Abstract
There is a lack of prospective data about second-line treatments for metastatic pancreatic ductal adenocarcinoma patients. This is partially due to recent changes in first-line chemotherapy treatments. Despite this dearth of information, 50.0% of the patients who experience failure with first-line folinic acid, 5-fluorouracil, irinotecan and oxaliplatin (folfirinox) treatment are eligible for additional chemotherapy. In this setting, gemcitabine is widely used without any standard recommendations available. The present study evaluated 42 patients who received gemcitabine subsequent to a first-line treatment of folfirinox between January 2008 and December 2012 at the Centre Léon Bérard (Lyon, France). Clinical data, biological data and tumor characteristics were retrospectively analyzed to identify prognostic factors for successful treatment with gemcitabine. In total, 11 patients (26.2%) experienced control of their cancer with gemcitabine treatment. However, there was no predictive marker for their response to the drug. The median overall survival was 3.6 months from gemcitabine initiation [95% confidence interval (CI), 2.1–5.1]. The median length of gemcitabine treatment was 1.5 months (95% CI, 0.3–13.3). Among the 11 patients who were successfully treated with gemcitabine, 6 were resistant to first-line folfirinox treatment. Patients who were non responsive to folfirinox had a higher probability of success with gemcitabine compared with patients that responded to folfirinox (54.5 vs. 21.4%, respectively; P=0.061). The present study did not identify any clinical or biological marker with a predictive value for successful gemcitabine treatment. Furthermore, successful gemcitabine treatment was not correlated with patients' response to first-line folfirinox treatment. This suggests an absence of cross-resistance in the chemotherapy protocols and provides evidence for effective cancer treatment with the second-line gemcitabine therapy.
Keywords: adenocarcinoma, pancreas, gemcitabine, second-line chemotherapy, predictive factors
Introduction
Pancreatic adenocarcinoma is the sixth cause of cancer-associated mortalities worldwide (1). The majority of patients with pancreatic adenocarcinoma present with an unresectable tumor or with metastatic disease, which lead to a 5-year survival rate of ~5%. Since the mortality rate remains close to the incidence rate, this is a particularly dreaded form of cancer (1). For years, gemcitabine was the front-line chemotherapy for the advanced disease due to its effects on quality of life and overall survival (OS) (2,3). In 2011, folinic acid, fluorouracil (5-FU), irinotecan and oxaliplatin (folfirinox) was observed to produce better outcomes compared with those of the standard gemcitabine chemotherapy (median OS, 11.1 vs. 6.8 months, respectively; P<0.001) (4). Henceforth, folfirinox became the first-line treatment for patients with advanced pancreatic adenocarcinoma who had a good Eastern Cooperative Oncology Group (ECOG) performance status (5). More recently, Von Hoff et al reported an improved outcome when nanoparticle albumin-bound (nab)-paclitaxel was combined with gemcitabine (GemBrax) compared with that of gemcitabine treatment alone (6).
When gemcitabine was the standard first-line treatment, an oxaliplatin-based chemotherapy was usually proposed as the second-line chemotherapy (7). Indeed, based on promising results from phase II studies (8–10), a randomized-phase III study demonstrated that the median survival time upon failure of first-line gemcitabine treatment increased to 21 weeks with oxaliplatin/folinic acid/5-fluorouracil treatment and best supportive care (BSC) compared with only 10 weeks in patients receiving BSC alone (7). Other studies have reported different experiences with second-line treatments subsequent to gemcitabine, with modest efficacy for patients who still have a good ECOG performance status (Table I).
Table I.
Studies available about second-line therapies after a first-line treatment of gemcitabine for pancreatic cancer.
| Author, year | Trial | First-line chemotherapy | Second-line chemotherapy | Patients, n | Response rate, % | Median PFS, months (range) | Median OS, months (range) | PFS at 6 months, % | OS at 6 months, % | OS at 1 year, % | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pelzer et al, 2011 | Phase III CONKO-003 | GEM | OFF vs. FF | 46 | 4.82 vs. 2.3 | 23 vs. 10 | 10 vs. 0 | (7) | |||
| Tsavaris et al, 2005 | Phase II | GEM | 5-FU/AF/OX | 30 | 23.3 (PR) 30 (SD) | 5.3 (3–4.7) | 5.8 (4.7–7.7) | (8) | |||
| Cantore et al, 2004 | Phase II | GEM-containing treatment | IRI plus OX | 30 | 10 | 4.1 (0.7–13.1) | 5.9 | 23.3 | (9) | ||
| Demols et al, 2006 | Phase II | GEM | GEMOX | 33 | 22.6 (PR) | 4.2 | 6 (0.5–21) | (10) | |||
| Boeck et al, 2006 | Phase II | GEM | Pemetrexed | 52 | 3.8 19.2 (SD) | 1.6 (0.25–14.5) | 4.7 (0.25–19.6) | (14) | |||
| Kulke et al, 2007 | Phase II | GEM | CAP plus erlotinib | 30 | 10 (PR) | 3.4 | 6.5 | 26 | (15) | ||
| Xiong et al, 2008 | Phase II | GEM | CAP plus OX | 41 | 2.6 (PR) 26 (SD) | 2.3 | 5.7 | 44 | 21 | (37) | |
| Reni et al, 2008 | Retrospective study | GEM | PEFG | 46 | 24 (PR) | 5 | 8.3 | 34 | 26 | (38) | |
| Boeck et al, 2007 | Phase II | GEM | CAP | 39 | 0 (PR) 15 (SD) | 2.3 (0.5–45.1) | 7.6 (0.7–45.1) | (39) | |||
| Reni et al, 2006 | Phase II | GEM | Raltitrexed plus OX | 41 | 24 (PR) 11 (SD) | 1.8 | 5.2 | 14.6 | 12 | (40) | |
| Burris et al, 2005 | Phase II | Any prior treatment | Rubitecan | 58 | 7 | 3 | 17 | 9 | (41) | ||
| Jacobs et al, 2004 | Phase III | Any prior treatment | Rubitecan | 196 | 11 (PR) 38 (SD) | 1.9 | 3.5 | (42) | |||
| Ulrich-pur et al, 2003 | Phase II | GEM | IRI plus raltitrexed vs. raltitrexed | 38 | 16 vs. 0 | 4 vs. 2.5 | 6.5 vs. 4.3 | (43) | |||
| Kozuch et al, 2001 | Retrospective study | Any prior treatment | G-FLIP | 34 | 24 (PR) 21 (SD) | 3.9 | 10.3 | (44) | |||
| Altwegg, 2012 | Retrospective study | GEM | All second-line therapies | 80 | 40 (PR+SD) | 3.4 | 5.8 | 13.6 | (45) | ||
| Wang-Gillam et al, 2016 | Phase III NAPOLI-1 | GEM-containing treatment | Nanoliposomal IRI plus FF vs. FF | 417 | 16 (PR) vs. 1 (PR) | 3.1 vs. 1.5 | 6.1 vs. 4.2 | (46) |
OFF, oxaliplatin, 5-fluorouracil and folinic acid; 5-FU/FA or FF, 5-fluorouracil and folinic acid; CAP, capecitabine; OX, oxaliplatin; IRI, irinotecan; PEFG, cisplatin, epirubicin, gemcitabine and 5-fluorouracil; G-FLIP, gemcitabin, folinic acid, 5-flurorouracil and irinotecan; GEM, gemcitabin; GEMOX, gemcitabine and oxaliplatin; 5-FU, 5-fluorouracil; PFS, progression-free survival; OS, overall survival; PR, partial response; SD, stable disease.
