesis at which MPC genes are silenced. In Ezh2mKO mice, EZH2-mediated effects on gene expression are perturbed during satellite cell quiescence and activation, whereas increased activation of EZH2 by TNF in the inflamed muscle of wild-type mice occurs in MPCs that were previously activated and proliferating. Recent findings highlight that the stage of muscle regeneration determines how EZH2 activity affects myogenesis46. Although ablation of Ezh2 in Ezh2mKO mice produces severe defects in regenerative muscle and impairs MPC proliferation, mice in which floxed EZH2 is deleted by Cre recombinase driven by the myogenin promoter show no regenerative or proliferative defects46. In this mouse line, Ezh2 deletion occurs only in the later stages of myogenesis.
The discovery that TNF also has epigenetic effects on muscle regeneration by targeting NOTCH1 adds to the complex picture of how pro-inflammatory cytokines regulate regeneration. NOTCH1 is a transmembrane receptor that is expressed in muscle and other cells, in which it has a central role in regulating development. NOTCH1-mediated effects on myogenesis are mainly attributable to the inhibition of MYOD, myogenin and myocyte-specific enhancer factor 2C (MEF2C) expression47, which leads to the increased proliferation and reduced differentiation of MPCs48. The finding that the exposure of muscle to TNF increases EZH2-mediated H3K27 methylation at the NOTCH1 promoter49 identified a mechanism through which TNF could influence myogenesis in a manner that reinforced TNF-induced silencing of PAX7. By decreasing the expression of a protein that reduces the activity of transcription factors necessary for MPC differentiation, TNF-induced silencing of NOTCH1 could reduce satellite cell numbers.
Together, these findings show that the two cytokines that can activate macrophages to the M1-biased phenotype in injured muscle, IFNγ and TNF, also have important roles in regulating the proliferation and differentiation of MPCs. Many of the effects are caused by EZH2-mediated silencing of myogenic-regulatory genes. The findings also show that epigenetic events regulating myogenesis differ in injured and non-injured muscles. This view is further supported by analysis of the effects of a satellite cell-specific deletion of the histone lysine-specific demethylase 6A UTX on muscle regeneration50. UTX demethylates H3K27 and can thereby activate genes silenced by EZH2. The importance of this demethylation is established in MPCs in vitro, in which UTX is essential for activating myogenin expression, which is required for terminal differentiation51. However, satellite cell-specific deletion of UTX showed no histologically discernible effect on healthy muscle structure, although it caused severe defects in regeneration following injury50. Whether this distinction is attributable to differences in compensatory mechanisms for H2K27 demethylation that are available in healthy developing muscle but not in mature injured muscle, or whether it reflects another mechanism, is unknown.
Muscle–immune cell interactions during the transition to the terminal differentiation stage of muscle regeneration
Collectively, the net effects of increasing TNF-induced and IFNγ-induced signalling at the early stage of regeneration drives macrophages to an M1-biased state of activation, and regulates proliferation and early differentiation of MPCs. This complex but well-tuned system permits the expansion of the population of MPCs, some of which return to the reserve population of satellite cells, whereas others differentiate and grow into fully differentiated muscle fibres. Early histological observations that relied on macrophage phenotyping on the basis of the level of expression of CD68 and CD163 showed that the peak number of CD68hiCD163− phagocytic M1 macrophages that occurred 2 days post injury was replaced by a population of non-phagocytic CD68lowCD163+ M2 macrophages that reached peak numbers approximately 4 to 7 days post injury, coinciding with the expression of genes that are markers of terminal differentiation52. This suggested that the transition from an initial immune response, dominated by phagocytic M1-biased macrophages, to a type 2 immune environment, dominated by M2-biased macrophages, could be functionally coupled to the transition in stages of myogenesis.
Although numerous signalling systems interact to influence the coordinated transitions in the immune environment and the stages of myogenesis, modifications in cytokine production over the course of regeneration are particularly important. For example, marked increases in the expression of IL-10 accompany the transition of macrophages from an M1 to an M2 phenotype in injured muscle and correspond to the transition of regeneration from the proliferative stage to the differentiation and growth stage of myogenesis42,53,54 (FIG. 4). IL-10-induced activation of macrophages drives them to become an M2-biased subpopulation that functions in immunoregulation and extracellular matrix deposition, and is characterized by elevated expression of IL-10 and transforming growth factor-β (TGFβ)55. As has now been clearly shown, the transition from an M1-biased to an M2-biased macrophage phenotype in injured muscle is necessary for normal regeneration. Depletion of F4/80+ macrophages at the time of the M1-biased to M2-biased macrophage phenotype switch in regenerating muscle significantly slowed muscle growth, repair and regeneration, and disrupted the normal expression patterns of transcription factors that regulate regeneration56. Similar reductions in the growth of muscle fibres in muscle damaged by CTX injections occurred if CD11b+ inflammatory cell numbers were reduced at the stage of peak muscle regeneration57. However, the transition in phenotype did not occur in injured muscles in which IL-10 expression was ablated, and the loss of the IL-10-mediated phenotype switch caused impairments of regeneration resembling those caused by deletion of F4/80+ or CD11b+ cells42.
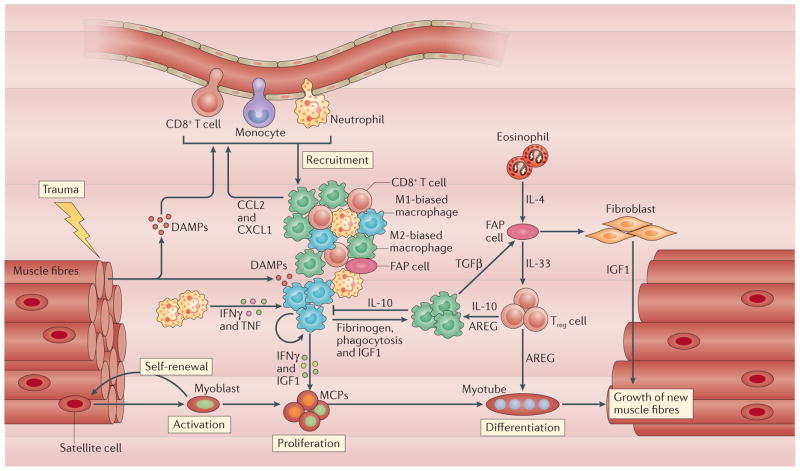
Figure 4. Interactions between myeloid cells, lymphoid cells, fibro-adipogenic progenitor cells and myogenic precursor cells determine the course of muscle growth and regeneration.
Trauma to muscle can cause the release of molecules containing damage-associated molecular patterns (DAMPs), such as high mobility group protein B1 (HMGB1), that activate and recruit immune cells to the site of trauma127,128. This corresponds to the activation of satellite cells to enter the cell cycle and to proliferate. The release of cytokines and chemokines, especially CC-chemokine ligand 2 (CCL2) and CXC-chemokine ligand 1 (CXCL1), from neutrophils, resident macrophages and CD8+ T cells promotes further recruitment of immune cells to an inflammatory response driven by interferon-γ (IFNγ) and tumour necrosis factor (TNF). Elevations in those pro-inflammatory cytokines sustain the initial inflammatory response but also act directly on myogenic precursor cells (MPCs) through epigenetic mechanisms to regulate their expression of myogenic-regulatory genes that control MPC development. Macrophages biased towards the M1 phenotype also release insulin-like growth factor 1 (IGF1), which increases MPC proliferation and further expands the MPC population. M1 macrophage populations then undergo a shift to a population that is biased towards the M2 pro-regenerative phenotype. Several ligands have important roles in promoting this phenotypic shift, including fibrinogen129, amphiregulin (AREG) and interleukin-10 (IL-10) produced by regulatory T (Treg) cells, and IL-10 and IGF1 produced by macrophages. In addition, phagocytosis of cellular debris also contributes to the macrophage phenotypic switch. AREG release by Treg cells increases the expression of myogenic-regulatory factors that drive the later stages of muscle differentiation. This influence is further enhanced by the recruitment of Treg cells to regenerated muscle by IL-33 released by fibro-adipogenic progenitor (FAP) cells and possibly by macrophages. FAP cell proliferation and differentiation into cells with phagocytic or fibrogenic phenotypes are influenced by the release of transforming growth factor-β (TGFβ) from macrophages and by IL-4 released by eosinophils. Those macrophages that differentiate to become fibroblasts then contribute to the extracellular matrix production that supports muscle repair and the release of IGF1, which increases the growth of fully differentiated muscle.
