Abstract
Pulmonary enteric adenocarcinoma is a markedly rare pathological type of lung adenocarcinoma. As the pancreas is a relatively uncommon site for metastasis, the present case is even more unusual. A 62-year-old male was admitted to hospital following the identification of masses in the left chest wall, right abdominal wall and right upper limb, but with no respiratory symptoms. Computed tomography (CT) of the chest revealed a lump in the lung and a mass in the left chest wall, and 18F-fluorodeoxyglucose (18F-FDG) uptake by the lumps was increased. An enhanced abdominal CT revealed a hypodense and homogeneous mass on the head of the pancreas, which was slightly enhanced compared with normal pancreatic tissue. In addition, the 18F-FDG uptake of the lesion was increased and the standardized uptake value (SUV) delayed was not evidently decreased compared with SUVearly. A number of other abnormal metabolic lesions were also identified using positron emission tomography/CT, whereas no abnormal 18F-FDG uptake was identified in the gastrointestinal organ. Furthermore, rectocolonoscopy was performed to exclude diagnosis of metastatic colorectal adenocarcinoma. The hematoxylin- and eosin-stained smears of the masses in the right lung and left chest demonstrated an enteric pattern, which shared morphological and immunohistochemical (IHC) features with those of colorectal adenocarcinoma. The IHC detection revealed that the lesions in the right lung were positive for cytokeratin 7 (CK7), and negative for CK20 and thyroid transcription factor 1 (TTF-1), and the expression of caudal type homeobox 2 (CDX2) was weakly positive; the masses in the left chest wall were positive for CK7, negative for TTF-1, and CK20 and CDX2 were weakly expressed.
Keywords: pulmonary adenocarcinoma, enteric differentiation, pancreatic metastasis
Introduction
Pulmonary enteric adenocarcinoma (PEAC), a rare pathological type of primary lung adenocarcinoma, shares similar pathological morphology and immunohistochemical (IHC) markers with those of metastatic colorectal carcinoma (MCC). In 1991, Tsao and Fraser (1) initially proposed the name ‘intestinal type of lung adenocarcinoma’; subsequently, it has been referred to as ‘pulmonary intestinal-type adenocarcinoma’ or ‘pulmonary adenocarcinoma with enteric (or intestinal) differentiation’. In 2005, PEAC was classified as pulmonary mucinous adenocarcinoma by Yousem (2) and Inamura et al (3). The International Association for the Study of Lung Cancer (IASLC)/American Thoracic Society (ATS)/European Respiratory Society (ERS) International Multidisciplinary Classification of Lung Adenocarcinoma, published in 2011, confirmed the definition of PEAC (4). PEAC is a rare pathological type. Between 1 May 2005 and 8 October 2015, <20 PEAC-related references have been recorded by PubMed (www.ncbi.nlm.nih.gov/pubmed) and Wanfang (http://g.wanfangdata.com.cn/) (2,3,5–18).
Pancreatic neoplasms are mainly primary ductal adenocarcinoma; the incidence of intrapancreatic metastases is <10%: Frequencies reported by Smith et al (19) and Shi et al (20) are between 1.8 and 7.6%, and between 2 and 5%, respectively. ~6% of pancreatic metastases originated from the lung cancer (21). The occurrence of pancreatic metastases is a consequence of the terminal stage of the disease and the invasion of numerous other organs (19).
Case presentation
A 62-year-old man with a ~20-year history of smoking 20 cigarettes/day was admitted to Ruijin Hospital (Shanghai, China) on 28 September, 2015 owing to the discovery of masses in the left chest wall and right abdominal wall for ~1 month and another in the subcutaneous tissue of the right upper limb for ~1 week, but with no respiratory symptoms. The patient had a history of hypertension and diabetes for >10 years, but no history of malignancy. Blood pressure and blood glucose were managed in a comparatively normal range. Informed consent was obtained from the patient.
Masses were palpated in the subcutaneous tissue of the left upper chest wall, right lower abdominal wall and right upper extremity during the physical examination. The masses were between 1.0×1.0 and 1.5×1.0 cm, without redness, swelling or fever, had an irregular shape, with a hard or pliable texture, and were difficult to move and not tender. Lungs were clear to auscultation bilaterally and without evidently dry or moist rales. Abdominal signs and symptoms were negative. No abnormality was identified in the extremities or following neurological examination.
Laboratory data identified that carbohydrate antigen 19–9 (CA19-9) was at a normal level; however, carcinoembryonic antigen levels were increased compared with normal levels.
