Abstract
Objective
Initial success with chimeric antigen receptor–modified T cell therapy for relapsed/refractory acute lymphoblastic leukemia is leading to expanded use through multicenter trials. Cytokine release syndrome, the most severe toxicity, presents a novel critical illness syndrome with limited data regarding diagnosis, prognosis, and therapy. We sought to characterize the timing, severity, and intensive care management of cytokine release syndrome after chimeric antigen receptor–modified T cell therapy.
Design
Retrospective cohort study.
Setting
Academic children’s hospital.
Patients
Thirty-nine subjects with relapsed/refractory acute lymphoblastic leukemia treated with chimeric antigen receptor–modified T cell therapy on a phase I/IIa clinical trial (ClinicalTrials.gov number NCT01626495).
Interventions
All subjects received chimeric antigen receptor–modified T cell therapy. Thirteen subjects with cardiovascular dysfunction were treated with the interleukin-6 receptor antibody tocilizumab.
Measurements and Main Results
Eighteen subjects (46%) developed grade 3–4 cytokine release syndrome, with prolonged fever (median, 6.5 d), hyperferritinemia (median peak ferritin, 60,214 ng/mL), and organ dysfunction. Fourteen (36%) developed cardiovascular dysfunction treated with vasoactive infusions a median of 5 days after T cell therapy. Six (15%) developed acute respiratory failure treated with invasive mechanical ventilation a median of 6 days after T cell therapy; five met criteria for acute respiratory distress syndrome. Encephalopathy, hepatic, and renal dysfunction manifested later than cardiovascular and respiratory dysfunction. Subjects had a median of 15 organ dysfunction days (interquartile range, 8–20). Treatment with tocilizumab in 13 subjects resulted in rapid defervescence (median, 4 hr) and clinical improvement.
Conclusions
Grade 3–4 cytokine release syndrome occurred in 46% of patients following T cell therapy for relapsed/refractory acute lymphoblastic leukemia. Clinicians should be aware of expanding use of this breakthrough therapy and implications for critical care units in cancer centers.
Keywords: cell therapy, leukemia, multiple organ failure, shock, tocilizumab
Up to 90% of children with newly diagnosed B-precursor acute lymphoblastic leukemia (B ALL), the most common childhood malignancy, survive (1–3). However, survival remains poor for those with relapsed/refractory disease (4, 5). Newly reported strategies using chimeric antigen receptor (CAR)-modified T cells directed against the B cell antigen cluster of differentiation 19 (CD19) effectively treat relapsed/refractory B ALL in children (6–8). CD19 CAR therapy involves genetically engineering a patient’s T cells to target CD19-expressing B cells (both normal and malignant) (9). CAR binding results in T cell activation, antigen-mediated cell killing, and T cell proliferation. Robust expansion and sustained proliferation of the modified T cell population can eradicate the cancer and appears to sustain remission in many patients (6–8, 10, 11). In a phase I/IIa trial of CD19 CAR T cells (CTL019) in relapsed/refractory B ALL, we observed a 90% complete remission rate with a 6-month event-free survival of 67% and overall survival of 78% (8).
One unique, significant toxicity following CAR therapy is cytokine release syndrome (CRS) (9, 12, 13). CRS is a systemic inflammatory response syndrome (SIRS) related to activated T cell proliferation with release of high levels of inflammatory cytokines (8, 12, 13). CRS typically peaks during maximal in vivo T cell proliferation (6, 13), which occurs days after CAR T cell infusion. CRS can result in profound shock and multiple organ dysfunction syndrome (MODS) with rapid evolution to life-threatening critical illness. Early recognition and appropriate therapy can control the risk of mortality due to CRS.
Due to promising results from early phase trials in pediatric relapsed/refractory B ALL and CTL019 receipt of the Food and Drug Administration Breakthrough Therapy status, CAR T cell therapy use is expanding through numerous multicenter trials. Thus, the occurrence of CRS is expected to rise. Unique therapeutic considerations are relevant for CRS to minimize toxicity while preserving the antileukemic activity of the CAR T cells. We characterized the time course, clinical phenotype, and intensive care management of severe (grade 3–4) CRS following CTL019 therapy in pediatric patients with relapsed/refractory B ALL. Since emerging evidence supports an important role for interleukin (IL)-6 in CRS (6–9, 12, 13), we report the effects of cytokine-directed immunotherapy with the humanized monoclonal anti-IL-6 receptor antibody, tocilizumab, on CRS clinical manifestations.
METHODS
We conducted an analysis of CRS for 39 consecutive subjects treated with CTL019 through an institutional review board–approved phase I/IIa clinical trial (ClinicalTrials.gov number NCT01626495) at The Children’s Hospital of Philadelphia between April 2012 and September 2014. All subjects or their guardians provided written informed consent for the phase I/IIa trial, including approval for future data collection and analysis.
