Abstract
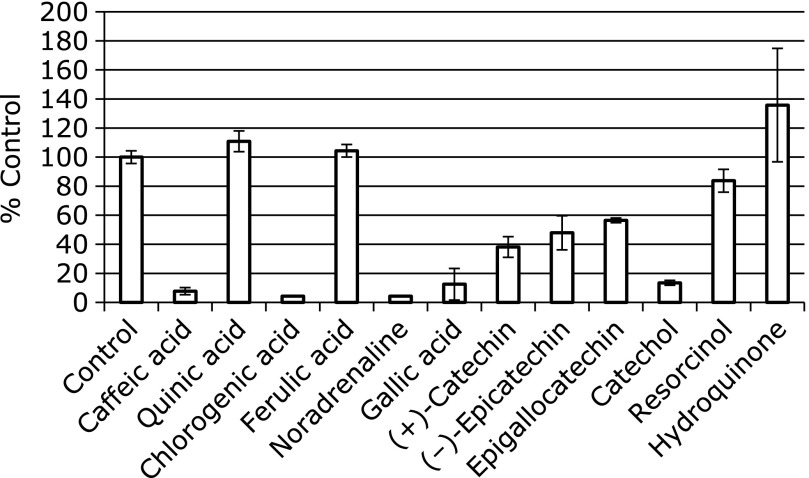
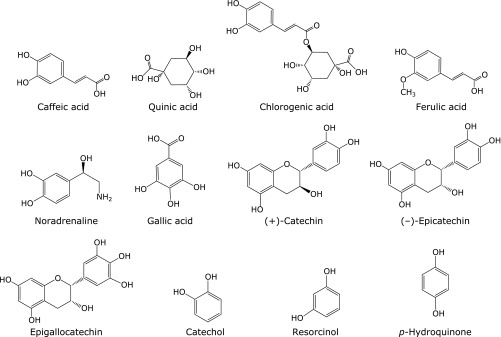
Caffeic acid and (+)-catechin, which are abundantly contained in coffee and tea, are typical polyphenols. In order to know the relative magnitudes of antioxidant activity, effects by caffeic acid, (+)-catechin and their derivatives on the formation of 4-POBN/carbon-centered linoleic acid-derived radical adducts were examined in the control reaction mixture of linoleic acid with FeCl3 at 30°C for 168 h. In the presence of 1.0 mM of the polyphenols, peak to peak heights of the third ESR signal resulted in 7.7 ± 2.4% (n = 3) (caffeic acid), 145 ± 13% (n = 3) (quinic acid), 4.4 ± 0.0% (n = 3) (chlorogenic acid), 104 ± 4.4% (n = 3) (ferulic acid), 4.3 ± 0.0% (n = 3) (noradrenaline), 12.5 ± 10.9% (n = 3) (gallic acid), 38.1 ± 7.1% (n = 3) [(+)-catechin], 47.9 ± 11.7% (n = 3) [(–)-epicatechin], 56.5 ± 1.6% (n = 3) (epigallocatechin), 13.5 ± 1.7% (n = 3) (catechol) and 83.7 ± 7.8% (n = 3) (resorcinol) of the control reaction mixture. All the compounds with catechol moiety exerted potent inhibitory effects on the radical formation except for (+)-catechin, (–)-epicatechin and epigallocatechin. (+)-Catechin, (–)-epicatechin and epigallocatechin may not exert the inhibitory effect as much possibly because they are less stable compared with caffeic acid. The resorcinol moiety in these molecules may also weaken their antioxidant activity.
Keywords: iron chelators, caffeic acid, chlorogenic acid, (–)-epicatechin, epigallocatechin
Introduction
Iron is present in the human body in great quantity in the form of heme and non-heme proteins. It plays a crucial role in electron transfer, cellular respiration, cell proliferation and differentiation, and regulation of gene expression.(1) On the other hand, iron exposure is directly associated with the pathogenesis of many disorders, such as atherosclerosis, cancer and inflammation, possibly via the production of free radicals.(2,3)
Chlorogenic acid, caffeic acid (CA), noradrenaline and gallic acid are typical catechol compounds. Of the catechols, chlorogenic acid and CA are found naturally in various agricultural products such as coffee beans, potatoes, and apples.(4,5) Chlorogenic acid is an ester of CA with quinic acid. Chlorogenic acid and CA have been known to be inhibitors of formation of hydroxyl radical in the reaction of 3-hydroxyanthranilic acid and hydrogen peroxide with ferric ions,(6) lipid peroxidation and formation of lipid-derived radicals.(7,8) Chlorogenic acid and CA also act as scavengers of superoxide, hydroxyl and peroxy radicals.(9,10) It was reported that noradrenaline and dopamine provide an antioxidant defense in the brain against oxidatative stress.(11) The chemically induced LDL oxidation is reduced by galloyl derivatives.(12) Their antioxidative activities appear to be achieved through inhibiting the formation of the free radical by catechol moiety which has iron ion chelating activity.(13)
Catechins such as (+)-catechin, (–)-epicatechin (EC), epigallocatechin (EGC) and epigallocatechingallate (EGCG) are also typical catechol derivatives. They are tricyclic phenols (flavonoids) found in green tea. Catechins exert protective effects against oxidative damage of erythrocyte membrane,(14) ethanol-induced fatty livers,(15) cardiovascular diseases,(16,17) inflammatory,(18) and cancer.(19) Catechins decreases 4-POBN/radical adducts formed in bile of rats after transplantation of ethanol-induced fatty livers.(15)
The radical scavenging activities of (+)-catechin and CA found in the two common beverages, coffee and tea, were investigated in detail in terms of their reaction with the stable radical 2,2-diphenyl-1-picrylhydrazyl in methanol.(20,21) Meanwhile, the polyphenols are strong antioxidants due to their ability to chelate transition metals like iron as well as their radical scavenging activities.(22) In order to examine the effect of their chelating ability on the formation of lipid-derived free radical in the reaction of linoleic acid with iron ions, we used electron spin resonance (ESR), high performance liquid chromatography-electron spin resonance (HPLC-ESR) and high performance liquid chromatography-electron spin resonance-mass spectrometries (HPLC-ESR-MS) and conducted the comparative study on CA and catechins in antioxidative activities.(23)
Materials and Methods
Chemicals
Caffeic acid, catechol, resorcinol, gallic acid, ferulic acid, quinic acid, chlorogenic acid, and (+)-catechin were purchased from Tokyo Kasei Kogyo, Ltd. (Tokyo, Japan). Ferric chloride (FeCl3) was from Wako Pure Chemical Industries, Ltd. (Tokyo, Japan). Linoleic acid and (–)-epicatechin were obtained from Sigma Aldrich Co. (St. Louis, MO). Noradrenaline was from Nacalai Tesque, Inc. (Tokyo, Japan). Epigallocatechin was purchased from Kishida Chemical Co., Ltd. (Osaka, Japan). Water used in these experiments was purified by passing through AUTOPURE WT101UV (Nihon Millipore Kogyo K.K., Yonezawa, Japan) after distillation.