Although IL-10 directly deactivates the M1 phenotype by inhibiting the expression of pro-inflammatory cytokines, it also induces the macrophage transition from the M1-biased to the M2-biased phenotype through mechanisms that may influence muscle regeneration. The discovery of a mechanism through which IL-10 mediates changes in macrophage phenotype came, unexpectedly, from studies of metabolic pathways. In particular, AMP-activated protein kinase (AMPK) has regulatory roles in inflammation and metabolism whereby it switches off ATP-consuming pathways in cells when ATP levels are low58. As M1-biased macrophages are more glycolytic and M2-biased macrophages more oxidative59, AMPK levels differ according to the state of macrophage polarization. For example, anti-inflammatory cytokines, such as IL-10 and TGFβ, rapidly activate AMPK, whereas stimulation with pro-inflammatory stimuli deactivates AMPK60. Conversely, AMPK regulates M1 to M2 polarization. Knockdown of AMPK increased the expression of pro-inflammatory cytokines, whereas overexpression of AMPK α-subunit 1 (AMPKα1) in macrophages reduced TNF but increased IL-10 expression61. These regulatory functions of AMPK also affect macrophage phenotype in regenerating muscle, thereby influencing macrophage-dependent regeneration. Myeloid cell-specific deletion of AMPKα1 in mice experiencing a CTX-induced muscle injury led to increased muscle damage, and slowed repair and growth37. In addition, bone marrow-derived macrophages (BMDMs) from AMPKα1-mutant mice showed less induction of CD163, CD206 and TGFβ1 expression following stimulation with IL-10 or IL-4 than did wild-type BMDMs37. Collectively, these findings support a model in which IL-10 promotes the M2-biased phenotype, increasing AMPK activity, which drives the production of anti-inflammatory cytokines that further support type 2 immunity.
Much of the recent investigation of myeloid cell-derived signals that affect the early stages of muscle regeneration has focused on chemokines and cytokines. However, myeloid cells also provide growth factors that influence the course and success of muscle regeneration and that affect macrophage phenotype. Unexpectedly, insulin-like growth factor 1 (IGF1) released by M1-biased macrophages is a strong mitogen for MPCs in muscle, and myeloid cell-specific deletion of IGF1 slows muscle growth following injury62. Although muscle fibres themselves are rich sources of IGF1 (REF. 63) and IGF1 is a well-known mitogen for MPCs64,65, muscle-derived IGF1 may not be available following severe muscle damage. In this case, local delivery of IGF1 by macrophages precisely at injury sites may promote regeneration. However, myeloid cell-derived IGF1 also influences muscle regeneration through an autocrine effect on macrophages themselves (FIG. 4). Loss of IGF1 in myeloid cells disrupts the transition of macrophages from an M1-biased to an M2-biased phenotype in injured muscle62, which can contribute to slowed growth of injured muscle.
Other subtle factors coordinate changes in the innate immune environment with stages of myogenesis. For many years, those studying muscle regeneration noted that perturbations that slow phagocytic removal of apoptotic cells and other debris were associated with delayed regeneration17,19,57,66,67. On the one hand, defective regeneration among large amounts of persistent debris was not surprising; the prolonged accumulation of debris could provide a physical obstacle to the regeneration. On the other hand, the phagocytic removal of debris also has more specific regulatory roles, in addition to creating space for repair. For example, macrophage phagocytosis of apoptotic neutrophils suppressed the expression of TNF, and phagocytosis of necrotic or apoptotic neutrophils increased the expression of TGFβ, which indicates a shift towards an M2-biased phenotype68,69. Similarly, phagocytosis of necrotic MPCs isolated from muscles reduced TNF and increased TGFβ production by muscle-derived macrophages57. Thus, signalling activated by phagocytosis can promote a shift in macrophage phenotype that supports muscle regeneration.
Once again, the phenotypic switch associated with macrophage phagocytosis is attributable, at least in part, to AMPK-mediated signalling. Phagocytosis of apoptotic cells by macrophages increased AMPK activity70, and elevations in AMPK activity increased phagocytosis70 and biased macrophages towards an M2 phenotype61. Similarly, phagocytosis of apoptotic MPCs by BMDMs caused an AMPK-influenced shift towards an M2-biased phenotype; unlike BMDMs from wild-type mice, BMDMs from AMPKα1-mutant mice showed no induction of CD163, CD206 or TGFβ expression after phagocytosis of apoptotic MPCs37. These in vitro observations, combined with the finding that defects in the regeneration of CTX-injured muscle in AMPKα1-mutant mice were partially rescued by the transplantation of bone marrow-derived cells from wild-type mice, show that regeneration is improved by AMPK-mediated signalling that is initiated by phagocytosis in macrophages.
Although these findings show that immunoregulatory signals that are activated by macrophage apoptosis have considerable effects on muscle regeneration, observations in other systems indicate that phagocytosis of apoptotic bodies may have longer-term effects on the response of muscle to injury. Most interestingly, recent discoveries show that phagocytosis of apoptotic bodies in infected tissues can generate a form of immunological memory in macrophages that facilitates their ability to quickly recognize and respond to apoptotic cells and tissue damage71. This suggests that sterile injuries in tissues, including muscle, that lead to phagocytosis of apoptotic cells by macrophages could also condition macrophage memory and influence the ability of macrophages to respond to subsequent injuries.
Muscle–immune cell interactions during the terminal differentiation and growth stage of muscle regeneration
Beginning with the earliest observations that changes in macrophage polarization coincided with muscle regeneration, CD163 was identified as a specific marker of the macrophage phenotype that predominated during the terminal differentiation and growth stage of regeneration52. Now, we know that CD163 also regulates macrophage phenotype and muscle regeneration. CD163 is a transmembrane glycoprotein that is constitutively expressed by macrophages72, although its expression is strongly influenced by cytokines. For example, TNF downregulates CD163 expression73, whereas IL-10 is a powerful inducer of CD163 expression72–74. CD163 facilitates tissue regeneration by binding haemoglobin–haptoglobin complexes, which enables the complexes to be internalized and degraded75,76. This is important in regeneration because haemolysis in injured tissue causes local, toxic elevations of haemoglobin that amplify damage77. CD163 binding also increases the expression of IL-10, which further promotes its anti-inflammatory effects78. Presumably, immunoregulatory functions of membrane-bound CD163 improve muscle regeneration because systemic ablation of CD163 exacerbated muscle damage caused by ischaemia, slowed muscle growth and delayed the normal myogenic programme required for regeneration79. However, injury also caused the release of the extracellular domain of CD163 into the serum79, and this soluble CD163 inactivated the pro-inflammatory cytokine TNF-related weak inducer of apoptosis (TWEAK; also known as TNFSF12)79,80. As soluble CD163 reverses the changes in NF-κB activation induced by TWEAK in ischaemic muscle, and TWEAK directly activates NF-κB and promotes MPC proliferation, part of the pro-regenerative effect of CD163 may occur by preventing perturbations in myogenesis induced by TWEAK. However, this protective effect of CD163 must be placed in the context of apparently complex findings concerning the role of TWEAK signalling in muscle regeneration. On the one hand, systemic ablation of the TWEAK receptor FGF-inducible 14 (FN14; also known as TNFRSF12A) slowed the regeneration of CTX-injured muscle81, but, on the other hand, systemic deletion of TWEAK improved muscle growth following injury82.