In the phase III study by Conroy et al (4), 47% of patients who experienced treatment failure with folfirinox received a second-line therapy. In the phase III study by Von Hoff et al (6), 38% of the nab-paclitaxel and gemcitabine treatment group, and 42% of the gemcitabine alone group received second-line chemotherapy. Notably, 6% of the gemcitabine group received second-line treatments of nab-paclitaxel and gemcitabine, and these patients had a longer survival rate than the patients receiving gemcitabine treatment alone (median survival, 9.4 vs. 6.8 months, respectively; P<0.001).
Several scenarios could account for these data. First, patients usually experience a decline in their ECOG performance status, which may limit the therapeutic options for second-line treatment (11). Second, the survival benefit of second-line treatments is clinically questionable. Certain data are in favor of second-line therapy (12), whereas others do not encourage its use (13). Based on phase II data, a median survival of 4–6 months after the initiation of second-line treatment may be achieved with salvage chemotherapy in selected patients (14–16).
Folfirinox is currently the first-line treatment for metastatic pancreatic cancer in our center (Centre Léon Bérard, Lyon, France). For second-line therapy, patients are usually treated with gemcitabine alone when clinical trials are unavailable. The present study aimed to retrospectively analyze this treatment approach, which has not been validated in a phase III randomized study, in order to evaluate the clinical benefit of this strategy and to identify any clinical or biological characteristics that could predict the treatment outcome.
Patients and methods
Patients
The present study retrospectively reviewed 42 consecutive cases of advanced-stage pancreatic adenocarcinoma in patients who were treated with gemcitabine as the second-line chemotherapy (following initial treatment with folfirinox) between January 2008 and December 2012 at the Centre Léon Bérard (Lyon, France). Folfirinox was administered in accordance with the study regimen reported by Conroy et al (4): Treatment was provided for 6 months (12 cycles) or until disease progression or unmanageable toxicity occurred. When 6 months of folfirinox was achieved, discontinuation was proposed. At the point of disease progression, folfirinox reintroduction was considered for first-line continuation and this treatment was included in the total number of folfirinox cycles that were received. Clinical (age, sex, history of recent diabetes, thromboembolic events, and body mass index and its variation during chemotherapy) and biological [hemoglobin, total bilirubin, lymphocyte levels, carbohydrate antigen 19–9 (CA19-9) and carcinoembryonic antigen (CEA) levels, and their variation during chemotherapy] data, as well as tumor characteristics [pancreas localization, Union for International Cancer Control staging (17) at diagnosis, number of metastatic sites, presence of skip metastasis and computed tomography (CT)-scan evaluation every 2 months] were collected prior to or during gemcitabine treatment to elucidate any parameter with a predictive value on survival. Patients who had previous surgery and adjuvant chemotherapy were excluded. All patients provided their informed consent for inclusion in the study and an external Ethics Committee (Comité de Protection des Personnes LYON SUD-EST IV) gave its approval for the project on 16th December 2015.
The side effects of gemcitabine were graded using the Common Toxicity Criteria defined (Common Terminology Criteria for Adverse Events scale developed by Eastern Cooperative Oncology Group and the National Cancer Institute with last version 4.03 released in 2010; http://evs.nci.nih.gov/ftp1/CTCAE/About.html). Tumor objective response was monitored every 2 months with a CT-scan using the Response Evaluation Criteria in Solid Tumors (18), physical examination and assessment of blood tumor markers. Control of the disease was defined as achieving a complete response, partial response or disease stabilization while on chemotherapy. OS was measured from the initiation of treatment to the date of mortality for any reason or to the last follow-up assessment.
Statistical analysis
Descriptive statistics are presented as medians and ranges for the quantitative data, and as proportions for the qualitative data. Survival data are presented as Kaplan-Meier curves. OS was defined as the time from the initiation of gemcitabine chemotherapy to mortality (whatever the cause). Patients alive at the last date of follow-up were censored.