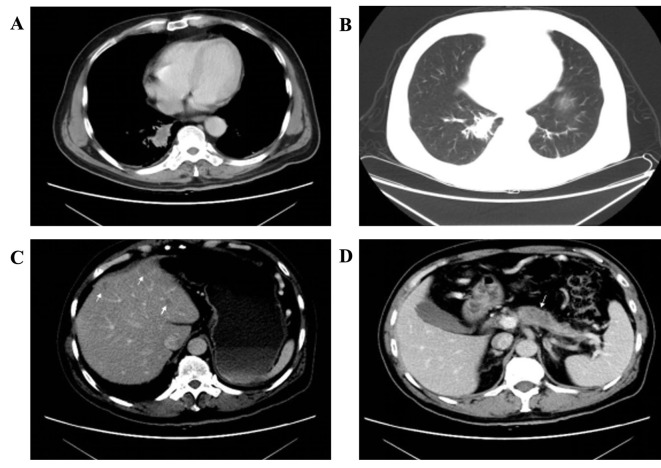
Computed tomography (CT) of the chest identified a 2.8×1.5 cm parenchymal lump with burrs and mild effusion in the lower lobe of the right lung closing to the vertebral column, with mild pleural thickening, but no evidently swollen mediastinal lymph nodes observed (Fig. 1A and B). A 1.7×1.4 cm mass with irregular margin was revealed in the subcutaneous tissue of the left chest. An abdominal enhanced CT identified that there were multiple cystic-hypodense lesions with no enhancement in the liver, but no dilatation was observed in the intrahepatic bile duct (Fig. 1C). A hypodense and homogeneous mass with relatively explicit margin was revealed on the head of the mild atrophic pancreas and the mass was enhanced compared with normal pancreatic tissue (Fig. 1D). No dilatation of the pancreatic duct was identified. Furthermore, the bilateral adrenal glands were swollen. No other focus was identified.
Figure 1.
CT characteristics of lesions in different organs. (A) Mediastinal window demonstrating the parenchyma lung lump and the mild pleural thickening. (B) Pulmonary window demonstrating the lump with burrs and mild effusion. (C) An enhanced CT image demonstrating a number of cystic-hypodense lesions with no enhancement in the liver. (D) Enhanced CT image demonstrating that a hypodense and homogeneous mass with relatively explicit margin, which is suspect for metastatic tumor, is on the head of the pancreas, and the mass is hypoattenuated compared with the normal pancreatic tissue. CT, computed tomography.
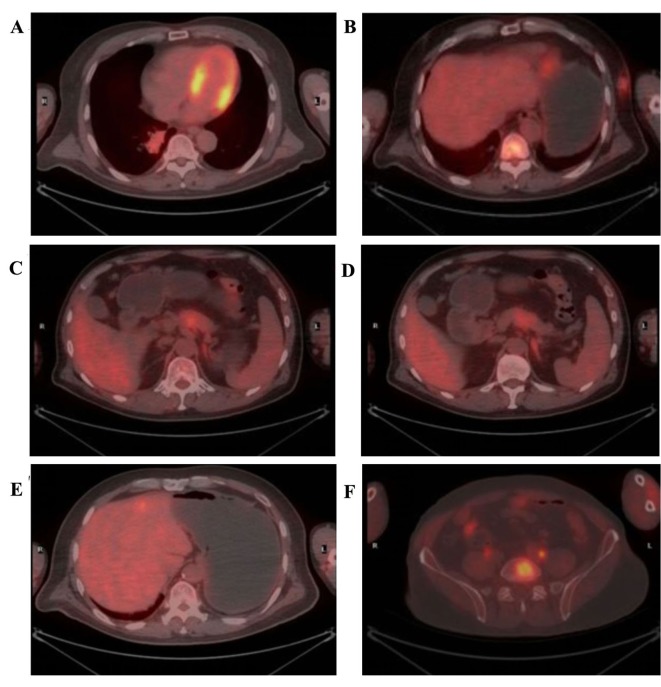
Positron emission tomography (PET)/CT was performed to determine the metabolic status of the tumor. The standardized uptake values (SUV) max of the masses in the right lung and left chest were 6.2 and 4.7, respectively (Fig. 2A and B). In the pancreatic lesion, which was a suspected metastatic tumor, the SUVearly was 3.7, and the SUVdelayed was not markedly decreased compared with the SUVearly (Fig. 2C and D). However, the 18F-fluorodeoxyglucose (18F-FDG) uptake by the pancreatic duct was not increased. The SUVmax of the lesions in the left lobe of the liver and left adrenal exceeded normal levels (Fig. 2E and F). In addition, abnormally metabolic lesions were identified in many other organs; however, no abnormal 18F-FDG uptake was identified in the gastrointestinal organ.
Figure 2.
Positron emission tomography/computed tomography characteristics of the lesions. (A) 18F-FDG uptake in the lung lump increases. SUVmax is 6.2. (B) 18F-FDG uptake in the chest mass increases. SUVmax is 4.7. (C) The early 18F-FDG uptake in the pancreas increases. SUVearly is 3.7. (D) The delayed 18F-FDG uptake in the pancreas is not markedly decreased. (E) 18F-FDG uptake in the left lobe of the liver increases. SUVmax is 4.1. (F) 18F-FDG uptake in the left adrenal gland increases. SUVmax is 3.1. 18F-FDG, 18F-fluorodeoxyglucose; SUV, standardized uptake value.
To exclude a diagnosis of colorectal adenocarcinoma, rectocolonoscopy had been performed and no anomaly was identified.
