Abstract
Over the past decades, organic-inorganic hybrid polymers have been applied in different fields, including the adsorption of pollutants from wastewater and solid-state separations. In this review, firstly, these compounds are classified. These compounds are prepared by sol-gel method, self-assembly process (mesopores), assembling of nanobuilding blocks (e.g., layered or core-shell compounds) and as interpenetrating networks and hierarchically structures. Lastly, the adsorption characteristics of heavy metals of these materials, including different kinds of functional groups, selectivity of them for heavy metals, effect of pH and synthesis conditions on adsorption capacity, are studied.
Keywords: organic-inorganic hybrid polymer, heavy metal ion, wastewater treatment, adsorption, sol-gel method, self-assembly process, nanobuilding blocks, interpenetrating networks
1. Introduction
A wide variety of toxic inorganic and organic chemicals are discharged into the environment as industrial wastes, causing serious water, air, and soil pollution. Water pollution caused by toxic heavy metal ions has become a serious environmental problem. Heavy metals (such as Pt, Pd, Ag, Cu, Cd, Pb, Hg, Ni, Co, Zn, etc.) are natural constituents of the earth crust and present in the environment as a result of weathering and erosion of parent rocks [1]. In addition to natural sources, they are introduced in ecosystems through wastewaters originating from anthropogenic sources such as chemical manufacturing, metal finishing, welding, alloys manufacturing, painting, mining, extractive metallurgy, plating, tannery and battery industry and using metal-containing fertilizers and pesticides [2].
These toxic metal ions, even at low concentrations, have deteriorated water resources and drinking water and easily accumulated in the human body throughout the food chain, causing a variety of diseases and disorders [3]. So, it is necessary to remove these metal ions from industrial effluents for their subsequent safe disposal.
The removal of heavy metal ions from wastewaters has been a subject of extensive industrial research. At the same time, some of them (e.g., Pt and Au) are precious and can be recycled and reused for extensive applications [4,5]. The recovery of heavy or valuable metals from water or wastewaters can often result in considerable cost savings and have both ecological and economic benefits.
Different methods, such as precipitation [6], solvent extraction [7] chemical and electrochemical techniques [8], ion-exchange methods [9] ultrafiltration [10] and reverse osmosis [11,12], flotation [13] and coagulation [14] have been developed for the removal of toxic metal ions from industrial effluents and wastewaters. However, most of these processes are unacceptable, owing to the disposal of sludge, their high cost, low efficiency and inapplicability to a wide range of pollutants [15].
Adsorption is a well-known separation method and recognized as one of efficient and economic methods for water decontamination applications. In addition, owing to the reversible nature of most adsorption processes, the adsorbents can be regenerated by suitable desorption processes for multiple uses [16], and many desorption processes are of low maintenance cost, high efficiency, and ease of operation [17].
However, the major problem in this field is to select novel types of adsorbents. A number of adsorbents such as activated carbon [18], zeolites [19,20], clays [21,22] and agricultural residues [23–25] have been used for the removal of heavy metal ions. However, the major disadvantages of these adsorbents are their low adsorption capacities, their relatively weak interactions with metallic ions and difficulties of separation and regeneration of some of them from water. Ion-exchange resins can remove metal ions substantially; however, they have low selectivities and show a high degree of swelling combined with poor mechanical stability [26].
To overcome these limitations, more recently, promising organic-inorganic hybrid polymers have been used for the removal of toxic species from wastewater [27–35]. In these compounds, the functional variation of organic materials is combined with advantages of a thermally stable and robust inorganic substrate, resulting in strong binding affinities toward selected metal ions and relatively high metal ion adsorption capacities. Functionalized hybrid polymeric materials as adsorbent are regarded as one of the most effective techniques because metal ions can be chemically bonded by the organic-inorganic polymer hybrids. These kinds of materials often present the best properties of each of its components in a synergic way and have high performances of physical, chemical and mechanical properties [36].
Organic-inorganic hybrid polymeric materials are currently intensely studied [37] for their efficient applications. The intrinsic multifunctional character of these materials makes them potentially useful in multiple fields. Different forms of organic-inorganic hybrids have been intensively studied due to their interesting properties resulting in a number of applications such as electroanalytical applications [38], their extensive applications as membranes for ultra-and nanofiltration [39,40], superhydrophobic surfaces [41,42], highly transparent films [43,44], pH sensitive composites [45], solar cells [46,47], electrolyte [48], molecular shuttles [49], semiconductors [50], gas separation [51,52], catalysts [53], biosensors [54], drug delivery systems [55], coatings for corrosion protection [56], adsorbents of toxic compounds [27–35], Fire retardant polymers [57], biomaterials for osteo-reconstructive surgery [58], materials for telecommunications or information displays [59], etc.
This review describes classifications and synthesis methods of organic-inorganic hybrid polymers and particular attention will be focused on application of them for the adsorption of heavy metals from water as well as their performances and mechanisms.
2. Classification and Synthesis of Organic-Inorganic Hybrid Polymers
Organic-inorganic hybrid polymers are classes of materials whose structure includes both organic and inorganic units that interact with each other at the molecular level. These materials are divided into two classes on the basis of interaction between organic and inorganic components. In class I, organic and inorganic are embedded and there are weak interactions, such as hydrogen bonding, van der Waals, π–π or weak electrostatic interactions between them and in class II, these two components are bonded together through strong covalent or coordinative bonds [37]. Organic-inorganic hybrid polymers are obtained through (1) sol-gel process; (2) self-assembly process; (3) assembling or dispersion of nanobuilding blocks; (4) hierarchical structures [37] and interpenetrating networks [60].