Control reaction mixture
In the control reaction mixture, there were 50 mM phosphate buffer (pH 7.4), 0.1 M 4-POBN, 0.89 mM linoleic acid, 0.38 M acetonitrile and 20 µM FeCl3 in a quartz test tube (100 mm long × 8 mm i.d.). 4-POBN is a spin-trapping agent. The reactions were performed at 30°C for 168 h. After the reaction, the reaction mixture was applied to the ESR (or HPLC-ESR or HPLC-ESR-MS).
ESR studies
The ESR experiments were carried out on a JES-FR 30 Free Radical Monitor (JEOL Ltd., Tokyo, Japan). Operating conditions of the ESR spectrometer were: power, 4 mW; modulation width, 0.1 mT; time constant, 0.3 s. Magnetic fields were calculated by the splitting of MnO (ΔH3–4 = 8.69 mT).
HPLC-ESR chromatography
An HPLC used in the HPLC-ESR consisted of a model 7125 injector (Reodyne, Cotati, CA), a model L-7100 pump (Hitachi Ltd., Ibaraki, Japan). A semi-preparative column (300 mm long × 10 mm i.d.) packed with TSKgel ODS-120T (TOSOH Co., Tokyo, Japan) was used. Flow rate was 2.0 ml/min throughout the HPLC-ESR experiments. For the HPLC-ESR, two solvents were used: solvent A, 50 mM acetic acid; solvent B, 50 mM acetic acid/acetonitrile (20/80, v/v). A following combination of isocratic and linear gradient was used: 0–40 min, 100% A to 20% A (linear gradient); 40–60 min, 80% B (isocratic). The eluent was introduced into a model JES-FR30 Free Radical Monitor (JEOL Ltd.). The ESR spectrometer was connected to the HPLC with a Teflon tube, which passed through the center of the ESR cavity. The operating conditions of the ESR spectrometer were: power, 4 mW; modulation width, 0.2 mT; time constant, 1 s. The magnetic field was fixed at the third peak in the double-triplet ESR spectrum (αN = 1.58 mT and αHβ = 0.26 mT) of the 4-POBN radical adducts (Fig. 1).
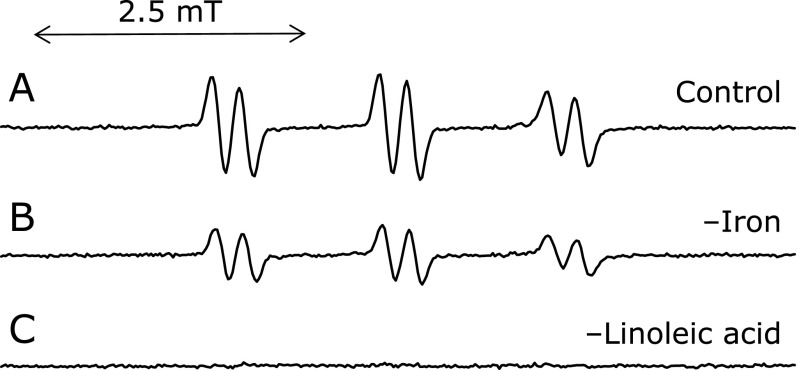
Fig. 1.
ESR Spectra of the control reaction mixtures. The reaction and ESR condition were as described in materials and methods section. In the control reaction mixture, there were 50 mM phosphate buffer (pH 7.4), 0.1 M 4-POBN, 0.89 mM linoleic acid, 0.38 M acetonitrile and 20 µM FeCl3. A, control reaction mixture. B, without 20 µM FeCl3. C, without 0.89 mM linoleic acid.
HPLC-ESR-MS chromatography
The HPLC and ESR conditions were as described in the HPLC-ESR. The mass spectrometer (MS) used in the HPLC-ESR-MS was a model M-1200 HS electrospray ionization (ESI)-MS (Hitachi Ltd.). The operating conditions of the ESI-MS were: nebulizer, 180°C; aperture1, 120°C; N2 controller pressure, 19.6 N/cm2; drift voltage, 70 V; multiplier, 2,000 V; needle voltage, 4,000 V; polarity, positive; resolution, 48. The mass spectra were obtained by introducing the eluent from the ESR detector into the ESI-MS system just before the peak was eluted. The flow kept at 50 µl/min while the eluent was introducing into the ESI-MS.
