Abstract
Strategies for the treatment of cancer remain unsatisfactory due to the poor understanding of the complicated underlying molecular mechanisms of carcinogenesis. A number of types of cancer exhibit a marked association with dietary habits and lifestyles. Therefore, the modulation of dietary habits or lifestyles may be an effective strategy for preventing the formation and progression of cancer. Proteins and polypeptides from soybean have been developed as healthcare products due to their marked activity in inhibiting the progression of cancer at various stages. Lunasin, containing 43 amino acid residues, is one such example of a soybean-derived polypeptide that has been demonstrated to exhibit marked anti-cancer activity. In the present review, studies of the underlying molecular mechanisms and potential advantages of lunasin in the prevention and treatment of cancer have been examined, to provide a theoretical reference for the development of natural product-based agents or healthcare products for the prevention and treatment of cancer.
Keywords: lunasin, polypeptide, cancer prevention, dietary habit, healthcare product, anti-cancer mechanism
1. Introduction
Cancer is well-known to be one of the leading causes of mortality worldwide (1). However, the therapy of various types of cancer remains unsatisfactory due to the poor understanding of the complicated underlying molecular mechanisms of carcinogenesis. A number of epidemiological studies have demonstrated that a number of types of cancer exhibit a marked association with dietary habits and lifestyles (2–4). A statistical study has demonstrated that ~3.07 million individuals in China were diagnosed with cancer in 2012, which accounts for 1/5 of the total number of patients with cancer worldwide (5). Furthermore, ~2.21 million patients succumbed to cancer in China, which is ~25% of the total mortality worldwide due to cancer (5). Epidemiological evidence has demonstrated that cancer in 35% of patients exhibits a marked association with lifestyle, particularly diet (2). Therefore, the adjustment of dietary habits and lifestyles may be an effective strategy to prevent carcinogenesis. Cancer cell- and tumor-bearing animal models have demonstrated that the consumption of foods containing natural compounds with anti-cancer activity is able to markedly reduce the risk of cancer, and increase the sensitivity of cancer cells to treatment (6). A number of phytochemicals, including resveratrol, quercetin and flavonoids, have been demonstrated to exhibit marked anti-cancer activity (7–9). Similarly, foodborne proteins and polypeptides have attracted attention due to their specific advantages as anti-cancer substances (10,11). Compared with certain small-molecule drugs, polypeptides exhibit characteristics of increased affinity, marked ability of specific targeting and decreased toxicity; furthermore, polypeptides exhibit increased permeability in tissues compared with protein-based drugs (12). Therefore, polypeptides have been recognized as potential natural anti-cancer substances for inhibiting the development and progression of cancer at different stages (13).
Following extensive exploitation and utilization of natural products, soybean and other associated products have aroused interest from consumers due to their health-promoting benefits (14). Previous studies have demonstrated that increased soybean consumption in Asian populations is associated with decreased incidence of osteoporosis, cardiovascular disease and cancer (15–17). Furthermore, soybean products may reduce the risk of developing prostate and breast cancer (18,19). Soybean was demonstrated to contain a variety of bioactive compounds, including protease inhibitors, lunasin, sitosterol, saponins and isoflavones, with significant anti-cancer activity (20,21). Bowman-Birk protease inhibitor (BBI) and isoflavones have been investigated extensively (18,20,22). Similarly, lunasin, isolated and extracted from soybean or other similar plants, has been reported as a polypeptide with a clear function to inhibit chemical-induced carcinogenesis (14). In the present review, studies of the underlying molecular mechanisms and corresponding advantages of lunasin for the prevention and treatment of cancer have been systematically examined to provide a theoretical reference, for the development of natural product-based agents or healthcare products, for the prevention and treatment of cancer.
2. Structure of lunasin
Lunasin, a polypeptide with molecular mass of 5.5 kDa, is able to enter mammalian cells within min and target nuclei within 18 h (23). Lunasin contains 43 amino acid residues, which comprise a fragment with an unknown function (residues 1–22), a helical structure (residues 23–31) that is similar to the conserved structure of chromatin-binding protein for binding to histones H3/H4, a cell adhesion module of Arg-Gly-Asp (RGD) (residues 32–34) that functions as an extracellular matrix to promote the intracellular accessibility of lunasin and a critical fragment of nine aspartic acids at the C-terminus that is involved in anti-mitotic functions (23). The full sequence of lunasin is SKW QH QQD SCR KQL QGV NLT PCE KHI MEK IQG RDD DDD DDD DD and the fragments are depicted in Fig. 1 (14).