Efficacy data for the first 25 pediatric subjects treated in the CTL019 trial have been previously published (8), although details regarding time course, organ dysfunction severity, and response to critical care interventions have not been reported. Patients with relapsed/refractory CD19+ ALL who were ineligible for allogeneic hematopoietic stem cell transplant (HSCT) or who had relapsed after allogeneic HSCT were eligible for the phase I/IIa trial. Exclusion criteria and details of T cell therapy for the parent trial are provided in the Supplemental Methods (Supplemental Digital Content 1, http://links.lww.com/CCM/C151).
Data were abstracted from the medical record (Supplemental Methods, Supplemental Digital Content 1, http://links.lww.com/CCM/C151), and were anchored to the first day of CTL019 infusion (day 0). Subjects were followed for 28 days. We used published criteria to define organ dysfunction (14), modified as described in the Supplemental Methods (Supplemental Digital Content 1, http://links.lww.com/CCM/C151). Severity of illness was measured by the Pediatric Index of Mortality (PIM)-2 (15). Inotrope scores were calculated using dosages of dopamine, epinephrine, and norepinephrine normalized to dopamine dosage in μg/kg/min (16). To characterize CRS severity, we have developed a descriptive grading scale (Table 1) (17).
TABLE 1.
Definition of Cytokine Release Syndrome Grading System
| Grade | Description |
|---|---|
| 1 | Mild: treated with supportive care (antipyretics, antiemetics) |
| 2 | Moderate: requiring hospitalization or IV therapies with some signs of organ dysfunction |
| 3 | Severe,: requiring therapies such as IV fluid boluses or low dose vasopressors for hypotension, coagulopathy requiring cryoprecipitate or plasma transfusion, hypoxemia requiring high flow oxygen therapy or noninvasive mechanical ventilation |
| 4 | Life threatening: requiring therapies such as high dose vasopressors, hypoxemia requiring invasive mechanical ventilation |
Adapted with permission from Porter et al (17). Adaptations are themselves works protected by copyright. So in order to publish this adaptation, authorization must be obtained both from the owner of the copyright in the original work and from the owner of copyright in the translation or adaptation.
Statistical analysis was performed using STATA (Version 12.1; StataCorp, College Station, TX). Descriptive data are presented as means ± SD or medians with interquartile ranges (IQRs) for continuous variables and proportions for categoric variables. Differences between medians were analyzed using Wilcoxon signed rank test. Changes in vital signs over time were compared using Wilcoxon signed rank test. Kaplan-Meier and Cox regression analysis were used to evaluate the association of ferritin levels and time to resolution of organ dysfunction (Supplemental Methods, Supplemental Digital Content 1, http://links.lww.com/CCM/C151). Statistical significance was defined as a p value of less than 0.05.
RESULTS
The initial 39 subjects with B ALL treated on the phase I/IIa trial of CTL019 were included in this analysis. The median age was 11 years (range, 5–22). Thirty-six subjects (92%) developed symptoms attributable to CRS: two with grade 1 (8%), 16 with grade 2 (41%), seven with grade 3 (18%), and 11 with grade 4 (28%). Subject characteristics by CRS grade were similar (Table 2).
TABLE 2.
Demographic Comparison of Subjects With Grade 0–2 Versus Grade 3–4 Cytokine Release Syndrome
| Characteristic | Grade 0–2 CRS (n = 21) | Grade 3–4 CRS (n = 18) |
|---|---|---|
| Age, median (IQR) | 11 (8–15) | 11 (9–16) |
|
| ||
| Gender (% male) | 57 | 44 |
|
| ||
| Weight, kg, median (IQR) | 35.2 (22.5–52.3) | 39.5 (34.5–48) |
|
| ||
| Height, cm, median (IQR) | 141 (125–157) | 142 (136–156) |
|
| ||
| Race, n (%) | ||
| White | 18 (86) | 14 (78) |
| Black | 2 (10) | 1 (6) |
| Asian | 0 (0) | 2 (11) |
| Other | 1 (5) | 1 (6) |
|
| ||
| Ethnicity, n (%) | ||
| Hispanic | 3 (14) | 2 (11) |
| Not Hispanic | 18 (86) | 16 (89) |
|
| ||
| Relapsed after allogeneic hematopoietic stem cell transplant, n (%) | 13 (62) | 10 (56) |
CRS = cytokine release syndrome, IQR = interquartile range.
Five of seven subjects with grade 3 and all subjects with grade 4 CRS were treated in the ICU for management of CRS-related organ dysfunction. The median time from CTL019 infusion to ICU admission was 5.6 (IQR, 3.7–6.2) days. Median PIM-2 score at ICU admission was 1.75 (IQR, 1.40–4.85), and median ICU length of stay was 7.8 (IQR, 2.9–14.9) days.
CRS was characterized by prolonged high fevers, tachycardia, and myalgias. Fever duration was longer in those with grade 3–4 CRS compared to those with grade 0–2 CRS: 7 (IQR, 4–9) versus 5 (IQR, 2–6) days (p = 0.04). In subjects with grade 3–4 CRS, fever peaked a median of 5 days (IQR, 3–7) after CTL019 infusion, and the median peak heart rate was 170 beats/min (IQR, 156–186). All patients with fever underwent infectious evaluations, yet only one of 18 patients with grade 3–4 CRS had an identified infection within 1 week of CTL019 therapy.