Results and Discussion
ESR Spectra of the control reaction mixtures
ESR spectrum of the control reaction mixture (without FeCl3 or linoleic acid) was measured (Fig. 1). A prominent ESR spectrum (αN = 1.58 mT and αHβ = 0.26 mT) was observed in the control reaction mixture (Fig. 1A). The ESR signals remained unchanged for the control reaction in the absence of light [90 ± 6% (n = 3) of the control reaction mixture], suggesting that light is not involved in the radical formation. For the reaction mixture without iron, the ESR signal decreased to 53 ± 3% (n = 3) of the control reaction mixture (Fig. 1B), suggesting that iron ions were involved in the radical formation. ESR peaks were hardly observed in the absence of linoleic acid (Fig. 1C). The result indicates that the radicals formed in the control reaction mixture are derived from linoleic acid.
Time course of the ESR peak heights
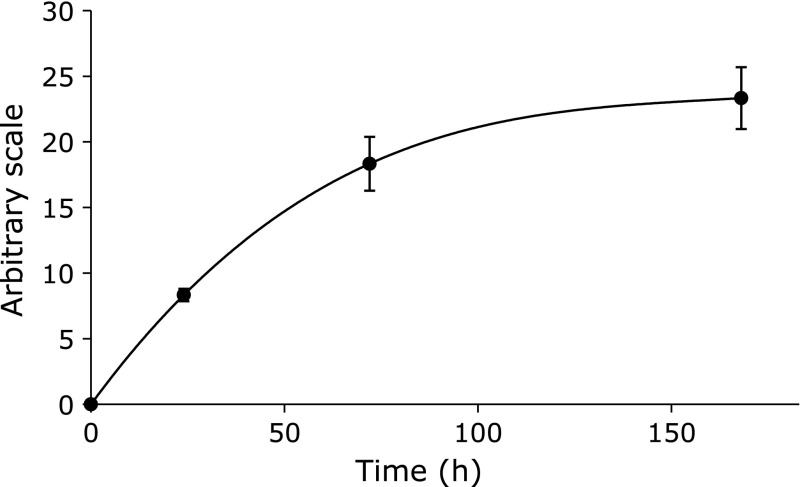
Time course experiments of the ESR peak height were performed for the control reaction mixture (Fig. 2). No ESR peak was observed at 0 h. The ESR peak height gradually increased and reached plateau at 168 h.
Fig. 2.
Time course of the ESR peak heights of control reaction mixture. The reaction and ESR condition were as described in materials and methods section except for reaction time. Reaction times were 0, 24, 72 and 168 h. The data represent the mean ± SD of independent three measurements. In the control reaction mixture, there were 50 mM phosphate buffer (pH 7.4), 0.1 M 4-POBN, 0.89 mM linoleic acid, 0.38 M acetonitrile and 20 µM FeCl3.
HPLC-ESR analyses
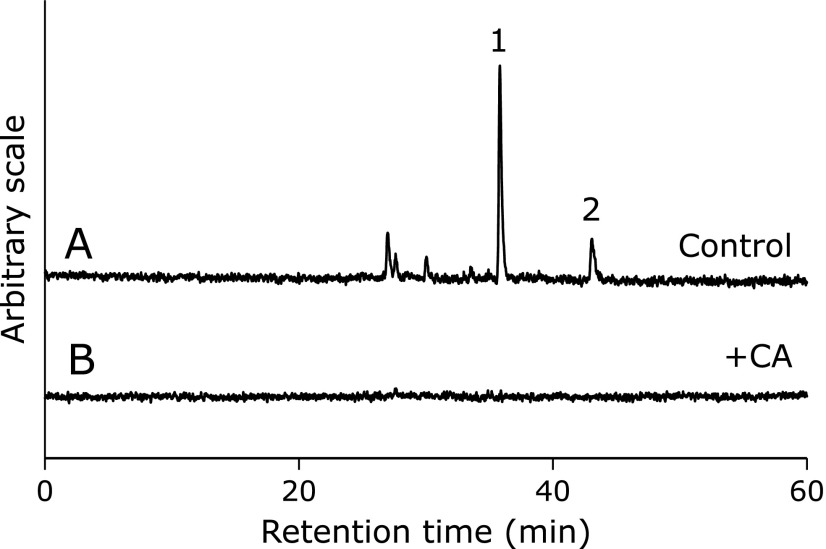
The HPLC-ESR analyses were performed for the control reaction mixture. On the HPLC-ESR elution profile of the control reaction mixture, two prominent peaks (peak 1 and peak 2) were observed at the retention times of 35.8 min (peak 1) and 43.1 min (peak 2) (Fig. 3A).
Fig. 3.
HPLC-ESR analyses. The reaction and HPLC-ESR conditions were as described in materials and methods section. In the control reaction mixture, there were 50 mM phosphate buffer (pH 7.4), 0.1 M 4-POBN, 0.89 mM linoleic acid, 0.38 M acetonitrile and 20 µM FeCl3. A, control reaction mixture. B, with 1 mM CA.
