Abstract
The present study aimed to investigate the occurrence and clinical features of cases of multiple primary cancers including colorectal cancer (MPCC). The medical records of patients with colorectal cancer (CRC) who underwent surgery at the Third Xiangya Hospital of Central South University (Changsha, China) between August 2007 and August 2014 were retrospectively analyzed. Patients with MPCCs were identified and mutation analyses were performed on colon specimens. The results revealed that among 1,311 patients with CRC, 59 had MPCC (including 35 cases of ≥1 CRC with ≥1 other cancer type, and 24 cases with multiple CRCs and no other primary cancers). Foci occurred on the right side of the colon (n=32), in the rectum (n=28), and on the left side of the colon (n=24). MPCCs were synchronous in 24 patients, metachronous in 32 patients, and both in 3 patients. Age of onset and presence of polyps were identified as significantly different between MPCC and CRC overall (P<0.05); however, sex or adenoma incidence were not observed to differ significantly between groups. Mutation incidence rates in 26 specimens were 11.54% for KRAS proto-oncogene GTPase (KRAS) G13D, 3.85% for KRAS Q61R and 3.85% B-Raf proto-oncogene serine/threonine kinase V600E. Mutations of exon 21 of the epithelial growth factor receptor gene, including L858R and L861Q, and of KRAS G12V were not detected. In conclusion, the likelihood of occurrence of MPCC is closely associated with the age of onset and the presence of polyp(s). Routine examination of multiple systems is necessary for patients with CRC to avoid missed diagnosis and misdiagnosis. Further study is required to demonstrate the molecular mechanism of CRC in cases of multiple primary cancers.
Keywords: colorectal cancer, multiple primary colorectal cancers, multiple colorectal cancers, synchronous cancer, metachronous cancer
Introduction
Colorectal cancer (CRC) is one of the most common malignant tumor types in humans. The annual incidence of CRC worldwide is >1,000,000 cases, which is greater than the incidence of all other cancer types, with the exception of lung and breast cancer (1). In 2011, there were 310,244 new cases of CRC in China, accounting for 9.2% of all new cases of cancer; additionally, there were 149,722 mortalities due to CRC in 2011, accounting for 7.09% of all cancer-associated mortalities (2). Evidence indicates that the incidence of CRC has increased each year between 1998 and 2007 and has continued to increase thereafter (3). It has long been recognized that the development of CRC is associated with the accumulation of certain genetic mutations, including components of the epidermal growth factor receptor (EGFR) signaling pathway (4). Evidence has also suggested that mutations in KRAS proto-oncogene GTPase (KRAS) and B-Raf proto-oncogene serine/threonine kinase (BRAF) are associated with a worse patient prognosis in CRC (5). Therefore, detection of EGFR, KRAS and BRAF mutations is important in studies of CRC.
The term ‘multiple primary cancers’ refers to cases in which independent primary malignant tumors occur simultaneously or successively in one or multiple organs in the same individual. Based on cancer registry records from between 1986 and 1995 at the Cancer Institute Hospital of The Japanese Foundation for Cancer Research (Tokyo, Japan), Ueno et al (6) reported that among 24,498 patients with tumors, 1,281 (5.2%) had multiple primary cancers, and out of 1,587 cases of CRC, there were 142 (8.9%) cases of multiple primary cancer. Therefore, increasing the understanding of multiple primary cancers and CRCs, and strengthening the screening of patients for whom CRC is suspected is expected to greatly reduce the rate of misdiagnosis and missed diagnosis, provide novel strategies for treatment and thus improve the prognosis for CRC patients.
The purpose of the present study was to investigate the pathogenesis of cases of multiple primary cancer that included ≥1 CRC (MPCC) and provide evidence to aid the prevention and treatment of CRC, including in cases of MPCC.
Subjects and methods
Patients
A total of 1,311 patients who received surgical treatment for CRC at the Third Xiangya Hospital of Central South University (Changsha, China) between August 2007 and August 2014 were included in this study. Patient data was obtained from medical records and is presented in Table I. All patients had signed informed consent forms and the study received approval from Xiangya Hospital Ethics Committee. A subgroup of these patients was confirmed by pathological and/or cytological examinations to have MPCC.