Lymphoid cell regulation of muscle regeneration
Interest in immune cell regulation of muscle regeneration has focused on myeloid cells because of their vast preponderance in regenerating muscle. However, just as for myeloid cells, early histological observations showed that CD4+ and CD8+ lymphoid cells appeared in elevated numbers in injured and regenerating muscle83. However, those observations were not followed up for nearly two decades, and only recently came to the fore as we developed an understanding of the immunobiology of muscle.
Perhaps most surprisingly, Treg cells have an important regulatory role in muscle regeneration. This improbable finding emerged from the observation that forkhead box protein 3 (FOXP3)-expressing CD4+ T cells accumulated in injured muscle with kinetics similar to those of M2 macrophages, reaching peak numbers of approximately 107 cells per litre of muscle at 4 days post injury84. Furthermore, depletion of Treg cells during muscle regeneration slowed repair, prolonged inflammation and perturbed the expression of myogenic transcription factors84, resembling the effects of depleting F4/80+ macrophages during muscle regeneration56. These effects of Treg cell depletion may be partly mediated by the disruption of their regulation of macrophage phenotype; Treg cell depletion impaired the normal transition from the M1-biased to the M2-biased macrophage phenotype in the regenerating muscle. However, Treg cells can also have effects on regeneration84 that may be influenced by Treg cell-derived IL-10 (REFS 10,85) or amphiregulin84.
The most interesting story concerning the regulatory interactions between Treg cells and muscle may remain to be discovered. As Treg cells that reside in healthy muscle11 have the ability to regulate the immune response and to directly influence muscle differentiation, they are ideally situated to act as monitors that coordinate immune cell interactions with muscle during regeneration. They may also provide muscle with a memory. Sequencing of T cell receptors (TCRs) from muscle-derived Treg cells showed identical paired complementarity-determining region 3α (CDR3α) and CDR3β in multiple mice assayed after injury84. However, the conserved CDR3 sequences did not occur in splenic Treg cells or in conventional T cells from muscle. This introduces the possibility not only that there is a common antigen or antigens driving Treg cell activation in regenerating muscle, but also that there may be memory Treg cells that regulate the response to commonly encountered TCR ligands produced by muscle damage.
Immune cell interactions with FAP cells in muscle
As presented above, inflammatory cells influence muscle regeneration through direct, instructive interactions that can affect gene expression in MPCs. However, they also promote regeneration through permissive interactions in which they influence the myogenic environment so that regeneration can proceed. A particularly important example of these permissive interactions is provided by the role of myeloid cells in influencing the function of a muscle-resident population of mesenchymal cells that express platelet-derived growth factor receptor-α (PDGFRα); these cells are known as fibro-adipogenic progenitor cells (FAP cells)86,87.
Similarly to satellite cells and muscle-resident macrophages, FAP cells in healthy muscle are quiescent but become rapidly activated following injury or intense exercise. Resembling the kinetics of macrophages and satellite cells, they reach peak numbers 3 days post injury, declining to non-injured control levels by day 14 (REF. 87). Much of the time course of the expansion and decline of FAP cell populations following injury is determined by myeloid cells. For example, IL-4 derived from eosinophils may increase FAP cell proliferation and bias them towards a phagocytic phenotype at early stages post injury32. FAP cells are then eliminated by apoptosis that is driven by TNF released from M1-biased macrophages31. However, during their few days of pro-fibrotic activity, they modulate the developmental programme of MPCs to influence the course of muscle regeneration31,88.
A particularly delicate interplay between macrophages and FAP cells regulates the production of the extracellular matrix scaffold that is necessary for muscle regeneration. FAP cells that are activated to the fibrogenic phenotype are regulated by M2-biased macrophages that release TGFβ, which prevents TNF-mediated induction of FAP cell apoptosis and leads to the expansion of the FAP cell population and their differentiation into a fibrogenic phenotype31 (FIG. 5). As fibrogenic FAP cells are primary producers of connective tissue in injured muscle89, these macrophage-mediated effects have a key role in determining the quantity and quality of connective tissue production. In addition, TGFβ-mediated activation of dermal fibroblasts increases the expression of genes such as those encoding collagen type 1 and connective tissue growth factor90,91, which amplifies the profibrotic effects of the cytokine. However, another subpopulation of M2-biased macrophages, referred to as M2a macrophages in some nomenclature schemes, also determines the process of connective tissue production in injured muscles, and they have been identified in increased numbers in regenerative and ageing muscle11,35,37,42. M2a macrophages secrete IL-10, and express CD206 and arginase 1 (ARG1)54. ARG1 has an important role in fibrosis via its metabolism of arginine to yield polyamines, which stimulate fibroblast proliferation, and ornithine, which is metabolized to produce the proline that is required for collagen production92,93. Thus, the net effect of M2-biased macrophage function during the later stages of regeneration is to support the production of connective tissue, which is necessary for the complete structural and functional recovery of injured muscle.
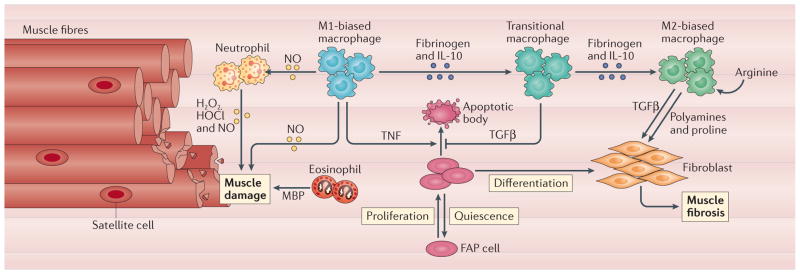
Figure 5. Dysregulation of the immune response to muscle injury increases muscle damage and fibrosis.
If muscle experiences chronic trauma, or if the inflammatory response to muscle injury is perturbed by disease or ageing, immune cells can exacerbate fibre damage or fibrosis. Under conditions that amplify or prolong the initial inflammatory response, free radicals produced by neutrophils or macrophages can promote muscle membrane lysis and fibre death. In particular, neutrophil production of superoxide (O2−), hydrogen peroxide (H2O2), hypochlorous acid (HOCl), nitric oxide (NO) and peroxynitrite (ONOO−) by NADPH oxidase, myeloperoxidase and inducible nitric oxide synthase can greatly increase muscle damage130. In addition, in mdx mouse skeletal muscle, which experiences chronic inflammation, there are persistently higher numbers of eosinophils that can cause further damage of muscle fibres through the release of major basic protein (MBP)131. Chronic muscle damage also increases the occurrence of a transitional macrophage population that can lead to pathological increases in the number of fibro-adipogenic progenitor (FAP) cells and their profibrotic activities because transforming growth factor-β (TGFβ) production by transitional macrophages counters the negative regulation of their numbers by tumour necrosis factor (TNF) released by M1 macrophages. In addition, chronic muscle inflammation leads to an M2-biased macrophage phenotype in which the levels of expression and activity of arginase are highly elevated, leading to high levels of polyamines that can further expand fibroblast populations and the production of proline, which can increase connective tissue production. IL-10, interleukin-10.
What happens when things go wrong?
The efficiency and reliability of muscle regeneration following acute injury is attributable to natural selection for processes that provide rapid recovery following trauma. By contrast, there is little selective pressure for mechanisms to regenerate muscle that has been subjected to chronic damage because pathologies that give rise to chronic muscle damage are uncommon. Lacking a system adapted to chronic injuries, immune cell modulation of muscle regeneration can go awry, leading to muscle fibrosis and impaired regeneration. These regenerative defects are most thoroughly shown in the mdx mouse model of DMD. DMD and mdx dystrophy involve a mechanically weakened cell membrane, which leads to chronic muscle damage and inflammation94. Although numerous pathological features arise from chronic muscle injury, disruptions of the normal immune response to muscle damage exacerbate the pathology95. Most prominently, chronic injury causes the alteration of macrophages to phenotypes for which the normal regulation of connective tissue production is disrupted. At early stages of chronic damage, these macrophages express both M1 and M2 phenotypic markers and, unlike M2-biased macrophages from acutely injured wild-type muscle, these transitional macrophages are unable to induce FAP cell apoptosis, leading to increased connective tissue deposition31 (FIG. 5). In addition, M2-biased macrophages in advanced stages of mdx dystrophy shift to a highly fibrogenic state in which there is enhanced ARG1 activity that drives pathological fibrosis in dystrophic muscle96. Despite their importance in muscular dystrophy, the mechanisms that underlie the phenotypic specialization of macrophages in chronically injured muscle are unknown.