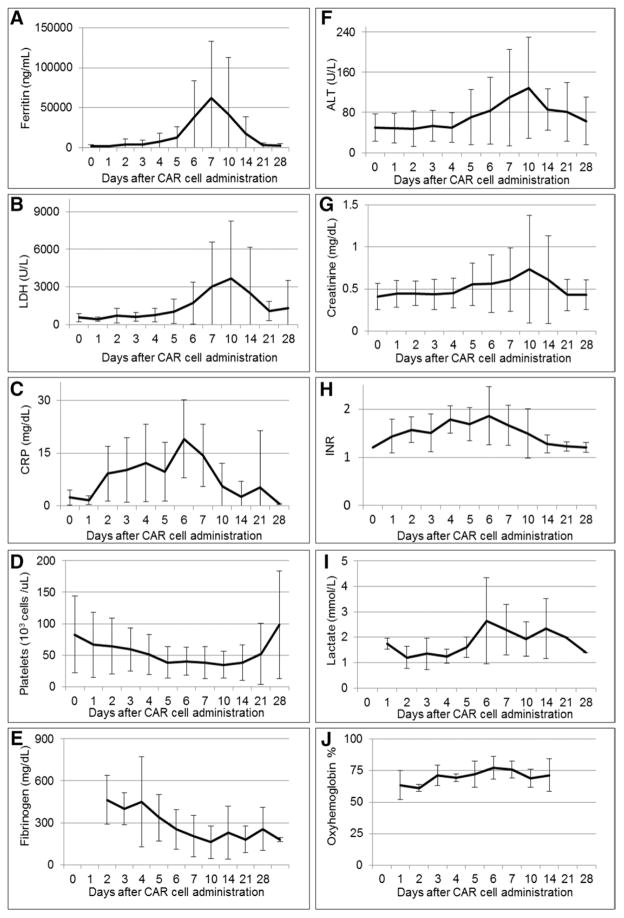
The evolution of tachycardia and hypotension (Supplemental Fig. 1, Supplemental Digital Content 1, http://links.lww.com/CCM/C151) and laboratory and inflammatory marker abnormalities (Fig. 1) in the CRS grade 3–4 subjects were tracked. Subjects with grade 3–4 CRS developed a macrophage activation syndrome/hemophagocytic lymphohistiocytosis (MAS/HLH)-like clinical picture: 12 subjects met MAS/HLH diagnostic criteria (with ≥ five of eight diagnostic criteria present), three had four criteria, and three had three criteria (Supplemental Table 1, Supplemental Digital Content 1, http://links.lww.com/CCM/C151). All had fever, cytopenias, and peak ferritin levels greater than 1,123 pmol/L (500 ng/mL), 10 had fibrinogen less than 4.4 μmol/L (150 mg/dL, measured in 16), and cryoprecipitate was administered to seven for coagulopathy. The median peak ferritin level was 135,300 pmol/L (60,214 ng/mL [IQR, 27,000–292,000 pmol/L or 12,000–130,000 ng/mL]).
Figure 1.
Laboratory trends after chimeric antigen receptor (CAR) cell administration in grade 3 and 4 cytokine release syndrome patients. Means and SD are presented. A, Ferritin (n = 17). B, Lactate dehydrogenase (LDH) (n = 18). C, C-reactive protein (CRP) (n = 16). D, Platelet count (n = 18). E, Fibrinogen (n = 16). F, Alanine aminotransferase (ALT) (n = 18). G, Creatinine (n = 18). H, International normalized ratio (INR) (n = 14). I, Lactate (n = 15). J, Central venous oxyhemoglobin saturation (n = 14).
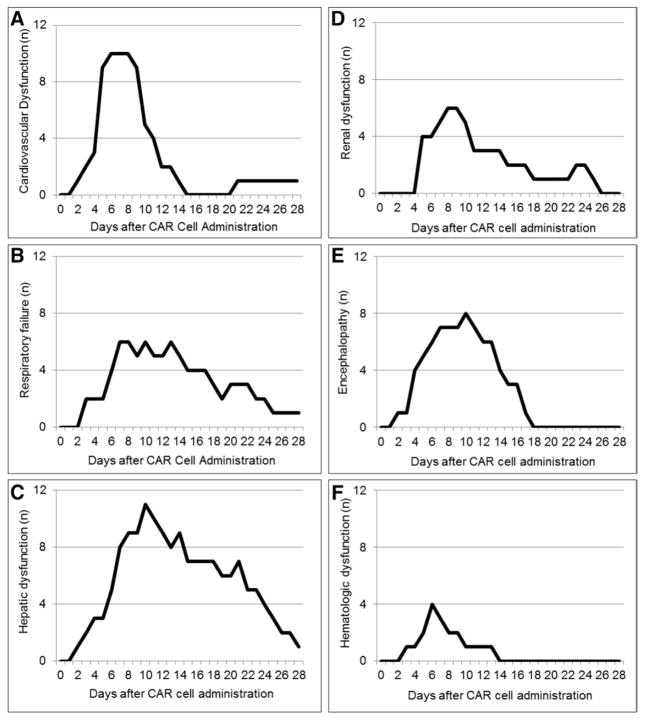
The time course of organ dysfunction is presented in Figure 2. Subjects had a median of 15 (IQR, 8–20) days of organ dysfunction. Fourteen subjects developed MODS, with MODS onset a median of 5 days (IQR, 4–7) after CTL019 infusion. Time from organ dysfunction onset to resolution was longer for subjects with peak ferritin values greater than 11,200 pmol/L by day 5 after CTL019 infusion (hazard ratio, 0.17; 95% CI, 0.04–0.67; p = 0.011; Supplemental Fig. 2, Supplemental Digital Content 1, http://links.lww.com/CCM/C151).