HPLC-ESR-MS analyses of peaks 1 and 2
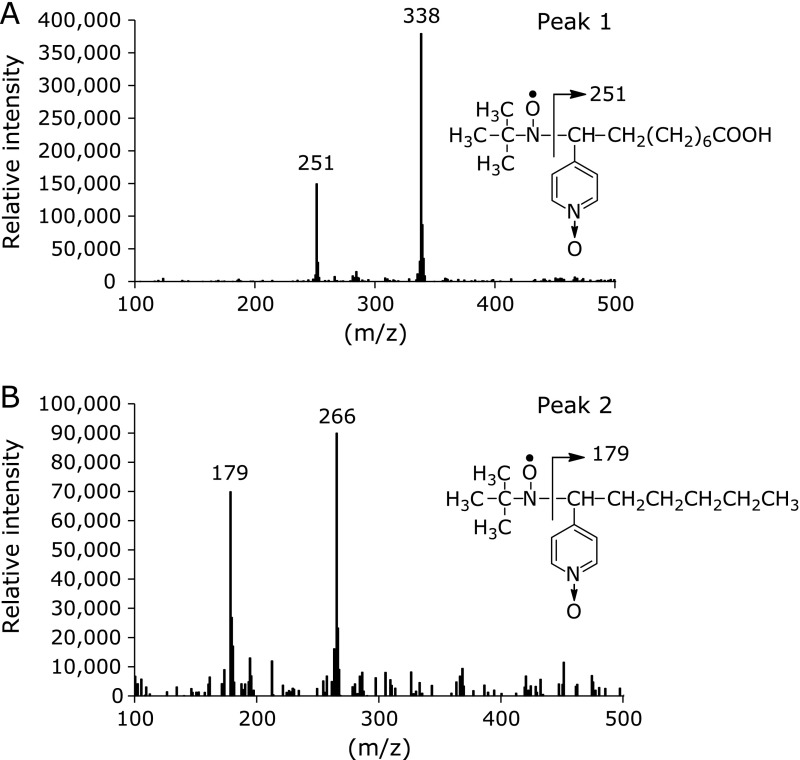
In order to find out what kinds of radicals were formed in the control reaction mixture, HPLC-ESR-MS analyses were performed for peaks 1 and 2. Ions at m/z 251 and m/z 338 were observed in HPLC-ESR-MS analysis of the peak 1 (Fig. 4A), suggesting that peak 1 compound was 4-POBN/7-carboxyheptyl radical adduct. The ion m/z 338 corresponds to the protonated molecular ion of the 4-POBN/7-carboxyheptyl radical adduct, [M + H]+. A fragment ion at m/z 251 corresponds to the loss of [(CH3) 3C(O)N] from the protonated molecular ion. HPLC-ESR-MS analysis of peak 2 gave ions at m/z 179 and m/z 266 (Fig. 4B), suggesting that peak 2 was 4-POBN/pentyl radical adduct. The ion m/z 266 corresponds to the protonated molecular ion of the 4-POBN/pentyl radical adduct, [M + H]+. A fragment ion at m/z 179 corresponds to the loss of [(CH3) 3C(O)N] from the protonated molecular ion.
Fig. 4.
HPLC-ESR-MS analyses of the peak 1 and peak 2. The reaction and HPLC-ESR-MS conditions were as described in materials and methods section. In the control reaction mixture, there were 50 mM phosphate buffer (pH 7.4), 0.1 M 4-POBN, 0.89 mM linoleic acid, 0.38 M acetonitrile and 20 µM FeCl3. A, peak 1. B, peak 2.
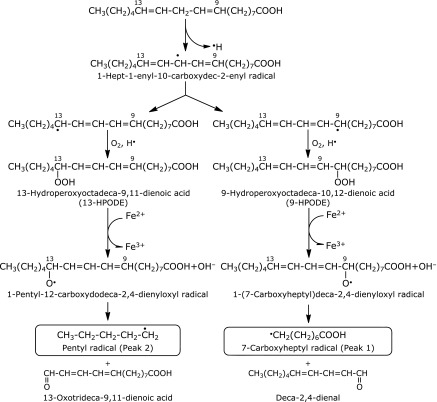
Our previous studies have also shown the formation of the 7-carboxyheptyl and pentyl radicals in the reaction mixture of linoleic acid with soya bean lipoxygenase and 13-hydroperoxyoctadeca-9,11-dienoic acid (13-HPODE) with ferrous ions (or cytochrome c or haematin).(8,24–26)
We proposed a scheme to account for the formation of the 7-carboxyheptyl radical and pentyl radical (Fig. 5). As the ESR signal decreased to 53 ± 3% (n = 3) of the control reaction mixture for the reaction mixture without iron (Fig. 1B), iron complexes appear to catalyze the formation of 13-hydroperoxyoctadeca-9,11-dienoic acid (13-HPODE) and 9-hydroperoxyoctadeca-10,12-dienoic acid (9-HPODE) through the hydrogen atom abstraction at 11 carbon. Iron complexes such as iron(IV)-oxo and iron(III)-superoxo may initiate the O2-activation chemistry by abstraction of an H atom from the substrate.(27,28) Product analysis and spin-trapping studies provided evidence for the formation of 1-pentyl-12-carboxydodeca-2,4-dienyloxyl radical and 1-(7-carboxyheptyl)deca-2,4-dienyloxyl radical through the reaction of 13-HPODE and 9-HPODE with ferrous ions.(29–31) The ferrous ions may form in the following equilibrium to a small extent.
Fig. 5.
A possible reaction path for the formation of 7-carboxyheptyl radical (peak 1) and pentyl radical (peak 2) in the control reaction mixture.
| Fe3+ ⇄ Fe2+ |
The β scissions of 1-pentyl-12-carboxydodeca-2,4-dienyloxyl and 1-(7-carboxyheptyl)deca-2,4-dienyloxyl radicals resulted in the formation of pentyl radical and 7-carboxyheptyl radical.(24–26,32–35) The pentyl radical could be a precursor of pentane.(35)
Effect of CA on the reaction
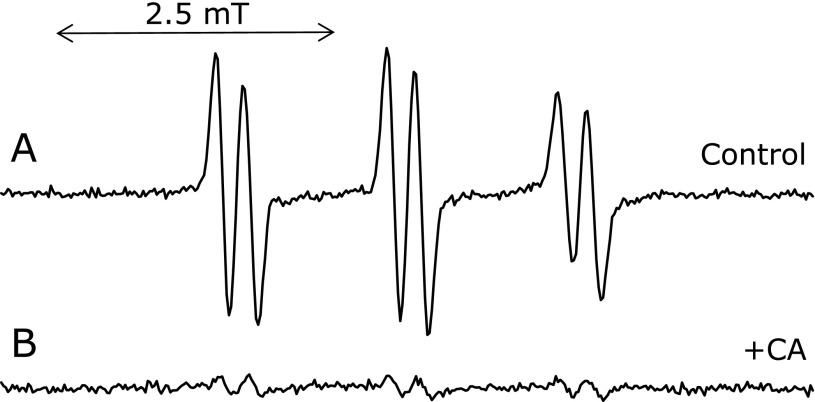
An ESR spectrum was measured for the control reaction mixture with 1.0 mM CA (Fig. 6B). The ESR peak height sharply decreased to 7.7 ± 2.4% (n = 3) of the control reaction mixture on addition of 1 mM CA.