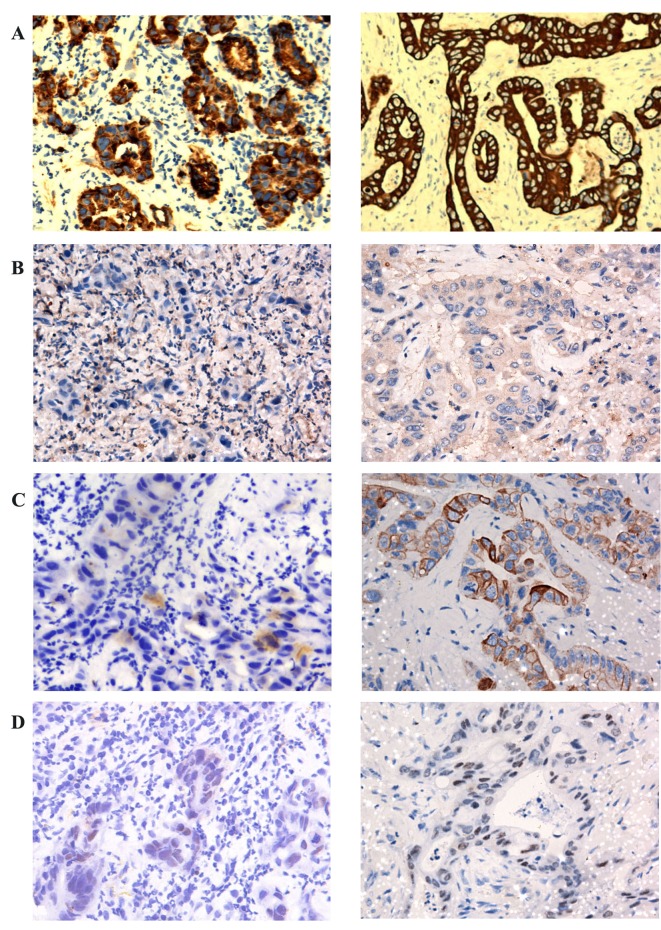
The neoplasm samples in the lower lobe of the right lung and subcutaneous tissue of the left chest wall were obtained using CT-guided fine-needle aspiration (FNA) and endoscopic ultrasound (EUS)-guided FNA respectively. The cytological examination of the two masses, which were stained using hematoxylin and eosin, identified that the two masses possessed similar pathological characteristics: Glandular or cribriform structures with luminal necrosis, tall-columnar oncocytes with eosinophilic cytoplasm, tall or ovoid nuclei arranging in pseudostratified pattern and quantities of inflammatory cells infiltrating the mesenchyma with fibrotic hyperplasia (Fig. 3). It was not possible to easily distinguish the pathological features of the two lesions from those of gastrointestinal adenocarcinoma. The antibodies used for IHC detection are listed in Table I. The tumor cells of the lung neoplasm was positive for cytokeratin 7 (CK7), negative for CK20, thyroid transcription factor 1 (TTF-1) and CA19-9, and the expression of caudal type homeobox 2 (CDX2) was weakly positive; by comparison, those of the chest neoplasm was positive for CK7, negative for TTF-1, and CA19-9, and CK20 and CDX2 were weakly expressed (Table II; Fig. 4).
Figure 3.
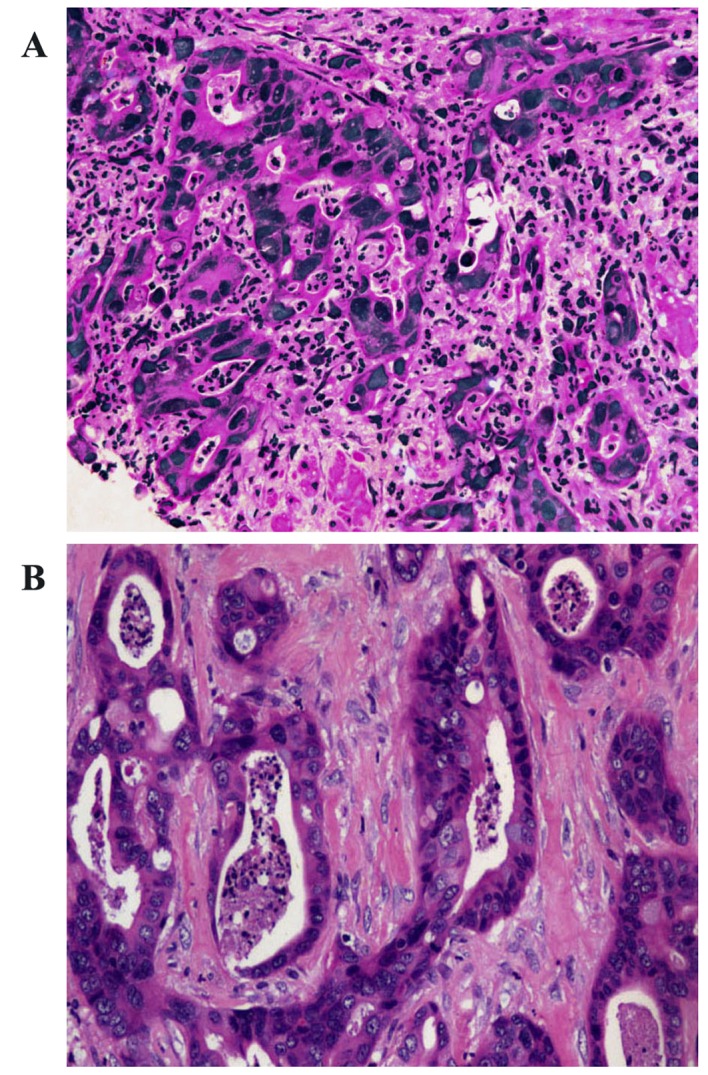
Histopathological section stained with hematoxylin and eosin (H&E). (A) The lung neoplasm and (B) the chest mass magnification were characterized by glandular structures with cellular debris resulting in ‘dirty necroses’ in glandular cavities, tall-columnar cells with pseudostratified and atypical nuclei. However, the atypical karyokinesis and dirty necroses of the chest neoplasm were more marked than that of the lung neoplasm (H&E, ×200).