Table I.
Clinical epidemiological characteristics of 1,311 colorectal cancer patients following surgery.
| Variable | n | % |
|---|---|---|
| Sex | ||
| Male | 761 | 58.00 |
| Female | 550 | 42.00 |
| Age, years | ||
| ≤35 | 63 | 4.80 |
| 36–59 | 600 | 45.77 |
| ≥60 | 648 | 49.43 |
| Pathological type | ||
| Tubular adenocarcinoma | 1,143 | 86.98 |
| Mucinous adenocarcinoma | 89 | 6.77 |
| Signet-ring cell carcinoma | 6 | 0.46 |
| Other | 76 | 5.79 |
| Lesion site | ||
| Rectum | 669 | 50.11 |
| Left side of the colon | 278 | 20.83 |
| Right side of the colon | 388 | 29.06 |
Group inclusion and exclusion criteria
CRC group
Patients were required to have pathologically confirmed CRC and to previously have received surgical treatment at the Third Xiangya Hospital. Patients with metastatic or recurrent cancers, and those whose cancers were not pathologically confirmed, were excluded.
CRC with other cancer (CCOC) group
Patients were selected according to the modification of diagnostic criteria from Warren and Gates (7) in combination with clinical observations: i) The patient must have multiple primary cancers including ≥1 CRC and ≥1 primary cancer at another location; ii) each primary tumor must be pathologically confirmed to be malignant and have its own specific pathological morphology; iii) the possibility of the subsequent tumors having arisen from the first primary tumor via recurrence or metastasis must be excluded.
Multiple CRCs (MCC) group
Patients were selected according to the diagnostic criteria of Lee et al (8) in combination with clinical observations: i) The patient must have ≥2 primary cancers in the colon/rectum, with no primary tumors at other sites, and each tumor must be pathologically confirmed to be malignant; ii) cancer foci must be independent of each other, with a transitional zone composed of heterotypic cell glands between tumor foci and the normal intestinal wall; iii) the possibility of metastasis, recurrence and submucosal diffusion of the primary cancer must be excluded; iv) there must be no recurrence of previous surgical anastomotic stoma, and new foci must be clearly distinct from previous surgical incisions; v) patients must not have been diagnosed with familial adenoma or enteropathic arthritis-induced cancer.
DNA isolation and mutation assessment
Hematoxylin and eosin staining was implemented to confirm that specimens contained >80% cancer cells, and areas enriched with malignant cells were identified prior to DNA extraction by two independent pathologists. DNA was extracted from formalin-fixed, paraffin-embedded tissue specimens using a QIAamp DNA FFPE Tissue Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions and stored at −80°C until use.
Mutation hotspots in EGFR (L858R and L861Q), KRAS (G12V, G13D and Q61R) and BRAF (V600E) were detected. Polymerase chain reaction (PCR) amplification was performed as follows: 1 min of initial denaturation at 95°C; 35 cycles of amplification consisting of 30 sec at 94°C, 40 sec at 57°C and 30 sec at 72°C, with a final additional elongation at 72°C for 7 min. Used primers are as follows: EGFR L858R/L861Q forward primer, 5′-CCAGGAACGTACTGGTGAAA-3′; EGFR L858R/L861Q reverse primer, 5′-TGACCTAAAGCCACCTCCTT-3′; KRAS12/13 forward primer, 5′-TATAAGGCCTGCTGAAAATGACTG-3′; KRAS12/13 reverse primer, 5′-TATTCGTCCACAAAATGATTCTGA-3′; KRAS61 forward primer, 5′-AATTGATGGAGAAACCTGTCTCTT-3′; KRAS61 reverse primer, 5′-TCCTCATGTACTGGTCCCTCATT-3′; BRAF V600E forward primer, 5′-CTTCATAATGCTTGCTCTGATAGG-3′; BRAF V600E reverse primer, 5′-GCATCTCAGGGCCAAAAATT-3′. Non-template control was included in each batch during the experiment, and sequencing was performed using ABI 3500xL Genetic Analyzer (Applied Biosystems, Carlsbad, CA, USA).