Fig. 6.
Effect of CA on the ESR spectra of the control reaction mixture. The reaction and ESR conditions were as described in materials and methods section. In the control reaction mixture, there were 50 mM phosphate buffer (pH 7.4), 0.1 M 4-POBN, 0.89 mM linoleic acid, 0.38 M acetonitrile and 20 µM FeCl3. A, control reaction mixture. B, control reaction mixture with 1 mM CA.
In order to understand the effect of CA on respective radical formation, the control reaction mixture and control reaction mixture with 1.0 mM CA were analyzed using HPLC-ESR. On the HPLC-ESR elution profile of the control reaction mixture, two prominent peaks (peak 1 and peak 2) were observed at the retention times of 35.8 min (peak 1) and 43.1 min (peak 2) (Fig. 3A). The respective peaks disappeared when the control reaction mixture was added with 1 mM CA (Fig. 3B). Caffeic acid inhibited the formation of both radicals. Caffeic acid forms a chelate complex with iron ions.(6) Therefore, the polyphenols possibly inhibit the following three steps (Fig. 5), i.e., step 1, the reaction of linoleic acid with iron complexes such as iron(IV)-oxo and iron(III)-superoxo to form 1-hept-1-enyl-10-carboxydec-2-enyl radical,(27,28) step 2, the reaction between 13-HPODE and 1-pentyl-12-carboxydodeca-2,4-dienyloxyl radical, and step 3, the reaction between 9-HPODE and 1-(7-carboxyheptyl)deca-2,4-dienyloxyl radicals because iron ions participate in the three reactions. It has previously been shown that the step 2 is inhibited by the polyphenols.(8)
Effect of CA on the reaction in the presence of EDTA
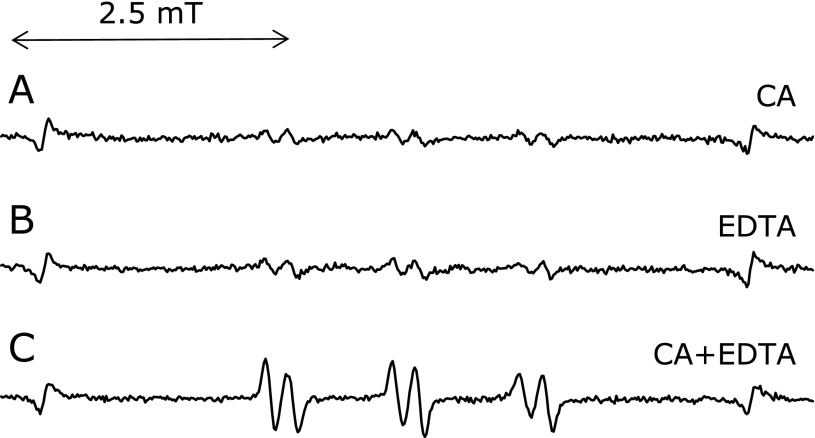
ESR spectra were measured for the control reaction mixture with 1.0 mM CA in the presence of 1 mM EDTA (Fig. 7). On adding 1 mM CA, the ESR peak height sharply increased to 254 ± 21% (n = 3) of the control reaction mixture in the presence of EDTA. Caffeic acid enhanced the generation of the radicals in the presence of 1 mM EDTA. It has been reported that caffeic acid enhanced hydroxyl radicals and t-butylhydroperoxide-derived radicals in the presence of EDTA.(6) The enhancement is possibly due to EDTA-ferric ion complexes being reduced by CA.
Fig. 7.
Effect of CA on an ESR spectra of the control reaction mixtures. The reaction and ESR conditions were as described in materials and methods section. In the control reaction mixture, there were 50 mM phosphate buffer (pH 7.4), 0.1 M 4-POBN, 0.89 mM linoleic acid, 0.38 M acetonitrile and 20 µM FeCl3. A, control reaction mixture with 1 mM CA. B, control reaction mixture with 1 mM EDTA. C, control reaction mixture with 1 mM CA and 1 mM EDTA.
Effect of several iron chelators on the reaction
In order to investigate the effects of several iron chelators on the radical formation, ESR spectra were measured for the control reaction mixture with 1 mM some iron chelators (Fig. 8). In the presence of the iron chelators, the peak heights of the ESR signals greatly decreased to 7.7 ± 2.4% (n = 3) (CA), 4.4 ± 0.0% (n = 3) (chlorogenic), 4.3 ± 0.0% (n = 3) (noradrenaline), 12.5 ± 10.9% (n = 3) (gallic acid), and 13.5 ± 1.7% (n = 3) (catechol) of the control reaction mixture, respectively. Meanwhile, the ESR peak heights decreased slightly to 38.1 ± 7.1% (n = 3) [(+)catechin], 47.9 ± 11.7% (n = 3) (epicatechin), 56.5 ± 1.6% (n = 3) (epigallocatechin). However, ESR peak heights were unchanged for the following compounds ; 110 ± 7.1% (n = 3) (quinic acid), 104 ± 4.3% (n = 3) (ferulic acid), 83.7 ± 7.8% (n = 3) (resorcinol) and 135 ± 39.0% (n = 3) (p-hydroquinone). The contribution of the direct radical-scavenging reaction of the phenolic compounds to the inhibitory effect seems to be small in these reactions. Of the compounds examined here, only compounds with catechol moiety, which were reported to be potent iron chelators,(6) showed the inhibitory effect. Furthermore, caffeic acid enhanced the generation of the radicals in the presence of 1 mM EDTA. Thus, the inhibitory effects seem to be due to the chelation of iron ions. Polyphenols with catechol moiety also exerted inhibitory effects on radical formations in the other reactions through the chelation of ion ions.(6,8) Since the three positional isomers of benzenediol, catechol, resorcinol and hydroquinone scavenged 1O2 to the same degree,(36) 1O2 does not seem to be involved in the radical formation.