2.1. Sol-Gel Process
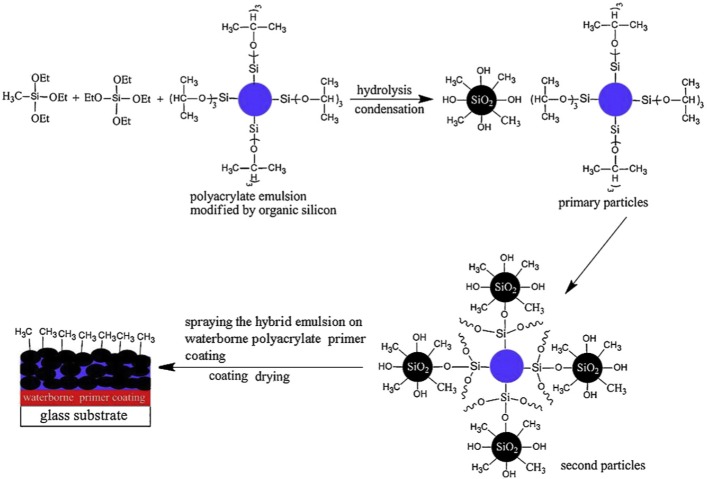
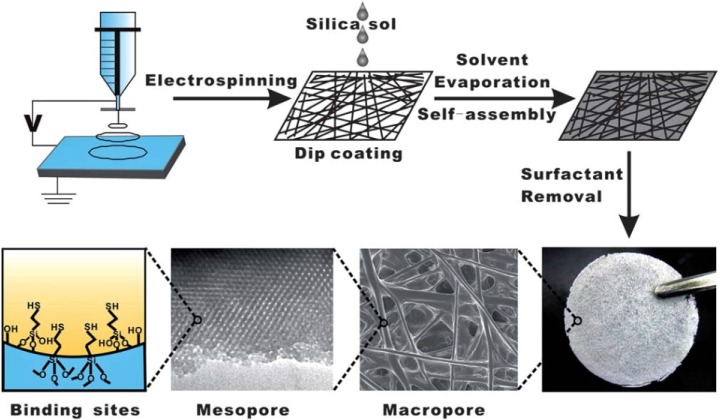
In 1846, Ebelmanl and Graham reported that the hydrolysis of tetraethylorthosilicate (TEOS), under acidic conditions produced SiO2 in the form of fibres [61,62]. In 1950s, Brady et al. [63] produced a number of phenylsilsesquioxane-alkylsilsesquioxane copolymers that were the first successful commercial organic-inorganic hybrid polymers. The sol-gel process is a cheap and low-temperature method for producing transparent and homogenous solid materials with high purity from small molecules and for controlling the chemical composition of products. Compounds produced by the sol-gel process have many applications in superhydrophobic surfaces [41] (shown in Scheme 1), electrolyte [48], biosensors [54], corrosion protection [56], etc.
Scheme 1.
Schematic superhydrophobic surfaces prepared through the sol-gel derived organic-inorganic hybrid emulsion. Reprinted with permission from [41]. Copyright 2011 Elsevier.
This process is carried out in water and organic solvents and its precursors are usually metal halides [64] (in organic solvents) and metal alkoxides such as Si(OR)4, SiR′(OR)3, Ti(OR)4, etc. [41,65–67] (Scheme 1). As seen in Scheme 2 [68], these materials are subject to a series of hydrolysis (or non-hydrolytic process in organic solvents) and condensation reactions that through nucleophilic substitution mechanisms result in sol formation. Sol is a colloidal solution in which individual particles interact weakly with each other and due to cross-linking reactions, converts into an integrated network (wet gel). This structure with further drying processes converts to gel.
Scheme 2.
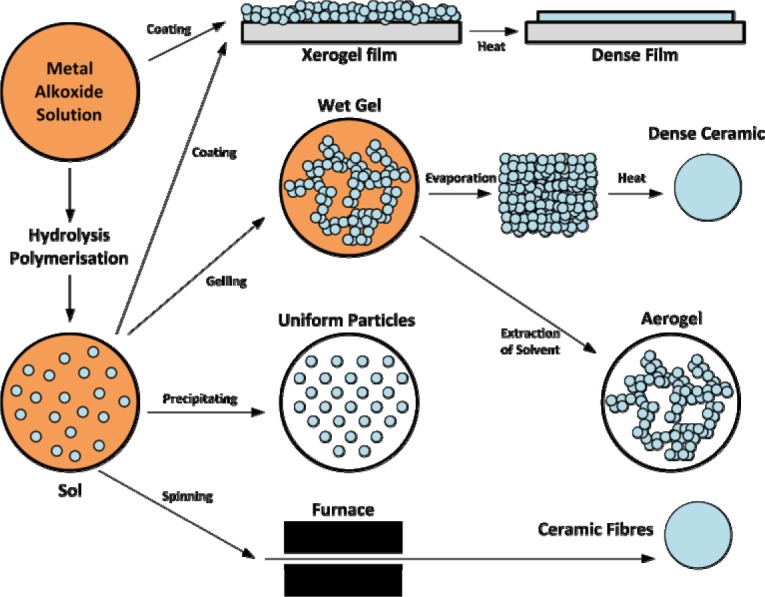
Description of technologies and products of the sol-gel process [68].
In spite of silicon alkoxides, hydrolysis reactions of other metal alkoxides in water are too fast. The hydrolysis reaction of silicon alkoxides, is typically acid- or base-catalyzed [69]. As reported [70], silica networks formed under acid-catalyzed conditions are dense and those of formed in the presence of base are porous and loose.