Figure 1.
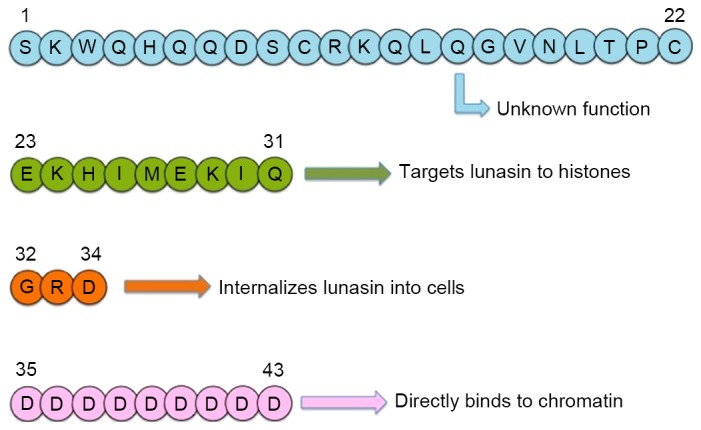
Sequence and structure of the fragments of lunasin. Figure previously published in (14).
3. Characterization of lunasin
Lunasin has been identified in soybean, barley, wheat and rye, and has been subjected to corresponding comparative analysis (24,25). However, due to the genotypic impact of different species, the lunasin content varies between species (26). The lunasin content in soybean, barley, wheat and rye was determined to be between 0.5 and 8.1, between 0.013 and 0.099, between 0.2 and 0.3, and between 0.045 and 0.150 mg/g seed, respectively (14,25,26). Therefore, soybean and wheat are recognized as the principal sources of lunasin (14).
The common extraction procedures for lunasin include crushing, defatting, extraction, dialysis, centrifugation and determination (14). This extraction method usually results in a crude protein containing lunasin, which requires further purification. Ion-exchange column chromatography has been used for the purification of lunasin extracted from soybean and barley in previous studies, and the principal material used in the ion-exchange column is bio-gel resin AG1-X4 with a particle size of between 100 and 200 mesh, and the volume of the ion-exchange column is 5.0×50 cm with a filled height of 40 cm (27,28). In addition, gel electrophoresis, western blotting and mass spectrometry are also used for the qualitative analysis and quantitative determination of lunasin (29,30).
4. Molecular mechanisms and advantages of lunasin, in the prevention and treatment of cancer
Advantages of lunasin in the prevention and treatment of cancer
An important characteristic of the ideal agents or health-promoting products for the prevention and treatment of cancer is their marked bioactivity in the inhibition of cancer cell generation and growth. Following oral administration of lunasin, it is absorbed into the bloodstream and reaches target tissues or organs in an active and stable state (31). Animal studies using rats fed on a soybean diet rich in lunasin demonstrated that the marked stability of lunasin enables it to reach target tissues and organs following administration, thus serving an important role in the prevention of cancer (24,32). The bioactivity of lunasin has been investigated in humans, and results have demonstrated that 4.5% lunasin at full activity is able to enter the plasma of healthy volunteers in 30 min (33). In spite of the marked bioactivity of lunasin following administration, another study has demonstrated that BBI is required to protect lunasin from digestion (34). This protective role provides the favorable conditions required for lunasin to perform its functions. In addition, its marked stability following administration may be due to its specific secondary or tertiary structure, which remains unclear and requires further study. These studies provide the scientific basis for understanding the functions of lunasin in the prevention and treatment of cancer, and provide a rationale for the development of lunasin-based health-promoting products or drugs used in the treatment of cancer.
Molecular mechanisms of lunasin in the prevention and treatment of cancer
A previous study has demonstrated that lunasin is able to suppress the proliferation and migration of cancer cells caused by chemical carcinogens without any impact on wild-type cells (35). The potential underlying molecular mechanisms of lunasin in the prevention and treatment of cancer have been systematically investigated. Lunasin exhibits anti-mitotic effects, and may prevent the mutation caused by the chemical carcinogen 7,12-dimethylaminobenzaldehyde (DMAB) and viral oncogene early region 1A (E1A) in mammals (36). When DMAB or methylcholanthrene is present alone, lunasin at between 10 nmol/l and 10 µmol/l, was able to inhibit the mutation of cells by between 62 and 90% (36). In retinoblastoma (Rb) and DMAB-induced mouse embryonic fibroblasts, lunasin was able to inhibit the expression of oncogenes, the acetylation of histone H3 and the migration of tumor cells, and regulate the cell cycle to induce apoptosis, thereby inhibiting tumor formation (36,37). In human breast cancer MDA-MB-231 cells, lunasin was able to reduce the expression of cyclin D1/D3, and down-regulate protein kinase Bα(PKBα) and activator protein 1 (AP-1), thereby inhibiting cell proliferation and inducing apoptosis (38,39). In human leukemia L1210 cells, lunasin was able to reduce cell proliferation and induce apoptosis (40,41). Similarly, in DMAB-induced mouse skin tumors, lunasin was able to decrease the number of tumors generated and delay the generation of tumors in skin (42,43). Therefore, lunasin has been identified as a promising polypeptide for preventing tumor formation induced by chemical carcinogens.