Fig. 8.
ESR peak heights of the control reaction mixtures with various iron chelators. The ESR spectra were observed for control reaction mixtures with 1 mM CA (or quininc acid, or chlorogenic acid, or ferulic acid, or noradrenaline, or gallic acid, or (+)-catechin, or (–)-epicatechin, or epigallocatechin, or catechol, or resorcinol, or hydroquinone). The reaction and ESR conditions were as described in materials and methods section. In the control reaction mixture, there were 50 mM phosphate buffer (pH 7.4), 0.1 M 4-POBN, 0.89 mM linoleic acid, 0.38 M acetonitrile and 20 µM FeCl3. The data represent the mean ± SD of independent three measurements.
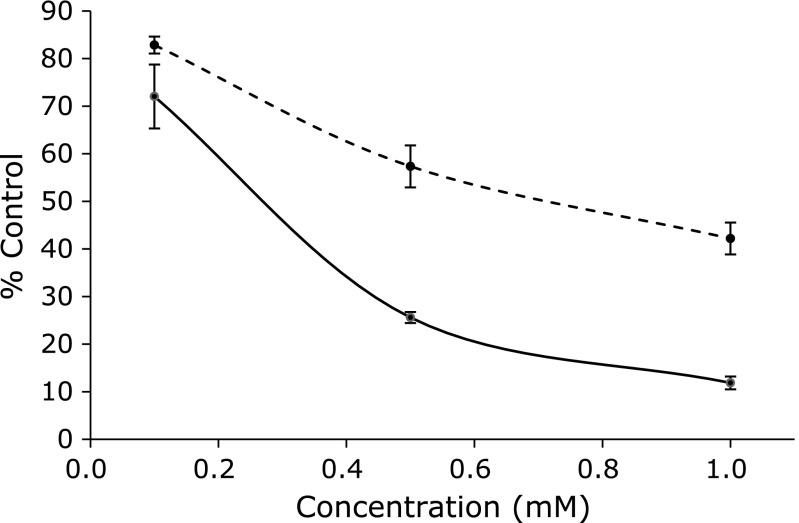
Caffeic acid, catechol, noradrenaline, chlorogenic, and gallic acid exert potent inhibitory effect on the formation of pentyl radical and 7-carboxyheptyl radical in the reaction of linoleic acid with iron ions. Of the compounds examined (Fig. 9), all these compounds, which exerted inhibitory effect on the formation of pentyl radical and 7-carboxyheptyl radical, have catechol moiety in the molecules. Interestingly, (+)-catechin, (–)-epicatechin, and epigallocatechin did not exert inhibitory effect on the formation of pentyl radical and 7-carboxyheptyl radical as much in spite of the catechol moiety in the molecules. That is also the case for the several different concentrations of (–)-epicatechin and CA (Fig. 10).
Fig. 9.
Chemical structures of compounds examined here.
Fig. 10.
Inhibitory effects by CA and (–)-epicatechin on the radical formation in the reaction of linoleic acid with iron ion. The ESR spectra were observed for control reaction mixture with various concentrations of CA [or (–)-epicatechin]. In the control reaction mixture, there were 50 mM phosphate buffer (pH 7.4), 0.1 M 4-POBN, 0.89 mM linoleic acid, 0.38 M acetonitrile and 20 µM FeCl3. The reaction and ESR conditions were as described in materials and methods section. CA, solid line. (–)-Epicatechin, broken line. The data represent the mean ± SD of independent three measurements.
Effects of (+)-catechin, (–)-epicatechin, and epigallocatechin on the reaction
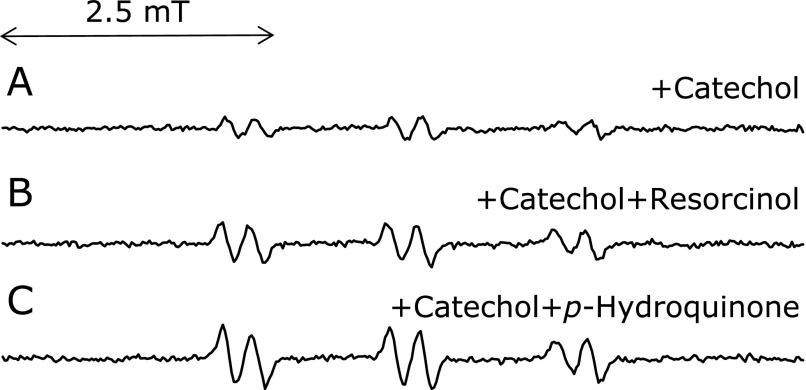
In clarifying why (+)-catechin, (–)-epicatechin, and epigallocatechin did not exert the inhibitory effect as much on the radical formation, resorcinol moiety effect on the radical formation was examined. Addition of resorcinol to the control reaction mixture with catechol resulted in minimal enhancement of the radical formation (150 ± 24% of the control with catechol) (n = 3) (Fig. 11B). That was also the case for the addition of p-hydroquinone instead of resorcinol (182 ± 57% of the control with catechol) (n = 3) (Fig. 11C). The enhancement is presumably due to ferric ions being reduced by p-hydroquinone or resorcinol.
Fig. 11.
Effects of resorcinol (or p-hydroquinone) on the ESR peak height of the control reaction mixture with 1 mM catechol. Effects of resorcinol (or p-hydroquinone) on the ESR peak height of the control reaction mixture with 1 mM catechol were examined. The reaction and ESR conditions were as described in materials and methods section. In the control reaction mixture, there were 50 mM phosphate buffer (pH 7.4), 0.1 M 4-POBN, 0.89 mM linoleic acid, 0.38 M acetonitrile and 20 µM FeCl3. A, control reaction mixture with 1 mM catechol. B, control reaction mixture with 1 mM catechol in the presence of 1 mM resorcinol. C, control reaction mixture with 1 mM catechol in the presence of 1 mM p-hydroquinone.