Table I.
Antibodies applied in the study of PEAC.
| Antigen | Antibody | Catalog no. | Dilution |
|---|---|---|---|
| CK7 | OV-TL 12/30 | M7018 | 1:100 |
| TTF-1 | 8G7G3/1 | M3575 | 1:50 |
| CK20 | Ks20.8 | M7019 | 1:50 |
| CDX2 | EPR2764Y | M3617 | 1:100 |
| P63 | DAK-p63 | M7317 | 1:200 |
| CK5/6 | D5/16B4 | M7237 | 1:100 |
| CK19 | A53-B/A2.26 | M0888 | 1:100 |
| AE1/AE3 Cytokeratin | AE1/AE3 | M3515 | 1:200 |
| CK903 | 34βE12 | M0630 | 1:50 |
| CA19–9 | 1116-NS-19-9 | M3517 | 1:50 |
| Ki67 | MIB-1 | M7240 | 1:100 |
Antigen retrieval was via EDTA (pH 9.0) or citric acid (pH 6.0). All antibodies were from Dako, Agilent Technologies, Inc. (Santa Clara, CA, USA). PEAC, pulmonary enteric adenocarcinoma; CK, cytokeratin; TTF-1, thyroid transcription factor 1; CDX2, caudal type homeobox 2; Ki67, proliferation marker protein Ki67.
Table II.
Immunohistochemical markers of right lung lump and left chest mass.
| Lump in right lung | Mass in left chest | |
|---|---|---|
| CK7 | + | + |
| TTF-1 | − | − |
| CK20 | − | Weakly positive |
| CDX2 | Weakly positive | Weakly positive |
| p63 | − | − |
| CK5/6 | − | − |
| CK19 | + | + |
| Cytokeratin | ||
| AE1/AE3 | + | + |
| CK903 | + | + |
| CA19–9 | − | − |
| Ki67 | 30% | 40% |
AE1/AE3, CK903 and CK19 markers denote origination in the tumor epithelium. p63 and CK5/6 are the specific markers for squamous carcinoma. CK7, TTF-1, CK20 and CDX2 are the primary markers to distinguish pulmonary enteric adenocarcinoma from metastatic colorectal carcinoma and primary lung adenocarcinoma. CK, cytokeratin; TTF-1, thyroid transcription factor 1; CDX2, caudal type homeobox 2; CA19-9, cancer antigen 19–9; Ki67, proliferation marker protein Ki67.
Figure 4.
Immunohistochemical results. Right lung neoplasm (left) and left chest neoplasm (right) were stained for (A) CK7, (B) TTF-1, (C) CK20 and (D) CDX2. IHC markers of right lung neoplasm: CK7 (+), TTF-1 (−), CK20 (−), CDX2 (weakly positive). IHC markers of left chest neoplasm: CK7 (+), TTF-1 (−), CK20 (weakly positive) CDX2 (weakly positive). IHC, immunohistochemical; CK, cytokeratin; TTF-1, thyroid transcription factor 1; CDX2, caudal type homeobox 2 (IHC, ×200).
Discussion
Pulmonary enteric adenocarcinoma
According to the 2011 IASLC/ATS/ERS International Multidisciplinary Lung Adenocarcinoma Classification, PEAC was defined as a lung adenocarcinoma with enteric differentiation of >50% (4). The enteric pattern exhibits the features of colorectal adenocarcinoma, which has glandular, papillary and/or cribriform structures with luminal necrosis, tall-columnar cells with pseudostratified and atypical nuclei and eosinophilic cytoplasm; in contrast with metastatic colorectal adenocarcinoma (MCA), PEAC exhibits a histological component that resembled primary lung adenocarcinoma (PLA), including lepidic growth (4). PEAC is consistently positive for CK7 (CK7-negative cases may occur) and positive for TTF-1 in ~50% of cases, and exhibits >1 IHC marker of enteric differentiation, including CDX2, CK20 and mucin 2 (MUC2) (4). The IASLC/ATS/ERS Classification pointed out that the tumor not exhibiting IHC markers of enteric differentiation should be regarded as lung adenocarcinoma with enteric morphology rather than PEAC (4).
According to the references published in PubMed and Wanfang between 1 May 2005 and 8 October 2015, CK7, TTF-1, CK20 and CDX2 are the principal IHC markers for distinguishing between PEAC, MCA and PLA.
In MCC and PLA, the expression of IHC markers is markedly consistent. Yousem (2) demonstrated that all lesions in cases of MCA shared the same immunoprofile, which was negative for TTF-1 and CK7, but markedly positive for CK20 and CDX2 (75–100%). Inamura et al (3) identified that TTF-1 and CK7 were all negative in the 14 samples of MCC; however, CDX2 and CK20 were markedly positive in 12 cases. Montezuma et al (22) performed IHC detection in 25 cases of MCC and 198 cases of PLA, revealing that all MCC cases were positive for CK20, but negative for TTF-1, and CK7 was negative or weakly expressed; all PLA cases were strongly positive for TTF-1, a number were positive for CK7, and only a limited number were weakly positive for p63. TTF-1, which belongs to the NK-2 homeobox family, is positive in PLA, but negative in MCC, therefore it is recognized as the best single stain for PLA (23).