Statistical analysis
The medical records of the overall population of patients with CRC and the subset of patients with MPCC were analyzed using SPSS 13.0 software (SPSS Inc., Chicago, IL, USA). Numerical data were analyzed with the χ2 test. Measurement data are presented as the mean ± standard deviation. The two-sample t-test was used for comparisons between groups. P<0.05 was considered to indicate a statistically significant difference.
Results
Patient selection
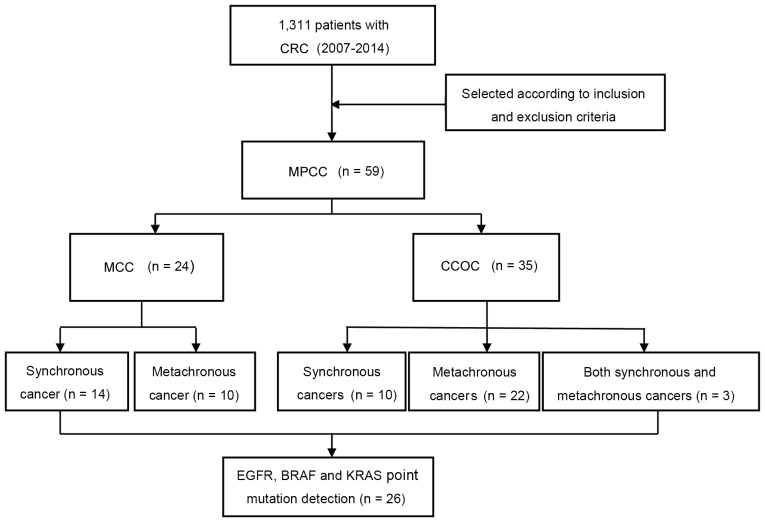
A flow diagram illustrating the selection of patients in the study is presented in Fig. 1. A total of 1,311 patients underwent surgery for CRC during the study period. Of these, 59 patients had MPCC (including 35 with CCOC and 24 with MCC).
Figure 1.
Flowchart of patient selection. CRC, colorectal cancer; MPCC, multiple primary cancers including CRC; MCC, multiple CRCs; CCOC, CRC and other primary cancer; EGFR, epidermal growth factor receptor; BRAF, B-Raf proto-oncogene serine/threonine kinase; KRAS, KRAS proto-oncogene GTPase.
Clinical epidemiological characteristics of CRC patients following surgery
The clinical epidemiological characteristics of the 1,311 patients who underwent CRC surgery are presented in Table I. The overall patient population consisted of 761 males and 550 females (male:female ratio, 1.38:1), with a mean age of 58.36±13.07 years (range, 20–91 years).
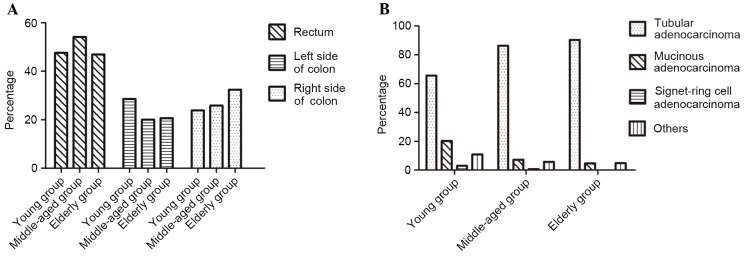
Patients were divided into three age groups: Young (≤35 years), middle-aged (36–59 years), and elderly (≥60 years). In the young group, 47.62% of patients had rectal cancer, 23.81% had cancer of the right side of the colon, and 28.57% had cancer of the left side of the colon. In the middle-aged group, 54.17% of patients had rectal cancer, 20.00% had cancer of the left side of the colon, and 25.83% had cancer of the right side of the colon. In the elderly group, 46.91% of patients had rectal cancer, 20.68% had cancer of the left side of the colon, and 32.41% had cancer of the right side of the colon.
Among the total study population, 1,143 (86.98%) patients had tubular adenocarcinoma, 89 (6.77%) had mucinous adenocarcinoma, 6 (0.46%) had signet-ring cell cancer, and 76 (5.79%) had undifferentiated carcinoma or other cancer types. For cases in which the tumors were of the same histological type, the highest-level pathological diagnosis was included in the statistics. Of the total 1,311 patients, 3 patients had different pathological types between two CRC foci and therefore were retrospectively included in the statistical analysis. A significant difference in pathological type between age groups was identified (P<0.05; Fig. 2).