Patients were defined as ‘responders’ [responders group (RG)] to gemcitabine chemotherapy if the disease was under control (complete or partial response, or tumor stability) during the first evaluation of treatment efficacy (2 months after initiation). Patients were defined as ‘non-responders’ if disease progression or mortality was experienced prior to the first evaluation [non-responders group (NRG)]. The association between response (yes/no) and potential predictive factors was studied using the χ2 or Fisher's exact tests for qualitative variables, and the nonparametric Wilcoxon test for quantitative variables. Due to the small sample size, a multivariate analysis was not performed. In all cases, P<0.05 was considered to indicate a statistically significant difference, and a P-value between 0.05 and 0.1 was considered to indicate a trend. All statistical analyses were performed using SAS version 9.3 software (SAS Institute, Cary, NC, USA). The statistical methods used in the present study were reviewed by Miss Nadia Oussaid (a biomedical statistician at the Centre Léon Bérard).
Results
Patient characteristics
Among the 42 patients enrolled in the present study, 22 (52.4%) were males (Table II). The majority of patients (90.5%) had a good performance status (PS) (0 or 1) at diagnosis. In total, 50.0% of the tumors were localized in the pancreatic head, 26.2% in the body and the remaining tumors in the tail. In total, 5 patients (12.0%) had a recent diagnosis of diabetes (<2 years), while 8 patients (19.1%) had jaundice at their first consultation; the obstruction was relieved through an endoscopic retrograde cholangiopancreatography with stent insertion (14.3%) or biliodigestive surgical derivation (4.8%). At diagnosis, 95.2% of patients had metastatic cancer (stage 4): 28 patients (70.0%) had 1 metastatic site; 11 patients (27.5%) had 2 metastatic sites; and 1 patient had 3 metastatic sites. In addition, 5 patients (12.0%) had lung metastasis without any liver localization.
Table II.
Clinical characteristics of patients with pancreatic adenocarcinoma (n=42).
| Characteristic | Patients, n (%) |
|---|---|
| Median age (range), years | 63.5 (47–76) |
| Sex ratio, male/female | 22/20 |
| TNM stage at diagnosis, n (%) | |
| 4 | 40 (95.2) |
| 3 | 0 (0.0) |
| 2 | 2 (4.8) |
| Metastatic sites at diagnosis, n (%) | |
| 0 | 2 (4.8) |
| 1 | 28 (66.6) |
| 2 | 11 (26.2) |
| 3 | 1 (2.4) |
| TNM stage at the start of second-line gemcitabine, n (%) | |
| 4 | 41 (97.6) |
| 3 | 0 (0.0) |
| 2 | 1 (2.4) |
TNM, tumor-node-metastasis.
Treatments
First-line folfirinox treatment
On average, 9.4 cycles of folfirinox were administered as the first-line treatment (range, 2–36 cycles). A total of 21 patients received 6 months of folfirinox and discontinued chemotherapy for 3.2 months (range, 0–20 months) before disease progression occurred. Overall, folfirinox treatment induced disease stability or a partial response in 27 patients (69.2%). During the first-line treatment, 16 patients (38.1%) had progressed prior to receiving 6 months of folfirinox, and 7 patients (16.7%) had tumor progression at the 6-month evaluation. Second-line chemotherapy was introduced for patients whose disease had progressed (83.3%) or who experienced toxicity (14.3%); a single patient (2.4%) requested early treatment discontinuation following 8 cycles (without limiting toxicity and despite partial response). In total, 5 patients (12.5%) received >6 months of folfirinox: 2 did not have discontinuation and experienced progressive disease following 16 and 17 cycles of treatment, respectively (oxaliplatin was interrupted due to neurotoxicity following 14 cycles and 10 cycles, respectively), while 3 patients had a therapeutic interruptions. Of these 3 patients, 1 patient received folfirinox again following an interruption of 4 months, with long disease control; the other 2 patients had a 6-month and a 20-month interruption, respectively, prior to reintroduction of first-line chemotherapy with folfiri (folinic acid, 5-fluorouracil, irinotecan) only (due to residual neurotoxicity to oxaliplatin), resulting in no benefit on disease control. The disease control offered by folfirinox reintroduction led to another 4-month therapeutic interruption following 12 additional cycles. The disease was controlled again at the third time of folfirinox treatment, but progression occurred following 9 cycles.
Second-line gemcitabine treatment
Gemcitabine treatment was initiated with a median time of 1.75 months (range, 0–20 months) upon termination of folfirinox treatment. Overall, gemcitabine treatment was well tolerated (Table III). A single grade 4 thrombocytopenia was recorded. In total, 6 patients experienced a maximum of grade 2 toxicity (4 had thrombocytopenia, 1 had arthromyalgia and 2 had anemia). In addition, 4 other patients had a maximum of grade 3 neutropenia, and 1 patient discontinued gemcitabine treatment due to asthenia.
Table III.
Treatment characteristics in pancreatic adeno-carcinoma patients (n=42).
| Treatment | Patients, n (%) |
|---|---|
| Folfirinox followed by GEM, total number of chemotherapy regimens received | |
| 2 | 42 (100.0) |
| 3 | 17 (40.5) |
| 4 | 3 (7.0) |
| Folfirinox response at 2 months | |
| Disease controlled (disease stabilization or partial response) | 28 (66.6) |
| Disease progression | 12 (28.6) |
| NA | 2 (4.8) |
| Treatment interruption following first-line therapy | |
| Patients, n (%) | 12 (28.6) |
| Time to second-line therapy, months | 3.4 |
| GEM response at 2 months | |
| Disease controlled (disease stabilization or partial response) | 11 (26.0) |
| Disease progression | 31 (74.0) |
| GEM toxicity, maximum grade observed | |
| 1 | 5 (11.9) |
| 2 | 6 (14.3) |
| 3 | 6 (14.3) |
| Treatment interruption | 1 (2.4) |
GEM, gemcitabine; NA, not available.