Just as there are few selective pressures associated with chronic damage, few selective pressures are associated with regenerating old muscle in organisms that are beyond reproductive age. Lacking natural selection for maintaining normal muscle interactions with the immune system in old muscle, those interactions become dysregulated and contribute to defects in muscle regeneration. Ageing and senescent mammals show a progressive loss of the ability of muscle to regenerate following injury97, and this is partially attributable to defects in immune cell interactions with ageing muscle. For example, age-related perturbations in signalling between FAP cells and Treg cells may contribute to slower regeneration of injured muscle in ageing animals. In contrast to young mice in which Treg cell numbers increase within the first 2 days following injury, no Treg cell increase occurs in old injured muscle98. This defect was attributed to lower levels of IL-33 production by FAP cells. IL-33, which is expressed by macrophages, dendritic cells and fibroblasts98–100, is a ligand for IL-1 receptor-like 1 (IL-1RL1; also known as ST2), which is expressed by CD4+ T cells and macrophages. Currently, FAP cells are the only known source of IL-33 in muscle, and their low level of IL-33 expression in old muscle is associated with reductions in Treg cell recruitment and proliferation in injured tissue98. However, other factors must underlie the age-dependent defect in the regeneration of old muscle. Although the defect in IL-33 expression occurs in old mice (aged 22 to 24 months; equivalent to ~68 years old in humans), defective IL-33 expression also occurs in 6-month-old mice98 (equivalent to ~28 years old in humans)101, which do not experience regenerative defects caused by muscle senescence.
Prospects, challenges and future directions
Although recent discoveries illuminate interactions between the immune system and muscle that affect regeneration, have they brought us closer to developing therapies for improving muscle regeneration in humans? The close coupling between the stages of inflammation and the stages of muscle regeneration provides a ‘springboard’ for preclinical approaches that explore whether manipulating leukocyte populations can promote muscle growth and regeneration. For example, many deficiencies in the functional recovery of muscle that are caused by ischaemia when a tourniquet is applied to a limb can be prevented by injection of BMDMs directly into the injured muscle102. However, these functional and structural improvements only occurred if the BMDMs were activated with IFNγ and TLR4 ligand before injection, and not when muscles were injected with non-activated BMDMs. This could reflect a pro-regenerative effect that is specifically associated with the M1-biased phenotype. Interestingly, the beneficial effects of delivering BMDMs were achieved when the cells were injected 24 hours after tourniquet removal102, which suggests that the treatment may have value for improving muscle healing after tourniquet applications following acute trauma on battlefields or roads.
Transplantation of macrophages into muscle can also improve muscle regeneration and function in chronic disease. In an attempt to improve the engraftment of wild-type MPCs in diseased muscle, dystrophic mdx mice were co-injected with PAX7+ MPCs and F4/80+ macrophages; this led to better engraftment and dispersal of transplanted MPCs than did injection of MPCs alone103. Macrophages in the treated tissue 5 days post injection were primarily CD206+, which indicates their polarization towards the M2 phenotype in vivo. In the context of stem cell-based therapeutics for muscular dystrophies, this is a particularly thought-provoking outcome because it suggests that suppression of type 2 innate immunity could reduce the engraftment and dispersal of transplanted stem cells.
Our growing knowledge about the influence of the immune system on muscle regeneration has been used in a particularly creative way for developing implantable materials to repair volumetric muscle loss (VML), in which there is a traumatic loss of muscle caused by surgery, blast injuries, car accidents or other severe trauma. Much work in this area focuses on the design and production of synthetic or natural scaffolding materials that are implanted to provide a structure on which regeneration proceeds. Predictably, the immune response to implanted material from natural sources is a major determinant of whether the scaffold is compatible and supports regeneration, or undergoes a foreign-body reaction. However, less predictably, biological scaffolds used for repair of VML that were processed so that their implantation promoted an innate type 2 immune response yielded more successful regeneration than did scaffolds that produced a pro-inflammatory response104,105. In fact, skeletal muscle scaffolds that were successfully used for the repair of VML increased the numbers of CD163+ cells and ARG1+ cells, increased IL-10 expression and reduced IFNγ expression in the implanted scaffold106. Although the mechanisms through which some implantable materials drive a type 2 immune response associated with better engraftment are unknown, high-throughput screening for selecting materials for surgical implantation now includes in the selection criteria assaying the ability of the test material to influence polarization to the M2-biased phenotype107.
Despite the encouraging findings obtained from manipulating inflammatory cell numbers or phenotype in regenerative muscle in the few examples highlighted in this Review, they reveal the large gap between what we have learned about specific, molecular mechanisms that coordinate muscle regeneration with the immune response and what we have applied to improve regeneration in preclinical and clinical interventions. Learning more about the specific regulatory mechanisms through which the immune system promotes or hinders muscle regeneration can help us to bridge that gap. However, even with that more refined knowledge, achieving appropriate spatial and temporal targeting of therapeutic substances to injured muscle will certainly be a major challenge to developing effective treatments. This point was emphasized by the finding that ablation of CD11b+ cells from muscle at day 1 post injury caused substantial defects in muscle regeneration, although their ablation at day 4 did not affect regeneration26. Similarly, although endogenous IL-10 expressed in injured or diseased muscle promotes regeneration42,53, the delivery of supraphysiological levels of IL-10 to injured muscle at earlier stages of repair slows regeneration108. This tells us that the time window for immune cell-based interventions to improve muscle repair may be small. Not only will missing the target window lead to ineffective treatment effects, poor timing could also be detrimental. Nevertheless, the field of muscle immunobiology is very young, and the coming years will bring unexpected insights into regulatory processes that may be manipulated to improve recovery from muscle injury or disease. The burgeoning interactions between developmental biologists, immunologists, physiologists, systems biologists, clinicians and material scientists whose work focuses on the immunobiology of muscle regeneration will facilitate the translation of these new findings into clinically useful tools.
Acknowledgments
The author is supported by National Institutes of Health grants 1RO1AR066036, 1RO1AG041147, 1RO1AR062579 and 1R21AR066817. The author apologizes to scientists whose work was not included in this Review because of limitations on the length of the Review.
Abbreviation
- Satellite cells
A population of muscle stem cells that are committed to the myogenic lineage and normally reside in a quiescent state at the surface of fully differentiated muscle fibres. They can be activated by muscle injury, leading them to proliferate and then either return to quiescence, fuse with existing muscle fibres or continue to differentiate to form new muscle fibres
- Myotubes
During muscle development and regeneration, postmitotic, mononucleated muscle cells fuse with neighbouring postmitotic muscle cells to form long, cylindrical, multinucleated myotubes. Eventually myotubes can grow to include hundreds of muscle nuclei, and they then undergo terminal differentiation to become mature muscle fibres
- mdx mice
Mutant mice that lack dystrophin, the deficient gene product in Duchenne muscular dystrophy (DMD), which is a progressive, lethal, muscle-wasting disease in humans. Both mdx dystrophy and DMD involve an early, acute onset of muscle damage and inflammation. However, subsequent DMD pathology is more severe than mdx pathology, in which there is an extensive period of remission following initial onset
- Macrophage memory
Cells of the innate immune system, including macrophages, can show changes in their response to immune challenges according to the conditions under which they differentiated or were previously activated. This ‘trained’ immunity or memory reflects epigenetic changes that influence signalling or metabolic pathways
- Fibro-adipogenic progenitor cells, (FAP cells)
A population of muscle-resident mesenchymal cells that are lineage-negative, lack expression of integrin α7 and express CD34 and stem cell antigen 1, and that have the ability to differentiate into fibroblasts or adipocytes. Following acute injury, FAP cells can release factors that increase muscle cell differentiation and that promote repair.