Figure 2.
Time course of organ dysfunction after chimeric antigen receptor (CAR) cell administration in grade 3 and 4 cytokine release syndrome patients. The number of patients with each organ dysfunction on each day after CAR cell administration is presented. A, Cardiovascular dysfunction—use of vasoactive drug to support hemodynamic status (dopamine > 5 μg/kg/min or any dobutamine, epinephrine, norepinephrine, vasopressin, or milrinone). B, Respiratory failure—invasive or noninvasive mechanical ventilation. C, Hepatic dysfunction—total bilirubin greater than or equal to 4 mg/dL or alanine aminotransferase greater than 2× upper limit of normal for age. D, Renal dysfunction—two-fold increase in baseline creatinine. E, Encephalopathy—altered mental status or seizure. F, Hematologic dysfunction—international normalized ratio greater than 2.
Fourteen subjects (36%) developed cardiovascular dysfunction: 13 had profound fluid-refractory vasoplegic shock treated with α-agonist infusions and one had cardiomyopathy with diminished left ventricular systolic function supported with milrinone. Cardiovascular dysfunction developed a median of 5 days (IQR, 5–7) after CTL019 infusion, with a median duration of 4 days (IQR, 2–6). Hypotension was always preceded by fever and did not occur in the outpatient setting as we admitted all patients with fevers after CTL019. Shock was catecholamine resistant in 10 of 14 subjects, requiring more than one vasoactive agent. Vasopressin was used as a third or fourth agent in four subjects. Peak daily inotrope scores for those with grade 3 versus 4 CRS are shown in Supplemental Figure 3 (Supplemental Digital Content 1, http://links.lww.com/CCM/C151). The total IV fluid volume administered in the 72 hours surrounding onset of shock was 270 mL/kg (IQR, 215–351). The median total output over the same time period was 127 mL/kg (IQR, 101–221), resulting in a substantial net positive fluid balance for most subjects.
The most common organ dysfunction was hepatic dysfunction (most commonly transaminitis), present in 25 subjects (64%) (Supplemental Table 2, Supplemental Digital Content 1, http://links.lww.com/CCM/C151), with a later onset than cardiovascular dysfunction (Fig. 2). Renal dysfunction was common and could be prolonged; acute kidney injury (AKI) was present in 18 subjects (46%), but was generally mild with only nine subjects developing stage 2 or 3 AKI. No subjects received renal replacement therapy. Five subjects (13%) developed pancreatitis.
Thirty-three percent developed encephalopathy, manifesting as profound confusion, delirium, hallucinations, aphasia, and/or seizure, and lasting a median of 7 days (IQR, 2–9). Encephalopathy typically occurred later in the CRS course while fever was resolving and was self-limited with return to baseline neurologic status in all subjects by day 18. Diagnostic evaluation consisted of head CT (n = 6), brain MRI (n = 3), and continuous electroencephalogram monitoring (n = 4). Results of the diagnostic evaluation were nonspecific (Supplemental Results, Supplemental Digital Content 1, http://links.lww.com/CCM/C151).
Eight subjects developed respiratory failure: six supported with invasive mechanical ventilation (median duration, 8 d [IQR, 7–12]) and two supported only with noninvasive mechanical ventilation. Five subjects met criteria for pediatric acute respiratory distress syndrome (PARDS) (18), and two were supported with high frequency oscillatory ventilation. Respiratory variables for the six intubated subjects are presented in the Supplemental Table 3 (Supplemental Digital Content 1, http://links.lww.com/CCM/C151).