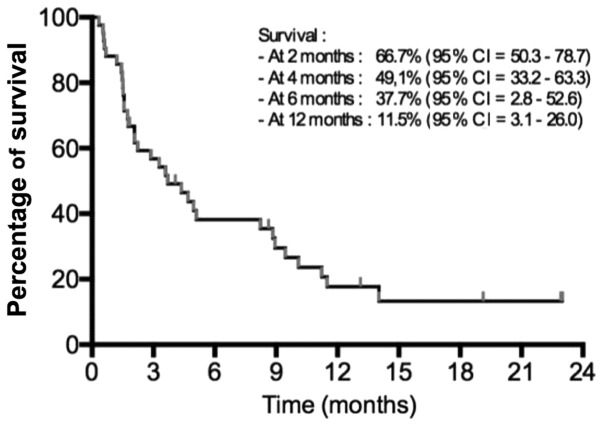
The median follow-up for the second-line treatment was 5.8 months (range, 0.3–25.5 months). From the 42 patients, 39 (92.9%) succumbed at the cut-off analysis time. The median OS was 3.6 months [95% confidence interval (CI), 2.1–5.1] after starting the second-line chemotherapy (Fig. 1). The median OS from the diagnosis was 13.4 months (range, 3.3–30.7 months).
Figure 1.
Overall survival following second-line chemotherapy initiation.
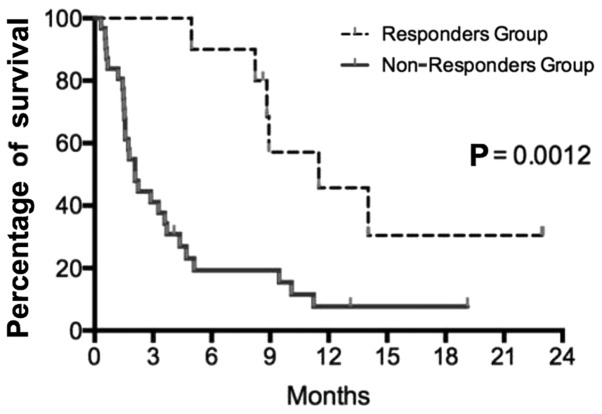
A median of 4.5 gemcitabine infusions were administered (range, 1–40 infusions); the median length of the treatment was 1.5 months (range, 0.3–13.3 months). After 2 months of gemcitabine chemotherapy, only 11 patients (26.2%) had the disease under control (mainly stable disease) and continued with treatment. Disease control at the first evaluation was the only identified significant prognostic factor for OS (P=0.0012) (Fig. 2). A total of 31 patients (74.0%) experienced disease progression at the first evaluation.
Figure 2.
Survival following gemcitabine initiation in the responders and non- responders groups. Log-rank analyses identified the first evaluation as a surrogate marker for survival.
Clinical, biological and tumor data analyses
The present study attempted to identify predictive biological or clinical characteristics explaining gemcitabine's efficacy. General characteristics such as PS were evaluated. This parameter was not different between the two groups; all the patients had a PS of 0–1 in the RG, and 27/31 patients (87.0%) had the same score in the NRG (P=0.350). The body mass index prior to the second-line treatment or its variation during therapy did not differ between the RG and NRG. The overall median body mass index preceding the second-line chemotherapy was 23.34 kg/m2 (range, 17.20–36.20 kg/m2). Similarly, the age at diagnosis and sex were not different between the two groups.
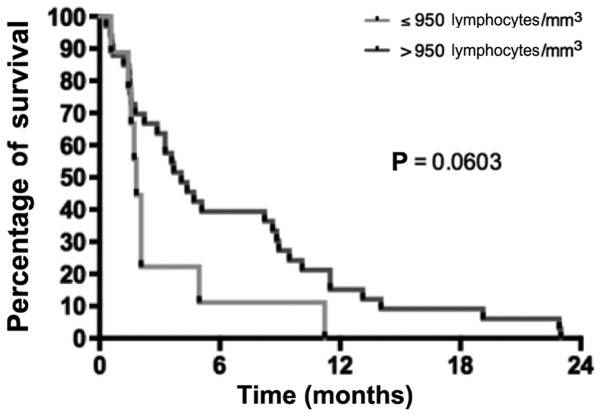
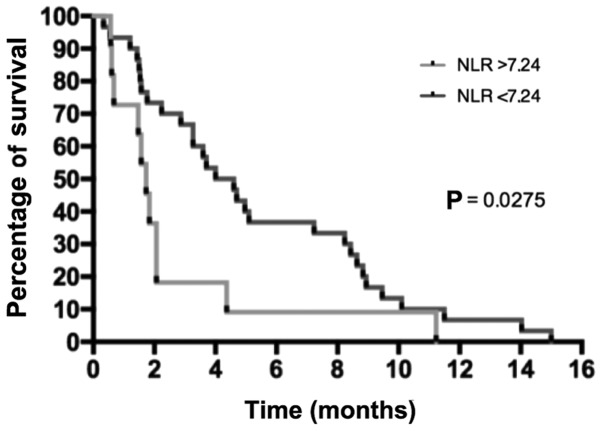
Various biological parameters were also compared between the two groups. The albumin level at diagnosis tended to be higher in the RG than in the NRG (median, 41 g/l in RG vs. 35 g/l in NRG; P=0.063). The lymphocyte levels prior to the second-line chemotherapy were not different between the NRG and RG (the mean lymphocyte levels were 1,349.5 and 1,593.8 lymphocytes/mm3, respectively; P=0.640). A value of 950 lymphocytes/mm3 was used as the cut-off to separate RG and NRG patients, and it was observed that patients with lymphocyte levels <950 lymphocytes/mm3 had poorer survival than patients with lymphocyte levels above this cut-off (Fig. 3). The neutrophil/lymphocyte ratio (NLR) preceding the second-line chemotherapy was 7.24 and 3.56 in RG and NRG, respectively, suggesting a trend between lower NLR and RG (P=0.200), while a significant higher survival percentage was observed in patients with a NLR <7.24 (P=0.030) (Fig. 4). The tumor burden was also analyzed. The number of metastatic sites prior to initiating the second-line treatment tended to be higher in the NRG than in the RG, although the difference was not significant (90.0% of RG patients had 1 metastatic site vs. 63.3% of patients in the NRG; P=0.420). There was also a trend for higher average CEA levels in the NRG than in the RG (156.3×10−6 vs. 44.3×10−6 g/l, respectively; P=0.330). The mean CA19-9 levels did not differ between the two groups (24,311.6 kU/l in the RG vs. 17,109 kU/l in the NRG; P=0.250).