In addition to the above-mentioned inhibition of cancer cell proliferation, epigenetic mechanisms have also been specifically analyzed during the application of lunasin. Acetylation is one of the most important modifications in cancer signaling pathways (44,45). DNA damage and the disruption of chromatin transcription demonstrate a marked association with the occurrence of acetylation of specific lysine residues on histones (46). Under normal circumstances, histones H3 and H4 are in a deacetylated, or suppressed state. Therefore, the decreased activity of histone acetyltransferases (HATs), including lysine acetyltransferase 2A/2B and p300, is consistent with the decreased incidence of cancer, including colorectal and breast cancer (47–49). In previous studies, lunasin was demonstrated to competitively inhibit HATs, thereby inhibiting acetylation and regulating the cell cycle (24,32). The underlying molecular mechanisms of the binding of lunasin to histones remain unclear; however, they may be associated with the helical structure formed by the amino acid residues at positions 23–31, and the specific spatial structure or orientation of lunasin (42). However, the inhibitory activity of histone acetylation was also markedly associated with the concentration of lunasin. Lunasin at a concentration of 10 nmol/l was able to decrease the acetylation of histones by between 20 and 25%; by contrast, lunasin at a concentration <1 µmol/l was able to decrease the acetylation of histones H3 and H4 by 80 and 74%, respectively (50). Lunasin was able to act on cells at the stage of division or transformation, which is described by the E1A-Rb-histone deacetylase (HDAC) model (Fig. 2) (51). The tumor suppressor protein Rb interacted with eukaryotic initiation factor to supplement HDAC and maintain the deacetylated state of core histones (52). E1A resulted in the inactivation of Rb, thus leading to the disassociation of the Rb-HDAC complex and the exposure of acetylated histones (52). Following acetylation of HATs, lunasin and HATs competitively deacetylate histones to terminate transcription, thus leading to apoptosis. Therefore, lunasin is involved in the destruction of acetylation-deacetylation kinetics of histones (32). Deacetylated histones are absent from wild-type cells, so lunasin was not able to disrupt the proliferation or cause the apoptosis of wild-type cells. While monitoring the cell cycle process in the presence of lunasin, lunasin was able to be activated to selectively kill tumor cells following the transformation of the cells from wild-type cells to tumor cells, and it was demonstrated that lunasin exhibits a limited number of side effects compared with other drugs (32,53).
Figure 2.
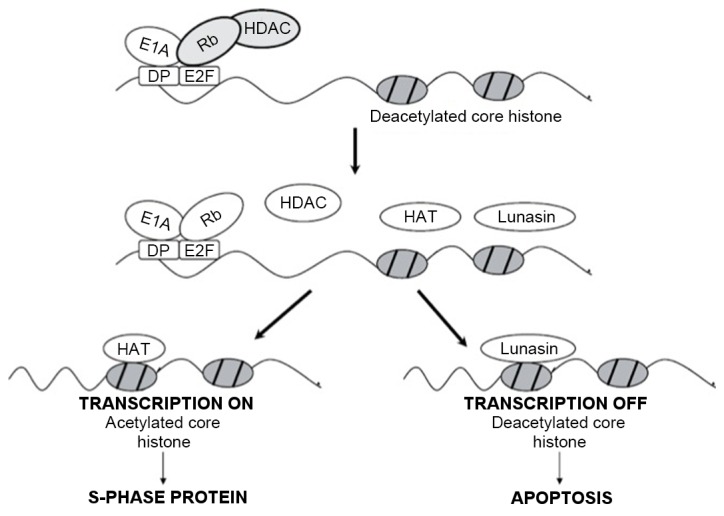
E1A-Rb-HDAC model describing the inhibition of E1A-induced cell transformation in the presence of lunasin. Figure previously published in (51). E1A, early region 1A; Rb, retinoblastoma; HDAC, histone deacetylase; DP, dimerization partner; E2F, transcription factor E2F; HAT, histone acetyltransferase.