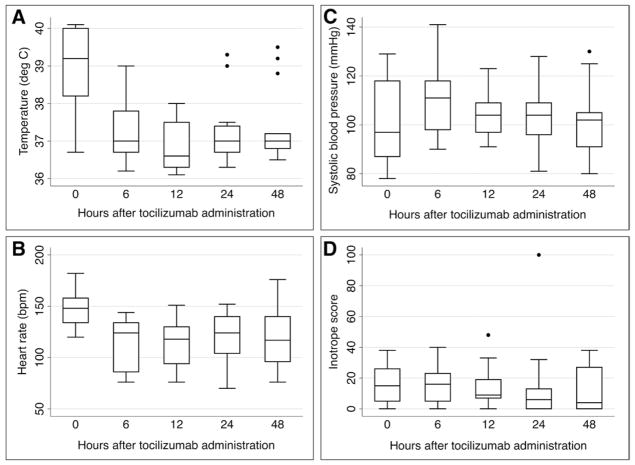
Thirteen of fourteen subjects with cardiovascular dysfunction were treated with tocilizumab. Nine subjects received one dose, and four subjects received two or three doses of tocilizumab due to partial response or recurrence of symptoms after initial dose. The first dose of tocilizumab was administered a median of 5 days after CTL019 infusion. Fever and tachycardia improved rapidly after tocilizumab administration (Fig. 3). Subjects defervesced a median of 4 hours (IQR, 2–5) after tocilizumab administration. Concomitant with this, their overall clinical appearance markedly improved within hours of tocilizumab administration. Nevertheless, catecholamine-dependent shock resolved over days rather than hours (Fig. 3D), with complete resolution a median of 4 days (IQR, 2–4) after tocilizumab administration. Eight subjects were also treated with short courses of corticosteroids (median, 6.5 d) for refractory hypotension: five received hydrocortisone, two received methylprednisolone, and one received both. All four subjects treated with multiple doses of tocilizumab also received corticosteroids. All patients requiring tocilizumab and/or steroids for grade 4 CRS subsequently achieved disease remission and survived CRS.
Figure 3.
Changes in A, temperature; B, heart rate; C, systolic blood pressure; and D), inotrope score after tocilizumab administration (n = 14). p < 0.05 for all changes in heart rate and temperature compared to time 0 (tocilizumab administration).
DISCUSSION
Severe CRS was common in pediatric subjects treated with CAR T cell therapy: 46% developed grade 3–4 CRS, 36% developed MODS, 26% had fluid-refractory/catecholamine-resistant vasoplegic shock, and 13% developed PARDS. Profound shock in grade 4 CRS occurred several days following fever onset. Subsequent progression to AKI, coagulopathy, and respiratory failure ensued, followed by delayed onset of hepatic dysfunction and encephalopathy. Treatment with the IL-6 receptor antibody, tocilizumab, resulted in dramatic improvement in fever, tachycardia, and overall clinical appearance within hours. Cardiovascular dysfunction subsequently improved, with more protracted recovery of other organ dysfunctions. CAR T cell therapy–associated CRS represents a novel critical illness syndrome, with similarities in phenotype to other shock states such as severe sepsis and MAS/HLH. However, the need to ensure survival of the infused T cells drives unique therapeutic considerations for this syndrome.
The profound cardiovascular and respiratory dysfunction in subjects with these toxicities was striking. Subjects with shock often had significant and refractory hypotension, and vascular leak with net positive fluid balance of over 140 mL/kg (14% fluid overload) over the 3 days around the time of shock onset. Rapid recognition and treatment of hypotension with crystalloid and colloid fluids and prompt transfer to the ICU with initiation of vasoactive infusion support early during fluid resuscitation were keystones in the supportive care of these patients.
Encephalopathy was a prominent feature of CRS. In contrast to septic shock, in which altered mental status is indicative of inadequate perfusion, or MAS/HLH, in which neurologic abnormalities are typically related to active CNS disease, the encephalopathy seen in CRS presented later than shock, when perfusion was adequately restored. This could be cytokine related, including IL-6 (12, 19, 20), or could be some direct action of the CAR T cells, which cross into spinal fluid (9). Prior administration of tocilizumab did not prevent encephalopathy. The late timing of encephalopathy raised suspicion for a new pathologic process other than CRS. However, no alternative etiology was identified in any subject, and it self resolved within days of onset.
In CAR T cell therapy, the degree of immune activation generated from the in vivo T cell expansion, which is necessary for clinical benefit, resulted in a clinical phenotype in many subjects with features similar to severe septic shock or MAS/HLH. Comprehensive cytokine profiling during CRS demonstrates a pattern that mirrors that seen in MAS/HLH (Teachey et al [21]). Despite phenotypic similarities to SIRS/septic shock and MAS/HLH, the severe CRS we observed was unique because of the overall favorable ICU outcomes, particularly in this high-risk relapsed/refractory leukemia population. The reported mortality in pediatric septic shock with MODS or SIRS with MAS/HLH is 18–50% (22–24), whereas the subjects in this cohort all survived to ICU discharge. Another important distinction between CRS and other forms of refractory shock is related to adjunctive shock therapy. Corticosteroid administration remains in the practice parameters for treatment of refractory pediatric shock (25) and is a mainstay of MAS/HLH therapy (26, 27); however, corticosteroid therapy may inhibit CAR T cell therapy efficacy (10, 12, 13, 28).
