Abstract
Angiogenesis is essential for tumor growth and metastasis. CD105 is reportedly a specific marker for tumor angiogenesis. It has been demonstrated that monoclonal antibodies to CD105 have high affinity for activated endothelial cells. A relationship between metastasis and microvessel density (MVD), as an indicator of neovascularization, has been identified in patients with colorectal cancer as shown by the presence of monoclonal antibodies to CD105. However, data on potentially confounding factors such as lymphatic and vascular infiltration and tumor size are lacking. We further investigated the relationship between MVD and distant metastasis, along with potentially confounding clinicopathological factors, to more precisely characterize this relationship. In this retrospective study, we analyzed colorectal cancer specimens surgically or endoscopically resected from January to September 2009. We defined MVD as the number of microvessels stained by monoclonal antibodies to CD105 per ×400 field. Selected clinicopathological factors were analyzed and stepwise multivariate logistic regression was performed to identify independent risk factors for distant metastasis. We analyzed 129 lesions. The median follow-up time was 34 months (range, 6–85 months) in patients with distant metastasis and 61 months (range, 60–86 months) in those without distant metastasis. At the time of resection or during subsequent follow-up, 32 patients had distant metastases. The MVD was significantly greater in patients with than without distant metastases (mean ± standard deviation: 10.4±4.9 vs. 7.6±3.3, P=0.008; Welch's t-test). Stepwise multivariate logistic regression indicated that MVD, regional lymph node metastasis, and tumor size were independent risk factors for distant metastases. Combining assessment of monoclonal antibodies to CD105-positive MVD with assessment of regional lymph node metastasis and tumor size may help to identify patients who need more intensive surveillance after surgery for colorectal cancer.
Keywords: CD105, angiogenesis, microvessel density, distant metastasis, colorectal cancer
Introduction
Colorectal cancer is a major cause of morbidity and mortality worldwide (1). It is the third most common malignancy and second most common cause of cancer-related death in developed countries (2). Intensive surveillance after colorectal cancer surgery improves survival because early diagnosis of recurrence increases the frequency of its curative resection (3). Therefore, identification of the risk factors for distant metastasis would optimize the treatment of colorectal cancer.
Tumor angiogenesis (the formation of new blood vessels) is essential for tumor growth and metastasis (2,4). The development of new blood vessels is a complex phenomenon involving growth factors, extracellular matrix enzymes, endothelial cell migration and proliferation, lumen formation, and anastomosis with other vessels (5,6). New blood vessel formation facilitates entry of malignant cells into the circulation, thus increasing the probability of metastasis (6,7). Assessment of microvessel density (MVD), a means of quantifying neovascular vessels, involves staining tissues with pan-endothelial antibodies to antigens such as CD34, CD31, and von Willebrand factor (6,8). However, these pan-endothelial markers are also expressed in normal tissues (9). Currently, CD105 is the immunohistochemical marker most frequently used to identify activated endothelial cells (10). CD105 is reportedly strongly expressed in endothelial cells of tissues participating in angiogenesis.
We believe that reevaluating the association between tumor angiogenesis as assessed by monoclonal antibodies to CD105 and distant metastasis is essential for optimal treatment of colorectal cancer. The purpose of our study was to identify associations between the presence or absence of distant metastasis and potential clinicopathological risk factors for tumor angiogenesis.
Materials and methods
Patients and clinical data
We retrospectively studied 129 T1, T2, T3, and T4 colorectal cancers specimens resected at the Showa University Northern Yokohama Hospital from January 2009 to September 2009. Written informed consent was obtained from all patients before endoscopic therapy or laparoscopic/open surgery. This study was approved by the Ethics Committee of Showa University Hospital.
The inclusion criteria were as follows: i) Colorectal cancers that had been surgically resected or endoscopically resected prior to surgery; and ii) cases without distant metastasis during follow-up of at least 5 years after surgery. The exclusion criteria were as follows: Patients who had i) two or more cancers excluding colorectal mucosal cancer; or ii) received chemotherapy and/or radiotherapy prior to surgery (because these factors affect the causal relationship with distant metastasis.).
