Abstract
Colorectal cancer (CRC) is an aggressive disease in which patients usually die due to its metastatic progression to the liver. Up to date, irinotecan is one of the most used chemotherapeutic agents to treat CRC metastasis with demonstrated efficacy. However, the severity of the side effects constitute the main limitation to its use in the treatment. Consequently, new complementary therapies are being developed to avoid these adverse effects while maintaining the efficacy of the antitumoral drugs. Ocoxin oral solution (OOS®) is a nutritional mixture containing biologically active compounds with demonstrated antitumoral and immunomodulatory effects. Thus, we aimed to analyze the effect of OOS® as a suitable complement to irinotecan therapy in the treatment of CRC metastasis to the liver. First, the effect of OOS®, irinotecan and the combination of both on the viability of C26 cells was tested in vitro and in vivo. Second, the expression of caspase-3, Ki67 and the macrophage infiltration by F4/80 marker was quantified in liver tissue sections by immunohistochemistry. Finally, mRNA microarray study was carried out on tumor cells isolated from tumor-bearing livers collected from mice subjected to the above treatments. Our results show that OOS® administered as a complementary therapy to irinotecan reduced tumor cell viability in vitro. Moreover, irinotecan administered either alone or in combination with 100 µl OOS® from the 7th day after tumor cell inoculation decreased the metastatic growth in the liver. Besides, several genes with binding and catalytic activities showed to be deregulated by RNA microarray analysis. In conclusion, OOS®, when administered as a complement to irinotecan, reduced the metastatic development of colorectal cancer to the liver. Additionally, the overall health state of the animals improved. These results point out OOS® as a potential supplement to the anti-tumoral treatments used in clinical settings in patients suffering from disseminated colorectal cancer.
Keywords: ocoxin oral solution, nutrient mixture, colorectal cancer, liver, metastasis
Introduction
Colorectal cancer (CRC) is one of the leading causes of cancer deaths in the world. However, those patients usually die to the metastatic progression, mainly to the liver, of the primary tumor. Among the various therapies used in the clinical setting to treat metastatic CRC, irinotecan is one of the most widely utilized as a chemotherapeutic agent for the treatment of this disease (1). Irinotecan is a camptothecin derivative that exerts its antitumor activity by breaking single strands of DNA inhibiting its re-ligation and, thus, blocking the DNA synthesis. As a result, cell cycle is arrested and tumor cells go into apoptosis. Irinotecan has widely demonstrated its efficacy improving the survival time of metastatic CRC patients.
Nevertheless, irinotecan exerts its effects on the tumor microenvironment as a whole, affecting not only to tumor cells but also to resident and recruited host cells. Indeed, irinotecan might also affect the recruitment of inflammatory cells including Kupffer cells, the resident macrophage population of the liver, which coordinate inflammatory networks by secreting multiple cytokines and growth factors, thereby promoting tumor cell adhesion, migration and finally, the metastatic progression (2). Additionally, the action of irinotecan on the stromal compartment might be responsible, at least in part, for the undesired side effects.
In order to diminish the adverse effects of irinotecan, different combinations of anticancer drugs or compounds with irinotecan are currently being tested (1). Several studies have shown that the use of biologically active compounds in combination with anti-tumoral drugs not only improve their efficacy, but also decrease their side effects. Gol'dberg et al (2008) described that glycyrrhizic acid extracted from licorice root, which has as an anti-inflammatory and immunomodulatory substance, improves the efficacy of cytostatic therapies such as cyclophosphamide that inhibits the growth and development of metastasis in lung tumor (3). Also, the slowdown of the disease progression has been observed after the addition of certain vitamins to the diet of patients undergoing chemotherapy (4). In fact, some authors have described that melatonin and vitamin C and E decrease the extent of DNA lesions on human lymphocytes and gastric mucosa after infection with Helicobacter pylori (4,5).
In the present study, we utilized the nutritional supplement OOS® which contains green tea, licorice extract vitamins, minerals and aminoacids. This compound has probed to possess antitumoral and immunomodulatory effects (6,7) and to potentiate the antiproliferative effect of standard chemotherapeutic agents in acute myeloid leukemia (8). Márquez et al (2016) showed that this nutrient mixture slows down the metastatic progression of CRC to the liver in an experimental model of metastatic development to the liver (7). Thus, OOS® might be a suitable complement to tumor therapies, such as irinotecan, in the treatment of disseminated CRC. Hence, the present study aims to evaluate the benefits of OOS® as a complement to irinotecan therapy in order to improve the overall status of metastatic CRC patients by reducing the side effects, and thereby, improving their quality of life.
Materials and methods
Animals
Balb/c mice (male, 8-weeks old) were obtained from Janvier Labs (Paris, France). The animals were kept in the animal facility of EHU/UPV and had access to standard chow and water ad libitum. All the proceedings were approved by the Ethics Committee for Animal Experimentation (CEEA) of the Basque Country University in accordance with institutional, national and international guidelines regarding the protection and care of animal use for scientific purposes.
Cell lines
Murine colorectal cancer C26 cells (ATCC, LGC Standards S.L.U. Barcelona, Spain) syngenic with Balb/cByJ mice were used.
Cells were cultured under standard conditions in RPMI-1640 medium (Life technologies, Madrid, Spain) supplemented with 10% fetal bovine serum (FBS), penicillin (100 U/ml), streptomycin (100 µg/ml) and amphotericin B (0.25 µg/ml) (Life technologies). Cells were passed at a confluency of 90%.
In vitro viability assay
The viability was quantified by means of PrestoBlue® cell viability reagent following manufacturer's instructions (Life Technologies). To do so, 5×104 cells/ml were cultured with RPMI-1640 medium (Life technologies) in collagen type I (1 mg/ml) (Sigma-Aldrich, St. Louis, MO, USA) precoated plates. After a 48 h incubation with either OOS® (1:100 (V/Vf)), irinotecan 50 µM or a combination of both (1:100 (V/Vf) and 50 µM), a 1:100 dilution of Presto Blue® was added in RPMI-1640 to the cell cultures for 2 h. Then, the absorbance was quantified with a Fluoroskan Ascent (Thermo Labsystems, Waltham, MA, USA). Cell number was quantified respect standard line.