Previous studies have demonstrated that chronic inflammation and oxygen free radicals are involved in degenerative diseases, inflammatory diseases, cardiovascular diseases and cancer (54,55). Therefore, anti-inflammation or antioxidation may be an important intervention strategy for the prevention and treatment of cancer (56). Lunasin was demonstrated to exhibit certain anti-inflammatory effects. i) Lunasin was able to inhibit the production of interleukin 6 (IL-6), tumor necrosis factor α and prostaglandin E2 (PGE2) in lipopolysaccharide (LPS)-induced RAW264.7 cells (57), and suppress the generation of IL-6 and IL-8, as well as suppress the secretion of matrix metalloproteinases in cultured rheumatoid arthritis synovial fibroblasts through the inhibition of nuclear factor kB activity (58). ii) Lunasin was also able to induce the inducible nitric oxide synthase/nitric oxide signaling pathway through adjustment of the cyclo-oxygenase 2/PGE2 ratio (59). iii) Lunasin was able to perform its antioxidant function through decreasing LPS-induced reactive oxygen species production (57), and prevent the oxidative damage of DNA by inhibiting the generation of hydroxyl free radicals (60). The anti-inflammatory and antioxidant functions of lunasin improve its potential use in the prevention and treatment of cancer.
Synergistic prevention and treatment of cancer using lunasin with other anti-cancer compounds
In order to achieve a synergistic effect on the prevention and treatment of cancer, the combinatorial application of two or more drugs is a commonly used strategy, which is able to increase the treatment efficacy and decrease the toxicity of the drugs (6). In the past two decades, various studies have demonstrated that aspirin exhibits clear inhibitory activity in chemical-induced carcinogenesis (61,62). However, the side effects of aspirin, including peptic ulcers, mucosal injury and bleeding, are evident (63). In order to use aspirin for its anti-carcinogenic effects and reduce its side effects, a number of studies have been conducted to explore the possibility of combinatorial application of aspirin and lunasin (37,39). As demonstrated by previous studies, the combinatorial application of aspirin and lunasin does not result in any safety issues (37,39). In addition, the combinatorial application of aspirin and lunasin resulted in a synergistic effect on the inhibition of proliferation and induction of apoptosis in human breast cancer MDA-MB-231 cells (39). This effect is primarily due to the down-regulation of PKBα, proto-oncogene Fos and AP-1 signaling genes through modulation of the genes that code for G1 and S phase regulatory proteins, thereby achieving a marked decrease in the side effects of aspirin (39). Furthermore, similar results have been observed in DMAB-induced fibroblast tumors in mice (37). However, the potential advantage of increasing anti-cancer activity and decreasing the side effects requires further study in animals and humans, and the underlying molecular mechanisms for the synergistic effects of lunasin and aspirin on the prevention and treatment of cancer require further clarification. Such studies may be of benefit for the development of a novel combinatorial treatment of cancer.
5. Conclusions
Numerous studies have demonstrated that lunasin may serve a role in the prevention and treatment of various types of cancer. However, the underlying molecular mechanisms of the prevention and treatment of different types of cancer or cancer stages, and the enhanced bioavailability of lunasin during oral administration remain unclear, and require further investigation or clarification using genomic or proteomic methods. In addition, the optimal fragment length, secondary or tertiary structure, or specific spatial orientation of lunasin in the prevention and treatment of cancer requires clarification. Furthermore, lunasin primarily exists in soybean, which is a principal agricultural product in China. Due to the potential activity in the prevention and treatment of cancer, and the prevention of cardiovascular diseases and inflammatory diseases, polypeptides, including lunasin from soybean, require investigation for the development of medical or healthcare products.
Acknowledgements
The present review was supported by the Natural Science Foundation of Hubei Province (grant no. 2014CFB613 received by X.C.), the Chutian Scholar Program, Hubei Superior Discipline Group of Physical Education and Health Promotion, and Outstanding Youth Scientific and Technological Innovation Team (grant no. T201624 received by N.C.) from Hubei Provincial Department of Education, and the National Natural Science Foundation of China (grant no. 31171738).