Footnotes
Competing interests statement
The author declares no competing interests.
References
- 1.World Health Organization. Global Status Report on Road Safety 2015. World Health Organization; 2015. p. 340. [Google Scholar]
- 2.Herridge MS, et al. One year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348:683–693. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 3.Chargé SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004;84:209–238. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- 4.Sambasivan R, et al. PAX7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development. 2011;138:3647–3656. doi: 10.1242/dev.067587. [DOI] [PubMed] [Google Scholar]
- 5.Lepper C, Partridge TA, Fan CM. An absolute requirement for PAX7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development. 2011;138:3639–3646. doi: 10.1242/dev.067595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, Kardon G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development. 2011;138:3625–3637. doi: 10.1242/dev.064162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Megeney LA, Kablar B, Garrett K, Anderson JE, Rudnicki MA. MYOD is required for myogenic stem cell function in adult skeletal muscle. Genes Dev. 1996;10:1173–1183. doi: 10.1101/gad.10.10.1173. [DOI] [PubMed] [Google Scholar]
- 8.George RM, et al. Numb-deficient satellite cells have regeneration and proliferation defects. Proc Natl Acad Sci USA. 2013;110:18549–18554. doi: 10.1073/pnas.1311628110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinez CO, et al. Regulation of skeletal muscle regeneration by CCR2-activating chemokines is directly related to macrophage recruitment. Am J Physiol Regul Integr Comp Physiol. 2010;299:R832–R842. doi: 10.1152/ajpregu.00797.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Villalta SA, et al. Regulatory T cells suppress muscle inflammation and injury in muscular dystrophy. Sci Transl Med. 2014;6:258ra142. doi: 10.1126/scitranslmed.3009925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, Wehling-Henricks M, Samengo G, Tidball JG. Increases of M2a macrophages and fibrosis in aging muscle are influenced by bone marrow aging and negatively regulated by muscle-derived nitric oxide. Aging Cell. 2015;14:678–688. doi: 10.1111/acel.12350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Honda H, Kimura H, Rostami A. Demonstration and phenotypic characterization of resident macrophages in rat skeletal muscle. Immunology. 1990;70:272–277. [PMC free article] [PubMed] [Google Scholar]
- 13.Brigette M, et al. Muscle resident macrophages control the immune cell reaction in a mouse model of notexin-induced myoinjury. Arthritis Rheum. 2010;62:268–279. doi: 10.1002/art.27183. [DOI] [PubMed] [Google Scholar]
- 14.Krippendorf BB, Riley DA. Distinguishing unloading- versus reloading-induced changes in rat soleus muscle. Muscle Nerve. 1993;16:99–108. doi: 10.1002/mus.880160116. [DOI] [PubMed] [Google Scholar]
- 15.Belcastro AN, Arthur GD, Albisser TA, Raj DA. Heart, liver, and skeletal muscle myeloperoxidase activity during exercise. J Appl Physiol. 1996;80:1331–1335. doi: 10.1152/jappl.1996.80.4.1331. [DOI] [PubMed] [Google Scholar]
- 16.Fielding RA, et al. Acute phase response in exercise. III. Neutrophil and IL-1β accumulation in skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 1993;265:R166–R172. doi: 10.1152/ajpregu.1993.265.1.R166. [DOI] [PubMed] [Google Scholar]
- 17.Lu H, Huang D, Ransohoff RM, Zhou L. Acute skeletal muscle injury: CCL2 expression by both monocytes and injured muscle is required for repair. FASEB J. 2011;25:3344–3355. doi: 10.1096/fj.10-178939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warren GL, et al. Chemokine receptor CCR2 involvement in skeletal muscle regeneration. FASEB J. 2005;19:413–415. doi: 10.1096/fj.04-2421fje. [DOI] [PubMed] [Google Scholar]
- 19.Shireman PK, et al. MCP-1 deficiency causes altered inflammation with impaired skeletal muscle regeneration. J Leukoc Biol. 2007;81:775–785. doi: 10.1189/jlb.0506356. [DOI] [PubMed] [Google Scholar]
- 20.Contreras-Shannon V, et al. Fat accumulation with altered inflammation and regeneration in skeletal muscle of CCR2−/− mice following ischemic injury. Am J Physiol Cell Physiol. 2007;292:C953–C967. doi: 10.1152/ajpcell.00154.2006. [DOI] [PubMed] [Google Scholar]
- 21.Sun D, et al. Bone marrow-derived cell regulation of skeletal muscle regeneration. FASEB J. 2009;23:382–395. doi: 10.1096/fj.07-095901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang J, et al. CD8 T cells are involved in skeletal muscle regeneration through facilitating MCP-1 secretion and GR1high macrophage infiltration. J Immunol. 2014;193:5149–5160. doi: 10.4049/jimmunol.1303486. [DOI] [PubMed] [Google Scholar]
- 23.Collins RA, Grounds MD. The role of tumor necrosis factor-α (TNFα) in skeletal muscle regeneration. Studies in TNFα−/− and TNFα−/−/LTα−/− mice. J Histochem Cytochem. 2001;49:989–1001. doi: 10.1177/002215540104900807. [DOI] [PubMed] [Google Scholar]
- 24.Warren GL, et al. Physiological role of tumor necrosis factor-α in traumatic muscle injury. FASEB J. 2002;16:1630–1632. doi: 10.1096/fj.02-0187fje. [DOI] [PubMed] [Google Scholar]
- 25.Cheng M, Nguyen MH, Fantuzzi G, Koh TJ. Endogenous interferon-γ is required for efficient skeletal muscle regeneration. Am J Physiol Cell Physiol. 2008;294:C1183–C1191. doi: 10.1152/ajpcell.00568.2007. [DOI] [PubMed] [Google Scholar]
- 26.Wang H, et al. Altered macrophage phenotype transition impairs skeletal muscle regeneration. Am J Pathol. 2014;184:1167–1184. doi: 10.1016/j.ajpath.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the TH1/TH2 paradigm. J Immunol. 2000;164:6166–6173. [Google Scholar]
- 28.Locati M, Mantovani A, Sica A. Macrophage activation and polarization as an adaptive component of innate immunity. Adv Immunol. 2013;120:163–184. doi: 10.1016/B978-0-12-417028-5.00006-5. [DOI] [PubMed] [Google Scholar]
- 29.Mills CD. Anatomy of a discovery: M1 and M2 macrophages. Front Immunol. 2015;6:212. doi: 10.3389/fimmu.2015.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lemos DR, et al. Nilotinib reduces muscle fibrosis in chronic muscle injury by promoting TNF-mediated apoptosis of fibro/adipogenic progenitors. Nat Med. 2015;21:786–794. doi: 10.1038/nm.3869. This investigation delineates how dysregulated interactions between macrophages and FAP cells in chronic injury can contribute to muscle fibrosis. [DOI] [PubMed] [Google Scholar]
- 32.Heredia JE, et al. Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell. 2013;153:376–388. doi: 10.1016/j.cell.2013.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-γ. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 34.Lehtonen A, Matikainen S, Julkunen I. Interferons up-regulate STAT1, STAT2, and IRF family transcription factor gene expression in human peripheral blood mononuclear cells and macrophages. J Immunol. 1997;159:794–803. [PubMed] [Google Scholar]
- 35.Villalta SA, Deng B, Rinaldi C, Wehling-Henricks M, Tidball JG. IFNγ promotes muscle damage in the mdx mouse model of Duchenne muscular dystrophy by suppressing M2 macrophage activation and inhibiting muscle cell proliferation. J Immunol. 2011;187:5419–5428. doi: 10.4049/jimmunol.1101267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Varga T, et al. Highly dynamic transcriptional signature of distinct macrophage subsets during sterile inflammation, resolution, and tissue repair. J Immunol. 2016;196:4771–4782. doi: 10.4049/jimmunol.1502490. [DOI] [PubMed] [Google Scholar]