Figure 2.
Characteristics of lesions in CRC patients of different ages. (A) Constituent ratios of lesion locations in CRC patients of different ages. (B) Pathological types of CRC in patients of different ages.
Comparisons of age of onset, sex and pathological types between patients with MPCC and CRC
There was a significant difference in the age of onset between the 59 patients with MPCC and the 1,311 patients with CRC (P<0.05); however, no significant difference was observed in sex between them (P>0.05). Among the 1,311 patients with CRC, 318 (24.265%) had adenoma and 446 (34.02%) had polyp(s). By comparison, 19 (30.20%) of the 59 patients with MPCC had adenoma and 33 (55.93%) had polyps. There was no significant difference between the CRC and MPCC cohorts with regard to the proportion of patients with adenoma (P=0.166); however, a significant difference was observed in the proportions of patients with polyp(s) (P=0.001; Table II).
Table II.
Comparison of 1,311 patients with CRC and 59 patients with MPCC.
| Variable | MPCC | All CRC | P-value |
|---|---|---|---|
| Total, n | 59 | 1,311 | – |
| Age, years | 0.035 | ||
| Mean ± SD | 62.11±11.74 | 58.36±13.07 | |
| Range | 40–81 | 20–91 | |
| Sex | 0.747 | ||
| Male | 33 | 761 | |
| Female | 26 | 550 | |
| Complication | |||
| Adenoma | 19 | 318 | 0.166 |
| Polyp(s) | 33 | 446 | 0.001 |
CRC, colorectal cancer; MPCC, multiple primary cancers including CRC; SD, standard deviation.
Occurrence and clinical features of patients with MPCC
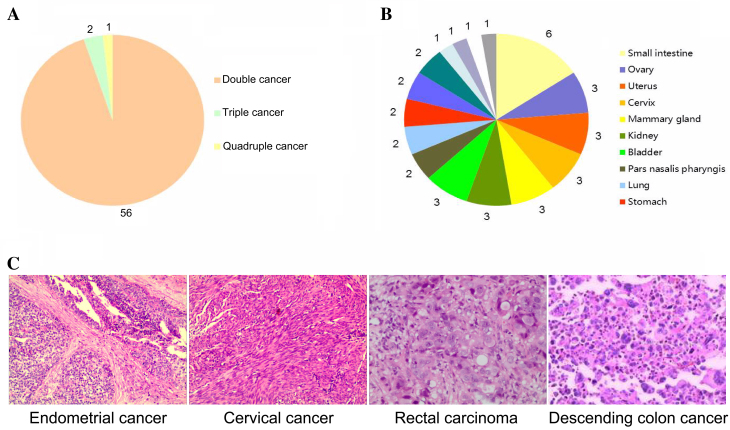
According to the inclusion criteria, 59 (4.50%) patients, consisting of 33 (55.93%) males and 26 (44.07%) females with a mean age of 62.11±11.74 years (range, 40–81 years) had MPCC. Among these patients, 56 patients (94.92%) had double cancer, 2 (3.39%) had triple cancer, and 1 (1.69%) had quadruple cancer (Fig. 3A).
Figure 3.
Occurrence and clinical characteristics of 59 cases of multiple primary cancers with CRC. (A) Numbers of double, triple and quadruple cancer cases; values indicate absolute numbers of cases. (B) Locations of 38 extracolonic primary malignant lesions in 35 patients with CRC and ≥1 other type of primary cancer; values indicate absolute numbers of lesions. (C) Histological images (hematoxylin and eosin staining; magnification, ×100) from one patient with quadruple cancers, synchronous and metachronous, including two CRCs. CRC, colorectal cancer.