Stability of (–)-epicatechin was compared with CA. We determined the quantity of the remaining (–)-epicatechin (or CA) after 24 h incubation of control reaction mixture at 30°C. Caffeic acid and (–)-epicatechin decreased to 73 ± 9% (n = 3) (CA) and 62 ± 7% (n = 3) [(–)-epicatechin] of the initial concentration, respectively. (–)-Epicatechin appears to be a little unstable compared with CA.
Thus, (+)-catechin, (–)-epicatechin, and epigallocatechin cannot exert the inhibitory effect as much potentially because it has less stability and resorcinol moieties of the catechins.
Acknowledgments
We would like to thank Shenli Hew from the Department of Clinical Research Center, Wakayama Medical University, for proofreading and editing the manuscript.
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Boldt DH. New perspectives on iron: an introduction. Am J Med Sci. 1999;318:207–212. doi: 10.1097/00000441-199910000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Emerit J, Beaumont C, Trivin F. Iron metabolism, free radicals, and oxidative injury. Biomed Pharmacother. 2001;55:333–339. doi: 10.1016/s0753-3322(01)00068-3. [DOI] [PubMed] [Google Scholar]
- 3.Jomova K, Valko M. Advances in metal-induced oxidative stress and human disease. Toxicology. 2011;283:65–87. doi: 10.1016/j.tox.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Liu RH. Health-promoting components of fruits and vegetables in the diet. Adv Nutr. 2013;4:384S–392S. doi: 10.3945/an.112.003517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rostagno MA, Celeghini RMS, Debien ICN, Nogueira GC, Meireles MAA. Coffee in Health and Disease Prevention. London: Elsevier Inc.; 2015. Phenolic compounds in coffee compared to other beverages; pp. 137–142. [Google Scholar]
- 6.Iwahashi H, Ishii T, Sugata R, Kido R. The effects of caffeic acid and its related catechols on hydroxyl radical formation by 3-hydroxyanthranilic acid, ferric chloride, and hydrogen peroxide. Arch Biochem Biophys. 1990;276:242–247. doi: 10.1016/0003-9861(90)90033-u. [DOI] [PubMed] [Google Scholar]
- 7.Yoshino M, Murakami K. Interaction of iron with polyphenolic compounds: application to antioxidant characterization. Anal Biochem. 1998;257:40–44. doi: 10.1006/abio.1997.2522. [DOI] [PubMed] [Google Scholar]
- 8.Iwahashi H. Some polyphenols inhibit the formation of pentyl radical and octanoic acid radical in the reaction mixture of linoleic acid hydroperoxide with ferrous ions. Biochem J. 2000;346 (Pt 2):265–273. [PMC free article] [PubMed] [Google Scholar]
- 9.Kono Y, Kobayashi K, Tagawa S, et al. Antioxidant activity of polyphenolics in diets. Rate constants of reactions of chlorogenic acid and caffeic acid with reactive species of oxygen and nitrogen. Biochim Biophys Acta. 1997;1335:335–342. doi: 10.1016/s0304-4165(96)00151-1. [DOI] [PubMed] [Google Scholar]
- 10.Terao J, Karasawa H, Arai H, Nagao A, Suzuki T, Takama K. Peroxyl radical scavenging activity of caffeic acid and its related phenolic compounds in solution. Biosci Biotechnol Biochem. 1993;57:1204–1205. doi: 10.1271/bbb.57.1204. [DOI] [PubMed] [Google Scholar]
- 11.Liu J, Mori A. Monoamine metabolism provides an antioxidant defense in the brain against oxidant- and free radical-induced damage. Arch Biochem Biophys. 1993;302:118–127. doi: 10.1006/abbi.1993.1189. [DOI] [PubMed] [Google Scholar]
- 12.Baratto MC, Tattini M, Galardi C, et al. Antioxidant activity of galloyl quinic derivatives isolated from P. lentiscus leaves. Free Radic Res. 2003;37:405–412. doi: 10.1080/1071576031000068618. [DOI] [PubMed] [Google Scholar]
- 13.Guo Q, Zhao B, Li M, Shen S, Xin W. Studies on protective mechanisms of four components of green tea polyphenols against lipid peroxidation in synaptosomes. Biochim Biophys Acta. 1996;1304:210–222. doi: 10.1016/s0005-2760(96)00122-1. [DOI] [PubMed] [Google Scholar]
- 14.Ferrali M, Signorini C, Caciotti B, et al. Protection against oxidative damage of erythrocyte membrane by the flavonoid quercetin and its relation to iron chelating activity. FEBS Lett. 1997;416:123–129. doi: 10.1016/s0014-5793(97)01182-4. [DOI] [PubMed] [Google Scholar]
- 15.Zhong Z, Connor HD, Froh M, et al. Polyphenols from Camellia sinenesis prevent primary graft failure after transplantation of ethanol-induced fatty livers from rats. Free Radic Biol Med. 2004;36:1248–1258. doi: 10.1016/j.freeradbiomed.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 16.Renaud S, de Lorgeril M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet. 1992;339:1523–1526. doi: 10.1016/0140-6736(92)91277-f. [DOI] [PubMed] [Google Scholar]
- 17.Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. 1993;342:1007–1011. doi: 10.1016/0140-6736(93)92876-u. [DOI] [PubMed] [Google Scholar]
- 18.Middleton E Jr, Kandaswami C. Effects of flavonoids on immune and inflammatory cell functions. Biochem Pharmacol. 1992;43:1167–1179. doi: 10.1016/0006-2952(92)90489-6. [DOI] [PubMed] [Google Scholar]