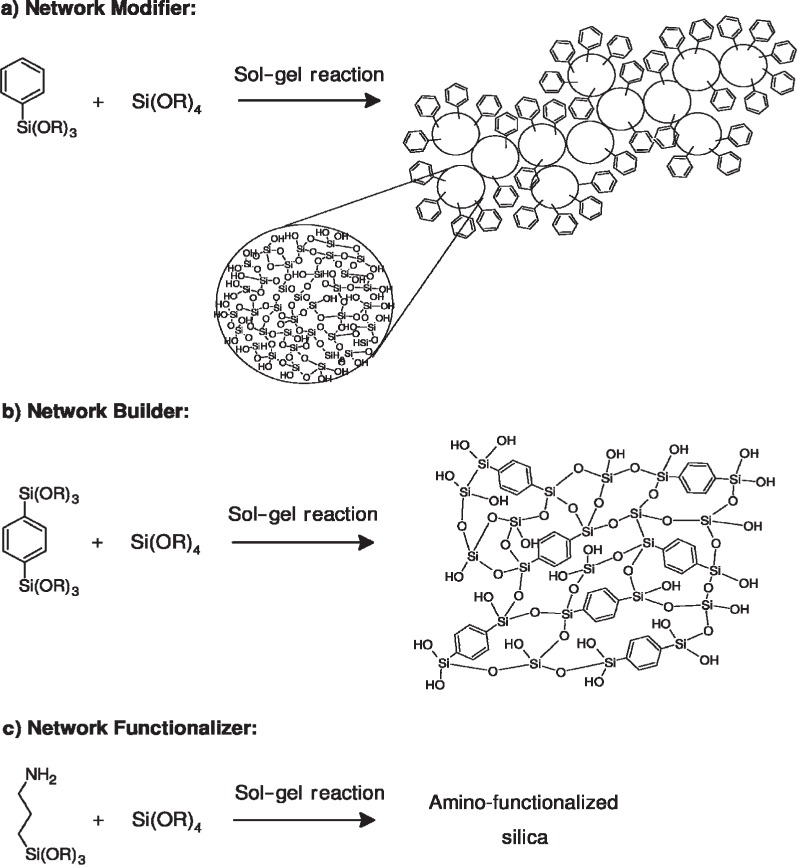
A suitable way for modifications of properties of materials obtained from this method is the introduction of organic groups (R) into their structure [71]. For example, the Si–C bond does not hydrolyze in most sol-gel processes and organic groups can be incorporated covalently into the network of inorganic gel using silsesquioxanes [(R–SiO1.5)n (n = even number)], such as R′(OR)2Si–R–Si(OR)3 compounds or derivatives of tetrafunctional silicon alkoxide [organically modified silicates (ORMOSILs)], such as TEOS, R′Si(OR)3, etc. [71]. If an organic group remains stable and does not hydrolyze during sol-gel processes (e.g., alkyl groups) it is defined as a network modifier. On the other hand, if it can react with another monomers or with itself (e.g., –CH=CH2) it is named as a network builder. Moreover, if it is a reactive functional group, such as –NH2, it is specified as a network functionalizer [72] (Scheme 3). These materials belong to class II interactions of organic-inorganic hybrid polymers.
Scheme 3.
Organically functionalized trialkoxysilanes, R′Si(OR)3, used as (a) modifier; (b) builder and (c) functionalizer of silica-based network through the sol-gel process. Reproduced with permission from [72]. Copyright 2006 WILEY-VCH Verlag GmbH.
Another example of using sol-gel processes is the synthesis of microporous zeolites (or molecular sieves). These materials are framework aluminosilicate and have a three-dimensional structure with high internal surface area and an ordered crystalline structure [73]. Zeolites with high silica contents are synthesized in the presence of bulky organic alkylammonium cations known as structure-directing agents or templates [74].
Synthetic zeolites are microporous (diameter of their pores < 2 nm) and have many applications such as membranes, catalysts, catalyst supports, gas storage, etc. [73]. In recent years, great efforts have been made on the direct preparation of hybrid organic-inorganic zeolites [75,76]. The incorporation of organic moieties within the pores or in the framework of microporous zeolites has developed their catalytic activity [77], molecular sieving property [78] and adsorption capacity [79].
2.2. Self-Assembly Process
In 1992, researchers of Mobil Oil Company discovered a new type of mesoporous silicas, so-called M41S [80,81]. M41S family includes hexagonal MCM–41, cubic MCM–48, and lamellar MCM–50 [82] (Figure 1). MCM is an abbreviation of “Mobil Crystalline Materials”.
Figure 1.
Representation of mesoporous M41S compounds including (a) MCM–41; (b) MCM–48 and (c) MCM–50. Reproduced with permission from [82]. Copyright 1999 WILEY-VCH Verlag GmbH.
Notwithstanding their amorphorous pore walls, these organic-inorganic hybrid compounds exhibit a long-range ordered array of uniform and controllable mesopores with sizes ranging between 2–10 nm [82]. Mesostructure compounds have high specific surface areas (greater than 1000 m2/g) [82] and the pore size of these materials enables them to adsorb much larger molecules than is possible for zeolites. These compounds are used for catalysis [83], gas adsorption [84], etc. M41S type compounds were synthesized using a silica source (e.g., TEOS) in the presence of long-chain alkyltrimethylammonium halide surfactants as templates or structure-directing agents [80]. These compounds formed through a base-catalysed hydrolytic sol-gel process. Mesostructures can be synthesized using different ionic or non-ionic surfactants or water-soluble polymers as templates [85,86]. Other types of ordered mesoporous silicas are Santa Barbara Amorphous (SBA) (e.g., SBA–15 [87]), Michigan State University (MSU) (e.g., MSU–2 [88]), folded sheets mechanism (FSM) (e.g., FSM–16 [89]), etc., respectively.