Relevant clinicopathological data (age, gender, tumor size, location and depth, lymphatic infiltration, vascular infiltration, regional lymph node metastasis, poorly differentiated or mucinous carcinoma components, adjuvant chemotherapy, and distant metastases) were extracted from an electronic record system and reviewed. This study included 72 men (55.8%) and 57 women (44.2%) aged 31 to 87 years (median, 66 years). Distant metastasis was defined as metastasis to another organ (such as the liver, lung, bone, brain, or peritoneum) and/or nonregional lymph node metastasis.
CD105 immunostaining
Immunohistochemical staining was performed on 4- to 5-µm-thick formalin-fixed and paraffin-embedded sections obtained from each specimen. Sections containing the most invasive areas of colorectal cancer were selected. Staining was performed automatically (BONDIII; Leica Biosystems Melbourne, Melbourne, Victoria, Australia), using a Bond Polymer Refine Detection kit (Leica Biosystems, Newcastle, UK), with mouse monoclonal antibodies to CD105 (clone SN6h; Dako, Kyoto, Japan; working dilution 1:500 for 15 min with bond wash solution buffer) (11).
Stainability of monoclonal antibodies to CD105 and pan-endothelial antibodies
To assess whether monoclonal antibodies to CD105 are specific for new tumor blood vessels, we performed a pilot study comparing microvessels between normal and cancerous areas by staining with monoclonal antibodies to CD105, CD31, CD34, and von Willebrand factor. The tissues were examined under high magnification (x400).
Pathological analysis
Tumor size was measured after formalin fixation. The specimens were assessed according to World Health Organization criteria (12) and the Japanese Society for Cancer of the Colon and Rectum guidelines (13). The histological grade and presence of regional lymph node metastasis were investigated by examining hematoxylin and eosin (H&E)-stained specimens. Lymphatic infiltration was evaluated by examining H&E-stained sections and/or sections immunostained with anti-D2-40 antibody (Dako North America, Carpinteria, CA, USA). Vascular infiltration was evaluated using double staining with H&E and/or Victoria blue (Muto Pure Chemicals, Tokyo, Japan). The histological grade was assigned according to the least differentiated tumor component. Poorly differentiated or mucinous carcinoma components were classified as present if any part of the lesion contained either of these features (14).
Assessment of MVD
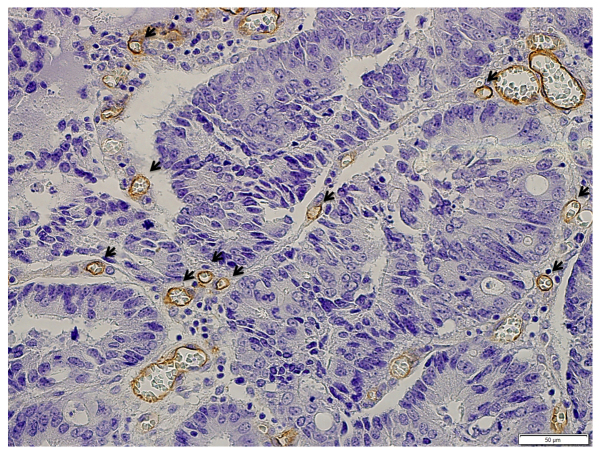
As previously reported, vessels stained with monoclonal antibodies to CD105 show three basic morphological features: Round-shape, sinusoid-like, and small vessels without discernible lumens (endothelial sprouts) (6). Only vessels with discernible lumens of a caliber smaller than approximately eight blood cells and without thick muscular walls were counted (15) (Fig. 1). The procedure used conforms with the international consensus method for assessing intratumoral MVD (16). After scanning the immunostained section at low magnifications (x40 and ×100), the three areas with the greatest number of distinctly highlighted microvessels (so-called hot spots) were selected. Microvessels at ×400 magnification were enumerated without knowledge of the patient's status. MVD is expressed as the mean number of vessels per high-power field (x400) in the three selected hot spots (6).
Figure 1.
Microvessels immunohistochemically stained with monoclonal antibodies to CD105. Monoclonal antibodies to CD105 create a brown circumference around angiogenetic vessels. As indicated by the arrows, vessels were counted.
Outcome measures and statistical analysis
Univariate analysis was first performed to identify associations between distant metastases and potential risk factors (17). The clinicopathological factors listed above were then analyzed using Fisher's exact test and Welch's t-test. A P-value of <0.05 was considered to indicate a statistically significant difference (14).