There were 122 primary cancer foci among the 59 patients with MPCC
These foci were located in the large intestine [n=84; including the rectum (n=28), left side of the colon (n=24), and right side of the colon (n=32)], small intestine (n=6), ovary (n=3), uterus (n=3), cervix (n=3), mammary gland (n=3), kidney (n=3), bladder (n=3), nasopharynx (n=2), lung (n=2), stomach (n=2), ureter (n=2), prostate (n=2), esophagus (n=1), thyroid gland (n=1), liver (n=1) and the left iliac fossa (n=1; Fig. 3B).
As well as the 59 pathologically confirmed primary CRC foci (i.e. 1 per patient) included in the cohort, the 63 additional primary cancer foci were located in the digestive system [n=35; including the large intestine (n=25), small intestine (n=6), stomach (n=2), esophagus (n=1), and liver (n=1)], reproductive system [n=14; including the mammary gland (n=3), ovary (n=3), uterus (n=3), cervix (n=3), and prostate (n=2)], urinary system [n=8; including the kidney (n=3), bladder (n=3), and ureter (n=2)], respiratory system [n=4; including the nasopharynx (n=2), and lung (n=2)], endocrine system (n=1) and hematological system (n=1).
Of the 59 patients with MPCC, 24 (1.83% of total CRC cohort) patients had synchronous cancer, 32 (2.44% of total CRC cohort) patients had metachronous cancer, and 3 (0.23% of total CRC cohort) patients had both. There was 1 patient with quadruple cancer, including both synchronous and metachronous cancers, comprising 2 CRCs, 1 cervical cancer and 1 endometrial cancer (Fig. 3C).
Among the 24 patients with synchronous cancer, 18 patients (75.00%) were diagnosed with a synchronous cancer simultaneously with the diagnosis of CRC (before or during the CRC surgery), and 2 patients were diagnosed with the second cancer ~1 month after surgery. A total of 6 patients (25.00%) in the synchronous cancer group were misdiagnosed.
Among the 32 patients with metachronous cancer, the mean disease onset interval (between two cancer diagnoses) was 49.25 months (range, 7 months to 20 years). For the 10 patients with metachronous cancers in the MCC group, the mean disease onset interval was 23.80 months (range, 7 months to 6 years). In the 22 patients with metachronous cancers in the CCOC group, the mean disease onset interval was 60.86 months (range, 7 months to 20 years; Table III).
Table III.
Intervals between onset of each cancer in 32 patients with metachronous multiple primary cancers including CRC.
| Disease onset interval, years | |||||||
|---|---|---|---|---|---|---|---|
| Group | Total, n | <1 | ≥1-<3 | ≥3-<5 | ≥5-<10 | ≥10 | Mean interval, months |
| MCC | 10 | 5 (50) | 4 (40) | 0 (0) | 1 (10) | 0 (0) | 23.80 |
| CCOC | 22 | 5 (23) | 6 (27) | 4 (18) | 4 (18) | 3 (14) | 60.86 |
| Total | 32 | 10 (31) | 10 (31) | 4 (13) | 5 (16) | 3 (9) | 49.25 |
Data are presented as number of patients (%). CRC, colorectal cancer; CCOC, CRC and other primary cancer; MCC, multiple CRCs and no other primary cancer types.
Detection of point mutations
The common CRC-associated point mutation loci of the EGFR, KRAS and BRAF genes were analyzed in available specimens from 26 patients with MPCC. Mutations of KRAS G12V and in exon 21 of the EGFR gene (including L858R and L861Q) were not detected. The incidence rates were 11.54% for KRAS G13D, 3.85% for KRAS Q61R and 3.85% for BRAF V600E (Table IV).
Table IV.
Point mutations detected among 26 specimens from patients with multiple primary cancers including a colorectal cancer.
| Incidence of mutation | ||
|---|---|---|
| Gene and mutation locus | n | % |
| EGFR L858R | 0 | 0.00 |
| EGFR L861Q | 0 | 0.00 |
| KRAS G12V | 0 | 0.00 |
| KRAS G13D | 3 | 11.54 |
| KRAS Q61R | 1 | 3.85 |
| BRAF V600E | 1 | 3.85 |
EGFR, epidermal growth factor receptor; KRAS, KRAS protooncogene GTPase; BRAF, B-Raf proto-oncogene, serine/threonine kinase.