Experimental development of colorectal cancer metastasis to the liver
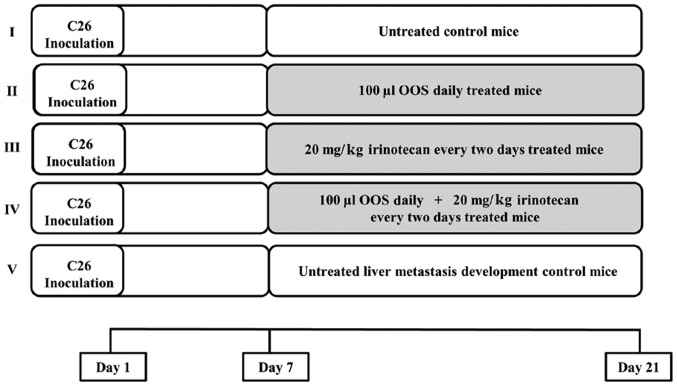
Balb/c mice were anesthetized with an intraperitoneal (i.p.) administration of Nembutal (50 mg/kg). Then, the spleen was exposed to carry out an intrasplenic (i.s.) inoculation of C26 cells (1.5×105 cells) in the inferior pole. Seven days later, the animals were divided into 5 treatment groups as shown in Fig. 1. Briefly, a) group I was constituted by mice receiving no treatment; b) group II comprised mice treated with an oral daily dose of 100 µl of OOS®; c) group III mice included mice treated with an i.p. dose of 20 mg/kg irinotecan once every two days; d) group IV consisted in mice receiving an oral daily dose of 100 µl of OOS® and an i.p. administration of 20 mg/kg of irinotecan once every two days; and e) group V was constituted by untreated mice sacrificed at different time points begining the 7th day after tumor cell inoculation in order to monitor tumor development. Two weeks after the initiation of the treatments, mice were sacrificed by cervical dislocation and the livers were collected and fixed in Zinc solution (Panreac, Barcelona, Spain). Finally, they were embedded in paraffin. Tumor occupied area was quantified in five 7 µm-thick sections per liver, separated 500 µm from each other and calculated as the area occupied by tumor foci per section of liver tissue. At least 6 mice per group were used per each experiment. Tumor area was quantified by ImageJ Software. Results were expressed as % liver area occupied by total tumor burden.
Figure 1.
Experimental animal groups treatment pattern. Seven days after tumor cell inoculation, at least 6 mice per group were divided into 5 experimental groups as follows: group I, untreated mice; group II, mice treated with a daily oral administration of 100 µl of OOS®; group III, mice treated with a i.p. dose of 20 µg/ml of irinotecan every two days; group IV, mice treated with both, a daily oral dose of 100 µl of OOS® and 20 µg/ml of irinotecan every two days; group V, untreated mice used to control the metastatic development. All mice were treated for a period of 14 days.
Immunohistological analysis of liver tissue sections
The expression levels of the apoptotic marker caspase-3, the proliferation marker Ki67 antigen, and the grade of macrophage infiltration by F4/80 marker was analyzed in liver tissue sections by means of immunohistological analysis. To do so, after antigens were retrieved in liver tissue, endogenous peroxidase and unspecific binding were blocked with 3% H2O2 and 5% FBS, respectively. Then, tissue sections were incubated with either specific antibodies against caspase-3 (ab4051; 1:100; Abcam, Cambridge, UK), Ki67 (ab16667; 1:100; Abcam), or F4/80 (MCA497R; 1:100; AbD Serotec, Oxford, UK). Finally, tissue was incubated with the specific biotinylated secondary antibodies and the antigen expression was revealed by horseradish peroxidase (HRP)-conjugated streptavidin (Life technologies, Carlsbad, CA, USA) and 2-Solution DAB kit (Life technologies) following the manufacturer's instructions. Antigen expression levels were quantified by ImageJ software (NIH, Bethesda, MD, USA). Results were expressed as the mean positive area per tumor foci area in at least 6 liver sections for each treatment.
Microarray mRNA analysis from tumor explants
Mice were inoculated with C26 cells as previously described and subjected to the same treatment protocol (Fig. 1). Then, mice were sacrificed and tumor explants were collected from livers of tumor-bearing mice and cut into small pieces in a petri dish, incubated with trypsin-EDTA 0.05% solution (Thermo Fisher Scientific, Inc., Madrid, Spain) and centrifuged. Cell pellets were resuspended in complete culture medium and supplemented with 0.1 µg/ml gentamicin (Sigma-aldrich, St. Louis, MO, USA). Once they were grown to confluency, cells were lysed and total RNA was extracted by the TRIzol® reagent (Life Technologies) and chloroform method. Total RNA was further purified by means of PureLink® RNA Mini kit (Life technologies) following manufacturer's instructions.
Afterwards, RNA integrity was analyzed by using a Eukaryote Total RNA Nano Assay with the Lab-chip in the Agilent 2100 Bioanalyzer in combination with Agilent RNA 6000 Nano Chips. Later, mRNA was labeled using the Agilent protocol ‘One-Color Microarray-Based Gene Expression Analysis. Low Input Quick Amp Labeling’ that uses the ‘Low Input Quick Amp Labeling kit, One-Color’. In order to generate labelled cDNA, mRNA was retrotranscribed with the AffinityScript Reverse Transcriptase (AffinityScript RT) in presence of Cy3-CTP. These samples were manually hybridized using ‘SureHyb’ hybridization chambers (Agilent Technologies, Inc., Santa Clara, CA, USA) according to the manufacturer's guidelines for the Agilent mRNA array and were washed according to the protocol of Agilent with ozone-barrier slide covers. Then the slides were scanned using the DNA microarrays scanner G2535CA of Agilent Technologies with the Agilent Scan control Software (v. 8.5.1.) (default settings). Finally, a feature extraction of the scanned images was made by using the Agilent Feature Extraction Software (v. 10.7.3.1) (Agilent Technologies, Inc.). Gene analysis was carried out with the PANTHER (v.10.0) analysis system (9,10).
Statistical analysis
Each assay was repeated three times, and the results are expressed as the mean ± SD of all of them. The statistical analysis was performed with the Student's two-tailed unpaired t-test. To carry out a statistical analysis of the comparative microarray assay, the MultiExperiment Viewer (MeV) vs. 4.9.0 application was used. In order to analyze the profiles between control and treated samples, LIMMA (Lineas Models for Microarray Data) and RANKPRODUCT methods were used. The criterion for significance was P<0.05 for all comparisons.
Results
In vitro effect of the combined therapy of OOS® and irinotecan in the viability of C26 cells
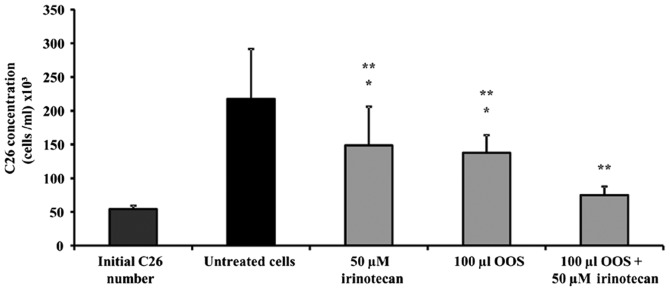
In order to analyze the effect of OOS® as a complement to irinotecan therapy, the viability of C26 cells was evaluated in presence of OOS®, irinotecan and a combination of both. To do so, C26 cells were cultured on type I collagen for 24 h before the addition of 1:100 OOS® (V/Vf), irinotecan 50 µM alone, or the combination of both. Then, the viability was measured as described in Material and Methods. As shown in Fig. 2, irinotecan and OOS® alone reduced the viability of C26 cells by 31.66 and 36.80%, respectively. After the combined treatment, the viability of C26 cells was reduced as much as by 65%, showing a synergistic effect on tumor cell viability when both complement and irinotecan were added simultaneously (Fig. 2).