References
- 1.Razzaghi H, Quesnel-Crooks S, Sherman R, Joseph R, Kohler B, Andall-Brereton G, Ivey MA, Edwards BK, Mery L, Gawryszewski V, Saraiya M. Leading causes of cancer mortality-Caribbean region, 2003–2013. MMWR Morb Mortal Wkly Rep. 2016;65:1395–1400. doi: 10.15585/mmwr.mm6549a3. [DOI] [PubMed] [Google Scholar]
- 2.Manson MM. Cancer prevention-the potential for diet to modulate molecular signalling. Trends Mol Med. 2003;9:11–18. doi: 10.1016/S1471-4914(02)00002-3. [DOI] [PubMed] [Google Scholar]
- 3.Zhang SF, Wang XL, Yang XQ, Chen N. Autophagy-associated targeting pathways of natural products during cancer treatment. Asian Pac J Cancer Prev. 2014;15:10557–10563. doi: 10.7314/APJCP.2014.15.24.10557. [DOI] [PubMed] [Google Scholar]
- 4.Kou X, Kirberger M, Yang Y, Chen N. Natural products for cancer prevention associated with Nrf2-ARE pathway. Food Science and Human Wellness. 2013;2:22–28. doi: 10.1016/j.fshw.2013.01.001. [DOI] [Google Scholar]
- 5.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 6.de Kok TM, van Breda SG, Manson MM. Mechanisms of combined action of different chemopreventive dietary compounds: A review. Eur J Nutr. 2008;47(Suppl 2):S51–S59. doi: 10.1007/s00394-008-2006-y. [DOI] [PubMed] [Google Scholar]
- 7.Chen D, Dou QP. Tea polyphenols and their roles in cancer prevention and chemotherapy. Int J Mol Sci. 2008;9:1196–1206. doi: 10.3390/ijms9071196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang CS, Ju J, Lu G, Xiao H, Hao X, Sang S, Lambert JD. Cancer prevention by tea and tea polyphenols. Asia Pac J Clin Nutr. 2008;17(Suppl 1):S245–S248. [PMC free article] [PubMed] [Google Scholar]
- 9.Fimognari C, Lenzi M, Hrelia P. Chemoprevention of cancer by isothiocyanates and anthocyanins: Mechanisms of action and structure-activity relationship. Curr Med Chem. 2008;15:440–447. doi: 10.2174/092986708783503168. [DOI] [PubMed] [Google Scholar]
- 10.Udenigwe CC, Aluko RE. Food protein-derived bioactive peptides: Production, processing, and potential health benefits. J Food Sci. 2012;77:R11–R14. doi: 10.1111/j.1750-3841.2011.02455.x. [DOI] [PubMed] [Google Scholar]
- 11.Jia S, Du Z, Jiang H, Huang X, Chen Z, Chen N. Daintain/AIF-1 accelerates the activation of insulin-like growth factor-1 receptor signaling pathway in HepG2 cells. Oncol Rep. 2015;34:511–517. doi: 10.3892/or.2015.4002. [DOI] [PubMed] [Google Scholar]
- 12.Bhutia SK, Maiti TK. Targeting tumors with peptides from natural sources. Trends Biotechnol. 2008;26:210–217. doi: 10.1016/j.tibtech.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 13.de Mejia EG, Dia VP. The role of nutraceutical proteins and peptides in apoptosis, angiogenesis and metastasis of cancer cells. Cancer Metastasis Rev. 2010;29:511–528. doi: 10.1007/s10555-010-9241-4. [DOI] [PubMed] [Google Scholar]
- 14.Liu J, Jia SH, Kirberger M, Chen N. Lunasin as a promising health-beneficial peptide. Eur Rev Med Pharmacol Sci. 2014;18:2070–2075. [PubMed] [Google Scholar]
- 15.McCue P, Shetty K. Health benefits of soy isoflavonoids and strategies for enhancement: A review. Crit Rev Food Sci Nutr. 2004;44:361–367. doi: 10.1080/10408690490509591. [DOI] [PubMed] [Google Scholar]
- 16.Woo HD, Park S, Oh K, Kim HJ, Shin HR, Moon HK, Kim J. Diet and cancer risk in the Korean population: A meta- analysis. Asian Pac J Cancer Prev. 2014;15:8509–8519. doi: 10.7314/APJCP.2014.15.19.8509. [DOI] [PubMed] [Google Scholar]
- 17.Chi F, Wu R, Zeng YC, Xing R, Liu Y, Xu ZG. Post-diagnosis soy food intake and breast cancer survival: A meta-analysis of cohort studies. Asian Pac J Cancer Prev. 2013;14:2407–2412. doi: 10.7314/APJCP.2013.14.4.2407. [DOI] [PubMed] [Google Scholar]