Discussion
The incidence of CRC has increased in recent years, and rectal cancers account for a larger proportion than colon cancers (9). In the present study, the lesions in middle-aged patients predominantly occurred in the rectum, whereas, in elderly patients, lesions most commonly occurred in the colon; cancer of the right side of the colon was more common than the left side in elderly patients. These results are consistent with previous reports (10,11). Adenocarcinoma is the predominant type of CRC; with age, patients possess an increasing risk of adenocarcinoma overall, whereas the risk of signet-ring cell carcinoma or mucinous adenocarcinoma decreases (12).
There is evidence that 17–19% of patients with new tumors have previously experienced malignant tumors (13,14). Multiple cancers of the head and neck are among the most extensively studied of recent years (15,16). A small number of studies have reported on multiple CRCs and other multiple primary cancers. The results of the present study indicate that the incidence of MPCC among CRC cases is 4.50%, which is consistent with the incidence reported in a previous series of studies (6,17–19). There was a significant difference in the age of onset between the 59 patients with MPCC and the 1,311 patients with CRC (P<0.05). A significant difference in the number of cases complicated by polyp(s) was identified between the 59 patients with MPCC and the overall population of 1,311 patients with CRC (P<0.05), indicating that the occurrence of MPCC is associated with the presence of polyp(s) (20,21).
Among the 59 patients with MPCC, the proportion of males (n=33; 55.93%) was higher than that of females (n=26; 44.07%), which is similar to the incidence rates of single primary CRC in males and females (22). Among the patients with MPCC, the proportion with metachronous cancers was greater than the proportion with synchronous cancers. Among patients with MPCC, double cancer was the most common type, and triple or greater multiples of cancers were rarely identified. Metachronous cancers predominantly occurred within 1–3 years, followed by within 5 years, after the diagnosis of CRC. In addition to the 59 primary foci in the large intestine, the other primary foci were mainly located in the digestive system, reproductive system, urinary system and respiratory system. This is consistent with the field cancerization theory (23); specifically, as the esophagus and gastrointestinal tract are the passage through which food passes, the lungs are the air channels, and the urinary system is the fluid waste channel, all of these organs are particularly exposed to carcinogens in food or air.
It is well known that the occurrence of cancer is closely associated with the accumulation of gene mutations, and patients with incipient cancer thus possess a high risk for secondary cancer (24,25). In this study, common point mutation loci of the EGFR, KRAS and BRAF genes in 26 specimens from patients with multiple cancers were analyzed. The rates of incidence were 11.54% for KRAS G13D and 3.85% for KRAS Q61R mutations, which were evidently lower than previously reported rates of 30–60% (5,26,27). The incidence rate of BRAF V600E mutation was 3.85%, which was also lower than previously reported (12.44%) (26). There is evidence that survival following surgery in patients who developed metachronous MPCCs tended to be better than that of patients with a single carcinoma (6). This suggests that the mutation profile of MPCC is likely to be associated with its prognosis. This hypothesis will be investigated further in a larger study by this group.
The findings of the present study suggest that, for patients with CRC, detailed medical records, physical examination and associated auxiliary examinations are required, and pathological examination of living tissue is necessary to determine whether a new foci is a primary cancer, metastatic cancer or recurrent cancer. Thus, treatment strategies can be developed with the purpose of lengthening the patient's survival time and improving their quality of life. Studies in patients with MPCC aid the development of novel strategies for investigating the occurrence and underlying mechanism of the development of this disease, and identifying molecular targets for its treatment.
Acknowledgements
This study was supported by National Natural Science Foundation of China (grant nos. 81301688, 81272192, 81572965); PhD Programs Foundation of Ministry of Education of China (grant nos. 20130162110050 and 20130162120093); Natural Science Foundation of Hunan Province (grant no. 2015JJ4053); Technology Project of Hunan Province (grant no. 2012SK3229); Post-doctoral Foundation of Central South University (grant no. 131425); 125 Talent Project/New Xiangya Project of the Third Xiangya Hospital of Central South University; the Fundamental Research Funds for the Central Universities of Central South University (grant no. 2016zzts561).