In recent years, attempts of scientists resulted in syntheses of non-silica mesoporous metal oxides such as TiO2, ZrO2, Al2O3, Nb2O5, SnO2, mixed oxides such as SiAlO3.5, SiTiO4, carbon nanocage, mesoporous carbon nitride, etc. [90]. The formation of mesoporous materials is depicted schematically in Figure 2, based on “liquid crystal templating” mechanism [91]. Through route (a) at surfactant concentrations above its critical micelle concentration (CMC), the self-assembly of precursor molecules on the space between micellar rods of lyotropic liquid crystal phase and creates walls between them. Through route (b) at surfactant concentrations below CMC, the mesostructure is formed as a cooperative self-assembly of the precursor and surfactant molecules [82,91].
Figure 2.

Synthesis of mesoporous compounds in the presence of template through (A) liquid-crystal template or (B) cooperative liquid-crystal mechanisms. Reprinted with permission from [91]. Copyright 2002 American Chemical Society.
To prevent phase separation, there should be attractive interactions between inorganic precursors and template molecules. Depending on reaction conditions, such as pH and chemicals, e.g., inorganic precursors and templates, six routes have been suggested for syntheses of ordered mesoporous compounds [92]. These routes include (a) S+I−; (b) S+X−I+; (c) S−M+I−; (d) S−I+; (e) S0I0 or N0I0 and (f) S0(XI)0 where S is the surfactant, I is the inorganic phase and X− is a mediator anionic species (usually a halide), M+ is a mediator cation, S0 is a neutral amine and N0 is a long-chain non-ionic template. Interactions between the charged inorganic phase and the head group of surfactants in routes (a–d) are mainly electrostatic forces; while those between neutral inorganic species and non-electrolyte template molecules are hydrogen bonds in routes (e) and (f) [92,93] (Figure 3). It is necessary to say that the inorganic precursor is neutral at its isoelectric point (e.g., at pH~2 for silica [94]) and has negative and positive electric charge at pH above and below this point, respectively.
Figure 3.
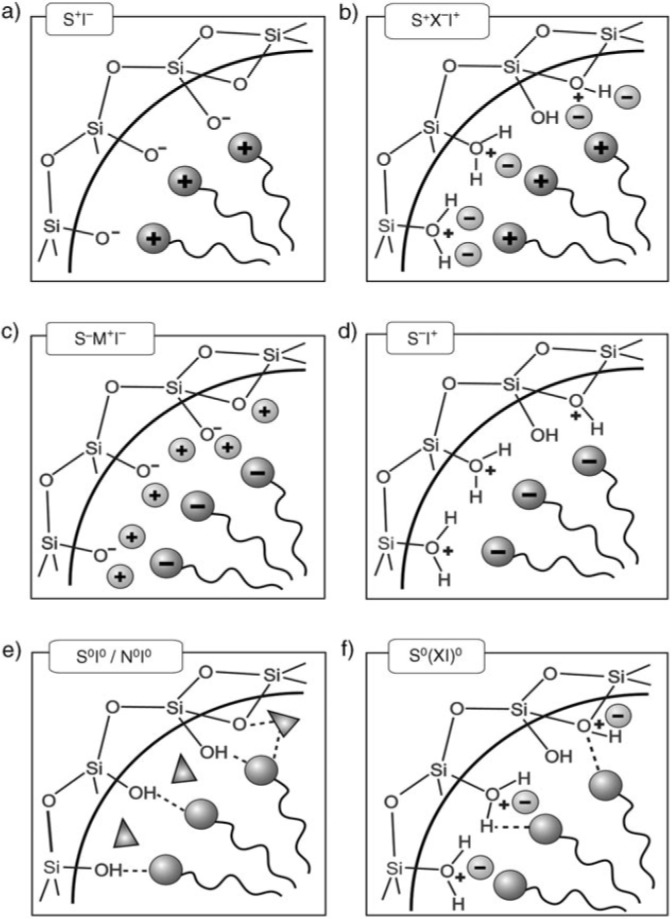
Different kinds of interactions between inorganic species and surfactant head groups through electrostatic interactions in (a) and (c) basic, (b) acidic and (d) neutral media or via hydrogen bonds between (e) unchared species or (f) ion pairs. Reproduced with permission from [93]. Copyright 2006 WILEY-VCH Verlag GmbH.
In recent years, mesoporous silicas have been used as supports for preparations of organic-inorganic hybrid polymers and have many applications in lasers [95], drug delivery [96], pollutant adsorption [36], etc. In these compounds, different organic functional groups are located on the robust, thermally stable and porous inorganic substrate.
As illustrated in Figure 4, these organosilica compounds are prepared through (a) grafting or post-synthetic functionalisation; (b) co-condensation or one-pot synthesis and (c) synthesis of periodic mesoporous organosilicas (PMOs) [93,97,98]. In grafting processes, an organosilica is synthesized through condensation reaction between Si–OH groups of the pore walls of mesoporous silica and appropriate reagents such as, chlorosilanes (e.g., ClSiR3), alkoxyorganosilanes [e.g., RSi(OR′)3] etc. In the co-condensation method, an organosilica compound is prepared in one step through co-condensation reactions of tetraalkoxysilanes (e.g., TEOS) and an appropriate reagent (e.g., terminal trialkoxyorganosilanes) in the presence of a template. Syntheses of PMOs are carried out through the hydrolysis and condensation of bridged organosilane precursors of the type (R′O)3 Si–R–Si(OR′)3 in the presence of a structure-directing agent. In this method, R organic group are incorporated uniformly and periodically in the pore walls of resulted organosilica [93,98].