Figure 3.
Survival as a function of the lymphocyte levels prior to second-line therapy.
Figure 4.
Survival as a function of NLR prior to second-line therapy. NLR, neutrophile/lymphocyte ratio.
Patients with primary resistance to folfirinox
Of the 11 patients in the RG, 6 exhibited primary resistance to folfirinox and had early disease progression. Patients whose disease progressed during folfirinox treatment had a higher probability of responding to gemcitabine (54.5 vs. 21.4%, respectively; P=0.061; Table IV). By contrast, only 3/15 patients whose disease was well controlled with folfirinox (20.0%) experienced any disease control with second-line gemcitabine treatment. In the RG, of the 6 patients who exhibited primary resistance to folfirinox, 2 received a prolonged benefit under gemcitabine treatment, and their cancer remained under control for 6 months.
Table IV.
Correlation between folfirinox and gemcitabine responses in pancreatic adenocarcinoma patients (n=42).
| Gemcitabine | ||||
|---|---|---|---|---|
| Folfirinox | Controlled disease, n (%) | Resistant disease, n (%) | Total, n (%) | Fisher's exact test |
| Controlled disease | 5 (45.5) | 22 (78.6) | 27 (69.2) | P=0.061 |
| Resistant disease | 6 (54.5) | 6 (21.4) | 12 (30.8) | |
Third-line treatments
Of the patients whose disease progressed while receiving gemcitabine, 19 patients (45.0%) were administered a third-line chemotherapy regimen and 3 patients received a fourth-line chemotherapy treatment (Table III). Among these patients, 15 received >1 cycle of therapy, while 8 patients received >3 cycles. During the third-line treatment, only 2 patients experienced prolonged disease control with gemcitabine-oxaliplatin (15 cycles prior to disease progression) and with folinic acid, 5-FU and oxaliplatin (14 cycles prior to discontinuation and surveillance). The latter patients experienced disease progression after 6 months of treatment, but were able to control the cancer with 5-FU and folinic acid combination prior to discontinuation and surveillance.
Discussion
There is a lack of prospective data about second-line treatments for metastatic pancreatic ductal adenocarcinoma (PDAC) patients. This is partially due to the recent changes in first-line chemotherapy treatments (4,19). Despite this dearth of information, a significant proportion of PDAC patients are eligible for second-line therapy. In the Action to Control Cardiovascular Risk in Diabetes 11 study, 47% of patients who received folfirinox were eligible for second-line chemotherapy, and the majority of them received gemcitabine (4). In the Metastatic Pancreatic Adenocarcinoma Clinical Trial, 38% of patients received second-line chemotherapy (19). The published data that suggest that second-line chemotherapy is beneficial are mainly derived from studies on gemcitabine-refractory patients (20,21).
When folfirinox treatment fails to improve a patient's cancer, gemcitabine monotherapy appears to be a convenient treatment option due to its safety profile (3); however, there are no prospective data available or studies planned to address its efficacy according to the website (https://clinicaltrials.gov/). The present study reports the findings on a single center cohort of patients who received second-line gemcitabine treatment for advanced-stage pancreatic adenocarcinoma. A survival of 3.6 months with second-line chemotherapy was observed, which is in agreement with previous published data (Table I) (7–10,14,15,22–31). Response to gemcitabine therapy at the first follow-up evaluation (at 2 months) impacted significantly the OS of the patients.
Notably, the present study demonstrated that gemcitabine was able to control cancer in patients who were resistant to folfirinox treatment, suggesting that there is no cross-resistance between folfirinox and gemcitabine regimens. Indeed, it was observed that patients who were resistant to first-line folfirinox treatment tended to respond well to gemcitabine treatment (54.5 vs. 21.4%, respectively). These data strengthen the argument for gemcitabine treatment, particularly if the patient displays primary resistance to folfirinox, and also support the requirement for more effective drug combinations.
Predictive factors of successful gemcitabine treatment were also analyzed; however, no predictive biological markers were identified, which may be due to the small cohort size. CA19-9 level did not display a predictive value during second-line treatment, whereas it does have a predictive value for first-line treatment (11). Other biomarkers were not evaluated, including the Glasgow prognostic score or its modified version, which is an inflammation-based prognostic score using standard laboratory measurements of albumin and C-reactive protein (32,33). This score is able to identify systemic inflammatory responses responsible for poor survival due to tumor growth stimulation and catabolic effects on the host's metabolism at every stage of the disease for resectable, unresectable and metastatic pancreatic cancer (33–35). The measurement of pre-treatment plasma circulating DNA KRAS mutation load and CA19-9 level has also been recently shown to be a strong prognostic factor for PDAC patients who receive gemcitabine or folfirinox (36). It has also been suggested that human equilibrative nucleoside transporter 1 (hENT1) expression may select patients for gemcitabine treatment (37). However, there are issues regarding the evaluation of its expression by immunohistochemistry, since two different antibodies are usually employed: 10D7G2 monoclonal murine antibody (not commercialized) and SP120 rabbit monoclonal antibody (commercialized by Ventana Medical Systems, Inc., Tucson, AZ, USA). All studies using the above murine antibody demonstrated a significant predictive value in response to gemcitabine in an adjuvant setting only (38–40) whereas those using the aforementioned rabbit monoclonal antibody did not (41). Data on advanced pancreatic cancer are scarce, with only two studies published to date, both of which used the SP120 rabbit monoclonal antibody to measure hENT1 expression, and no evidence of predictive value was identified (42,43). Thus, the role of hENT1 as a predictive marker of gemcitabine efficacy remains unclear, particularly in a metastatic pancreatic cancer setting.