- 18.Lee MM, Gomez SL, Chang JS, Wey M, Wang RT, Hsing AW. Soy and isoflavone consumption in relation to prostate cancer risk in China. Cancer Epidemiol Biomarkers Prev. 2003;12:665–668. [PubMed] [Google Scholar]
- 19.Yamamoto S, Sobue T, Kobayashi M, Sasaki S, S; Japan Public Health Center-Based Prospective Study on Cancer Cardiovascular Diseases G Tsugane. Soy, isoflavones, and breast cancer risk in Japan. J Natl Cancer Inst. 2003;95:906–913. doi: 10.1093/jnci/95.12.906. [DOI] [PubMed] [Google Scholar]
- 20.Messina M, Barnes S. The role of soy products in reducing risk of cancer. J Natl Cancer Inst. 1991;83:541–546. doi: 10.1093/jnci/83.8.541. [DOI] [PubMed] [Google Scholar]
- 21.Toyomura K, Kono S. Soybeans, soy foods, isoflavones and risk of colorectal cancer: A review of experimental and dpidemiological data. Asian Pac J Cancer Prev. 2002;3:125–132. [PubMed] [Google Scholar]
- 22.Clemente A, Mdel C Arques. Bowman-Birk inhibitors from legumes as colorectal chemopreventive agents. World J Gastroenterol. 2014;20:10305–10315. doi: 10.3748/wjg.v20.i30.10305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galvez AF, de Lumen BO. A soybean cDNA encoding a chromatin-binding peptide inhibits mitosis of mammalian cells. Nat Biotechnol. 1999;17:495–500. doi: 10.1038/8676. [DOI] [PubMed] [Google Scholar]
- 24.Jeong HJ, Jeong JB, Kim DS, Park JH, Lee JB, Kweon DH, Chung GY, Seo EW, de Lumen BO. The cancer preventive peptide lunasin from wheat inhibits core histone acetylation. Cancer Lett. 2007;255:42–48. doi: 10.1016/j.canlet.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 25.Jeong HJ, Lee JR, Jeong JB, Park JH, Cheong YK, de Lumen BO. The cancer preventive seed peptide lunasin from rye is bioavailable and bioactive. Nutr Cancer. 2009;61:680–686. doi: 10.1080/01635580902850082. [DOI] [PubMed] [Google Scholar]
- 26.de Mejia E Gonzalez, Vásconez M, de Lumen BO, Nelson R. Lunasin concentration in different soybean genotypes, commercial soy protein, and isoflavone products. J Agric Food Chem. 2004;52:5882–5887. doi: 10.1021/jf0496752. [DOI] [PubMed] [Google Scholar]
- 27.Jeong HJ, Lam Y, de Lumen BO. Barley lunasin suppresses ras-induced colony formation and inhibits core histone acetylation in mammalian cells. J Agric Food Chem. 2002;50:5903–5908. doi: 10.1021/jf0256945. [DOI] [PubMed] [Google Scholar]
- 28.Jeong HJ, Park JH, Lam Y, de Lumen BO. Characterization of lunasin isolated from soybean. J Agric Food Chem. 2003;51:7901–7906. doi: 10.1021/jf034460y. [DOI] [PubMed] [Google Scholar]
- 29.Rizzello CG, Nionelli L, Coda R, Gobbetti M. Synthesis of the cancer preventive peptide lunasin by lactic acid bacteria during sourdough fermention. Nutr Cancer. 2012;64:111–120. doi: 10.1080/01635581.2012.630159. [DOI] [PubMed] [Google Scholar]
- 30.Guijarro-Díez M, García MC, Crego AL, Marina ML. Off-line two dimensional isoelectrofocusing-liquid chromatography/mass spectrometry (time of flight) for the determination of the bioactive peptide lunasin. J Chromatogr A. 2014;1371:117–124. doi: 10.1016/j.chroma.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 31.Hsieh CC, Hernándz-Ledesma B, Jeong HJ, Park JH, de Lumen BO. Complementary roles in cancer prevention: Protease inhibitor makes the cancer preventive peptide lunasin bioavailable. PLoS One. 2010;5:e8890. doi: 10.1371/journal.pone.0008890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jeong JB, Jeong HJ, Park JH, Lee SH, Lee JR, Lee HK, Chung GY, Choi JD, de Lumen BO. Cancer-preventive peptide lunasin from Solanum nigrum L. inhibits acetylation of core histones H3 and H4 and phosphorylation of retinoblastoma protein (Rb) J Agric Food Chem. 2007;55:10707–10713. doi: 10.1021/jf062405u. [DOI] [PubMed] [Google Scholar]