Figure 2.
Effect of the combined treatment of irinotecan and OOS® in the viability of C26 cells. The viability of C26 cells was tested in the presence of OOS® (1:100), irinotecan (50 µM) or the combination of OOS® and irinotecan (1:100 and 50 µM) for 48 h. Then, the viability was quantified in untreated (black) and treated (grey) cells. Data are mean values ± SD from three different experiments. Differences in the viability of treated cells vs. untreated cells (**) and vs. initially cultured cells (*) were considered to be statistically significant at P<0.05.
Effect of the combined therapy of OOS® and irinotecan in the development of CRC metastasis to the liver
To assess the effect of OOS® as a complement to irinotecan therapy in the in vivo metastatic progression of CRC to the liver, C26 cells were i.s. inoculated to mice. Seven days later, mice were treated with a daily oral dose of 100 µl of OOS®, an i.p. dose of 20 mg/kg of irinotecan once every 2 days, or a combination of both for 2 weeks as described in Materials and methods (Fig. 1).
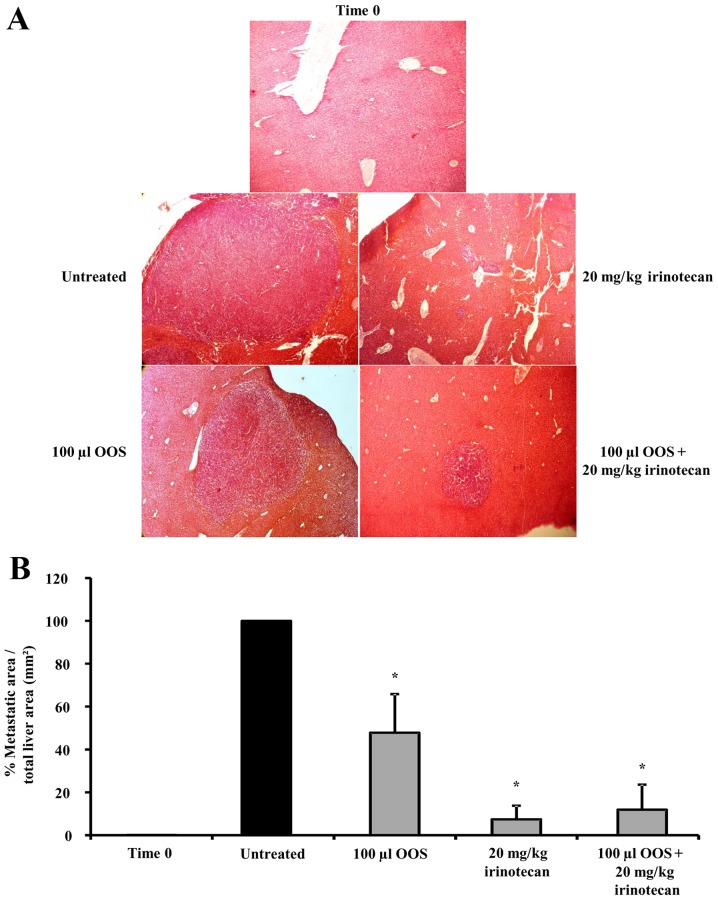
Three sentinel mice were sacrificed the 7th day after tumor cell inoculation in order to establish the tumor development at the time of treatment initiation. At that point in time, micrometastasis was detected in the livers collected from tumor-bearing mice (data not shown). Tumor occupied area was quantified in three 7 µm-thick sections per liver, as described in Material and Methods. In comparison with the untreated group I, II and III showed a significant reduction of 58 and 92% in the tumor-occupied area respectively (Fig. 3A and B). Interestingly, although no synergistic effect could be observed between the irinotecan treatment alone (group III) or in combination with OOS® (group IV), the supplemented therapy reduced tumor burden by 88% (Fig. 3A and B) and furthermore, eye observations indicated an improvement in the overall fitness of the animals according to the Mouse Grimace Scale (data not shown) (11).
Figure 3.
Effect of the combined treatment of irinotecan and OOS® in the development of CRC metastasis to the liver. C26 cells were i.s. inoculated into mice and seven days later they were divided into the five groups shown in Fig. 1 with at least 6 mice per group. Mice were either untreated or treated with OOS® (100 µl), irinotecan (20 mg/kg) or with the combined therapy (100 µl OOS® and 20 mg/kg irinotecan) as described in Material and Methods. (A) Images showing tumor foci grown in the liver of untreated and treated mice. Image original magnification was ×4. (B) The total tumoral burden was quantified and represented as the percentage of liver area occupied by the tumor. Differences were considered statistically significant at *P<0.05.