References concerning IHC markers of PEAC, deposited in the Wanfang database and PubMed between 1 May 2005 and 8 October 2015, were examined. As the studies by Geles et al (17) and Suzuki et al (18) lacked the specific information concerning patients and the results of IHC, these studies were excluded from the analysis. The results of the analysis demonstrated that, in the 41 cases of PEAC recorded, the positive rate of CK7 was 87.8%, the negative rates of CK20 and TTF-1 were 70.7 and 51.2%, respectively, and the positive rate of CDX2 ranged between 51.2 and 65.9% (Table III). CK7 and CK20 were identified to be comparatively accurate markers that may be used to distinguish PEAC from MCC, whereas TTF-1 possesses marked accuracy in distinguishing between PEAC and PLA. In addition to the aforementioned immune markers, PEAC and MCC are heterogeneous in the expression of MUC1, MUC2 and MUC5 (2). Lin et al (9) reported that villin was able to function as the IHC marker, which determined the occurrence of enteric differentiation in cases of PEAC; however, PEAC was not able to be distinguished from MCC on the basis of the expression pattern of villin in the brush border.
Table III.
Studies concerning IHC markers of PEAC.
| Author | No. of cases | TTF-1 | CK7 | CK20 | CDX2 | (Refs.) |
|---|---|---|---|---|---|---|
| Yousem | 6 | 6(+); 0(−) | 6(+); 0(−) | 0(+); 6(−) | 0(+); 6(−) | (2) |
| Inamura et al | 7 | 3(+); 4(−) | 7(+); 0(−) | 3(+); 4(−) | 5(+); 2(−) | (3) |
| Maeda et al | 1 | 1(+); 0(−) | 1(+); 0(−) | 0(+); 1(−) | NA | (5) |
| Li et al | 1 | 0(+); 1(−) | 0(+); 1(−) | 1(+); 0(−) | 1(+); 0(−) | (6) |
| Hatanaka et al | 1 | 0(+); 1(−) | 0(+); 1(−) | 1(+); 0(−) | 1(+); 0(−) | (7) |
| Qureshi et al | 1 | 0(+); 1(−) | 1(+); 0(−) | 1(+); 0(−) | 1(+); 0(−) | (8) |
| Lin et al | 1 | 0(+); 1(−) | 1(+); 0(−) | 1(+); 0(−) | NA | (9) |
| Stojsic et al | 2 | 0(+); 2(−) | 0(+); 2(−) | 2(+); 0(−) | 2(+); 0(−) | (10) |
| Wang et al | 9 | 4(+); 5(−) | 9(+); 0(−) | 2(+); 7(−) | 6(+); 3(+) | (11) |
| László et al | 1 | 0(+); 1(−) | 0(+); 1(−) | 1(+); 0(−) | 1(+); 0(−) | (12) |
| Handa et al | 1 | 1(+); 0(−) | 1(+); 0(−) | 0(+); 1(−) | 0(+); 1(−) | (13) |
| Metro et al | 1 | 0(+); 1(−) | 1(+); 0(−) | 0(+); 1(−) | 1(+); 0(−) | (14) |
| Wei et al | 4 | 2(+); 2(−) | 4(+); 0(−) | 0(+); 4(−) | NA | (15) |
| Wang et al | 5 | 3(+); 2(−) | 5(+); 0(−) | 5(−); 0(+) | 3(+); 2(−) | (16) |
| Totals | 41 | 20(+), 48.80%; | 36(+), 87.80%; | 12(+), 29.30%; | 21(+), 51.2–65.9%; | |
| 21(−), 51.20% | 5(−), 12.20% | 29(−), 70.70% | 14(−), 34.1–48.8%; 6NA |
Geles et al (17) conducted morphological, IHC and genetic tests on pulmonary mucinous adenocarcinoma; however, the number of PEAC patients and specific results from the IHC tests were not specified, therefore this study is not included. Although Suzuki et al (18) confirmed the number of PEAC cases, only CDX2 was examined, therefore the study was omitted. IHC, immunohistochemical; PEAC, pulmonary enteric adenocarcinoma; TTF-1, thyroid transcription factor 1; CK, cytokeratin; CDX2, caudal type homeobox 2; NA, not available.
To further confirm PEAC, in addition to the morphological detection of IHC markers, rectocolonoscopy must be performed to exclude MCA or MCC (10).
In the present case study, the right lung neoplasm and the left chest neoplasm exhibited similar morphological characteristics to an enteric pattern. Additionally, the two lesions possessed similar expressions of the IHC marker: CK7(+), TTF-1(−) and CDX-2 (weakly positive). Furthermore, rectocolonoscopy was performed and no anomaly was identified. These results led to the conclusion that the two neoplasms are of the same pathological type and the left chest neoplasm is a metastasis from the primary lung neoplasm. Furthermore, the rectocolonoscopy examination assisted in excluding the possibility of the presence of MCA or MCC, and the negative expression of CA19-9 demonstrates that the two masses are not the metastases of a pancreatic neoplasm.