Stepwise multivariate logistic regression was then performed to determine which combination of variables provided the best estimate of the relative risk of distant metastasis. Odds ratios and 95% confidence intervals (CIs) were calculated after accounting for potential confounders. All data are presented as the mean ± standard deviation. Furthermore, receiver operating characteristic (ROC) analysis was performed to determine the optimal cut-off value for MVD. All analyses were performed using JMP®12 (SAS Institute, Cary, NC, USA).
Results
Total lesions
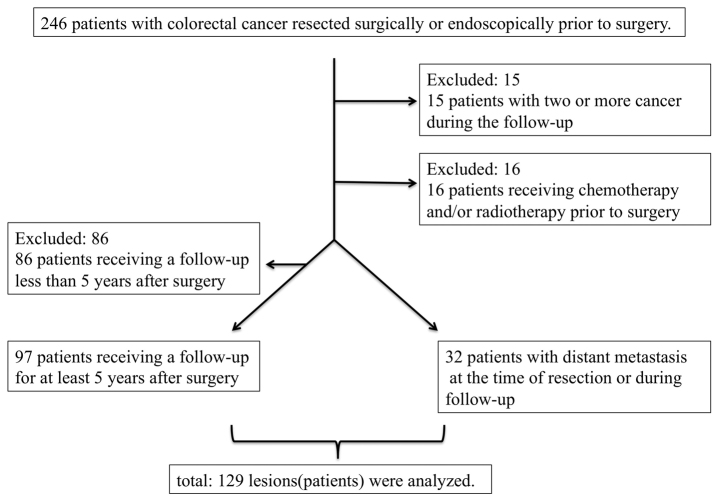
In this study, 129 lesions were analyzed. A flow chart showing application of the inclusion and exclusion criteria is shown in Fig. 2. The median follow-up time was 34 months (range, 6–85 months) in patients with distant metastasis and 61 months (range, 60–86 months) in patients without distant metastasis.
Figure 2.
Flow chart showing application of inclusion and exclusion criteria.
Distant metastases
At the time of resection or during follow-up, 32 patients had distant metastases (liver, n=13; lung, n=5; peritoneum, n=4; liver and peritoneum, n=3; lung and peritoneum, n=3; liver, lung and peritoneum, n=1; liver and bone, n=1; liver, lung, distant lymph node, and bone, n=1; and lung, kidney, and spleen, n=1). Thirteen patients had distant metastasis from the beginning of the study, and 19 patients had distant metastasis during follow-up.
Comparison of staining with monoclonal antibodies to CD105 and pan-endothelial antibodies for assessing microvessels
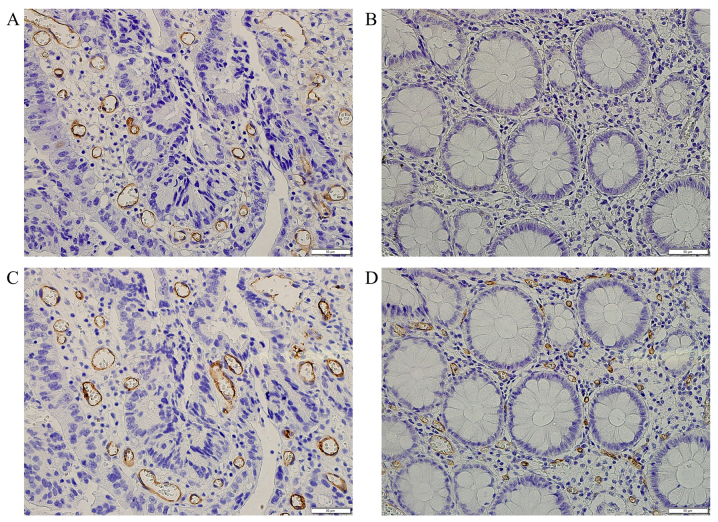
In normal areas, these vessels stained strongly with CD31, CD34, and von Willebrand factor antibodies, whereas they stained weakly or not at all with monoclonal antibodies to CD105. Fig. 3 shows typical examples of microvessel evaluation between normal and cancerous areas stained with monoclonal antibodies to CD105 and CD34.
Figure 3.
Comparison of immunohistochemical staining with monoclonal antibodies to CD105 and CD34 for quantitating microvessels between normal and cancerous areas. (A) CD105, in cancerous area; (B) CD105, in normal area; (C) CD34, in cancerous area; (D) CD34, in normal area. Microvessels stained with monoclonal antibodies to CD105 are either undetectable or only weakly present in normal areas whereas microvessels stained strongly with monoclonal antibodies to CD34 in normal areas at high magnification (x400).