Figure 4.
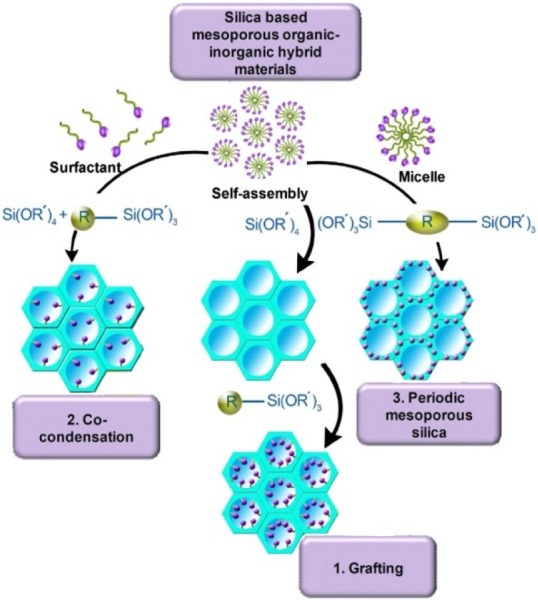
Different methods for the synthesis of organic-inorganic hybrid mesoporous silica: 1. Grafting, 2. Co-condensation or in situ grafting and 3. Periodic mesoporous silica. Reproduced wity permissiom from [93]. Copyright 2006 WILEY-VCH Verlag GmbH.
2.3. Assembling or Dispersion of Nanobuilding Blocks (NBBs)
The interest in nanoscale materials originates from this fact that their properties are a function of their size, composition, and structural order. Here, a series of compounds called as nanobuilding blocks (NBBs) are used for preparing organic-inorganic hybrid polymers. NBBs are capped with polymerizable ligands or connected through organic spacers, e.g., telechelic molecules or functional dendrimers [37]. These compounds maintain their integrity and fulfill certain (chemical or physical) functions in the final material. NBBs are classified as (1) clusters; (2) organically in situ- or post-functionalized nanoparticles; (3) layered compounds and (4) core-shell nanocompounds [37,99].
2.3.1. Clusters
Metallic clusters (e.g., polyoxometalates) are an ensemble of bound atoms and contain a group of two or more metal atoms that bind together by direct Metal-Metal bonding. Clusters are connected either (1) by the polymerization of their ligands (e.g., allyl) and are called cross-linked clusters or (2) by coordinative bonds between coordinating groups of organic ligands (called as a linker or spacer) to each metal center (called as a connector) of clusters [100,101]. In the latter case, a linker is a bi-, tri- or tetradentate organic ligand (e.g., 1,4-benzenedicarboxylate) with an organic spacer between coordinating groups [102]. For example, a one-dimensional polymer is built when a metal cluster links to just one coordinating group of two bidentate organic linkers and through them links to other metal clusters. Coordination of more than two coordinating groups to each metal center, optionally through tri- or tetradentate linkers, results in two- or three-dimensional polymers [100]. The three-dimensional polymers are called metal-organic frameworks (MOFs) and their metal centers are bridged in three dimensions (Supporting information Figure S1). MOFs along with cross-linked clusters are major types of cluster-based organic-inorganic hybrid polymers [103,104].
2.3.2. Organically in Situ- or Post-Functionalized Nanoparticles
In this method reactive organic groups are bound to the surface of inorganic moiety by strong ionic or covalent interactions. These organic groups are either grafted to preformed nanoparticles (called as post-functionalization) [105] or are incorporated during the synthesis of nanoparticles (called as in-situ-functionalization) [106]. This method is used for metal oxides (e.g., TiO2 [107]), chalcogenides (e.g., CdSe [108]), metallic nanoparticles (e.g., Au [109]) and polyoxometalates (e.g., γ-[SiW10O36]8− [110]), etc.
2.3.3. Layered Compounds
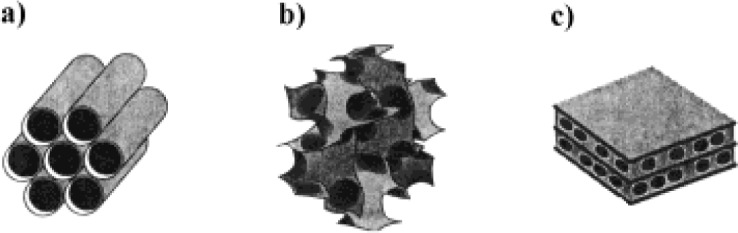
To improve the physical properties of polymers, inorganic compounds physically are dispersed in them (as fillers) in various forms such as platelets, spheres, fibers, etc. If the size of discrete inorganic components is in the range of 1–100 nm, the resulted organic-inorganic compound is called a nanocomposite [111]. Organic-inorganic hybrid nanocomposites are either as interpenetrating networks which will be explained in Section 2.4 [99] or layered inorganic components embedded into the organic matrix.