Glossary
Abbreviations
- CRC
colorectal cancer
- MPCC
multiple primary cancers including ≥1 colorectal cancer
- CCOC
colorectal cancer with other primary cancer type
- MCC
multiple CRCs and no other primary cancer types
- EGFR
epidermal growth factor receptor
- KRAS
KRAS proto-oncogene GTPase
- BRAF
B-Raf proto-oncogene serine/threonine kinase
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Zeng H, Zhang S, He J. Annual report on status of cancer in China, 2011. Chin J Cancer Res. 2015;27:2–12. doi: 10.1186/s40880-015-0001-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dai Z, Zheng RS, Zou XN, Zhang SW, Zeng HM, Li N, Chen WQ. Analysis and prediction of colorectal cancer incidence trend in China. Zhonghua Yu Fang Yi Xue Za Zh. 2012;46:598–603. (In Chinese) [PubMed] [Google Scholar]
- 4.Shen Y, Han X, Wang J, Wang S, Yang H, Lu SH, Shi Y. Prognostic impact of mutation profiling in patients with stage II and III colon cancer. Sci Rep. 2016;6:24310. doi: 10.1038/srep24310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kriegsmann M, Arens N, Endris V, Weichert W, Kriegsmann J. Detection of KRAS NRAS and BRAF by mass spectrometry-a sensitive, reliable, fast and cost-effective technique. Diagn Pathol. 2015;10:132. doi: 10.1186/s13000-015-0364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ueno M, Muto T, Oya M, Ota H, Azekura K, Yamaguchi T. Multiple primary cancer: An experience at the Cancer Institute Hospital with special reference to colorectal cancer. Int J Clin Oncol. 2003;8:162–167. doi: 10.1007/s10147-003-0322-z. [DOI] [PubMed] [Google Scholar]
- 7.Warren S, Gates O. Carcinoma of ceruminous gland. Am J Pathol. 1941;17:821–826.3. [PMC free article] [PubMed] [Google Scholar]
- 8.Lee TK, Barringer M, Myers RT, Sterchi JM. Multiple primary carcinomas of the colon and associated extracolonic primary malignant tumors. Ann Surg. 1982;195:501–507. doi: 10.1097/00000658-198204000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li M, Gu J. Changing patterns of colorectal cancer in China over a period of 20 years. World J Gastroenterol. 2005;11:4685–4688. doi: 10.3748/wjg.v11.i30.4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCashland TM, Brand R, Lyden E, de Garmo P. CORI Research Project: Gender differences in colorectal polyps and tumors. Am J Gastroenterol. 2001;96:882–886. doi: 10.1111/j.1572-0241.2001.03638.x. [DOI] [PubMed] [Google Scholar]
- 11.Lacopetta B. Are there two sides to colorectal cancer? Int J Cancer. 2002;101:403–408. doi: 10.1002/ijc.10635. [DOI] [PubMed] [Google Scholar]
- 12.Stewart SL, Wike JM, Kato I, Lewis DR, Michaud F. A population-based study of colorectal cancer histology in the United States, 1998–2001. Cancer. 2006;107(5 Suppl):S1128–S1141. doi: 10.1002/cncr.22010. [DOI] [PubMed] [Google Scholar]
- 13.Morton LM, Swerdlow AJ, Schaapveld M, Ramadan S, Hodgson DC, Radford J, van Leeuwen FE. Current knowledge and future research directions in treatment-related second primary malignancies. EJC Suppl. 2014;12:S5–S17. doi: 10.1016/j.ejcsup.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morton LM, Onel K, Curtis RE, Hungate EA, Armstrong GT. The rising incidence of second cancers: Patterns of occurrence and identification of risk factors for children and adults. Am Soc Clin Oncol Educ Book. 2014:e57–e67. doi: 10.14694/EdBook_AM.2014.34.e57. [DOI] [PubMed] [Google Scholar]