Vessels stained with monoclonal antibodies to CD105
Fig. 1 shows staining of microvessels with monoclonal antibodies to CD105, as indicated by the brown circle lines around angiogenetic vessels. Only vessels with discernible lumens, of smaller caliber than approximately eight red blood cells, and without thick muscular walls were counted.
MVD and distant metastasis
Table I shows the associations of selected clinicopathological factors with distant metastasis. The MVD was 10.4±4.9 (mean ± standard deviation) in patients with distant metastases from colorectal cancer and 7.6±3.3 in those without distant metastases; this difference was significant according to the univariate analysis (P=0.008, Welch's t-test).
Table I.
Relationships between selected clinicopathological factors and distant metastasis.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Clinicopathological factors | No. of cases with metastasis (n=32) | No. of cases without metastasis (n=97) | OR (95% CI) | P-value | OR (95% CI) | P-value |
| Aged (≥70 years old/<70) | 14/18 | 38/59 | 1.21 (0.54–2.54) | 0.68a | ||
| Genderd (male/female) | 18/14 | 54/43 | 1.02 (0.46–2.46) | 1.00a | ||
| Tumor sized (mean ± SD) | 54.1±28.3 | 34.6±18.3 | N/A | <0.001b | 1.03 (1.01–1.01) | 0.007c |
| Tumor locationd (rectum/colon) | 12/20 | 24/73 | 1.83 (0.78–4.78) | 0.18a | ||
| Tumor depthd (T2-T4/T1) | 31/1 | 66/31 | 14.56 (1.90–111.90) | <0.001a | ||
| Lymphatic infiltrationd (+/−) | 25/7 | 54/43 | 2.84 (1.12–7.12) | 0.035a | ||
| Vascular infiltrationd (+/−) | 28/4 | 59/38 | 4.51 (1.46–13.46) | 0.0048a | ||
| Regional lymph node metastasisd (+/−) | 21/11 | 21/76 | 6.91 (2.88–16.88) | <0.001a | 4.76 (1.87–12.87) | 0.001c |
| Por/Muc componentd (+/−) | 9/23 | 22/75 | 1.33 (0.54–3.54) | 0.63a | ||
| Adjuvant chemo therapyd (+/−) | 15/17 | 23/74 | 2.84 (1.23–6.23) | 0.024a | ||
| MVDd (mean ± SD) | 10.4±4.9 | 7.6±3.3 | N/A | 0.0081b | 1.14 (1.02–1.02) | 0.033c |
Fisher's exact test
Welch's t-test
Stepwise multivariate logistic regression
Values are expressed as percentage at the 95% confidence interval. No, number; OR, odds ratio; CI, confidence interval; SD, standard deviation; Por, poorly differentiated adenocarcinoma; Muc, mucinous carcinoma; N/A, not applicable; MVD, microvessel density.
Associations between clinicopathological factors other than MVD and distant metastasis
According to the univariate analysis, there were also statistically significant differences in tumor size, tumor depth (T2-4 tumors), lymphatic infiltration, vascular infiltration, regional lymph node metastasis, and having received adjuvant chemotherapy between patients with and without metastases from colorectal cancer (Welch's t-test and Fisher's exact test) (Table I).
Stepwise multivariate logistic regression
According to stepwise multivariate logistic regression, MVD, regional lymph node metastasis, and tumor size were independent risk factors for distant metastases (Table I).