Layered compounds are regular stacks of two-dimensional inorganic constituent. These compounds such as clay, are used as fillers in organic-inorganic hybrid composites. Some examples of nanocomposites are polyaniline-montmorillonite [112], polyaniline–V2O5 [113], polyvinyl alcohol-layered double hydroxide (LDH) [114], poly-N-vinyl carbazole-graphene oxide [115], etc. These compounds are incorporated in organic polymers as (a) a phase-separated structure in which the polymer can not intercalate within the inorganic layers and the inorganic component is dispersed as aggregates or particles within the polymer matrix; (b) an intercalated structure in which one or more polymer chains are inserted into the galleries (space between layers) of inorganic components and subsequently increase the interlayer spacing and (c) an exfoliated structure in which the insertion of polymer chains between inorganic sheets delaminates the layered compound [116] (Figure 5). Polymers can be intercalated to layered compounds by (1) solution; (2) melting polymers and (3) in situ polymerization of intercalated monomers [99]. However, some compounds (e.g., EDTA and malate) intercalate layer compounds [117,118] are purly nanocomposites and not organic-inorganic hybrid polymers.
Figure 5.
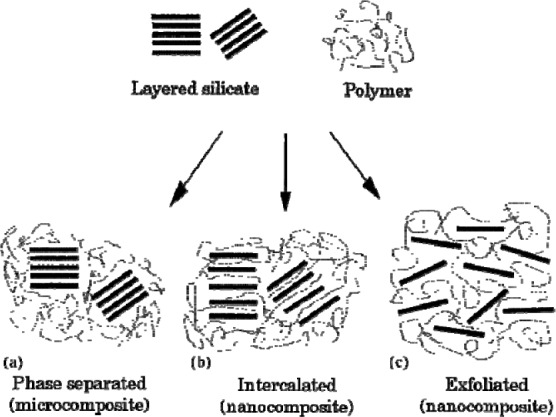
Schematic representation of different kinds of composites produced from interaction of layer compound with polymers: (a) phase separated; (b) intercalated and (c) exfoliated structures. Reprinted with permission from [116]. Copyright 2000 Elsevier.
2.3.4. Core-Shell Nanocomposites
Core-shell nanocomposites (CSNs) consist of a core region covered by a shell domain (Figure 6). A series of CSNs are polymer-coated inorganic compounds [119–121]. The encapsulation of an inorganic particles in a polymer shell are carried out through (1) the adsorption of monomers on the surface of inorganic moiety followed by their polymerization in the adsorbed layer [122,123] and (2) interaction of inorganic particle with preformed polymer as in situ growing inorganic components in a polymer matrix [124]. CSNs are also shown as core@shell. Some kinds of CSNs are completely inorganic in nature (e.g., CdS@TiO2 [125]).
Figure 6.
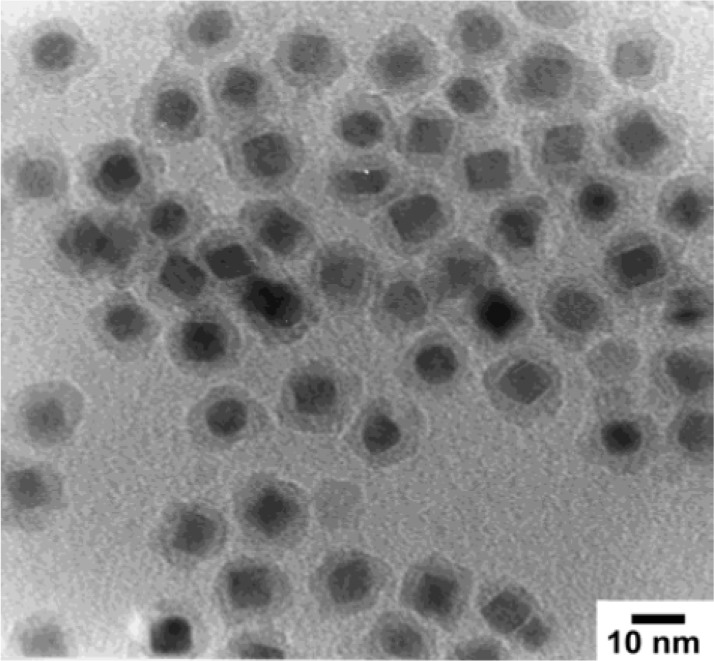
TEM of polystyrene-coated Fe2O3 particles. The polymer coatings are seen as a shell around the Fe2O3 cores. Reprinted with permission from [122]. Copyright 2003 American Chemical Society.
2.3.5. Hierarchical Structures
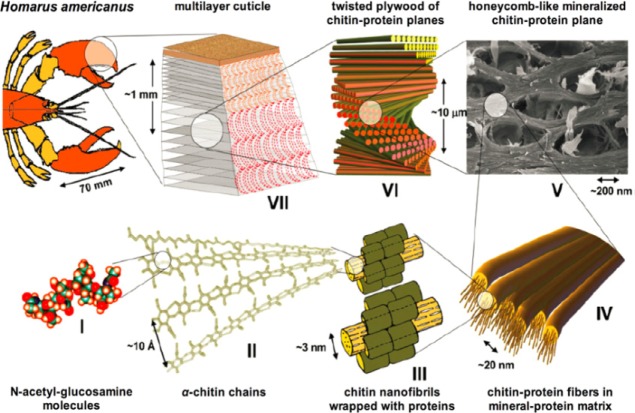
A hierarchy is a feature of self-assembly, where primary building blocks associate into more complex structures which are integrated into the next size level in the hierarchy. Self-assembly is emerging as an elegant, “bottom-up” method for the construction of nanostructured materials and is distinct from the template-directed assembly [126]. The feature of an integrated chemical, biological, etc. system is the assembly of components into a specific architecture that performs a certain function. Many examples of hierarchical structural designs are seen in nature and are characteristic of many self-assembling biological structures (e.g., lobster cuticle [127] and tendon [128]) (Figure 7).