- 37.Mounier R, et al. AMPKα1 regulates macrophage skewing at the time of resolution of inflammation during skeletal muscle regeneration. Cell Metab. 2013;18:251–264. doi: 10.1016/j.cmet.2013.06.017. This study shows that AMPKα activity has a functionally important role in regulating macrophage phenotype and muscle regeneration following acute injury. [DOI] [PubMed] [Google Scholar]
- 38.Londhe P, Davie JK. γ-interferon modulates myogenesis through the major histocompatibility complex class II transactivator, CIITA. Mol Cell Biol. 2011;31:2854–2866. doi: 10.1128/MCB.05397-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Londhe P, Davie JK. Interferon-γ resets muscle cell fate by stimulating the sequential recruitment of JARID2 and PRC2 to promoters to repress myogenesis. Sci Signal. 2013;6:ra107. doi: 10.1126/scisignal.2004633. This study clarifies the mechanisms through which IFNγ can exert epigenetic controls on muscle differentiation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morris AC, Beresford GW, Mooney MR, Boss JM. Kinetics of a γ-interferon response: expression and assembly of CIITA promoter IV and inhibition by methylation. Mol Cell Biol. 2002;22:4781–4791. doi: 10.1128/MCB.22.13.4781-4791.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mitchell CA, McGeachie JK, Grounds MD. Cellular differences in the regeneration of murine skeletal muscle: a quantitative histological study in SJL/J and BALB/c mice. Cell Tissue Res. 1992;269:159–166. doi: 10.1007/BF00384736. [DOI] [PubMed] [Google Scholar]
- 42.Deng B, Wehling-Henricks M, Villalta SA, Wang Y, Tidball JG. IL-10 triggers changes in macrophage phenotype that promote muscle growth and regeneration. J Immunol. 2012;189:3669–3680. doi: 10.4049/jimmunol.1103180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen SE, et al. Role of TNFα signaling in regeneration of cardiotoxin-injured muscle. Am J Physiol Cell Physiol. 2005;289:C1179–C1187. doi: 10.1152/ajpcell.00062.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Palacios D, et al. TNF/p38α/Polycomb signaling to PAX7 locus in satellite cells links inflammation to the epigenetic control of muscle regeneration. Cell Stem Cell. 2010;7:455–469. doi: 10.1016/j.stem.2010.08.013. This investigation clarifies the mechanisms through which TNF-mediated signalling could influence PAX7 expression and thereby affect myogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Juan AH, et al. Polycomb EZH2 controls self-renewal and safeguards the transcriptional identity of skeletal muscle stem cells. Genes Dev. 2011;25:789–794. doi: 10.1101/gad.2027911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Woodhouse S, Pugazhendhi D, Brien P, Pell JM. EZH2 maintains a key phase of muscle satellite cell expansion but does not regulate terminal differentiation. J Cell Sci. 2013;126:565–579. doi: 10.1242/jcs.114843. [DOI] [PubMed] [Google Scholar]
- 47.Wilson-Rawls J, Molkentin JD, Black BL, Olson EN. Activated notch inhibits myogenic activity of the MADS-Box transcription factor myocyte enhancer factor 2C. Mol Cell Biol. 1999;19:2853–2862. doi: 10.1128/mcb.19.4.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Conboy IM, Rando TA. The regulation of NOTCH signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. Dev Cell. 2002;3:397–409. doi: 10.1016/s1534-5807(02)00254-x. [DOI] [PubMed] [Google Scholar]
- 49.Acharyya S, et al. TNF inhibits NOTCH1 in skeletal muscle cells by EZH2 and DNA methylation mediated repression: implications in Duchenne muscular dystrophy. PLoS ONE. 2010;5:e12479. doi: 10.1371/journal.pone.0012479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Faralli H, et al. UTX demethylase activity is required for satellite cell-mediated muscle regeneration. J Clin Invest. 2016;126:1555–1565. doi: 10.1172/JCI83239. This study shows a functionally important role for UTX in regulating the expression of myogenic genes in regenerating muscle in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seenundun S, et al. UTX mediates demethylation of H3K27me3 at muscle-specific genes during myogenesis. EMBO J. 2010;29:1401–1411. doi: 10.1038/emboj.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.St Pierre BA, Tidball JG. Differential response of macrophage subpopulations to soleus muscle reloading after rat hindlimb suspension. J Appl Physiol. 1994;77:290–297. doi: 10.1152/jappl.1994.77.1.290. [DOI] [PubMed] [Google Scholar]
- 53.Villalta SA, et al. Interleukin-10 reduces the pathology of mdx muscular dystrophy by deactivating M1 macrophages and modulating macrophage phenotype. Hum Mol Genet. 2011;20:790–805. doi: 10.1093/hmg/ddq523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rigamonti E, et al. Requirement of inducible nitric oxide synthase for skeletal muscle regeneration after acute damage. J Immunol. 2013;190:1767–1777. doi: 10.4049/jimmunol.1202903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mantovani A, et al. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 56.Tidball JG, Wehling-Henricks M. Macrophages promote muscle membrane repair and muscle fibre growth and regeneration during modified muscle loading in mice in vivo. J Physiol. 2007;578:327–336. doi: 10.1113/jphysiol.2006.118265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arnold L, et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204:1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hardie DG, Scott JW, Pan DA, Hudson ER. Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett. 2003;546:113–120. doi: 10.1016/s0014-5793(03)00560-x. [DOI] [PubMed] [Google Scholar]
- 59.O’Neill LA, Hardie DG. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature. 2013;493:346–355. doi: 10.1038/nature11862. [DOI] [PubMed] [Google Scholar]
- 60.Zhu YP, Brown JR, Sag D, Zhang L, Suttles J. Adenosine 5′-monophosphate-activated protein kinase regulates IL-10-mediated anti-inflammatory signaling pathways in macrophages. J Immunol. 2015;194:584–594. doi: 10.4049/jimmunol.1401024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sag D, Carling D, Stout RD, Suttles J. Adenosine 5′-monophosphate-activated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. J Immunol. 2008;181:8633–8641. doi: 10.4049/jimmunol.181.12.8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tonkin J, et al. Monocyte/macrophage-derived IGF1 orchestrates murine skeletal muscle regeneration and modulates autocrine polarization. Mol Ther. 2015;23:1189–1200. doi: 10.1038/mt.2015.66. This investigation shows that macrophage-derived IGF1 could mediate both myogenesis and macrophage phenotype transitions in injured muscle in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tollefsen SE, Sadow JL, Rotwein P. Coordinate expression of insulin-like growth factor II and its receptor during muscle differentiation. Proc Natl Acad Sci USA. 1989;86:1543–1547. doi: 10.1073/pnas.86.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barton-Davis ER, Shoturma DI, Sweeney HL. Contribution of satellite cells to IGF1 induced hypertrophy of skeletal muscle. Acta Physiol Scand. 1999;167:301–305. doi: 10.1046/j.1365-201x.1999.00618.x. [DOI] [PubMed] [Google Scholar]
- 65.Musarò A, McCullagh KJ, Naya FJ, Olson EN, Rosenthal N. IGF1 induces skeletal myocyte hypertrophy through calcineurin in association with GATA-2 and NF-ATc1. Nature. 1999;400:581–585. doi: 10.1038/23060. [DOI] [PubMed] [Google Scholar]
- 66.Summan M, et al. Macrophages and skeletal muscle regeneration: a clodronate-containing liposome depletion study. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1488–R1495. doi: 10.1152/ajpregu.00465.2005. [DOI] [PubMed] [Google Scholar]