Effect of the combined OOS® plus irinotecan therapy on proliferation and apoptotic markers
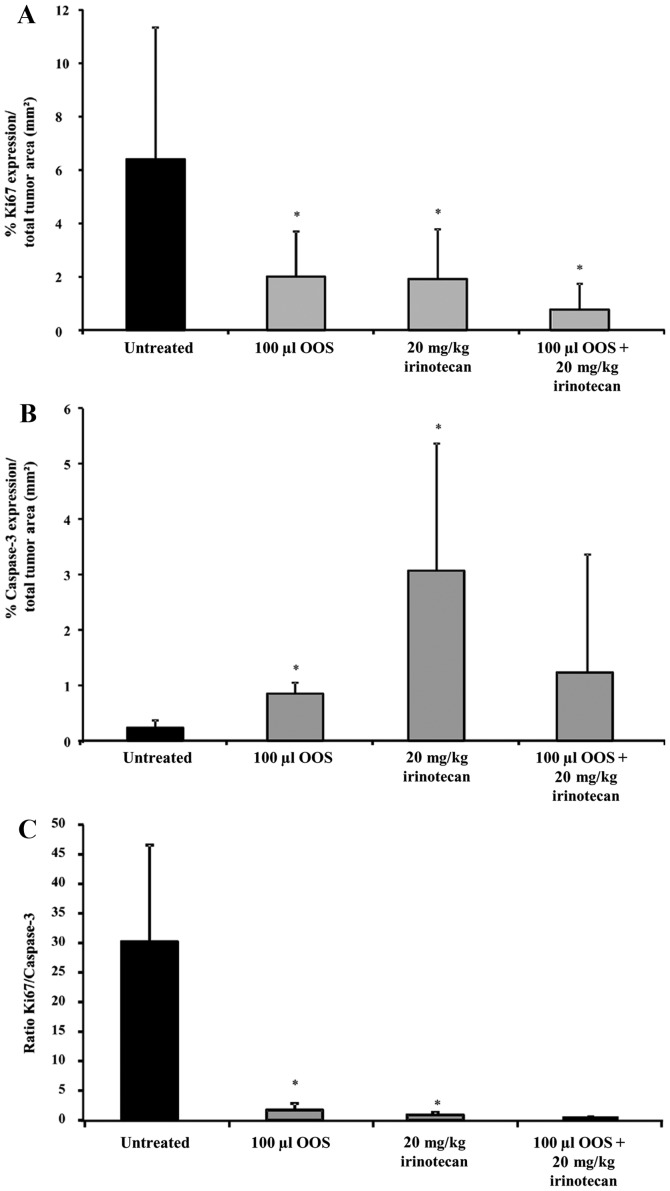
The administration of either OOS® or irinotecan alone have shown to have proapoptotic and antiproliferative effects. However, the effect of the combined treatment of OOS® and irinotecan in the metastatic progression of CRC to the liver is unknown. The expression level of the proliferative marker Ki67 and the apoptotic marker caspase-3 analyzed by immunohistochemistry showed that OOS® and irinotecan administered alone decreased Ki67 expression by 80% in the tumor foci of livers collected from treated mice compared to untreated mice (Fig. 4A). Also, the combined therapy decreased Ki67 expression up to 93% (Fig. 4A). To analyze the effect on apoptosis, caspase-3 expression levels were quantified and were found to be upregulated within tumor foci of mice treated with each one of the therapies (Fig. 4B). The differences between the tumor burden in the livers collected from mice subjected to the combined therapy were not significant. This result might be due to the small tumor burden in the liver. The ratio between proliferation and apoptosis markers diminished up to 90% in livers from mice under OOS® treatment and up to 99% in livers from mice under either irinotecan treatment alone or the combined treatment (Fig. 4C).
Figure 4.
Effect of the combined treatment of irinotecan and OOS® in the proliferation and apoptotic markers. The expression of Ki67 and caspase-3 in livers collected from tumor-bearing mice either untreated or treated with OOS® (100 µl), irinotecan (20 mg/kg) or the complementary therapy (100 µl OOS® and 20 mg/kg irinotecan) was analyzed by immunohistochemistry with at least 6 mice per group. (A) Ki67 expression was quantified in livers collected from untreated and treated C26-bearing mice as the percentage of the area positive for Ki67 expression within tumor foci respect to the total metastatic tumor area. (B) Caspase-3 expression was quantified in livers collected from untreated and treated C26-bearing mice as the percentage of the area positive for caspase-3 expression respect to the total metastatic tumor area. (C) The ratio between Ki67 and caspase-3 in livers was calculated from results shown in A and B. Differences were considered statistically significant at *P<0.05.
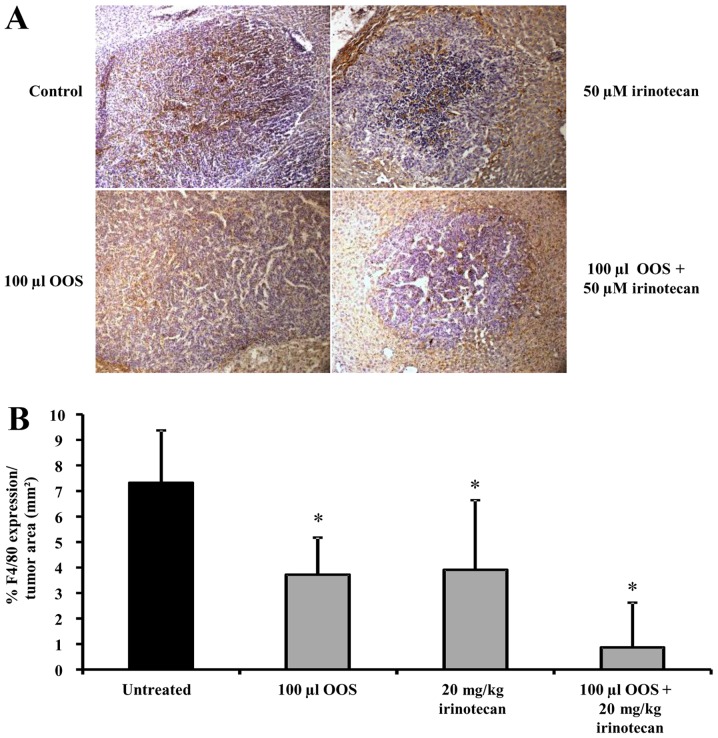
Effect of the combined therapy of OOS® and irinotecan on the macrophage infiltration into tumor foci in vivo
Since macrophage infiltration into tumor foci has been related to tumor progression and the development of chemoresistance, we analyzed the effect of OOS® and irinotecan either alone or in combination in the number of tumor-infiltrating macrophages. To do so, liver tissue sections were incubated with antibodies against F4/80, a marker for activated macrophages (Fig. 5A). As shown in Fig. 5B, the intratumoral level of F4/80 expression in the liver tissue collected from tumor bearing mice was reduced by 49 and 47% in mice treated with either 100 µl of OOS® or 20 mg/kg irinotecan alone, respectively. It is interesting to note that the infiltration of macrophages in those foci developed in livers of mice treated with OOS® and irinotecan in combination was further reduced by 88% showing a synergistic effect of both compounds (Fig. 5B).
Figure 5.
Effect of the combined treatment of irinotecan and OOS® on the tumor infiltration of macrophages in vivo. Expression level of F4/80 was analyzed in liver tissue by immunohistochemistry. (A) Images showing F4/80 expression (brown) and hematoxylin (purple) in liver tissue collected from untreated and treated mice. Image magnification was ×20. (B) F4/80 expression was quantified in livers collected from untreated and treated C26-bearing mice. Data are calculated as % of F4/80 expression per tumor foci area. At least 6 mice per group were used and differences were considered statistically significant at *P<0.05.
Metastatic tumor explants mRNA comparative microarray study
Total RNA was extracted from the tumor explants collected from tumor-bearing mice livers as described in Material and Methods. The microarray data were subjected to two different comparisons. First, the RNA expression levels were compared between tumor cells isolated from tumor explants collected from treated mice (either treated with OOS®, or irinotecan alone or the combination of both) vs. those in the tumor cells isolated from explants collected from untreated mice. And second, a comparison was made between RNA expression levels in those tumor cells isolated from explants collected from mice subjected to the combined treatment vs. those in the tumor cells isolated from explants collected from mice treated with irinotecan alone.