The two lesions are markedly positive for CK7 and negative for TTF-1. The aforementioned analysis identified that CK7 and CK20 are comparatively accurate markers used to distinct PEAC from MCC (MCC is typically negative for CK7 and positive for CK20, whereas PEAC is typically positive for CK and negative for CK20). TTF-1 possesses marked accuracy in distinguishing PEAC from PLA (PEAC is typically negative for TTF-1 and PLA is typically positive for TTF-1). As aforementioned, the IASLC/ATS/ERS Classification pointed out that the tumor not exhibiting IHC markers of enteric differentiation should be regarded as lung adenocarcinoma with enteric morphology rather than PEAC (4). As the two neoplasms exhibit the same pathological type and the metastases in the left chest are positive for CDX2, the uncertain CDX2 in the primary lung neoplasm is most likely to be positive. Therefore, the primary lung neoplasm is PEAC rather than primary lung adenocarcinoma with enteric morphology.
In addition, the 2011 IASLC/ATS/ERS Classification proposed that the enteric differentiation in lung adenocarcinoma should be >50% (4). However, the studies obtained from the PubMed and Wanfang databases did not demonstrate enteric differentiation of >50%. Furthermore, samples, obtained using CT-guided FNA, are limited, therefore a level of 50% is difficult to confirm, so the practical relevance of 50% requires reconsideration.
Metastasis to the pancreas
Pancreatic masses are typically primary neoplasms, and the incidence of pancreatic metastases reported in previous studies is <10%; however, pancreatic metastases are being observed with increased frequency at high-volume pancreatic surgeries (19,20). The underlying molecular mechanism for the occurrence of metastases in the pancreas is poorly understood. According to previous research, one reason may be the transformation of biological behavior of the tumor cells, which is a result of the alterations of certain molecules and genes during chemotherapeutic regimens (24). Another reason may lie in the fact that certain tumor cells may have a marked affinity for the parenchyma of the pancreas regardless of whether or not the neoplasm has been treated (24).
The definitive separation of primary and secondary pancreatic neoplasms depends primarily on the examination of pathological tissue, which is typically collected using EUS-guided FNA in living patients (25). Nevertheless, pathological samples of pancreatic metastases remain difficult to obtain. Furthermore, CT exhibits certain typical radiographic characteristics in separating the pancreatic metastases from primary pancreatic carcinomas (20).
The most common manifestation was hypodense or isodense mass on unenhanced CT. In terms of enhancement pattern, the homogeneous lesions with well-defined margins exhibited hypoattenuation, compared with the normal enhanced pancreas. The rare presentation was pancreatic infiltration with mild enhancement instead of focal mass, which is similar to focal pancreatitis (20). However, unlike pancreatic ductal adenocarcinoma (PDAC), the majority of metastases tended to be well circumscribed, although they lacked true capsules. Tan et al (26) identified other unique features of secondary alterations caused by PDAC that are rarely observed in pancreatic metastasis, including dilatation of the upstream pancreatic duct or pancreatic parenchymal atrophy; furthermore, multiple lesions occurring in the pancreas are strong evidence of pancreatic metastases. Shi et al (20) identified that pancreatic metastases from non-small cell lung cancer and gastrointestinal carcinoma exhibited similar CT imaging characteristics, and they hypothesized that this may have an association with pathological type, since the majority were adenocarcinoma.
PET/CT, combining metabolic detection with anatomical imaging, may reflect the metabolic level and proliferative index of the tumor. It has been reported that PET/CT is superior to CT in diagnosis of pancreatic cancer (27). Improved diagnostic value for pancreatic cancer may be obtained when 18F-FDG uptake of the anomaly is increased compared with that of normal parenchyma or that of normal liver. The threshold value of SUVmax in distinguishing pancreatic cancer from benign lesions has not yet been determined. Nishiyama et al (28) demonstrated that dual-phase 18F-FDG imaging may improve diagnostic efficacy in distinguishing pancreatic cancer from mass-forming pancreatitis, whereas Kato et al (29) indicated that differentiation between metastasis-free pancreatic cancer and mass-forming pancreatitis was difficult by 18F-FDG-PET/CT due to considerable overlap between the SUVmax of these two diseases.
With the exception of radiographic characteristics, Tan et al (26) proposed that the existence of other malignant lesions was important for the diagnosis of pancreatic metastasis. The presence of CA19-9 may also contribute to the correct diagnosis of pancreatic metastasis. CA19-9 possesses 81% sensitivity and 89% specificity for the diagnosis of primary pancreatic adenocarcinoma rather than secondary pancreatic lesions, particularly when the level of CA19-9 is increased progressively (20).
The present case has demonstrated typical radiographic characteristics of pancreatic metastases from PEAC, which differ from those of primary pancreatic lesions. The serum tumor marker CA19-9 in the present case was also at a normal level. Indeed, certain patients with primary pancreatic tumors have normal CA19-9 levels; however, if CA19-9 is maintained at a normal level, the possibility of the occurrence of primary pancreatic tumors decreases. Furthermore, it has been confirmed that the neoplasm in the left chest originated from a right lung neoplasm, and the IHC marker CA19-9 is negative in the two neoplasm, which further decreases the possibility of the identification of a primary pancreatic neoplasm. A number of other metastases have also been presented following examination using PET/CT; generally speaking, it is more likely that the metastases originate from one primary neoplasm rather than two. Although EUS-guided FNA was not applied in the present case, owing to the poor physical condition of the patient, according to the above analysis, the lesion in the pancreas is considered to be a metastasis originating from PEAC.