- 19.Brown JP. A review of the genetic effects of naturally occurring flavonoids, anthraquinones and related compounds. Mutat Res. 1980;75:243–277. doi: 10.1016/0165-1110(80)90029-9. [DOI] [PubMed] [Google Scholar]
- 20.León-Carmona JR, Alvarez-Idaboy JR, Galano A. On the peroxyl scavenging activity of hydroxycinnamic acid derivatives: mechanisms, kinetics, and importance of the acid-base equilibrium. Phys Chem Chem Phys. 2012;14:12534–12543. doi: 10.1039/c2cp40651a. [DOI] [PubMed] [Google Scholar]
- 21.Anissi J, El Hassouni M, Ouardaoui A, Sendide K. A comparative study of the antioxidant scavenging activity of green tea, black tea and coffee extracts: a kinetic approach. Food Chem. 2014;150:438–447. doi: 10.1016/j.foodchem.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 22.Khokhar S, Apenten RKO. Iron binding characteristics of phenolic compounds: some tentative structure–activity relations. Food Chem. 2003;81:133–140. [Google Scholar]
- 23.Iwahashi H. High performance liquid chromatography/electron spin resonance/mass spectrometry analyses of lipid-derived radicals. Methods Mol Biol. 2008;477:65–73. doi: 10.1007/978-1-60327-517-0_6. [DOI] [PubMed] [Google Scholar]
- 24.Iwahashi H, Nishizaki K, Takagi I. Cytochrome c catalyses the formation of pentyl radical and octanoic acid radical from linoleic acid hydroperoxide. Biochem J. 2002;361 (Pt 1):57–66. doi: 10.1042/0264-6021:3610057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iwahashi H, Albro PW, McGown SR, Tomer KB, Mason RP. Isolation and identification of α-(4-pyridyl-1-oxide)-N-tert-butylnitrone radical adducts formed by the decomposition of the hydroperoxides of linoleic acid, linolenic acid, and arachidonic acid by soybean lipoxygenase. Arch Biochem Biophys. 1991;285:172–180. doi: 10.1016/0003-9861(91)90346-k. [DOI] [PubMed] [Google Scholar]
- 26.Iwahashi H, Deterding LJ, Parker CE, Mason RP, Tomer KB. Identification of radical adducts formed in the reactions of unsaturated fatty acids with soybean lipoxygenase using continuous flow fast atom bombardment with tandem mass spectrometry. Free Radic Res. 1996;25:255–274. doi: 10.3109/10715769609149051. [DOI] [PubMed] [Google Scholar]
- 27.Nam W, Lee YM, Fukuzumi S. Tuning reactivity and mechanism in oxidation reactions by mononuclear nonheme iron(IV)-oxo complexes. Acc Chem Res. 2014;47:1146–1154. doi: 10.1021/ar400258p. [DOI] [PubMed] [Google Scholar]
- 28.Lee YM, Hong S, Morimoto Y, Shin W, Fukuzumi S, Nam W. Dioxygen activation by a non-heme iron (II) complex: formation of an iron(IV)–oxo complex via C–H activation by a putative iron(III)–superoxo species. J Am Chem Soc. 2010;132:10668–10670. doi: 10.1021/ja103903c. [DOI] [PubMed] [Google Scholar]
- 29.Iwahashi H, Hirai T, Kumamoto K. High performance liquid chromatography/electron spin resonance/mass spectrometry analyses of radicals formed in an anaerobic reaction of 9- (or 13-) hydroperoxide octadecadienoic acids with ferrous ions. J Chromatogr A. 2006;1132:67–75. doi: 10.1016/j.chroma.2006.07.028. [DOI] [PubMed] [Google Scholar]
- 30.Dix TA, Marnett LJ. Conversion of linoleic acid hydroperoxide to hydroxy, keto, epoxyhydroxy, and trihydroxy fatty acids by hematin. J Biol Chem. 1985;260:5351–5357. [PubMed] [Google Scholar]
- 31.Schreiber J, Mason RP, Eling TE. Carbon-centered free radical intermediates in the hematin- and ram seminal vesicle-catalyzed decomposition of fatty acid hydroperoxides. Arch Biochem Biophys. 1986;251:17–24. doi: 10.1016/0003-9861(86)90046-9. [DOI] [PubMed] [Google Scholar]
- 32.Davies MJ, Slater TF. Studies on the metal-ion and lipoxygenase-catalysed breakdown of hydroperoxides using electron-spin-resonance spectroscopy. Biochem J. 1987;245:167–173. doi: 10.1042/bj2450167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chamulitrat W, Mason RP. Alkyl free radicals from the beta-scission of fatty acid alkoxyl radicals as detected by spin trapping in a lipoxygenase system. Arch Biochem Biophys. 1990;282:65–69. doi: 10.1016/0003-9861(90)90087-f. [DOI] [PubMed] [Google Scholar]
- 34.Iwahashi H, Parker CE, Mason RP, Tomer KB. Radical adducts of nitrosobenzene and 2-methyl-2-nitrosopropane with 12,13-epoxylinoleic acid radical, 12,13-epoxylinolenic acid radical and 14,15-epoxyarachidonic acid radical Identification by h.p.l.c.-e.p.r. and liquid chromatography-thermospray-m.s. Biochem J. 1991;276 (Pt 2):447–453. doi: 10.1042/bj2760447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garssen GJ, Vliegenthart JFG, Boldingh J. An anaerobic reaction between lipoxygenase, linoleic acid and its hydroperoxides. Biochem J. 1971;122:327–332. doi: 10.1042/bj1220327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Asano M, Iwahashi H. Caffeic acid inhibits the formation of 7-carboxyheptyl radicals from oleic acid under flavin mononucleotide photosensitization by scavenging singlet oxygen and quenching the excited state of flavin mononucleotide. Molecules. 2014;19:12486–12499. doi: 10.3390/molecules190812486. [DOI] [PMC free article] [PubMed] [Google Scholar]