Tocilizumab, labeled for use in rheumatoid arthritis, is a monoclonal anti-IL-6 receptor antibody that blocks receptor binding of IL-6. IL-6 is a proinflammatory cytokine often elevated in patients with severe sepsis, particularly in nonsurvivors, and in MAS/HLH (29–31). Preclinical data suggests that IL-6 is not necessary for the efficacy of CAR T cell therapy, making this a better candidate for antibody therapy compared to therapies directed at interferon-γ or soluble IL2 receptor α, which could theoretically eliminate the CAR T cells (9). Thus far, treatment with tocilizumab at the time of grade 3–4 CRS does not seem to adversely impact the CTL019 cells or disease outcomes.
Given the rapid, dramatic clinical response to tocilizumab (6–8, 12) and lack of apparent side effects, tocilizumab has become a standard rescue therapy for CAR-mediated CRS within all ongoing clinical trials. However, a benefit to using tocilizumab to prevent CRS or as adjunctive therapy in mild CRS warrants specific trials. We administer tocilizumab as first line treatment for patients with grade 4 CRS and catecholamine-dependent shock to reverse shock and prevent worsening MODS (Supplemental Fig. 4, Supplemental Digital Content 1, http://links.lww.com/CCM/C151). The dose of tocilizumab we administer is the dose for treatment of rheumatoid arthritis: 12 mg/kg for patients less than 30 kg and 8 mg/kg for patients greater than or equal to 30 kg. Tocilizumab has a long half-life, but we have observed benefit with repeat dosing shortly after the first dose in some subjects, likely related to improved IL-6 receptor saturation. We use clinical signs and symptoms (especially refractory shock) to initiate tocilizumab therapy for CRS rather than cytokine levels. Cytokine levels are not rapidly available, nor is it known whether levels correlate with illness severity. Four patients in this cohort were treated with multiple tocilizumab doses due to ongoing or recurrent symptoms, raising the possibility that some patients may be partial or nonresponders to tocilizumab. These patients were also treated concomitantly with steroids due to the refractory CRS.
We consider corticosteroid treatment second line to tocilizumab for patients with partial response or recurrent symptoms, as corticosteroid therapy potentially could decrease the effectiveness of the CTL019 cells (10). Our current protocol recommends methylprednisolone, although a brief trial of hydrocortisone to rule out adrenal insufficiency may be considered. Corticosteroid therapy should be weaned rapidly after the patient stabilizes.
As CAR and other T cell therapies (including bispecific T cell engaging antibody therapy) expand, it is crucial for centers employing these therapies to be prepared to treat the associated CRS, which appears to be a class-specific toxicity (21). Disease burden and rapidity of onset may help predict which patients will develop severe CRS (8), and higher ferritin levels may identify patients expected to have longer organ dysfunction. A plan regarding CRS surveillance and the clinical threshold to administer tocilizumab should be in place, and adequate pharmacy supply of this antibody confirmed, prior to initiating T cell therapies. Communication with the ICU team that will be managing severe CRS is essential, including pro-active preparation for rapidly progressive shock, the rationale for limiting corticosteroid exposure, and the potential use of tocilizumab adjunctive therapy.
This descriptive cohort study has several limitations. The reported laboratory and hemodynamic abnormalities are limited by sampling bias as the most severely ill subjects had more measurements. The small sample size of subjects treated with multiple tocilizumab doses (n = 4) limited exploration of risk factors for ongoing refractory symptoms. The single-center design could limit generalizability, although the IL-6 blockade approach has been adopted in multiple centers. Growing clinical experience at other centers will allow for confirmation of our data, as well as future comparisons with different treatment strategies in larger, more diverse patient populations and may overcome some of these limitations.
CONCLUSIONS
As use of novel CAR therapy expands, the data from this large pediatric clinical experience may help others anticipate the clinical course of severe CRS and manage shock. Future investigation should include optimizing protocols for cytokine and corticosteroid therapy for CRS that maximize CRS treatment while preserving the CAR cell efficacy.
Supplementary Material
Acknowledgments
Supported, in part, by a grant from Novartis (to Dr. June), by grants from the National Institutes of Health (1R01CA165206 to Dr. June, R01CA102646 and R01CA116660 to Dr. Grupp, and R01CA193776 to Dr. Teachey), the Pennsylvania Department of Health (to Dr. Grupp), the Leukemia and Lymphoma Society, the Jeffrey Jay Weinberg Memorial Foundation, and the Children’s Hospital of Philadelphia Hematologic Malignancy Research Fund, a Stand Up to Cancer–St. Baldrick’s Pediatric Dream Team translational research grant (SU2C-AACR-DT1113), a St. Baldrick’s Foundation Scholar Award (to Dr. Maude and Dr. Barrett), and a Research Scholar Grant from the American Cancer Society (RSG-14-022-01-CDD to Dr. Teachey).