Optimal cut-off value for MVD for estimation of risk of distant metastasis
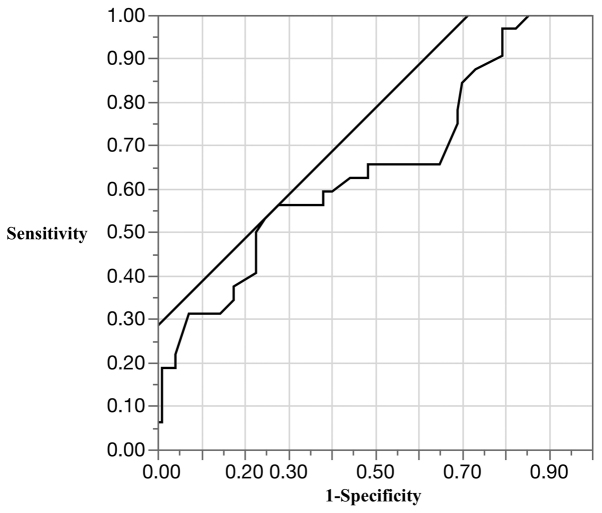
To facilitate clinical application of our findings, we determined the optimal cut-off value for MVD by ROC analysis (area under the curve, 0.65; cut-off value, 10 vessels/x400 field; sensitivity, 56.3%; specificity, 72.2%; and accuracy, 68.2%) (Fig. 4). Patients with cancers that had ≥10 vessels/x400 field of MVD were at significantly higher risk of distant metastasis than those with cancers that had <10 vessels/x400 field of MVD (P=0.005, Fisher's exact test).
Figure 4.
Receiver operating characteristic (ROC) analysis of microvessel density.
Discussion
In this study, we found that MVD, regional lymph node metastasis, and tumor size were significantly associated with distant metastases from colorectal cancer and that MVD is a more appropriate risk factor for distant metastasis than is lymphatic or vascular infiltration.
Because tumor growth and metastasis are dependent on angiogenesis (2,4), selected possible markers of angiogenesis have been investigated. It has been repeatedly found that CD105 is a better marker of tumor angiogenesis than pan-endothelial markers such as CD31, CD34, and von Willebrand factor (6,18,19). One of the reasons for this is that CD105 is an accessory receptor for transforming growth factor beta (TGF-β) (20,21). The main function of TGF-β is mediated through tyrosine/threonine kinase receptors on the cell surface, including TGF-β type II receptor, TGF-β type I receptor, and CD105. TGF-β is known to participate in angiogenesis by stimulating or inhibiting the activation of endothelial cells through a balance of activin-like kinase (ALK) 5 and ALK1 signaling. Phosphorylation of Smad1/5/8 after activation of ALK1 activates endothelial cells to migrate and proliferate (22). Immunostaining of tissue sections from various histological types of human tumors has shown that CD105 is strongly expressed in the endothelial cells of tumor blood vessels, but is either undetectable or only weakly present in blood vessels of most normal tissue (8). MVD estimated by monoclonal antibodies to CD105 can reportedly be used to differentiate between low-grade and high-grade dysplasia and between high-grade dysplasia and colorectal cancer. In contrast, MVD estimated by monoclonal antibodies to CD34 reportedly cannot be used to distinguish between these pathologies (9,20). MVD estimated by monoclonal antibodies to CD105 is inversely correlated with survival in patients with non-small cell lung cancer, hepatocellular cancer, and breast cancer, whereas MVD estimated by monoclonal antibodies to CD34 does not show this inverse correlation (8,20,23–25). Additionally, MVD estimated by monoclonal antibodies to CD105 correlates more strongly with the amount of vascular endothelial growth factor than does MVD estimated by monoclonal antibodies to CD31 or CD34 (20,24,26). Accordingly, the most frequently used immunohistochemical marker for identifying activated endothelial cells is CD105.
Angiogenesis is essential for tumor growth and metastasis. Therefore, angiogenesis is currently a target of cancer therapy. For example, bevacizumab is a humanized monoclonal antibody with a high affinity for circulating vascular endothelial growth factor A (27). Clinical trials of first- and second-line treatment of metastatic colorectal cancer have confirmed that the addition of bevacizumab to standard first-line chemotherapy regimens significantly improves patient outcomes (28–31); hence, it is important to investigate both the relationship between MVD and metastasis and potentially confounding clinicopathological factors. MVD and regional lymph node metastasis are reportedly risk factors for distant metastasis (6,17). However, data on potentially confounding factors such as lymphatic and vascular infiltration and tumor size are lacking. Another study has shown that lymphatic and vascular infiltration are risk factors for recurrence of colorectal cancer (32). We therefore expected that the stepwise multiple logistic regression analysis in the present study would confirm that lymphatic and vascular infiltration are risk factors for distant metastasis, but the analysis did not confirm this. One possible explanation for this apparent discrepancy is that generally, vascular infiltration reflects the risk of hematogenous metastasis and lymphatic infiltration reflects the risk of lymphatic metastasis. In this study, we investigated the risk of distant metastases, including hematogenous and lymphatic metastasis and peritoneal dissemination. One of the mechanisms of colorectal peritoneal dissemination is transcoelomic invasion by the primary cancer (33). Transcoelomic invasion implies prior primary tumor growth and current tumor growth. Angiogenesis is essential for tumor growth. Therefore, it is assumed that angiogenesis is essential for transcoelomic invasion. Moreover, it has been demonstrated that angiogenesis correlates with both hematogenous and lymphatic metastasis (7,17). Accordingly, it is conceivable that MVD is a more appropriate risk factor for distant metastasis than is lymphatic and vascular infiltration. This is a novel finding of the present study. Moreover, to facilitate the clinical application of our findings, we used an ROC curve to determine the optimal cut-off point for MVD (10 vessels/x400 field). The incidence of distant metastasis differed significantly between MVD above and below this cut-off point. The main objective of surveillance after curative resection of colorectal cancer is to detect recurrence early (32); intensive surveillance after curative resection of colorectal cancer reportedly improves prognosis (3). However, intensive surveillance is costly, and the optimal duration and frequency of surveillance has not yet been determined (32). Determination of appropriate risk factors for distant metastasis may help to identify patients who need intensive surveillance, thus improving the efficacy of follow-up.