The main limitations of the present study are its retrospective design and the small sample size. Another limitation is the use of a monotherapy treatment. Various clinicians use a dual-therapy regimen such as gemcitabine and cisplatin or GemBrax subsequent to first-line folfirinox treatment, despite the lack of evidence regarding its efficacy from prospective studies (31–33). In France, reimbursement for nab-paclitaxel is not yet available, thus limiting its prescription. Bertocchi et al (44), Portal et al (45) and Palacio et al (46) recently reported the results of a GemBrax regimen for second-line therapy following a gemcitabine- or pyrimidine-based treatment (including folfirinox). However, definitive conclusions could not be drawn due to the retrospective design of the studies and the lack of a control arm. New drug combinations with gemcitabine are currently under study in phase II trials as mentioned in https://clinicaltrials.gov/, but the choice of the control arm in future phase III studies remains open. Furthermore, second-line chemotherapy may have a significant effect on the OS results in phase III trials. Therefore, it appears to be essential to report these second-line treatments in phase III trials that evaluate first-line therapies in order to analyze any differences in the OS results.
In conclusion, the use of gemcitabine as a second-line treatment is well tolerated in PDAC patients, and may offer a small benefit to their OS (median, 3.6 months; 95% CI, 2.1–5.1), particularly in patients whose disease progressed during first-line folfirinox treatment. The management of metastatic PDAC patients has recently evolved, as the results of new chemotherapy regimens (folfirinox and GemBrax) have shown significant benefits over gemcitabine alone for first-line treatments (4,19). These therapeutic advances provide an opportunity for clinicians to explore new strategic approaches. Therefore, second-line treatments must be prospectively evaluated in order to draw formal conclusions about their efficacy.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Burris H, Storniolo AM. Assessing clinical benefit in the treatment of pancreas cancer: Gemcitabine compared to 5-fluorouracil. Eur J Cancer. 1997;33(Suppl 1):S18–S22. doi: 10.1016/S0959-8049(96)00324-3. [DOI] [PubMed] [Google Scholar]
- 3.Burris HA, III, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 4.Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 5.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the eastern cooperative oncology group. Am J Clin Oncol. 1982;5:649–655. doi: 10.1097/00000421-198212000-00014. [DOI] [PubMed] [Google Scholar]
- 6.von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pelzer U, Schwaner I, Stieler J, Adler M, Seraphin J, Dörken B, Riess H, Oettle H. Best supportive care (BSC) versus oxaliplatin, folinic acid and 5-fluorouracil (OFF) plus BSC in patients for second-line advanced pancreatic cancer: A phase III-study from the German CONKO-study group. Eur J Cancer. 2011;47:1676–1681. doi: 10.1016/j.ejca.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 8.Tsavaris N, Kosmas C, Skopelitis H, Gouveris P, Kopterides P, Loukeris D, Sigala F, Zorbala-Sypsa A, Felekouras E, Papalambros E. Second-line treatment with oxaliplatin, leucovorin and 5-fluorouracil in gemcitabine-pretreated advanced pancreatic cancer: A phase II study. Invest New Drugs. 2005;23:369–375. doi: 10.1007/s10637-005-1446-y. [DOI] [PubMed] [Google Scholar]
- 9.Cantore M, Rabbi C, Fiorentini G, Oliani C, Zamagni D, Iacono C, Mambrini A, Del Freo A, Manni A. Combined irinotecan and oxaliplatin in patients with advanced pre-treated pancreatic cancer. Oncology. 2004;67:93–97. doi: 10.1159/000080993. [DOI] [PubMed] [Google Scholar]
- 10.Demols A, Peeters M, Polus M, Marechal R, Gay F, Monsaert E, Hendlisz A, Van Laethem JL. Gemcitabine and oxaliplatin (GEMOX) in gemcitabine refractory advanced pancreatic adenocarcinoma: A phase II study. Br J Cancer. 2006;94:481–485. doi: 10.1038/sj.bjc.6602966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bauer TM, El-Rayes BF, Li X, Hammad N, Philip PA, Shields AF, Zalupski MM, Bekaii-Saab T. Carbohydrate antigen 19–9 is a prognostic and predictive biomarker in patients with advanced pancreatic cancer who receive gemcitabine-containing chemotherapy: A pooled analysis of 6 prospective trials. Cancer. 2013;119:285–292. doi: 10.1002/cncr.27734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagrial A, Chin VT, Sjoquist K, Chantrill LA, Yip D. Survival benefit of second-line chemotherapy in advanced pancreatic adenocarcinoma: A systematic review of the literature. J Clin Oncol. 2014;32:296. doi: 10.1200/jco.2014.32.3_suppl.296. [DOI] [Google Scholar]
- 13.Rahma OE, Duffy A, Liewehr DJ, Steinberg SM, Greten TF. Second-line treatment in advanced pancreatic cancer: A comprehensive analysis of published clinical trials. Ann Oncol. 2013;24:1972–1979. doi: 10.1093/annonc/mdt166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boeck S, Weigang-Köhler K, Fuchs M, Kettner E, Quietzsch D, Trojan J, Stötzer O, Zeuzem S, Lordick F, Köhne CH, et al. Second-line chemotherapy with pemetrexed after gemcitabine failure in patients with advanced pancreatic cancer: A multicenter phase II trial. Ann Oncol. 2007;18:745–751. doi: 10.1093/annonc/mdl463. [DOI] [PubMed] [Google Scholar]