Figure 7.
Hierarchical structure of lobster cuticle. Reproduced with permission from [127]. Copyright 2010 WILEY-VCH Verlag GmbH.
Also, the hierarchy has resulted in the discovery of versatile nanoparticles with many applications [129], synthetic bone implant materials [130], mesostructured solid electrolytes [131], TiO2–graphene composite [132], etc. For example, the incorporation of interconnected macropores in mesoporous films of TiO2–graphene composite (Supporting information Figure S2) augments the capacity of composite film for adsorption and photodegrading organic molecules and can be used for the treatment of waters.
2.4. Interpenetrating Networks (IPNs)
IPNs comprise organic and inorganic networks that are microscopically phase-separated. However, they seem uniform macroscopically and there are weak interactions between moieties of IPNs (class I interactions). These components are partially interlaced and, upon separating IPNs, chemical bonds are broken. IPNs are synthesized by (1) the formation of a secondary network in a primary one (a sequential two-step process); (2) the simultaneous formation of two networks and (3) IPN components connect to each other through covalent bonds in which the compound is named a dual organic-inorganic hybrid polymer (class II interactions) [99] (Figure 8). In the first method, IPNs are prepared by polymerization in a sol-gel network [133] or sol-gel process in the presence of preformed polymer [134]. In the third case, either bifunctional precursors are used or a preformed inorganic or organic polymer are functionalized by the required functional groups for the formation of other types of networks [135].
Figure 8.
Schematic representation of IPNs (a) without chemical bonds between moieties and (b) dual hybrid network. Reprinted with permission from [99]. Copyright 2003 Elsevier.
But, when three-dimensional frameworks such as zeolites or mesoporous silicates, are used as inorganic components and polymer is formed without crosslinking, the polymer chains could be partially or completely removed from the product. In this case, the composite is called pseudo-interpenetrating polymer network (PIPN) [136,137].
At the end of this section, it is noteworthy that the inclusion of metals in organic-inorganic hybrid polymers occurs at the molecular scale and the incorporation of metal complexes and metallic ions in polymers by coordination interactions does not produce organic-inorganic hybrid polymers [99]. For example, metal-centered polymers [138] and products of coordination of metallic ions to the polymer backbone [139] and polymerization of metal-coordinated monomers [140] are called metal-containing polymers [99].
3. Removal of Heavy Metals by Organic-Inorganic Hybrid Polymers
Based on the classifications of these kinds of compounds in the previous section, by analysis some comprehensive references, we study their synthesis methods, structure and mechanisms of heavy metal adsorption from water or wastewater.
3.1. Adsorption of Heavy Metals Using Materials Synthesized from Sol-Gel Method
As extensive researches have been done on producing silica-based compounds by the sol-gel method, these kinds of materials are discussed here. These compounds can be synthesized either directly through polymerization reaction of their precursors [141] or grafting appropriate functional groups on parent materials formed from sol-gel method such as silica gel and some examples [142–167] are given in Table 1.
Table 1.
Chemicals used for synthesizing adsorbents by sol-gel method, adsorbed heavy metals, maximum adsorption capacity, pH and temperature for adsorption of heavy metals from waters.
| Chemicals (interacting group) | Heavy metal [qmax, pH and t (°C)] | Isotherm | Reference |
|---|---|---|---|
| Pyromellitic acid dianhydride/phenylaminomethyl trimethoxysilane (–COOH) | Pb2+ (7.16 mmol/g, 5, room) | Langmuir | [142] |
| Pyromellitic acid dianhydride/phenylaminomethyl trimethoxysilane (–COOH) | Cu2+ (0.28 mmol/g, 4, room) | Langmuir | [143] |
| Pb2+ (1.56 mmol/g, 5, room) | Langmuir | ||
| 3-Thiocyanatopropyltriethoxysilane/TEOS (–SH) | Cd2+ (87.7 mg/g, 5, 25) | Langmuir | [144] |
| 3-[2-(2-Aminoethylamino)ethylamino]propyl-trimethoxysilane (Cd2+– imprinted hybrid sorbent) (–NH2) | Cd2+ (77.2 mg/g, 6, 25) | Langmuir | [145] |