Another problem is that the areas of a primary tumor with the highest MVD contain the cells that are most likely to disseminate systemically. These metastasizing cells are more likely to express the angiogenic phenotype than are cells escaping from areas with fewer microvessels. However, it has been shown that tumor cells that are not angiogenic may form dormant micrometastases that eventually switch to the angiogenic phenotype (34). In other words, dormant micrometastases may become angiogenic and grow long after resection of the original cancer (35). Thus, cancers with high MVD may harbor undetectable micrometastases, increasing the false-negative rate. This problem has not yet been resolved and thus further study is needed.
This study has some limitations. First, we excluded patients who had not been followed up for at least 5 years. Second, this was a single-center retrospective study with the inherent possibility of selection bias. Third, estimation of tumor budding in T1 colorectal cancer was not described. It was not an established outcome because only one patient had distant metastasis.
In conclusion, our analysis suggests that MVD estimated by monoclonal antibodies to CD105, regional lymph node metastasis, and tumor size are a more appropriate risk factors for distant metastasis from colorectal cancer than other potential confounding clinicopathological factors.
Acknowledgements
We would like to thank T. Nagai and Y. Sasaki of the Department of Pathology and Laboratory Medicine, Showa University School of Medicine for providing valuable assistance with the immunohistochemical analysis of the tissue samples. Edanz Group assisted with English language editing.
References
- 1.Haggar FA, Boushey RP. Colorectal cancer epidemiology: Incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg. 2009;22:191–197. doi: 10.1055/s-0029-1242458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cidon EU, Alonso P, Masters B. Markers of response to antiangiogenic therapies in colorectal cancer: Where are we now and what should be next? Clin Med Insights Oncol. 2016;10(Suppl 1):S41–S55. doi: 10.4137/CMO.S34542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Figueredo A, Rumble RB, Maroun J, Earle CC, Cummings B, McLeod R, Zuraw L, Zwaal C, Gastrointestinal Cancer Disease Site Group of Cancer Care Ontario's Program in Evidence-based Care Follow-up of patients with curatively resected colorectal cancer: A practice guideline. BMC Cancer. 2003;3:26. doi: 10.1186/1471-2407-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zetter BR. Angiogenesis and tumor metastasis. Annu Rev Med. 1998;49:407–424. doi: 10.1146/annurev.med.49.1.407. [DOI] [PubMed] [Google Scholar]
- 5.Kumar P, Wang JM, Bernabeu C. CD 105 and angiogenesis. J Pathol. 1996;178:363–366. doi: 10.1002/(SICI)1096-9896(199604)178:4<363::AID-PATH491>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 6.Romani AA, Borghetti AF, Del Rio P, Sianesi M, Soliani P. The risk of developing metastatic disease in colorectal cancer is related to CD105-positive vessel count. J Surg Oncol. 2006;93:446–455. doi: 10.1002/jso.20456. [DOI] [PubMed] [Google Scholar]
- 7.Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis-correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 8.Kumar S, Ghellal A, Li C, Byrne G, Haboubi N, Wang JM, Bundred N. Breast carcinoma: Vascular density determined using CD105 antibody correlates with tumor prognosis. Cancer Res. 1999;59:856–861. [PubMed] [Google Scholar]