The OOS® treatment alone provoked an alteration in the expression levels of 35 genes in contrast to the treatment with irinotecan alone, which altered the expression levels of 152 genes when compared to gene expression levels from untreated samples (Tables I and II). It is interesting to note that the irinotecan treatment supplemented with OOS® resulted in the reversal of most of the alterations observed when irinotecan was administered alone. That is, only 6 genes were altered in the cells collected from mice under the combined treatment (Table I). Two different patterns could be observed. 93% of the altered genes were downregulated in cells collected from mice treated with irinotecan alone when compared with control samples. In contrast, 85 and 83% of the altered genes in tumor cells isolated from mice treated with OOS® and OOS® plus irinotecan respectively showed an upregulation in their expression levels. Interestingly, only one gene presented an altered expression in every group. This gene resulted to be the one coding for Arglu1. When the gene expression levels were compared between irinotecan and combined treatment only 14 genes showed an altered expression. Interestingly all of them were upregulated (Table I).
Table I.
Ocoxin oral solution (OOS®), irinotecan and the combined therapy deregulated genes.
| Up | Down | Total | |
|---|---|---|---|
| OOS® vs. control | 30 | 5 | 35 |
| Irinotecan vs. control | 11 | 141 | 152 |
| OOS®+irinotecan vs. control | 5 | 1 | 6 |
| OOS®+irinotecan vs. irinotecan | 14 | 0 | 14 |
Table II.
Ocoxin Oral solution® (O), irinotecan (I) and the combined therapy (OI) deregulated genes.a
| Gene symbol | O vs. C | OI vs. C | I vs. C |
|---|---|---|---|
| 1700017G19Rik | ↓↓ | ||
| 1700086P04Rik | ↓ | ||
| 2700081O15Rik | ↑ | ||
| 2810454H06Rik | ↓↓↓ | ||
| 4930401B11Rik | ↓ | ||
| 4930556N13Rik | ↓↓ | ||
| 4933413J09Rik | ↓↓ | ||
| 4933415F23Rik | ↓ | ||
| 6430550D23Rik | ↓↓ | ||
| 9530003O04Rik | ↓ | ||
| A730028G07Rik | ↓↓↓ | ||
| Aak1 | ↓ | ||
| Adcy6 | ↓↓ | ||
| Afm | ↓↓↓ | ||
| Agxt2 | ↓↓ | ||
| AI481877 | ↓↓ | ||
| Ankk1 | ↑↑ | ||
| Apoe | ↓ | ||
| Aqp9 | ↓↓ | ||
| Arglu1 | ↓ | ↓ | ↓ |
| Atf6 | ↓↓↓ | ||
| B230334C09Rik | ↑↑ | ||
| BC030308 | ↑ | ||
| Bfar | ↓ | ||
| Bmp7 | ↑↑ | ||
| Bsph1 | ↓↓ | ||
| C030039L03Rik | ↓↓ | ||
| Cabp1 | ↑↑ | ||
| Cdh9 | ↑ | ||
| Cdk13 | ↑↑↑↑ | ||
| Cela3a | ↓↓↓ | ||
| Cfap61 | ↓ | ||
| Cfd | ↑↑ | ||
| Chil3 | ↑↑↑ | ||
| Cic | ↓↓ | ||
| Clcn7 | ↓↓ | ||
| Clec18a | ↓↓ | ||
| Clec2e | ↓ | ||
| Crygc | ↓↓ | ||
| Cyp19a1 | ↓ | ||
| Cypt15 | ↑↑↑ | ||
| Cypt2 | ↓ | ||
| Cyth4 | ↑↑↑↑ | ||
| D430018E03Rik | ↓↓ | ||
| D6Ertd527e | ↓↓ | ||
| Dlx6os1 | ↓ | ||
| Dnal1 | ↓ | ||
| Dock4 | ↓↓ | ||
| Dsc1 | ↓ | ||
| Dzip1 | ↑ | ||
| Ect2 | ↑↑↑ | ||
| Erich2 | ↓↓ | ||
| Fam71f2 | ↑↑↑ | ||
| Fanci | ↓ | ||
| Fat3 | ↓↓ | ||
| Fgr | ↓↓ | ||
| Fkbp6 | ↓ | ||
| Fuca2 | ↓↓ | ||
| Gja8 | ↓↓ | ||
| Glrx2 | ↓↓ | ||
| Gm10760 | ↓ | ||
| Gm16794 | ↓↓ | ||
| Gm38485 | ↑ | ||
| Gm5126 | ↓↓ | ||
| Gm5129 | ↓↓ | ||
| Gm5941 | ↓↓ | ||
| Gm8075 | ↓↓ | ||
| Gm9548 | ↓↓ | ||
| Gm9798 | ↓↓ | ||
| Gm9979 | ↑ | ||
| Gpr22 | ↓↓ | ||
| Hcrt | ↓↓ | ||
| Hist2h3c2 | ↓↓ | ||
| Hmcn1 | ↑ | ||
| Hras | ↓↓ | ||
| Il11 | ↑↑↑↑ | ||
| Il1b | ↑↑ | ||
| Kcnj12 | ↓ | ||
| Kif14 | ↓↓ | ||
| Klf11 | ↓↓↓ | ||
| Krtap19–2 | ↑↑↑ | ||
| LOC102641211 | ↑ | ||
| LOC105244659 | ↓↓ | ||
| Lppr3 | ↓↓↓ | ||
| Lrrc2 | ↓↓↓ | ||
| Ly6c1 | ↓↓↓ | ||
| Marcksl1 | ↑ | ||
| Marcksl1-ps4 | ↑ | ||
| Marveld2 | ↓↓ | ||
| Meg3 | ↓ | ||
| Ms4a1 | ↓↓ | ||
| Naprt | ↓ | ||
| Ncam1 | ↓↓ | ||
| Nctc1 | ↑↑ | ||
| Nfkbid | ↓↓ | ||
| Nkx6-1 | ↓↓ | ||
| Nol3 | ↑↑ | ||
| Nr1h3 | ↓ | ||
| Ntrk1 | ↑ | ||
| Nuf2 | ↑↑↑↑ | ||
| Olfr1110 | ↓↓↓ | ||
| Olfr328 | ↓↓↓ | ||
| Olfr549 | ↓↓ | ||
| Olfr945 | ↓↓ | ||
| Olfr97 | ↓↓ | ||
| Pggt1b | ↑↑↑ | ||
| Phka2 | ↓↓ | ||
| Pir | ↑ | ||
| Pyroxd2 | ↑↑ | ||
| Raver2 | ↓↓ | ||
| Recql5 | ↓ | ||
| Rgs16 | ↑↑ | ||
| Rian | ↓↓ | ||
| Rnf170 | ↓ | ||
| Rnf220 | ↓↓ | ||
| Sclt1 | ↓ | ||
| Scn10a | ↓↓ | ||
| Sel1l | ↓↓ | ||
| Sept8 | ↓↓↓ | ||
| Sf3a2 | ↓↓ | ||
| Sgk3 | ↓↓ | ||
| Shc3 | ↑ | ||
| Slc14a1 | ↑↑ | ||
| Slc1a2 | ↓ | ||
| Slc26a1 | ↓↓ | ||
| Slc5a9 | ↓↓ | ||
| Slc8b1 | ↓↓ | ||
| Snx14 | ↑↑ | ||
| Spice1 | ↓↓ | ||
| Ssh1 | ↑↑ | ||
| Stt3a | ↓↓ | ||
| Sucla2 | ↓↓ | ↓↓ | |
| Tanc1 | ↓↓ | ||
| Tmem29 | ↓↓ | ||
| Tmprss5 | ↓↓ | ||
| Tshz2 | ↓↓ | ||
| Tstd3 | ↑↑↑↑ | ||
| Ttbk1 | ↓ | ||
| Ttl | ↑↑ | ||
| Tuba8 | ↓ | ||