Footnotes
The sponsor had no role in the design of this current analysis; in the collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
For information regarding this article, FitzgeraldJ@email.chop.edu
Dr. Fitzgerald disclosed other support (A grant from Novartis supported the efforts of other authors for the parent clinical trial. Subjects from that trial were the cohort studied in this article) and disclosed off-label product use (Tocilizumab, an interleukin [IL]-6 receptor antibody, was used in the treatment of cytokine release syndrome [CRS]). Dr. Weiss disclosed off-label product use (Tocilizumab, an IL-6 receptor antibody, was used in the treatment of CRS). He is supported by National Institutes of Health (NIH) K23GM110496. His institution received funding from National Institute of General Medical Sciences K23GM110496 and ThermoFisher Scientific (Honorarium for lecture). Dr. Maude disclosed off-label product use (Tocilizumab, an IL-6 receptor antibody, was used in the treatment of CRS) and received funding from Novartis Pharmaceuticals. Dr. Barrett disclosed off-label product use (Tocilizumab, an IL-6 receptor antibody, was used in the treatment of CRS). Dr. Lacey disclosed off-label product use (Tocilizumab, an IL-6 receptor antibody, was used in the treatment of CRS). His institution received funding from Novartis. Dr. Melenhorst received support for article research from Novartis. Dr. Shaw disclosed off-label product use (Tocilizumab, an IL-6 receptor antibody, was used in the treatment of CRS). Her institution received funding from the Leukemia and Lymphoma Society and Novartis. Dr. Berg disclosed off-label product use (Tocilizumab, an IL-6 receptor antibody, was used in the treatment of CRS. Tocilizumab is not labeled or approved to specifically treat CRS) and other support (The Children’s Hospital of Philadelphia and University of Pennsylvania have received payment for intellectual property in regard to chimeric antigen receptor T cells against cluster of differentiation 19 [CD19] [CART19]. None was paid for this study to Dr. Berg’s knowledge. He did not receive any payment). Dr. June received support for article research from the NIH, disclosed off-label product use (CD19 CAR T cells [CTL019]: investigational use for leukemia), and received funding from Royalty income for intellectual property licensed by University of Pennsylvania to Novartis. His institution received funding from Novartis (sponsored research grant, which supported the clinical trial costs). Dr. Porter disclosed other support (Octata [data safety monitoring board (DSMB)], CIBMTR [DSMB], National Marrow Donor Program/Be the Match [Board of Directors]), disclosed off-label product use (CTL019, tocilizumab), and has a family disclosure (Spouse employment Genentech with salary, stock, and options). His institution received funding from Novartis (research support and royalties), Genentech (Spouse employment [stock, options, and salary]), and Novartis (research support and royalties). Dr. Frey disclosed off-label product use (describes an Investigational Product CART19/CTL019). Her institution received funding from Novartis. Dr. Grupp disclosed off-label product use (tocilizumab for CRS) and received funding (consulting for Novartis). His institution received funding from Novartis (research support). Dr. Teachey disclosed other support (American Cancer Society [Institution]. This is a research grant [He is the principal investigator (PI)]), received support for article research from the NIH, and disclosed off-label product use (Tocilizumab for CRS. This is an Food and Drug Administration approved agent, but is not labeled for this indication). His institution received funding from NIH (This is a research grant [He is the PI]), Novartis, and the Leukemia Lymphoma Society (This is a research grant [He is a co-investigator).
References
- 1.Hunger SP, Lu X, Devidas M, et al. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: A report from the children’s oncology group. J Clin Oncol. 2012;30:1663–1669. doi: 10.1200/JCO.2011.37.8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith MA, Seibel NL, Altekruse SF, et al. Outcomes for children and adolescents with cancer: Challenges for the twenty-first century. J Clin Oncol. 2010;28:2625–2634. doi: 10.1200/JCO.2009.27.0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ward E, DeSantis C, Robbins A, et al. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin. 2014;64:83–103. doi: 10.3322/caac.21219. [DOI] [PubMed] [Google Scholar]
- 4.Bhojwani D, Pui CH. Relapsed childhood acute lymphoblastic leukaemia. Lancet Oncol. 2013;14:e205–e217. doi: 10.1016/S1470-2045(12)70580-6. [DOI] [PubMed] [Google Scholar]
- 5.Raetz EA, Bhatla T. Where do we stand in the treatment of relapsed acute lymphoblastic leukemia? Hematology Am Soc Hematol Educ Program. 2012;2012:129–136. doi: 10.1182/asheducation-2012.1.129. [DOI] [PubMed] [Google Scholar]