- 9.Akagi K, Ikeda Y, Sumiyoshi Y, Kimura Y, Kinoshita J, Miyazaki M, Abe T. Estimation of angiogenesis with anti-CD105 immunostaining in the process of colorectal cancer development. Surgery. 2002;131(Suppl 1):S109–S113. doi: 10.1067/msy.2002.119361. [DOI] [PubMed] [Google Scholar]
- 10.Goldis DS, Sferdian MF, Tarţă C, Fulger LO, Totolici BD, Neamţu C. Comparative analysis of microvessel density quantified through the immunohistochemistry expression of CD34 and CD105 in rectal cancer. Rom J Morphol Embryol. 2015;56:419–424. [PubMed] [Google Scholar]
- 11.Ottaviano G, Cappellesso R, Mylonakis I, Lionello M, Favaretto N, Giacomelli L, Spoladore C, Marchese-Ragona R, Marino F, Staffieri A, et al. Endoglin (CD105) expression in sinonasal polyposis. Eur Arch Otorhinolaryngol. 2015;272:3367–3373. doi: 10.1007/s00405-014-3456-x. [DOI] [PubMed] [Google Scholar]
- 12.Hamilton SR. Carcinoma of the colon and rectum. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO Classification of Tumours of the Digestive System. IARC Press; Lyon: 2010. pp. 134–146. [Google Scholar]
- 13.Watanabe T, Itabashi M, Shimada Y, Tanaka S, Ito Y, Ajioka Y, Hamaguchi T, Hyodo I, Igarashi M, Ishida H, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2010 for the treatment of colorectal cancer. Int J Clin Oncol. 2012;17:1–29. doi: 10.1007/s10147-011-0315-2. [DOI] [PubMed] [Google Scholar]
- 14.Miyachi H, Kudo SE, Ichimasa K, Hisayuki T, Oikawa H, Matsudaira S, Kouyama Y, Kimura YJ, Misawa M, Mori Y, et al. Management of T1 colorectal cancers after endoscopic treatment based on the risk stratification of lymph node metastasis. J Gastroenterol Hepatol. 2016;31:1126–1132. doi: 10.1111/jgh.13257. [DOI] [PubMed] [Google Scholar]
- 15.Bosari S, Lee AK, DeLellis RA, Wiley BD, Heatley GJ, Silverman ML. Microvessel quantitation and prognosis in invasive breast carcinoma. Hum Pathol. 1992;23:755–761. doi: 10.1016/0046-8177(92)90344-3. [DOI] [PubMed] [Google Scholar]
- 16.Vermeulen PB, Gasparini G, Fox SB, Colpaert C, Marson LP, Gion M, Beliën JA, de Waal RM, van Marck E, Magnani E, et al. Second international consensus on the methodology and criteria of evaluation of angiogenesis quantification in solid human tumours. Eur J Cancer. 2002;38:1564–1579. doi: 10.1016/S0959-8049(02)00094-1. [DOI] [PubMed] [Google Scholar]
- 17.Saad RS, Liu YL, Nathan G, Celebrezze J, Medich D, Silverman JF. Endoglin (CD105) and vascular endothelial growth factor as prognostic markers in colorectal cancer. Mod Pathol. 2004;17:197–203. doi: 10.1038/modpathol.3800034. [DOI] [PubMed] [Google Scholar]
- 18.Seon BK, Takahashi N, Haba A, Matsuno F, Haruta Y, She XW, Harada N, Tsai H. Angiogenesis and metastasis marker of human tumors. Rinsho Byori. 2001;49:1005–1013. [PubMed] [Google Scholar]
- 19.Li C, Gardy R, Seon BK, Duff SE, Abdalla S, Renehan A, O'Dwyer ST, Haboubi N, Kumar S. Both high intratumoral microvessel density determined using CD105 antibody and elevated plasma levels of CD105 in colorectal cancer patients correlate with poor prognosis. Br J Cancer. 2003;88:1424–1431. doi: 10.1038/sj.bjc.6600874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nassiri F, Cusimano MD, Scheithauer BW, Rotondo F, Fazio A, Yousef GM, Syro LV, Kovacs K, Lloyd RV. Endoglin (CD105): A review of its role in angiogenesis and tumor diagnosis, progression and therapy. Anticancer Res. 2011;31:2283–2290. [PubMed] [Google Scholar]