| Ube2cbp | ↓↓ | ||
| Usp2 | ↑↑ | ||
| Usp42 | ↑ | ||
| Vmn1r19 | ↓↓ | ||
| Vps13c | ↓↓ | ||
| Xrcc4 | ↓↓ | ||
| Zdhhc21 | ↓↓↓ | ||
| Zfp318 | ↓↓↓ | ||
| Zpbp2 | ↓↓ | ||
| Zrsr2 | ↑↑ |
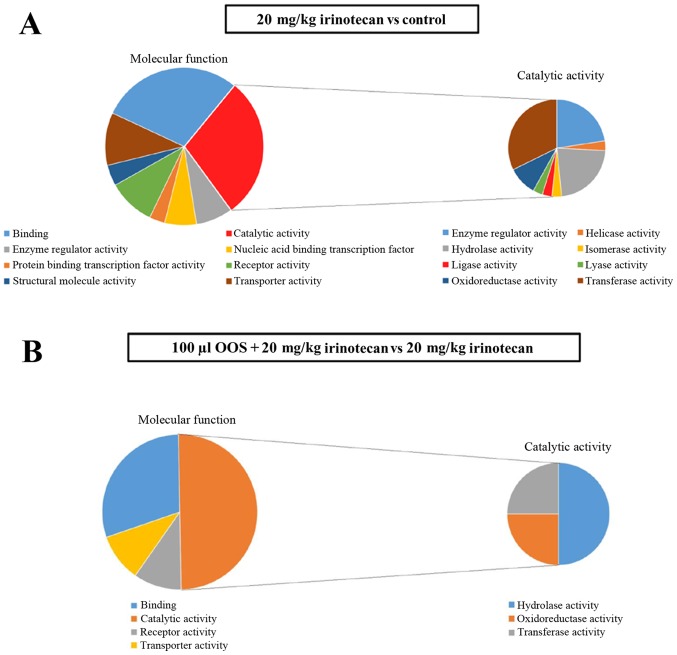
Next, the altered genes were classified using the PANTHER (v.10.0) analysis software according to their molecular functions. In irinotecan vs. control treatment, most of the genes were classified into two principal molecular functions, catalytic activity and binding activity (Fig. 6A). Some of the genes whose expression was altered were also included in other activities, such as, receptor activity and transporter activity among others. Furthermore, the genes altered in tumor cells isolated from mice treated with the irinotecan and classified under catalytic activity were analyzed in more detail. Those genes were included in three principal catalytic activities: Oxidoreductases, hydrolases and transferases. Moreover, most of the genes altered by the combined treatment were also included in the same three catalytic activities mentioned above. Additionally, the genes with binding activity were also altered when the combined treatment was administered (Fig. 6B). In general, the genes deregulated by the action of irinotecan fall into eight of the groups classified by their molecular functions as shown in the left panel of Fig. 6A. In contrast, those genes with altered expression by the complementary therapy fall only within four of the molecular categories as shown in the left panel of Fig. 6B.
Figure 6.
Metastatic tumor explant based mRNA microarray study. A microarray assay was carried out to detect differences in gene expression levels between tumor cells collected from mice treated under different protocols. Four tumor explants per experimental group were collected, each of them from one tumor bearing mouse. (A) A molecular functional gene analysis was carried out with the PANTHER (v.10.0) (9,10) analysis system for the untreated Control vs. irinotecan treatments. (B) A molecular functional gene analysis was carried out with the PANTHER (v.10.0) (9,10) analysis system for the OOS®+irinotecan vs. irinotecan treatments.
Discussion
Colorectal cancer is one of the leading causes of cancer deaths in the world due to the spread of the primary tumor to the liver. To date, irinotecan is one of the most used chemotherapeutic drugs for the treatment of liver metastasis of CRC, which increases patient's survival. However, these treatments generate diverse side effects which influence the quality of life of patients (1). Thus, different chemotherapeutic agents are being tested in combination with biologically active compounds to avoid these effects without comprising efficacy (12,13).
Certain nutrient mixtures have shown antitumoral effects in in vitro and in in vivo preclinical models (1–3). In this context, OOS® is a mixture which has shown to have promising antitumor results in different in vitro and in vivo cancer models (6–8); thus, it might be a suitable complement for irinotecan treatment in the progression of hepatic metastasis of CRC. Previously we have shown that OOS® slows down the metastatic progression of CRC to the liver in vivo (7). Thus, we aimed to evaluate this nutrient mixture as a candidate for a combined therapy with irinotecan, a chemotherapeutic agent used as a common therapy to treat this malignancy (1). According to our results, OOS® and irinotecan alone reduced the viability of C26 cells in vitro, which was further reduced when the combined treatment was applied. However, no synergistic effect was observed in vivo when OOS® was administered together with irinotecan. Nevertheless, the overall fitness state of the mice treated with OOS® plus irinotecan showed an improvement according to the Mouse Grimace Scale comparing to receiving irinotecan alone (data not shown) (11). That is, untreated and mice treated with irinotecan showed a narrowing of the orbital area, a tightly closed eyelid, and/or an eye squeeze, and more unkempt fur coat. The alteration in those parameters was observed to be diminished or absent in mice treated with the combined therapy. These results are in accordance with those carried out by Dayem-Uddin et al (2009) showing an improvement in the quality of patient's life suffering from different cancers after the addition of OOS® to chemotherapy or radiotherapy (14).