- 15.Kulke MH, Blaszkowsky LS, Ryan DP, Clark JW, Meyerhardt JA, Zhu AX, Enzinger PC, Kwak EL, Muzikansky A, Lawrence C, Fuchs CS. Capecitabine plus erlotinib in gemcitabine-refractory advanced pancreatic cancer. J Clin Oncol. 2007;25:4787–4792. doi: 10.1200/JCO.2007.11.8521. [DOI] [PubMed] [Google Scholar]
- 16.Boeck S, Heinemann V. Second-line therapy in gemcitabine-pretreated patients with advanced pancreatic cancer. J Clin Oncol. 2008;26:1178–1179. doi: 10.1200/JCO.2007.15.3304. [DOI] [PubMed] [Google Scholar]
- 17.Sobin LH, Gospodarowicz M, Wittekind C, editors. International Union Against Cancer (UICC), corp-author TNM Classification of Malignant Tumours. 7th. John Wiley & Sons, Ltd.; Chichester, West Sussex: 2009. [Google Scholar]
- 18.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European organization for research and treatment of cancer, national cancer institute of the United States, national cancer institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 19.Goldstein D, El-Maraghi RH, Hammel P, Heinemann V, Kunzmann V, Sastre J, Scheithauer W, Siena S, Tabernero J, Teixeira L, et al. nab-Paclitaxel plus gemcitabine for metastatic pancreatic cancer: Long-term survival from a phase III trial. J Natl Cancer Inst. 2015;107 doi: 10.1093/jnci/dju413. pii: dju413. [DOI] [PubMed] [Google Scholar]
- 20.Gresham GK, Wells GA, Gill S, Cameron C, Jonker DJ. Chemotherapy regimens for advanced pancreatic cancer: A systematic review and network meta-analysis. BMC Cancer. 2014;14:471. doi: 10.1186/1471-2407-14-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaddis N, Saif MW. Second-line treatment for pancreatic cancer. JOP. 2014;15:344–347. doi: 10.6092/1590-8577/2691. [DOI] [PubMed] [Google Scholar]
- 22.Xiong HQ, Varadhachary GR, Blais JC, Hess KR, Abbruzzese JL, Wolff RA. Phase 2 trial of oxaliplatin plus capecitabine (XELOX) as second-line therapy for patients with advanced pancreatic cancer. Cancer. 2008;113:2046–2052. doi: 10.1002/cncr.23810. [DOI] [PubMed] [Google Scholar]
- 23.Reni M, Cereda S, Mazza E, Passoni P, Nicoletti R, Balzano G, Zerbi A, Arcidiacono PG, Staudacher C, Di Carlo V. PEFG (cisplatin, epirubicin, 5-fluorouracil, gemcitabine) regimen as second-line therapy in patients with progressive or recurrent pancreatic cancer after gemcitabine-containing chemotherapy. Am J Clin Oncol. 2008;31:145–150. doi: 10.1097/COC.0b013e31814688f7. [DOI] [PubMed] [Google Scholar]
- 24.Boeck S, Wilkowski R, Bruns CJ, Issels RD, Schulz C, Moosmann N, Laessig D, Haas M, Golf A, Heinemann V. Oral capecitabine in gemcitabine-pretreated patients with advanced pancreatic cancer. Oncology. 2007;73:221–227. doi: 10.1159/000127413. [DOI] [PubMed] [Google Scholar]
- 25.Reni M, Pasetto L, Aprile G, Cordio S, Bonetto E, Dell'Oro S, Passoni P, Piemonti L, Fugazza C, Luppi G, et al. Raltitrexed-eloxatin salvage chemotherapy in gemcitabine-resistant metastatic pancreatic cancer. Br J Cancer. 2006;94:785–791. doi: 10.1038/sj.bjc.6603026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burris HA, III, Rivkin S, Reynolds R, Harris J, Wax A, Gerstein H, Mettinger KL, Staddon A. Phase II trial of oral rubitecan in previously treated pancreatic cancer patients. Oncologist. 2005;10:183–190. doi: 10.1634/theoncologist.10-3-183. [DOI] [PubMed] [Google Scholar]
- 27.Jacobs AD, Burris HA, Rivkin S, Ritch PS, Eisenberg PD, Mettinger KL. A randomized phase III study of rubitecan (ORA) vs. best choice (BC) in 409 patients with refractory pancreatic cancer report from a North-American multi-center study. J Clin Oncol. 2004;22:4013–4013. doi: 10.1200/jco.2004.22.14_suppl.4013. [DOI] [Google Scholar]
- 28.Ulrich-Pur H, Raderer M, Kornek G Verena, Schüll B, Schmid K, Haider K, Kwasny W, Depisch D, Schneeweiss B, Lang F, Scheithauer W. Irinotecan plus raltitrexed vs raltitrexed alone in patients with gemcitabine-pretreated advanced pancreatic adenocarcinoma. Br J Cancer. 2003;88:1180–1184. doi: 10.1038/sj.bjc.6600883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kozuch P, Grossbard ML, Barzdins A, Araneo M, Robin A, Frager D, Homel P, Marino J, DeGregorio P, Bruckner HW. Irinotecan combined with gemcitabine, 5-fluorouracil, leucovorin, and cisplatin (G-FLIP) is an effective and noncrossresistant treatment for chemotherapy refractory metastatic pancreatic cancer. Oncologist. 2001;6:488–495. doi: 10.1634/theoncologist.6-6-488. [DOI] [PubMed] [Google Scholar]
- 30.Altwegg R, Ychou M, Guillaumon V, Thezenas S, Senesse P, Flori N, Mazard T, Caillo L, Faure S, Samalin E, Assenat E. Second-line therapy for gemcitabine-pretreated advanced or metastatic pancreatic cancer. World J Gastroenterol. 2012;18:1357–1364. doi: 10.3748/wjg.v18.i12.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang-Gillam A, Li CP, Bodoky G, Dean A, Shan YS, Jameson G, Macarulla T, Lee KH, Cunningham D, Blanc JF, et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): A global, randomised, open-label, phase 3 trial. Lancet. 2016;387:545–557. doi: 10.1016/S0140-6736(15)00986-1. [DOI] [PubMed] [Google Scholar]