- 15.da Silva SD, Morand GB, Alobaid FA, Hier MP, Mlynarek AM, Alaoui-Jamali MA, Kowalski LP. Epithelial-mesenchymal transition (EMT) markers have prognostic impact in multiple primary oral squamous cell carcinoma. Clin Exp Metastasis. 2015;32:55–63. doi: 10.1007/s10585-014-9690-1. [DOI] [PubMed] [Google Scholar]
- 16.Jung YS, Lim J, Jung KW, Ryu J, Won YJ. Metachronous second primary malignancies after head and neck cancer in a Korean cohort (1993–2010) PLoS One. 2015;10:e0134160. doi: 10.1371/journal.pone.0134160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen HS, Sheen-Chen SM. Synchronous and ‘early’ metachronous colorectal adenocarcinoma: Analysis of prognosis and current trends. Dis Colon Rectum. 2000;43:1093–1099. doi: 10.1007/BF02236556. [DOI] [PubMed] [Google Scholar]
- 18.Takeuchi H, Toda T, Nagasaki S, Kawano T, Minamisono Y, Maehara Y, Sugimachi K. Synchronous multiple colorectal adenocarcinomas. J Surg Oncol. 1997;64:304–307. doi: 10.1002/(SICI)1096-9098(199704)64:4<304::AID-JSO10>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 19.Bertolaccini L, Olivero G. Hereditary non polyposis colorectal cancer (HNPCC). A clinical and genetic entity. Minerva Chir. 2002;57:63–72. [PubMed] [Google Scholar]
- 20.Hodadoostan MK, Reza F, Elham M, Alizade AH Mohammad, Molaie M, Mashaiekhy R, Doagoo SZ, Moosavy M, Malek FN, Zali MR. Clinical and pathology characteristics of colorectal polyps in Iranian population. Asian Pac J Cancer Prev. 2010;11:557–560. [PubMed] [Google Scholar]
- 21.Yoshida D, Kono S, Moore MA, Toyomura K, Nagano J, Mizoue T, Mibu R, Tanaka M, Kakeji Y, Maehara Y, et al. Colorectal polypectomy and risk of colorectal cancer by subsite: The Fukuoka colorectal cancer study. Jpn J Clin Oncol. 2007;37:597–602. doi: 10.1093/jjco/hym065. [DOI] [PubMed] [Google Scholar]
- 22.Papadopoulos V, Michalopoulos A, Baanis G, Papapolychroniadis K, Paramythiotis D, Fotiadis P, Berovalis P, Harlaftis N. Synchronous and metachronous colorectal carcinoma. Tech Coloproctol. 2004;8(Suppl 1):s97–s100. doi: 10.1007/s10151-004-0124-y. [DOI] [PubMed] [Google Scholar]
- 23.Brown SR, Finan PJ, Hall NR, Bishop DT. Incidence of DNA replication errors in patients with multiple primary cancers. Dis Colon Rectum. 1998;41:765–769. doi: 10.1007/BF02236266. [DOI] [PubMed] [Google Scholar]
- 24.Wood LD, Parsons DW, Jones S, Lin J, Sjöblom T, Leary RJ, Shen D, Boca SM, Barber T, Ptak J, et al. The genomic landscapes of human breast and colorectal cancers. Science. 2007;318:1108–1113. doi: 10.1126/science.1145720. [DOI] [PubMed] [Google Scholar]
- 25.Liang YH, Shao YY, Chen HM, Lai CL, Lin ZZ, Kuo RN, Cheng AL, Yeh KH, Lai MS. Young patients with colorectal cancer have increased risk of second primary cancers. Jpn J Clin Oncol. 2015;45:1029–1035. doi: 10.1093/jjco/hyv137. [DOI] [PubMed] [Google Scholar]
- 26.Negru S, Papadopoulou E, Apessos A, Stanculeanu DL, Ciuleanu E, Volovat C, Croitoru A, Kakolyris S, Aravantinos G, Ziras N, et al. KRAS, NRAS and BRAF mutations in Greek and Romanian patients with colorectal cancer: A cohort study. BMJ Open. 2014;4:e004652. doi: 10.1136/bmjopen-2013-004652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haley L, Tseng LH, Zheng G, Dudley J, Anderson DA, Azad NS, Gocke CD, Eshleman JR, Lin MT. Performance characteristics of next-generation sequencing in clinical mutation detection of colorectal cancers. Mod Pathol. 2015;28:1390–1399. doi: 10.1038/modpathol.2015.86. [DOI] [PMC free article] [PubMed] [Google Scholar]