Nowadays it is known that some nutritional complements suppress, among others, the hyper-proliferative processes during the development of a tumor (15–18). In line with these studies, the combined therapy of OOS® and irinotecan reduced Ki67 expression, an antigen expressed only in proliferative cells, in the metastatic liver tissue and increased caspase-3 expression, a protease expressed during apoptosis, when compared to the expression in the liver tissue collected from mice treated with the compounds alone. Moreover, the ratio of proliferative and apoptotic cells was diminished in livers from treated mice respect to those collected from untreated mice, even though the combined treatment did not show a synergistic effect with irinotecan in the in vivo metastatic progression. Further studies will show if a prolonged administration of irinotecan combined with OOS® would result in a visible reduction in the development of metastatic foci and an increase in the survival rate of mice under this treatment regime.
The complex microenvironment of solid tumors, comprised not only by tumor cells but also by the surrounding stromal components, has been associated with the induction of resistance to routine chemotherapies (19). This cross reactivity might be due to the interactions taking place between cancer cells and the multiple factors existing in the tumor microenvironment such as reactive oxygen species (ROS) and cytokines. These factors induce the recruitment of macrophages within the tumor foci (2), which influence the microenvironment towards one that favors tumor development. Thus, reducing the infiltration of stromal cells, such as tumor associated macrophages, into the tumor foci might impede or slow down tumor progression. In fact, a reduction in the number of tumor-associated macrophages is correlated with a better prognosis in several types of cancer.
Here we show that OOS® alone, as well as irinotecan, reduced the recruitment of macrophages to the tumor foci in the liver of treated mice, as shown by the reduced expression of F4/80. This infiltration was even lower when OOS® was administered simoultaneoulsy with irinotecan. As shown by others (20,21), irinotecan-induced colitis might be the result of an increase in an inflammatory response which, in turn, might damage normal tissues. In fact, macrophages are responsible for many of the inflammatory factors released into the liver and a reduced activation and recruitment of these cells might account for a reduction of irinotecan-induced side effects.
Additional studies in gene expression by gene array results have shown that irinotecan treatment significantly deregulates the expression level of 152 genes in the liver CRC metastasic explants while the combined therapy deregulated the expression of 14 genes only. Furthermore, 93% of the genes identified in tumor cells isolated from mice treated with irinotecan alone were downregulated. In contrast, the 100% of the altered genes in tumor cells isolated from mice treated with irinotecan plus OOS® were upregulated. This may indicate that OOS® could revert or modify the expression of the genes altered by irinotecan. These genes, whose expression was altered, were classified according to their molecular function, which turns to be in its majority binding and catalytic activity. Besides these, another biological and molecular activities were present, but in a shorter extent. Interestingly, the genes with catalytic activities which expression was altered in the tumor cells isolated from mice subjected to the combined therapy possess enzymatic functions such as oxidoreductases, hydrolases and transferases. On the one hand, the upregulation of oxidoreductases, a group of enzymes that transfer electrons between molecules, have previously been related to the induction of apoptosis and to the increase in the cytotoxicity of several antitumoral drugs (22,23). On the other hand, transferases are the enzymes responsible for the biosynthesis of glycoprotein and glycolipid sugar chains and it is described that cancer cells show an aberrant glycosylation in their surface. This could lead to abnormal ligand-receptor interactions, and more importantly, it may favor cancer cell proliferation, migration and invasion (24,25). At last, hydrolases have also been implicated in different cancer types and their downregulation has been associated with the development of chemoresistance of melanoma and colorectal cancer to cytotoxic drugs (26,27). In this way, the OOS® added to irinotecan might counteract the action of genes downregulated by irinotecan treatment. Future studies will show if these genes are responsible for the side effects or for the resistance to irinotecan, or both.
To sum up, the combination of OOS® with irinotecan results in a reduced tumor cell proliferation and macrophage infiltration at a greater extent than OOS® or irinotecan alone do. However, a synergistic effect of the complementary therapy could not be observed in the in vivo metastatic progression. Nevertheless, it was observed that the combined therapy improved the animal overall status. The subjacent mechanism could be mediated in part by the reversal, induced by the combined therapy, of the downregulation exerted by the action of irinotecan in the expression of those genes with catalytic and binding activities. Further studies will be performed to validate the exact role and implication of altered genes, and to identify their exact role either in the tumor development or in the improvement of drug-induced adverse effects. Therefore, OOS® may constitute a pharmacologically safe complementary compound for the treatment of cancer and its metastasis when administered together with irinotecan by improving the quality of life in patients suffering from CRC liver metastasis.
Acknowledgements
We thank Cristina Tobillas and Maria Jesus Fernandez for the excellent technical assistance. Additionally, we greatly appreciate the support of the Genomics and Proteomics Unit, the Analytical and High Resolution Microscopy Unit and the Animal Facilities from the Advance Research Facilities (SGIker) of the Basque Country University.
References
- 1.Fujita K, Kubota Y, Ishida H, Sasaki Y. Irinotecan, a key chemotherapeutic drug for metastatic colorectal cancer. World J Gastroenterol. 2015;21:12234–12248. doi: 10.3748/wjg.v21.i43.12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van den Eynden GG, Majeed AW, Illemann M, Vermeulen PB, Bird NC, Høyer-Hansen G, Eefsen RL, Reynolds AR, Brodt P. The multifaceted role of the microenvironment in liver metastasis: Biology and clinical implications. Cancer Res. 2013;73:2031–2043. doi: 10.1158/0008-5472.CAN-12-3931. [DOI] [PubMed] [Google Scholar]