- 21.Sica G, Lama G, Anile C, Geloso MC, La Torre G, de Bonis P, Maira G, Lauriola L, Jhanwar-Uniyal M, Mangiola A. Assessment of angiogenesis by CD105 and nestin expression in peritumor tissue of glioblastoma. Int J Oncol. 2011;38:41–49. [PubMed] [Google Scholar]
- 22.Park S, Sorenson CM, Sheibani N. PECAM-1 isoforms, eNOS and endoglin axis in regulation of angiogenesis. Clin Sci (Lond) 2015;129:217–234. doi: 10.1042/CS20140714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tabata M, Kondo M, Haruta Y, Seon BK. Antiangiogenic radioimmunotherapy of human solid tumors in SCID mice using (125)I-labeled anti-endoglin monoclonal antibodies. Int J Cancer. 1999;82:737–742. doi: 10.1002/(SICI)1097-0215(19990827)82:5<737::AID-IJC18>3.3.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka F, Otake Y, Yanagihara K, Kawano Y, Miyahara R, Li M, Yamada T, Hanaoka N, Inui K, Wada H. Evaluation of angiogenesis in non-small cell lung cancer: Comparison between anti-CD34 antibody and anti-CD105 antibody. Clin Cancer Res. 2001;7:3410–3415. [PubMed] [Google Scholar]
- 25.Yao Y, Pan Y, Chen J, Sun X, Qiu Y, Ding Y. Endoglin (CD105) expression in angiogenesis of primary hepatocellular carcinomas: Analysis using tissue microarrays and comparisons with CD34 and VEGF. Ann Clin Lab Sci. 2007;37:39–48. [PubMed] [Google Scholar]
- 26.Yao Y, Kubota T, Takeuchi H, Sato K. Prognostic significance of microvessel density determined by an anti-CD105/endoglin monoclonal antibody in astrocytic tumors: Comparison with an anti-CD31 monoclonal antibody. Neuropathology. 2005;25:201–206. doi: 10.1111/j.1440-1789.2005.00632.x. [DOI] [PubMed] [Google Scholar]
- 27.Allegra CJ, Yothers G, O'Connell MJ, Sharif S, Petrelli NJ, Colangelo LH, Atkins JN, Seay TE, Fehrenbacher L, Goldberg RM, et al. Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: Results of NSABP protocol C-08. J Clin Oncol. 2011;29:11–16. doi: 10.1200/JCO.2010.30.0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goel S, Duda DG, Xu L, Munn LL, Boucher Y, Fukumura D, Jain RK. Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev. 2011;91:1071–1121. doi: 10.1152/physrev.00038.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 30.Saltz LB, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: A randomized phase III study. J Clin Oncol. 2008;26:2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 31.Tebbutt NC, Wilson K, Gebski VJ, Cummins MM, Zannino D, van Hazel GA, Robinson B, Broad A, Ganju V, Ackland SP, et al. Capecitabine, bevacizumab, and mitomycin in first-line treatment of metastatic colorectal cancer: Results of the Australasian Gastrointestinal Trials Group Randomized Phase III MAX Study. J Clin Oncol. 2010;28:3191–3198. doi: 10.1200/JCO.2009.27.7723. [DOI] [PubMed] [Google Scholar]
- 32.Kobayashi H, Mochizuki H, Ishiguro M, Sugihara K. Risk factor for distant metastasis from colorectal cancer and surveillance. Frontier in Colorectal Cancer. 2008;4:264–268. (In Japanese) [Google Scholar]
- 33.Wang Y, Gong C, Yang L, Wu Q, Shi S, Shi H, Qian Z, Wei Y. 5-FU-hydrogel inhibits colorectal peritoneal carcinomatosis and tumor growth in mice. BMC Cancer. 2010;10:402. doi: 10.1186/1471-2407-10-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Folkman J. Seminars in Medicine of the Beth Israel Hospital, Boston. Clinical applications of research on angiogenesis. N Engl J Med. 1995;333:1757–1763. doi: 10.1056/NEJM199512283332608. [DOI] [PubMed] [Google Scholar]
- 35.Folkman J. Fighting cancer by attacking its blood supply. Sci Am. 1996;275:150–154. doi: 10.1038/scientificamerican0996-150. [DOI] [PubMed] [Google Scholar]