- 32.Proctor MJ, Horgan PG, Talwar D, Fletcher CD, Morrison DS, McMillan DC. Optimization of the systemic inflammation-based Glasgow prognostic score: A Glasgow Inflammation Outcome Study. Cancer. 2013;119:2325–2332. doi: 10.1002/cncr.28018. [DOI] [PubMed] [Google Scholar]
- 33.Glen P, Jamieson NB, McMillan DC, Carter R, Imrie CW, McKay CJ. Evaluation of an inflammation-based prognostic score in patients with inoperable pancreatic cancer. Pancreatology. 2006;6:450–453. doi: 10.1159/000094562. [DOI] [PubMed] [Google Scholar]
- 34.La Torre M, Nigri G, Cavallini M, Mercantini P, Ziparo V, Ramacciato G. The Glasgow prognostic score as a predictor of survival in patients with potentially resectable pancreatic adenocarcinoma. Ann Surg Oncol. 2012;19:2917–2923. doi: 10.1245/s10434-012-2348-9. [DOI] [PubMed] [Google Scholar]
- 35.Shimoda M, Katoh M, Kita J, Sawada T, Kubota K. The Glasgow prognostic score is a good predictor of treatment outcome in patients with unresectable pancreatic cancer. Chemotherapy. 2010;56:501–506. doi: 10.1159/000321014. [DOI] [PubMed] [Google Scholar]
- 36.Johansen JS, Vibat CRT, Hancock S, Chen I, Hassaine L, Samuelsz E, Collisson EA, Jensen VB, Lu T, Melnikova V, Erlander MG. Prognostic value of plasma circulating tumor (ct) DNA KRAS mutations and serum CA19-9 in unresectable pancreatic cancer (PC) patients; ASCO Meeting Abstracts; 2015.p. 4022. [Google Scholar]
- 37.Wei CH, Gorgan TR, Elashoff DA, Hines OJ, Farrell JJ, Donahue TR. A meta-analysis of gemcitabine biomarkers in patients with pancreaticobiliary cancers. Pancreas. 2013;42:1303–1310. doi: 10.1097/MPA.0b013e3182a23ae4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farrell JJ, Elsaleh H, Garcia M, Lai R, Ammar A, Regine WF, Abrams R, Benson AB, Macdonald J, Cass CE, et al. Human equilibrative nucleoside transporter 1 levels predict response to gemcitabine in patients with pancreatic cancer. Gastroenterology. 2009;136:187–195. doi: 10.1053/j.gastro.2008.09.067. [DOI] [PubMed] [Google Scholar]
- 39.Maréchal R, Bachet JB, Mackey JR, Dalban C, Demetter P, Graham K, Couvelard A, Svrcek M, Bardier-Dupas A, Hammel P, et al. Levels of gemcitabine transport and metabolism proteins predict survival times of patients treated with gemcitabine for pancreatic adenocarcinoma. Gastroenterology. 2012;143:664–674.e1-e6. doi: 10.1053/j.gastro.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 40.Greenhalf W, Ghaneh P, Neoptolemos JP, Palmer DH, Cox TF, Lamb RF, Garner E, Campbell F, Mackey JR, Costello E, et al. Pancreatic Cancer hENT1 expression and survival from gemcitabine in patients from the ESPAC-3 trial. J Natl Cancer Inst. 2014;106:djt347. doi: 10.1093/jnci/djt347. [DOI] [PubMed] [Google Scholar]
- 41.Sinn M, Riess H, Sinn BV, Stieler JM, Pelzer U, Striefler JK, Oettle H, Bahra M, Denkert C, Bläker H, Lohneis P. Human equilibrative nucleoside transporter 1 expression analysed by the clone SP 120 rabbit antibody is not predictive in patients with pancreatic cancer treated with adjuvant gemcitabine-Results from the CONKO-001 trial. Eur J Cancer. 2015;51:1546–1554. doi: 10.1016/j.ejca.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 42.Ormanns S, Heinemann V, Raponi M, Isaacson J, Laubender RP, Haas M, Kruger S, Kleespies A, Mann E, Bartosiewicz M, et al. Human equilibrative nucleoside transporter 1 is not predictive for gemcitabine efficacy in advanced pancreatic cancer: Translational results from the AIO-PK0104 phase III study with the clone SP120 rabbit antibody. Eur J Cancer. 2014;50:1891–1899. doi: 10.1016/j.ejca.2014.04.023. [DOI] [PubMed] [Google Scholar]
- 43.Poplin E, Wasan H, Rolfe L, Raponi M, Ikdahl T, Bondarenko I, Davidenko I, Bondar V, Garin A, Boeck S, et al. Randomized, multicenter, phase II study of CO-101 versus gemcitabine in patients with metastatic pancreatic ductal adenocarcinoma: Including a prospective evaluation of the role of hENT1 in gemcitabine or CO-101 sensitivity. J Clin Oncol. 2013;31:4453–4461. doi: 10.1200/JCO.2013.51.0826. [DOI] [PubMed] [Google Scholar]
- 44.Bertocchi P, Abeni C, Meriggi F, Rota L, Rizzi A, Di Biasi B, Aroldi F, Ogliosi C, Savelli G, Rosso E, Zaniboni A. Gemcitabine plus nab-paclitaxel as second-line and beyond treatment for metastatic pancreatic cancer: A single institution retrospective analysis. Rev Recent Clin Trials. 2015;10:142–145. doi: 10.2174/1574887110666150417115303. [DOI] [PubMed] [Google Scholar]
- 45.Portal A, Pernot S, Tougeron D, Arbaud C, Bidault AT, de la Fouchardière C, Hammel P, Lecomte T, Dréanic J, Coriat R, et al. Nab paclitaxel plus gemcitabine for metastatic pancreatic adenocarcinoma after Folfirinox failure: Results of an AGEO multicenter prospective cohort. Br J Cancer. 2015;113:989–995. doi: 10.1038/bjc.2015.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palacio S, Akunyili II, Ernani V, et al. Gemcitabine (Gem) and nab-paclitaxel (nab-P) in patients (pts) with refractory advanced pancreatic cancer. Am Soc Clin Oncol. 2015;(Suppl 3):S413. doi: 10.1200/jco.2015.33.3_suppl.413. [DOI] [Google Scholar]