- 67.Zhao W, Lu H, Wang X, Ransohoff RM, Zhou L. CX3CR1 deficiency delays acute skeletal muscle injury repair by impairing macrophage functions. FASEB J. 2016;30:380–393. doi: 10.1096/fj.14-270090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fadok VA, et al. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGFβ, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fadok VA, Bratton DL, Guthrie L, Henson PM. Differential effects of apoptotic versus lysed cells on macrophage production of cytokines: role of proteases. J Immunol. 2001;166:6847–6854. doi: 10.4049/jimmunol.166.11.6847. [DOI] [PubMed] [Google Scholar]
- 70.Bae HB, et al. AMP-activated protein kinase enhances the phagocytic ability of macrophages and neutrophils. FASEB J. 2011;25:4358–4368. doi: 10.1096/fj.11-190587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weavers H, Evans IR, Martin P, Wood W. Corpse engulfment generates a molecular memory that primes the macrophage inflammatory response. Cell. 2016;165:1658–1671. doi: 10.1016/j.cell.2016.04.049. This investigation shows that phagocytosis of apoptotic bodies by macrophages during Drosophila melanogaster development influences macrophage responsiveness to subsequent acute injuries. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schaer DJ, et al. Molecular cloning and characterization of the mouse CD163 homologue, a highly glucocorticoid-inducible member of the scavenger receptor cysteine-rich family. Immunogenetics. 2001;53:170–177. doi: 10.1007/s002510100304. [DOI] [PubMed] [Google Scholar]
- 73.Buechler C, et al. Regulation of scavenger receptor CD163 expression in human monocytes and macrophages by pro- and antiinflammatory stimuli. J Leukoc Biol. 2000;67:97–103. [PubMed] [Google Scholar]
- 74.Sulahian TH, et al. Human monocytes express CD163, which is upregulated by IL-10 and identical to p155. Cytokine. 2000;12:1312–1321. doi: 10.1006/cyto.2000.0720. [DOI] [PubMed] [Google Scholar]
- 75.Schaer DJ, Boretti FS, Schoedon G, Schaffner A. Induction of the CD163-dependent haemoglobin uptake by macrophages as a novel anti-inflammatory action of glucocorticoids. Br J Haematol. 2002;119:239–243. doi: 10.1046/j.1365-2141.2002.03790.x. [DOI] [PubMed] [Google Scholar]
- 76.Kristiansen M, et al. Identification of the haemoglobin scavenger receptor. Nature. 2001;409:198–201. doi: 10.1038/35051594. [DOI] [PubMed] [Google Scholar]
- 77.Moestrup SK, Moller HJ. CD163: a regulated hemoglobin scavenger receptor with a role in the anti-inflammatory response. Ann Med. 2004;36:347–354. doi: 10.1080/07853890410033171. [DOI] [PubMed] [Google Scholar]
- 78.Philippidis P, et al. Hemoglobin scavenger receptor CD163 mediates interleukin-10 release and heme oxygenase-1 synthesis: antiinflammatory monocyte-macrophage responses in vitro, in resolving skin blisters in vivo, and after cardiopulmonary bypass surgery. Circ Res. 2004;94:119–126. doi: 10.1161/01.RES.0000109414.78907.F9. [DOI] [PubMed] [Google Scholar]
- 79.Akahori H, et al. CD163 interacts with TWEAK to regulate tissue regeneration after ischaemic injury. Nat Commun. 2015;6:7792. doi: 10.1038/ncomms8792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bover LC, et al. A previously unrecognized protein-protein interaction between TWEAK and CD163: potential biological implications. J Immunol. 2007;178:8183–8194. doi: 10.4049/jimmunol.178.12.8183. [DOI] [PubMed] [Google Scholar]
- 81.Girgenrath M, et al. TWEAK, via its receptor FN14, is a novel regulator of mesenchymal progenitor cells and skeletal muscle regeneration. EMBO J. 2006;25:5826–5839. doi: 10.1038/sj.emboj.7601441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mittal A, et al. Genetic ablation of TWEAK augments regeneration and post-injury growth of skeletal muscle in mice. Am J Pathol. 2010;177:1732–1742. doi: 10.2353/ajpath.2010.100335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McLennan IS. Degenerating and regenerating skeletal muscles contain several subpopulations of macrophages with distinct spatial and temporal distributions. J Anat. 1996;188:17–28. [PMC free article] [PubMed] [Google Scholar]
- 84.Burzyn D, et al. A special population of regulatory T cells potentiates muscle repair. Cell. 2013;155:1282–1295. doi: 10.1016/j.cell.2013.10.054. This study shows that Treg cells can influence muscle regeneration and modulate macrophage phenotype following acute injury, and indicates that a muscle-specific Treg cell population is mainly responsible for these effects. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Castiglioni A, et al. FOXP3+ T cells recruited to sites of sterile skeletal muscle injury regulate the fate of satellite cells and guide effective tissue regeneration. PLoS ONE. 2015;10:e0128094. doi: 10.1371/journal.pone.0128094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Uezumi A, Fukada S, Yamamoto N, Takeda S, Tsuchida K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol. 2010;12:143–152. doi: 10.1038/ncb2014. [DOI] [PubMed] [Google Scholar]
- 87.Joe AW, et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12:153–163. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fiore D, et al. Pharmacological blockage of fibro/adipogenic progenitor expansion and suppression of regenerative fibrogenesis is associated with impaired skeletal muscle regeneration. Stem Cell Res. 2016;17:161–169. doi: 10.1016/j.scr.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 89.Uezumi A, et al. Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. J Cell Sci. 2011;124:3654–3664. doi: 10.1242/jcs.086629. [DOI] [PubMed] [Google Scholar]
- 90.Verrecchia F, Chu ML, Mauviel A. Identification of novel TGFβ /SMAD gene targets in dermal fibroblasts using a combined cDNA microarray/promoter transactivation approach. J Biol Chem. 2001;276:17058–17062. doi: 10.1074/jbc.M100754200. [DOI] [PubMed] [Google Scholar]
- 91.Holmes A, et al. CTGF and SMADs, maintenance of scleroderma phenotype is independent of SMAD signaling. J Biol Chem. 2001;276:10594–10601. doi: 10.1074/jbc.M010149200. [DOI] [PubMed] [Google Scholar]
- 92.Curran JN, Winter DC, Bouchier-Hayes D. Biological fate and clinical implications of arginine metabolism in tissue healing. Wound Repair Regen. 2006;14:376–386. doi: 10.1111/j.1743-6109.2006.00151.x. [DOI] [PubMed] [Google Scholar]
- 93.Witte MB, Barbul A. Arginine physiology and its implication for wound healing. Wound Repair Regen. 2003;11:419–423. doi: 10.1046/j.1524-475x.2003.11605.x. [DOI] [PubMed] [Google Scholar]
- 94.Petrof BJ, Shrager JB, Stedman HH, Kelly AM, Sweeney HL. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc Natl Acad Sci USA. 1993;90:3710–3714. doi: 10.1073/pnas.90.8.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wehling M, Spencer MJ, Tidball JG. A nitric oxide synthase transgene ameliorates muscular dystrophy in mdx mice. J Cell Biol. 2001;155:123–131. doi: 10.1083/jcb.200105110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wehling-Henricks M, et al. Arginine metabolism by macrophages promotes cardiac and muscle fibrosis in mdx muscular dystrophy. PLoS ONE. 2010;5:e10763. doi: 10.1371/journal.pone.0010763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Conboy IM, Rando TA. Aging, stem cells and tissue regeneration: lessons from muscle. Cell Cycle. 2005;4:407–410. doi: 10.4161/cc.4.3.1518. [DOI] [PubMed] [Google Scholar]