- 3.Gol'dberg ED, Amosova EN, Zueva EP, Razina TG, Krylova SG, Zorikov PS. Licorice preparations improve efficiency of chemotherapy and surgical treatment of transplanted tumors. Bull Exp Biol Med. 2008;145:252–255. doi: 10.1007/s10517-008-0063-0. [DOI] [PubMed] [Google Scholar]
- 4.Kontek R, Drozda R, Sliwiński M, Grzegorczyk K. Genotoxicity of irinotecan and its modulation by vitamins AC and E in human lymphocytes from healthy individuals and cancer patients. Toxicol In Vitro. 2010;24:417–424. doi: 10.1016/j.tiv.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 5.Arabski M, Kazmierczak P, Wisniewska-Jarosinska M, Poplawski T, Klupinska G, Chojnacki J, Drzewoski J, Blasiak J. Interaction of amoxicillin with DNA in human lymphocytes and H. pylori-infected and non-infected gastric mucosa cells. Chem Biol Interact. 2005;152:13–24. doi: 10.1016/j.cbi.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Hernández-Garcia S, González V, Sanz E, Pandiella A. Effect of oncoxin oral solution in HER2-overexpressing breast cancer. Nutr Cancer. 2015;67:1159–1169. doi: 10.1080/01635581.2015.1068819. [DOI] [PubMed] [Google Scholar]
- 7.Márquez J, Mena J, Hernandez-Unzueta I, Benedicto A, Sanz E, Arteta B, Olaso E. Ocoxin® oral solution slows down tumor growth in an experimental model of colorectal cancer metastasis to the liver in balb/c mice. Oncol Rep. 2016;35:1265–1272. doi: 10.3892/or.2015.4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diaz-Rodriguez E, Hernández-Garcia S, Sanz E, Pandiella A. Antitumoral effect of ocoxin on acute myeloid leukemia. Oncotarget. 2016;7:6231–6242. doi: 10.18632/oncotarget.6862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mi H, Muruganujan A, Casagrande JT, Thomas PD. Large-scale gene function analysis with the PANTHER classification system. Nat Protoc. 2013;8:1551–1566. doi: 10.1038/nprot.2013.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mi H, Poudel S, Muruganujan A, Casagrande JT, Thomas PD. PANTHER version 10: Expanded protein families and functions, and analysis tools. Nucleic Acids Res. 2016;44(D1):D336–D342. doi: 10.1093/nar/gkv1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langford DJ, Bailey AL, Chanda ML, Clarke SE, Drummond TE, Echols S, Glick S, Ingrao J, Klassen-Ross T, Lacroix-Fralish ML, et al. Coding of facial expressions of pain in the laboratory mouse. Nat Methods. 2010;7:447–449. doi: 10.1038/nmeth.1455. [DOI] [PubMed] [Google Scholar]
- 12.Mego M, Chovanec J, Vochyanova-Andrezalova I, Konkolovsky P, Mikulova M, Reckova M, Miskovska V, Bystricky B, Beniak J, Medvecova L, et al. Prevention of irinotecan induced diarrhea by probiotics: A randomized double blind, placebo controlled pilot study. Complement Ther Med. 2015;23:356–362. doi: 10.1016/j.ctim.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Mikalauskas S, Mikalauskiene L, Bruns H, Nickkholgh A, Hoffmann K, Longerich T, Strupas K, Büchler MW, Schemmer P. Dietary glycine protects from chemotherapy-induced hepatotoxicity. Amino Acids. 2011;40:1139–1150. doi: 10.1007/s00726-010-0737-6. [DOI] [PubMed] [Google Scholar]
- 14.Dayem-Uddin M, Islam M, Mahmood I, Gosh A, Khatun R, Kundu S. Findings of the 3-month supportive treatment with oncoxin solution beside the standard modalities of patients with different neoplastic diseases. TAJ. 2009;22:172–175. [Google Scholar]
- 15.Kim JA, Kim DH, Hossain MA, Kim MY, Sung B, Yoon JH, Suh H, Jeong TC, Chung HY, Kim ND. HS-1793, a resveratrol analogue, induces cell cycle arrest and apoptotic cell death in human breast cancer cells. Int J Oncol. 2014;44:473–480. doi: 10.3892/ijo.2013.2207. [DOI] [PubMed] [Google Scholar]
- 16.Gullett NP, Amin AR Ruhul, Bayraktar S, Pezzuto JM, Shin DM, Khuri FR, Aggarwal BB, Surh YJ, Kucuk O. Cancer prevention with natural compounds. Semin Oncol. 2010;37:258–281. doi: 10.1053/j.seminoncol.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 17.Mehta RG, Murillo G, Naithani R, Peng X. Cancer chemoprevention by natural products: How far have we come? Pharm Res. 2010;27:950–961. doi: 10.1007/s11095-010-0085-y. [DOI] [PubMed] [Google Scholar]
- 18.Cho YH, Yoon SY, Kim SN. Irinotecan monotherapy versus irinotecan-based combination as second-line chemotherapy in advanced gastric cancer: A meta-analysis. Cancer Res Treat. 2017;49:255–262. doi: 10.4143/crt.2015.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kachalaki S, Ebrahimi M, Khosroshahi L Mohamed, Mohammadinejad S, Baradaran B. Cancer chemoresistance; biochemical and molecular aspects: A brief overview. Eur J Pharm Sci. 2016;89:20–30. doi: 10.1016/j.ejps.2016.03.025. [DOI] [PubMed] [Google Scholar]
- 20.Stringer AM, Gibson RJ, Logan RM, Bowen JM, Yeoh AS, Laurence J, Keefe DM. Irinotecan-induced mucositis is associated with changes in intestinal mucins. Cancer Chemother Pharmacol. 2009;64:123–132. doi: 10.1007/s00280-008-0855-y. [DOI] [PubMed] [Google Scholar]
- 21.Stringer AM, Gibson RJ, Bowen JM, Logan RM, Ashton K, Yeoh AS, Al-Dasooqi N, Keefe DM. Irinotecan-induced mucositis manifesting as diarrhoea corresponds with an amended intestinal flora and mucin profile. Int J Exp Pathol. 2009;90:489–499. doi: 10.1111/j.1365-2613.2009.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kung HN, Weng TY, Liu YL, Lu KS, Chau YP. Sulindac compounds facilitate the cytotoxicity of β-lapachone by up-regulation of NAD(P)H quinone oxidoreductase in human lung cancer cells. PLoS One. 2014;9:e88122. doi: 10.1371/journal.pone.0088122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei D, Zhang X, Zou H, Wang L, Fu B, Wu X, Luo Z, Li X, Ge J, Li Y, et al. WW domain containing oxidoreductase induces apoptosis in gallbladder-derived malignant cell by upregulating expression of P73 and PUMA. Tumour Biol. 2014;35:1539–1550. doi: 10.1007/s13277-013-1213-1. [DOI] [PubMed] [Google Scholar]
- 24.Pinho SS, Reis CA. Glycosylation in cancer: Mechanisms and clinical implications. Nat Rev Cancer. 2015;15:540–555. doi: 10.1038/nrc3982. [DOI] [PubMed] [Google Scholar]
- 25.Sheta R, Woo CM, Roux-Dalvai F, Fournier F, Bourassa S, Droit A, Bertozzi CR, Bachvarov D. A metabolic labeling approach for glycoproteomic analysis reveals altered glycoprotein expression upon GALNT3 knockdown in ovarian cancer cells. J Proteomics. 2016;145:91–102. doi: 10.1016/j.jprot.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feferman L, Bhattacharyya S, Deaton R, Gann P, Guzman G, Kajdacsy-Balla A, Tobacman JK. Arylsulfatase B (N-acetylgalactosamine-4-sulfatase): Potential role as a biomarker in prostate cancer. Prostate Cancer Prostatic Dis. 2013;16:277–284. doi: 10.1038/pcan.2013.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brinkmann K, Zigrino P, Witt A, Schell M, Ackermann L, Broxtermann P, Schüll S, Andree M, Coutelle O, Yazdanpanah B, et al. Ubiquitin C-terminal hydrolase-L1 potentiates cancer chemosensitivity by stabilizing NOXA. Cell Rep. 2013;3:881–891. doi: 10.1016/j.celrep.2013.02.014. [DOI] [PubMed] [Google Scholar]