PEAC is an aggressive cancer, which is characterized by rapid growth and early metastatic behavior. To realize personalized therapy of primary lung carcinoma, further investigations are required for further understanding of PEAC. However, owing to a lack of cases of PEAC, it remains challenging to study PEAC. Pancreatic metastasis is also observed infrequently, but it typically affects the treatment decision, so increased efforts should be made to improve our knowledge of the underlying molecular mechanism of pancreatic metastasis and improve the radiographic diagnosis efficacy of PEAC since EUS-guided FNA is an invasive technique that should be limited in use.
Acknowledgements
The present study was supported by the Public Health Discipline Establishment in Shanghai (grant no. 12GWZX1002) and the Key Discipline Construction Project by Shanghai Health Development Planning(grant no. 2015ZB0503).
References
- 1.Tsao MS, Fraser RS. Primary pulmonary adenocarcinoma with enteric differentiation. Cancer. 1991;68:1754–1757. doi: 10.1002/1097-0142(19911015)68:8<1754::AID-CNCR2820680818>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 2.Yousem SA. Pulmonary intestinal-type adenocarcinoma does not show enteric differentiation by immunohistochemical study. Mod Pathol. 2005;18:816–821. doi: 10.1038/modpathol.3800358. [DOI] [PubMed] [Google Scholar]
- 3.Inamura K, Satoh Y, Okumura S, Nakagawa K, Tsuchiya E, Fukayama M, Ishikawa Y. Pulmonary adenocarcinomas with enteric differentiation: Histologic and immunohistochemical characteristics compared with metastatic colorectal cancers and usual pulmonary adenocarcinomas. Am J Surg Pathol. 2005;29:660–665. doi: 10.1097/01.pas.0000160438.00652.8b. [DOI] [PubMed] [Google Scholar]
- 4.Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger K, Yatabe Y, Powell CA, Beer D, Riely G, Garg K, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society: International multidisciplinary classification of lung adenocarcinoma: Executive summary; Proc Am Thorac Soc; 2011; pp. 381–385. [DOI] [PubMed] [Google Scholar]
- 5.Maeda R, Isowa N, Onuma H, Miura H. Pulmonary intestinal-type adenocarcinoma. Interact Cardiovasc Thorac Surg. 2008;7:349–351. doi: 10.1510/icvts.2007.168716. [DOI] [PubMed] [Google Scholar]
- 6.Li HC, Schmidt L, Greenson JK, Chang AC, Myers JL. Primary pulmonary adenocarcinoma with intestinal differentiation mimicking metastatic colorectal carcinoma: Case report and review of literature. Am J Clin Pathol. 2009;131:129–133. doi: 10.1309/AJCPB04XWICTFERL. [DOI] [PubMed] [Google Scholar]
- 7.Hatanaka K, Tsuta K, Watanabe K, Sugino K, Uekusa T. Primary pulmonary adenocarcinoma with enteric differentiation resembling metastatic colorectal carcinoma: A report of the second case negative for cytokeratin 7. Pathol Res Pract. 2011;207:188–191. doi: 10.1016/j.prp.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Qureshi A, Furrukh M. Enteric adenocarcinoma lung: A rare presentation in an Omani woman. BMJ Case Rep. 2013;2013 doi: 10.1136/bcr-2012-007667. bcr2012007667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin D, Zhao Y, Li H, Xing X. Pulmonary enteric adenocarcinoma with villin brush border immunoreactivity: A case report and literature review. J Thorac Dis. 2013;5:E17–E20. doi: 10.3978/j.issn.2072-1439.2012.06.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stojsic J, Kontic M, Subotic D, Popovic M, Tomasevic D, Lukic J. Intestinal type of lung adenocarcinoma in younger adults. Case Rep Pulmonol. 2014;2014:282196. doi: 10.1155/2014/282196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang CX, Liu B, Wang YF, Zhang RS, Yu B, Lu ZF, Shi QL, Zhou XJ. Pulmonary enteric adenocarcinoma: A study of the clinicopathologic and molecular status of nine cases. Int J Clin Exp Pathol. 2014;7:1266–1274. [PMC free article] [PubMed] [Google Scholar]
- 12.László T, Lacza A, Tóth D, Molnár TF, Kálmán E. Pulmonary enteric adenocarcinoma indistinguishable morphologically and immunohistologically from metastatic colorectal carcinoma. Histopathology. 2014;65:283–287. doi: 10.1111/his.12403. [DOI] [PubMed] [Google Scholar]
- 13.Handa Y, Kai Y, Ikeda T, Mukaida H, Egawa H, Kaneko M. Pulmonary enteric adenocarcinoma. Gen Thorac Cardiovasc Surg. 2015;64:749–751. doi: 10.1007/s11748-015-0569-0. [DOI] [PubMed] [Google Scholar]