- 98.Kuswanto W, et al. Poor repair of skeletal muscle in aging mice reflects a defect in local, interleukin-33-dependent accumulation of regulatory T cells. Immunity. 2016;44:355–367. doi: 10.1016/j.immuni.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Williams JW, et al. Transcription factor IRF4 drives dendritic cells to promote TH2 differentiation. Nat Commun. 2013;4:2990. doi: 10.1038/ncomms3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sato S, Yanagawa Y, Hiraide S, Iizuka K. Cyclic AMP signaling enhances lipopolysaccharide sensitivity and interleukin-33 production in RAW264.7 macrophages. Microbiol Immunol. 2016;60:382–389. doi: 10.1111/1348-0421.12381. [DOI] [PubMed] [Google Scholar]
- 101.Flurkey K, Currer JM, Harrison DE. In: The Mouse in Biomedical Research. 2. Fox JG, editor. Elsevier; 2007. pp. 637–672. [Google Scholar]
- 102.Rybalko V, Hsieh PL, Merscham-Banda M, Suggs LJ, Farrar RP. The development of macrophage-mediated cell therapy to improve skeletal muscle function after injury. PLoS ONE. 2015;10:e0145550. doi: 10.1371/journal.pone.0145550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lesault PF, et al. Macrophages improve survival, proliferation and migration of engrafted myogenic precursor cells into MDX skeletal muscle. PLoS ONE. 2012;7:e46698. doi: 10.1371/journal.pone.0046698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Badylak SF, Valentin JE, Ravindra AK, McCabe GP, Stewart-Akers AM. Macrophage phenotype as a determinant of biologic scaffold remodeling. Tissue Eng Part A. 2008;14:1835–1842. doi: 10.1089/ten.tea.2007.0264. [DOI] [PubMed] [Google Scholar]
- 105.Brown BN, Valentin JE, Stewart-Akers AM, McCabe GP, Badylak SF. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials. 2009;30:1482–1491. doi: 10.1016/j.biomaterials.2008.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fishman JM, et al. Immunomodulatory effect of a decellularized skeletal muscle scaffold in a discordant xenotransplantation model. Proc Natl Acad Sci USA. 2013;110:14360–14365. doi: 10.1073/pnas.1213228110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Beachley VA, et al. Tissue matrix arrays for high-throughput screening and systems analysis of cell function. Nat Methods. 2015;12:1197–1204. doi: 10.1038/nmeth.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Perdiguero E, et al. p38/MKP-1-regulated AKT coordinates macrophage transitions and resolution of inflammation during tissue repair. J Cell Biol. 2011;195:307–322. doi: 10.1083/jcb.201104053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Allbrook DB, Han MF, Hellmuth AE. Population of muscle satellite cells in relation to age and mitotic activity. Pathology. 1971;3:223–243. doi: 10.3109/00313027109073739. [DOI] [PubMed] [Google Scholar]
- 110.Schultz E. A quantitative study of the satellite cell population in postnatal mouse lumbrical muscle. Anat Rec. 1974;180:589–595. doi: 10.1002/ar.1091800405. [DOI] [PubMed] [Google Scholar]
- 111.Clarke MS, Feeback DL. Mechanical load induces sarcoplasmic wounding and FGF release in differentiated human skeletal muscle cultures. FASEB J. 1996;10:502–509. doi: 10.1096/fasebj.10.4.8647349. [DOI] [PubMed] [Google Scholar]
- 112.Fry CS, et al. Inducible depletion of satellite cells in adult, sedentary mice impairs muscle regenerative capacity without affecting sarcopenia. Nat Med. 2015;21:76–80. doi: 10.1038/nm.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tierney MT, et al. STAT3 signaling controls satellite cell expansion and skeletal muscle repair. Nat Med. 2014;20:1182–1186. doi: 10.1038/nm.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lieber RL, Thornell LE, Fridén J. Muscle cytoskeletal disruption occurs within the first 15 min of cyclic eccentric contraction. J Appl Physiol. 1996;80:278–284. doi: 10.1152/jappl.1996.80.1.278. [DOI] [PubMed] [Google Scholar]
- 115.Segawa M, et al. Suppression of macrophage functions impairs skeletal muscle regeneration with severe fibrosis. Exp Cell Res. 2008;314:3232–3244. doi: 10.1016/j.yexcr.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 116.Zhang L, et al. Chemokine CXCL16 regulates neutrophil and macrophage infiltration into injured muscle, promoting muscle regeneration. Am J Pathol. 2009;175:2518–2527. doi: 10.2353/ajpath.2009.090275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hardy D, et al. Comparative study of injury models for studying muscle regeneration in mice. PLoS ONE. 2016;11:e0147198. doi: 10.1371/journal.pone.0147198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Teixeira CF, Landucci EC, Antunes E, Chacur M, Cury Y. Inflammatory effects of snake venom myotoxic phospholipases A2. Toxicon. 2003;42:947–962. doi: 10.1016/j.toxicon.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 119.Gutiérez JM, Lomonte B. Phospholipase A2 myotoxins from Bothrops snake venoms. Curr Org Chem. 2004;8:1677–1690. doi: 10.1016/0041-0101(95)00085-z. [DOI] [PubMed] [Google Scholar]
- 120.Zuliani JP, Fernandes CM, Zamuner SR, Gutiérez JM, Teixeira CF. Inflammatory events induced by Lys-49 and Asp-49 phospholipases A2 isolated from Bothrops asper snake venom: role of catalytic activity. Toxicon. 2005;45:335–346. doi: 10.1016/j.toxicon.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 121.Zuliani JP, et al. Activation of cellular functions in macrophages by venom secretory Asp-49 and Lys-49 phospholipases A2. Toxicon. 2005;46:523–532. doi: 10.1016/j.toxicon.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 122.Gasanov SE, Dagda RK, Rael ED. Snake venom cytotoxins, phospholipase A2s, and Zn2+-dependent metalloproteinases: mechanisms of action and pharmacological relevance. J Clin Toxicol. 2014;4:1000181. doi: 10.4172/2161-0495.1000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hasty P, et al. Muscle deficiency and neonatal death in mice with a targeted mutation in the myogenin gene. Nature. 1993;364:501–506. doi: 10.1038/364501a0. [DOI] [PubMed] [Google Scholar]
- 124.Nabeshima Y, et al. Myogenin gene disruption results in perinatal lethality because of severe muscle defect. Nature. 1993;364:532–535. doi: 10.1038/364532a0. [DOI] [PubMed] [Google Scholar]
- 125.Warren GL, et al. Role of CC chemokines in skeletal muscle functional restoration after injury. Am J Physiol Cell Physiol. 2004;286:C1031–C1036. doi: 10.1152/ajpcell.00467.2003. [DOI] [PubMed] [Google Scholar]
- 126.Hu X, Ivashkiv LB. Cross-regulation of signaling pathways by interferon-γ: implications for immune responses and autoimmune diseases. Immunity. 2009;31:539–550. doi: 10.1016/j.immuni.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Giordano C, et al. Toll-like receptor 4 ablation in mdx mice reveals innate immunity as a therapeutic target in Duchenne muscular dystrophy. Hum Mol Genet. 2015;24:2147–2162. doi: 10.1093/hmg/ddu735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Henriques-Pons A, et al. Role of Toll-like receptors in the pathogenesis of dystrophin-deficient skeletal and heart muscle. Hum Mol Genet. 2014;23:2604–2617. doi: 10.1093/hmg/ddt656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Suelves M, et al. Plasmin activity is required for myogenesis in vitro and skeletal muscle regeneration in vivo. Blood. 2002;99:2835–2844. doi: 10.1182/blood.v99.8.2835. [DOI] [PubMed] [Google Scholar]
- 130.Nguyen HX, Lusis AJ, Tidball JG. Null mutation of myeloperoxidase in mice prevents mechanical activation of neutrophil lysis of muscle cell membranes in vitro and in vivo. J Physiol. 2005;565:403–413. doi: 10.1113/jphysiol.2005.085506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wehling-Henricks M, et al. Major basic protein-1 promotes fibrosis of dystrophic muscle and attenuates the cellular immune response in muscular dystrophy. Hum Mol Genet. 2008;17:2280–2292. doi: 10.1093/hmg/ddn129. [DOI] [PMC free article] [PubMed] [Google Scholar]