- 6.Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: A phase 1 dose-escalation trial. Lancet. 2015;385:517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maude SL, Teachey DT, Porter DL, et al. CD19-targeted chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Blood. 2015;125:4017–4023. doi: 10.1182/blood-2014-12-580068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19–28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6:224ra25. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kochenderfer JN, Dudley ME, Carpenter RO, et al. Donor-derived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation. Blood. 2013;122:4129–4139. doi: 10.1182/blood-2013-08-519413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124:188–195. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maude SL, Barrett D, Teachey DT, et al. Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer J. 2014;20:119–122. doi: 10.1097/PPO.0000000000000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldstein B, Giroir B, Randolph A. International Consensus Conference on Pediatric Sepsis: International pediatric sepsis consensus conference: Definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6:2–8. doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- 15.Slater A, Shann F, Pearson G. Paediatric Index of Mortality (PIM) Study Group: PIM2: A revised version of the Paediatric Index of Mortality. Intensive Care Med. 2003;29:278–285. doi: 10.1007/s00134-002-1601-2. [DOI] [PubMed] [Google Scholar]
- 16.Zuppa AF, Nadkarni V, Davis L, et al. The effect of a thyroid hormone infusion on vasopressor support in critically ill children with cessation of neurologic function. Crit Care Med. 2004;32:2318–2322. doi: 10.1097/01.ccm.0000146133.52982.17. [DOI] [PubMed] [Google Scholar]
- 17.Porter DL, Hwang WT, Frey NV, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015;7:303ra139. doi: 10.1126/scitranslmed.aac5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khemani RG, Smith LS, Zimmerman JJ, et al. Pediatric Acute Lung Injury Consensus Conference Group: Pediatric acute respiratory distress syndrome: Definition, incidence, and epidemiology: Proceedings from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med. 2015;16:S23–S40. doi: 10.1097/PCC.0000000000000432. [DOI] [PubMed] [Google Scholar]
- 19.Spooren A, Kolmus K, Laureys G, et al. Interleukin-6, a mental cytokine. Brain Res Rev. 2011;67:157–183. doi: 10.1016/j.brainresrev.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Weber J, Gunn H, Yang J, et al. A phase I trial of intravenous inter-leukin-6 in patients with advanced cancer. J Immunother Emphasis Tumor Immunol. 1994;15:292–302. doi: 10.1097/00002371-199405000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Teachey DT, Rheingold SR, Maude SL, et al. Cytokine release syndrome after blinatumomab treatment related to abnormal macrophage activation and ameliorated with cytokine-directed therapy. Blood. 2013;121:5154–5157. doi: 10.1182/blood-2013-02-485623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castillo L, Carcillo J. Secondary hemophagocytic lymphohistiocytosis and severe sepsis/ systemic inflammatory response syndrome/multi-organ dysfunction syndrome/macrophage activation syndrome share common intermediate phenotypes on a spectrum of inflammation. Pediatr Crit Care Med. 2009;10:387–392. doi: 10.1097/PCC.0b013e3181a1ae08. [DOI] [PubMed] [Google Scholar]
- 23.Kutko MC, Calarco MP, Flaherty MB, et al. Mortality rates in pediatric septic shock with and without multiple organ system failure. Pediatr Crit Care Med. 2003;4:333–337. doi: 10.1097/01.PCC.0000074266.10576.9B. [DOI] [PubMed] [Google Scholar]
- 24.Proulx F, Joyal JS, Mariscalco MM, et al. The pediatric multiple organ dysfunction syndrome. Pediatr Crit Care Med. 2009;10:12–22. doi: 10.1097/PCC.0b013e31819370a9. [DOI] [PubMed] [Google Scholar]
- 25.Brierley J, Carcillo JA, Choong K, et al. Clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock: 2007 update from the American College of Critical Care Medicine. Crit Care Med. 2009;37:666–688. doi: 10.1097/CCM.0b013e31819323c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carcillo JA, Simon DW, Podd BS. How we manage hyperferritinemic sepsis-related multiple organ dysfunction syndrome/macrophage activation syndrome/secondary hemophagocytic lymphohistiocytosis histiocytosis. Pediatr Crit Care Med. 2015;16:598–600. doi: 10.1097/PCC.0000000000000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trottestam H, Horne A, Aricò M, et al. Histiocyte Society: Chemoimmunotherapy for hemophagocytic lymphohistiocytosis: Long-term results of the HLH-94 treatment protocol. Blood. 2011;118:4577–4584. doi: 10.1182/blood-2011-06-356261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Porter DL, Levine BL, Kalos M, et al. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dalton HJ, Carcillo JA, Woodward DB, et al. Biomarker response to drotrecogin alfa (activated) in children with severe sepsis: Results from the RESOLVE clinical trial. Pediatr Crit Care Med. 2012;13:639–645. doi: 10.1097/PCC.0b013e318250ad48. [DOI] [PubMed] [Google Scholar]
- 30.Hall MW, Geyer SM, Guo CY, et al. Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network PICFlu Study Investigators: Innate immune function and mortality in critically ill children with influenza: A multicenter study. Crit Care Med. 2013;41:224–236. doi: 10.1097/CCM.0b013e318267633c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu XJ, Tang YM, Song H, et al. Diagnostic accuracy of a specific cytokine pattern in hemophagocytic lymphohistiocytosis in children. J Pediatr. 2012;160:984–990. e1. doi: 10.1016/j.jpeds.2011.11.046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.