- 33.Dia VP, Torres S, de Lumen BO, Erdman JW, Jr, De Mejia EG. Presence of lunasin in plasma of men after soy protein consumption. J Agric Food Chem. 2009;57:1260–1266. doi: 10.1021/jf803303k. [DOI] [PubMed] [Google Scholar]
- 34.Park JH, Jeong HJ, Lumen BO. In vitro digestibility of the cancer-preventive soy peptides lunasin and BBI. J Agric Food Chem. 2007;55:10703–10706. doi: 10.1021/jf072107c. [DOI] [PubMed] [Google Scholar]
- 35.Jiang Q, Pan Y, Cheng Y, Li H, Liu D, Li H. Lunasin suppresses the migration and invasion of breast cancer cells by inhibiting matrix metalloproteinase-2/-9 via the FAK/Akt/ERK and NF-kB signaling pathways. Oncol Rep. 2016;36:253–262. doi: 10.3892/or.2016.4798. [DOI] [PubMed] [Google Scholar]
- 36.Hsieh CC, Hernandez-Ledesma B, de Lumen BO. Soybean peptide lunasin suppresses in vitro and in vivo 7,12-dimethylbenz[a]anthracene-induced tumorigenesis. J Food Sci. 2010;75:H311–H316. doi: 10.1111/j.1750-3841.2010.01861.x. [DOI] [PubMed] [Google Scholar]
- 37.Hsieh CC, Hernandez-Ledesma B, de Lumen BO. Lunasin-aspirin combination against NIH/3T3 cells transformation induced by chemical carcinogens. Plant Foods Hum Nutr. 2011;66:107–113. doi: 10.1007/s11130-011-0229-1. [DOI] [PubMed] [Google Scholar]
- 38.Hernandez-Ledesma B, Hsieh CC, de Lumen BO. Relationship between lunasin's sequence and its inhibitory activity of histones H3 and H4 acetylation. Mol Nutr Food Res. 2011;55:989–998. doi: 10.1002/mnfr.201000632. [DOI] [PubMed] [Google Scholar]
- 39.Hsieh CC, Hernandez-Ledesma B, de Lumen BO. Lunasin, a novel seed peptide, sensitizes human breast cancer MDA-MB-231 cells to aspirin-arrested cell cycle and induced apoptosis. Chem Biol Interact. 2010;186:127–134. doi: 10.1016/j.cbi.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 40.Wang W, Bringe NA, Berhow MA, de Mejia E Gonzalez. beta-Conglycinins among sources of bioactives in hydrolysates of different soybean varieties that inhibit leukemia cells in vitro. J Agric Food Chem. 2008;56:4012–4020. doi: 10.1021/jf8002009. [DOI] [PubMed] [Google Scholar]
- 41.de Mejia EG, Wang W, Dia VP. Lunasin, with an arginine-glycine-aspartic acid motif, causes apoptosis to L1210 leukemia cells by activation of caspase-3. Mol Nutr Food Res. 2010;54:406–414. doi: 10.1002/mnfr.200900073. [DOI] [PubMed] [Google Scholar]
- 42.Galvez AF, Chen N, Macasieb J, de Lumen BO. Chemopreventive property of a soybean peptide (lunasin) that binds to deacetylated histones and inhibits acetylation. Cancer Res. 2001;61:7473–7478. [PubMed] [Google Scholar]
- 43.Hsieh EA, Chai CM, de Lumen BO, Neese RA, Hellerstein MK. Dynamics of keratinocytes in vivo using HO labeling: A sensitive marker of epidermal proliferation state. J Invest Dermatol. 2004;123:530–536. doi: 10.1111/j.0022-202X.2004.23303.x. [DOI] [PubMed] [Google Scholar]
- 44.Dwarakanath BS, Verma A, Bhatt AN, Parmar VS, Raj HG. Targeting protein acetylation for improving cancer therapy. Indian J Med Res. 2008;128:13–21. [PubMed] [Google Scholar]
- 45.Dalvai M, Bystricky K. The role of histone modifications and variants in regulating gene expression in breast cancer. J Mammary Gland Biol Neoplasia. 2010;15:19–33. doi: 10.1007/s10911-010-9167-z. [DOI] [PubMed] [Google Scholar]
- 46.Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- 47.Muraoka M, Konishi M, Kikuchi-Yanoshita R, Tanaka K, Shitara N, Chong JM, Iwama T, Miyaki M. p300 gene alterations in colorectal and gastric carcinomas. Oncogene. 1996;12:1565–1569. [PubMed] [Google Scholar]