- 14.Metro G, Valtorta E, Siggillino A, Lauricella C, Cenci M, Ludovini V, Minenza E, Prosperi E, Ricciuti B, Rebonato A, et al. Enteric-type adenocarcinoma of the lung harbouring a novel KRAS Q22K mutation with concomitant KRAS polysomy: A case report. Ecancermedicalscience. 2015;9:559. doi: 10.3332/ecancer.2015.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei QZ, Liu JH, Zhang ZX, Yang Q, Zhao T. Expression and significance of TTF-1, CK7, CK5/6, P63 and CD20 in mucin producing lung cancer. Clin J Diffic and Compl Cas. 2010;9:822–824. (In Chinese) [Google Scholar]
- 16.Wang CX, Xu Y, Liu B, Zhang J, Yu B, Shi SS, Zhou XJ. Clinicopathologic features and differential diagnosis of pulmonary intestinal-type adenocarcinoma. J Clin Exp Pathol. 2013;29:1101–1104. (In Chinese) [Google Scholar]
- 17.Geles A, Gruber-Moesenbacher U, Quehenberger F, Manzl C, Al Effah M, Grygar E, Juettner-Smolle F, Popper HH. Pulmonary mucinous adenocarcinomas: Architectural patterns in correlation with genetic changes, prognosis and survival. Virchows Arch. 2015;467:675–686. doi: 10.1007/s00428-015-1852-2. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki M, Yazawa T, Ota S, Morimoto J, Yoshino I, Yamanaka S, Inayama Y, Kawabata Y, Shimizu Y, Komatsu M, et al. High-grade fetal adenocarcinoma of the lung is a tumour with a fetal phenotype that shows diverse differentiation, including high-grade neuroendocrine carcinoma: A clinicopathological, immunohistochemical and mutational study of 20 cases. Histopathology. 2015;67:806–816. doi: 10.1111/his.12711. [DOI] [PubMed] [Google Scholar]
- 19.Smith AL, Odronic SI, Springer BS, Reynolds JP. Solid tumor metastases to the pancreas diagnosed by FNA: A single-institution experience and review of the literature. Cancer Cytopathol. 2015;123:347–355. doi: 10.1002/cncy.21541. [DOI] [PubMed] [Google Scholar]
- 20.Shi HY, Zhao XS, Miao F. Metastases to the pancreas: Computed tomography imaging spectrum and clinical features: A retrospective study of 18 patients with 36 metastases. Medicine (Baltimore) 2015;94:e913. doi: 10.1097/MD.0000000000000913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sweeney AD, Fisher WE, Wu MF, Hilsenbeck SG, Brunicardi FC. Value of pancreatic resection for cancer metastatic to the pancreas. J Surg Res. 2010;160:268–276. doi: 10.1016/j.jss.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 22.Montezuma D, Azevedo R, Lopes P, Vieira R, Cunha AL, Henrique R. A panel of four immunohistochemical markers (CK7, CK20, TTF-1 and p63) allows accurate diagnosis of primary and metastatic lung carcinoma on biopsy specimens. Virchows Arch. 2013;463:749–754. doi: 10.1007/s00428-013-1488-z. [DOI] [PubMed] [Google Scholar]
- 23.Travis WD, Rekhtman N. Pathological diagnosis and classification of lung cancer in small biopsies and cytology: Strategic management of tissue for molecular testing. Semin Respir Crit Care Med. 2011;32:22–31. doi: 10.1055/s-0031-1272866. [DOI] [PubMed] [Google Scholar]
- 24.Ballarin R, Spaggiari M, Cautero N, de Ruvo N, Montalti R, Longo C, Pecchi A, Giacobazzi P, de Marco G, D'Amico G, et al. Pancreatic metastases from renal cell carcinoma: The state of the art. World J Gastroenterol. 2011;17:4747–4756. doi: 10.3748/wjg.v17.i43.4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karakan T, Cengiz M, İbiş M, Akyürek N, Ünal S. Pancreatic metastasis in a case of small cell lung carcinoma diagnosed by EUS. Turk J Gastroenterol. 2015;26:53–55. doi: 10.5152/tjg.2015.3687. [DOI] [PubMed] [Google Scholar]
- 26.Tan CH, Tamm EP, Marcal L, Balachandran A, Charnsangavej C, Vikram R, Bhosale P. Imaging features of hematogenous metastases to the pancreas: Pictorial essay. Cancer Imaging. 2011;11:9–15. doi: 10.1102/1470-7330.2011.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang XY, Yang F, Jin C, Fu DL. Utility of PET/CT in diagnosis, staging, assessment of resectability and metabolic response of pancreatic cancer. World J Gastroenterol. 2014;20:15580–15589. doi: 10.3748/wjg.v20.i42.15580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishiyama Y, Yamamoto Y, Monden T, Sasakawa Y, Tsutsui K, Wakabayashi H, Ohkawa M. Evaluation of delayed additional FDG PET imaging in patients with pancreatic tumour. Nucl Med Commun. 2005;26:895–901. doi: 10.1097/00006231-200510000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Kato K, Nihashi T, Ikeda M, Abe S, Iwano S, Itoh S, Shimamoto K, Naganawa S. Limited efficacy of (18)F-FDG PET/CT for differentiation between metastasis-free pancreatic cancer and mass-forming pancreatitis. Clin Nucl Med. 2013;38:417–421. doi: 10.1097/RLU.0b013e3182817d9d. [DOI] [PubMed] [Google Scholar]