- 48.Gayther SA, Batley SJ, Linger L, Bannister A, Thorpe K, Chin SF, Daigo Y, Russell P, Wilson A, Sowter HM, et al. Mutations truncating the EP300 acetylase in human cancers. Nat Genet. 2000;24:300–303. doi: 10.1038/73536. [DOI] [PubMed] [Google Scholar]
- 49.Ionov Y, Matsui S, Cowell JK. A role for p300/CREB binding protein genes in promoting cancer progression in colon cancer cell lines with microsatellite instability; Proc Natl Acad Sci USA; 2004; pp. 1273–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jeong HJ, Jeong JB, Kim DS, de Lumen BO. Inhibition of core histone acetylation by the cancer preventive peptide lunasin. J Agric Food Chem. 2007;55:632–637. doi: 10.1021/jf062405u. [DOI] [PubMed] [Google Scholar]
- 51.Hernandez-Ledesma B, de Lumen BO. Lunasin: A novel cancer preventive seed Peptide. Perspect Medicin Chem. 2008;2:75–80. doi: 10.4137/pmc.s372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frolov MV, Dyson NJ. Molecular mechanisms of E2F-dependent activation and pRB-mediated repression. J Cell Sci. 2004;117:2173–2181. doi: 10.1242/jcs.01227. [DOI] [PubMed] [Google Scholar]
- 53.de Lumen BO. Lunasin: A novel cancer preventive seed peptide that modifies chromatin. J AOAC Int. 2008;91:932–935. [PubMed] [Google Scholar]
- 54.Kuper H, Adami HO, Trichopoulos D. Infections as a major preventable cause of human cancer. J Intern Med. 2000;248:171–183. doi: 10.1046/j.1365-2796.2000.00742.x. [DOI] [PubMed] [Google Scholar]
- 55.Allavena P, Garlanda C, Borrello MG, Sica A, Mantovani A. Pathways connecting inflammation and cancer. Curr Opin Genet Dev. 2008;18:3–10. doi: 10.1016/j.gde.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 56.Villegas I, Sanchez-Fidalgo S, Alarcón de la Lastra C. New mechanisms and therapeutic potential of curcumin for colorectal cancer. Mol Nutr Food Res. 2008;52:1040–1061. doi: 10.1002/mnfr.200700280. [DOI] [PubMed] [Google Scholar]
- 57.Hernandez-Ledesma B, Hsieh CC, de Lumen BO. Antioxidant and anti-inflammatory properties of cancer preventive peptide lunasin in RAW 264.7 macrophages. Biochem Biophys Res Commun. 2009;390:803–808. doi: 10.1016/j.bbrc.2009.10.053. [DOI] [PubMed] [Google Scholar]
- 58.Jia S, Zhang S, Yuan H, Chen N. Lunasin inhibits cell proliferation via apoptosis and reduces the production of proinflammatory cytokines in cultured rheumatoid arthritis synovial fibroblasts. Biomed Res Int. 2015;2015:346839. doi: 10.1155/2015/346839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang W, Dia VP, Vasconez M, de Mejia EG, Nelson RL. Analysis of soybean protein-derived peptides and the effect of cultivar, environmental conditions, and processing on lunasin concentration in soybean and soy products. J AOAC Int. 2008;91:936–946. [PubMed] [Google Scholar]
- 60.Jeong JB, De Lumen BO, Jeong HJ. Lunasin peptide purified from Solanum nigrum L. protects DNA from oxidative damage by suppressing the generation of hydroxyl radical via blocking fenton reaction. Cancer Lett. 2010;293:58–64. doi: 10.1016/j.canlet.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 61.Jacobo-Herrera NJ, Pérez-Plasencia C, Camacho-Zavala E, González GF, Urrutia EL, García-Castillo V, Zentella-Dehesa A. Clinical evidence of the relationship between aspirin and breast cancer (review) Oncol Rep. 2014;32:451. doi: 10.3892/or.2014.3270. [DOI] [PubMed] [Google Scholar]
- 62.Giovannucci E, Chan AT. Role of vitamin and mineral supplementation and aspirin use in cancer survivors. J Clin Oncol. 2010;28:4081–4085. doi: 10.1200/JCO.2009.27.3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Laine L. Review article: Gastrointestinal bleeding with low-dose aspirin-what's the risk? Aliment Pharmacol Ther. 2006;24:897–908. doi: 10.1111/j.1365-2036.2006.03077.x. [DOI] [PubMed] [Google Scholar]