| Cd2+-imprinted mercapto-functionalized silica gel (–SH) | Cd2+ (83.89 mg/g, 6, 27) | Experimental | [146] |
| Non-imprinted mercapto-functionalized silica gel (–SH) | Cd2+ (35.91 mg/g, 6, 27) | Experimental | – |
| Fe3+-imprinted cyanato-functionalized silica gel (–C≡N) | Fe3+ (35.6 mg/g, 3, 20) | Experimental | [147] |
| 3-Glycidyloxypropyltrimethoxysilane/potassium tert-butoxide/titanium isopropoxide (oxygen atoms of adsorbent) | Pb2+ (181.2 mg/g, 5.5, room) | Langmuir | [148] |
| Cu2+ (44.64 mg/g, 5.5, room) | Langmuir | ||
| Cd2+ (35.84 mg/g, 5.5, room) | Langmuir | ||
| Zr(OCH2CH2CH3)4/3-mercapto-1-propanesulfonic acid (–SH) | Pb2+ (0.36 mmol/g, neutral, room) | Experimental | [149] |
| Hg2+ (0.87 mmol/g, neutral, room) | Experimental | ||
| Ti(OCH2CH2CH3)4/3-mercapto-1-propanesulfonic acid (–SH) | Pb2+ (1.24 mmol/g, neutral, room) | Experimental | – |
| Hg2+ (1.41 mmol/g, neutral, room) | Experimental | ||
| 7-Amine-4-aza-heptyltrimethoxisilane/TEOS (amine group) | Pb2+ (36.64 mg/g, neutral, room) | Langmuir | [150] |
| 10-Amine-4-aza-decyltrimethoxisilane/TEOS (amine group) | Pb2+ (30.27 mg/g, neutral, room) | Langmuir | – |
| 3-Chloropropyltrimethoxysilane/TEOS (nitrogen center of sorbent) | Ni2+ (0.47 mmol/g, 4.5, room) | Langmuir | [151] |
| 3-Aminopropyl triethoxysilane/TEOS (–NH2) | Pb2+ (45.45 mg/g, 6, 25) | Langmuir | [152] |
| Cu2+ (35.71 mg/g, 6, 25) | Langmuir | – | |
| 3-Chloropropyltrimethoxysilane/TEOS (nitrogen center of sorbent) | Mn2+ (0.35 mmol/g, 4.5, room) | Langmuir | – |
| Amino-terminated dendrimer-like PAMAM polymer/silica gel (amino content 1.91 mmol/g) (–NH– and –NH2) | Cu2+ (78.7 mg/g, ethanolic, 25) | Langmuir | [153] |
| 2-Aminoethyl-3-aminopropyltrimethoxysilane/poly(dimethyl siloxane) (–NH/NH2) | Cu2+ (1.2 mmol/g, ethanolic, 25) | Langmuir | [154] |
| Ni2+ (0.37 mmol/g, ethanolic, 25) | |||
| Fe3+ (1.3 mmol/g, ethanolic, 25) | |||
| Bis[3-(triethoxysilyl)propyl]disulfide/TEOS (–S–S–) | Cd2+ (26.8 mg/g, 6, 25) | Langmuir | [155] |
| Pb2+ (56.7 mg/g, 6, 25) | Langmuir | ||
| Cu2+ (13.3 mg/g, 6, 25) | Langmuir | ||
| Salen(NEt2)2/silica gel (–OH and –N(C2H5)2) | Cu2+ (4.36 mg/g, 11, room) | Langmuir | [156] |
| Mn2+ (2.34 mg/g, 11, room) | Langmuir | ||
| Pb2+ (10.06 mg/g, 11, rom) | Langmuir | ||
| Zn2+ (11.3 mg/g, 11, room) | Langmuir | ||
| 2-Acrylamido-2-methylpropanesulfonic acid/silica gel (–OH, –NH– and –SO3H) | Cu2+ ( 0.66 mmol/g, ethanolic, 25) | Langmuir | [157] |
| Amino-terminated dendrimer-like polyamidoamine polymer/silica gel (–NH2) | Pd2+ (0.7 mmol/g, neutral, room) | Experimental | [158] |
| Pt4+ (0.41 mmol/g, neutral, room) | Experimental | ||
| Au3+ (0.12 mmol/g, neutral, room) | Experimental | ||
| Polyethylene glycol/3-mercaptopropyl trimethoxysilane (–SH) | Cu2+ (0.4 mmol/g, 4, 40) | Langmuir | [159] |
| Polyvinilalcohol/3-(2-aminoethylamino)propyl trimethoxysilane (–NH/NH2) | Pb2+ (67.6 mg/g, 5, 30) | Langmuir | [160] |
| Activated alumina/3-mercaptopropyl trimethoxysilane (–SH) | As(III) (9.28 mg/g, 7, room) | Experimental | [161] |
| N-[3-(trimethoxysilyl)propyl]-ethylenediamine/TEOS (–NH2) | Pt2+ (139 mg/g, 3.05, 25) | Langmuir–Freundlich | [162] |
| Escherichia coli/TEOS (immobilized E. coli cells) | Cd2+ (79.9 mg/g, 5.7–6.2, 25) | Langmuir | [163] |
| N,N-(dipropylcarbamothioyl)thiophene-2-carboxamide/TEOS (–S–, C=O and amine groups) | Cd2+ (3.89 mg/g, 7, 30) | Langmuir | [164] |
| SiNSSH/TEOS (via sulfur or nitrogen atoms of sorbent) | Hg2+ (8.52 mmol/, neutral, 25) | Langmuir | [165] |
| Pb2+ (1.90 mmol/g, neutral, 25) | Langmuir | ||
| Cu2+ (1.66 mmol/g, neutral, 25) | Langmuir | ||
| Ni2+ (1.44 mmol/g, neutral, 25) | Langmuir | ||
| Co2+ (1.26 mmol/g, neutral, 25) | Langmuir | ||
| 3-Aminopropyltriethoxysilane/TEOS (–NH2) | Ni2+ (31.29 mg/g, 4.5, 25) | Langmuir | [166] |
| Cd2+ (40.73 mg/g, 4.5, 25) | Langmuir | ||
| Pb2+ (96.79 mg/g, 4.5, 25) | Langmuir | ||
| 3-Chloropropyltrimethoxysilane/aniline/TEOS (–NH–) | Co2+ (0.32 mmol/g, 4.5, 25) | Experimental | [167] |
| Zn2+ (0.34 mmol/g, 4.5, 25) | Experimental | ||
| Cd2+ (0.12 mmol/g, 4.5, 25) | Experimental |
Zwitterionic hybrid polymers [142,143] were synthesized on the basis of ring-opening polymerization of pyromellitic acid dianhydride (PMDA) (a network builder) and phenylaminomethyl trimethoxysilane (PAMTMS), followed by the amination (Scheme 4).
Scheme 4.
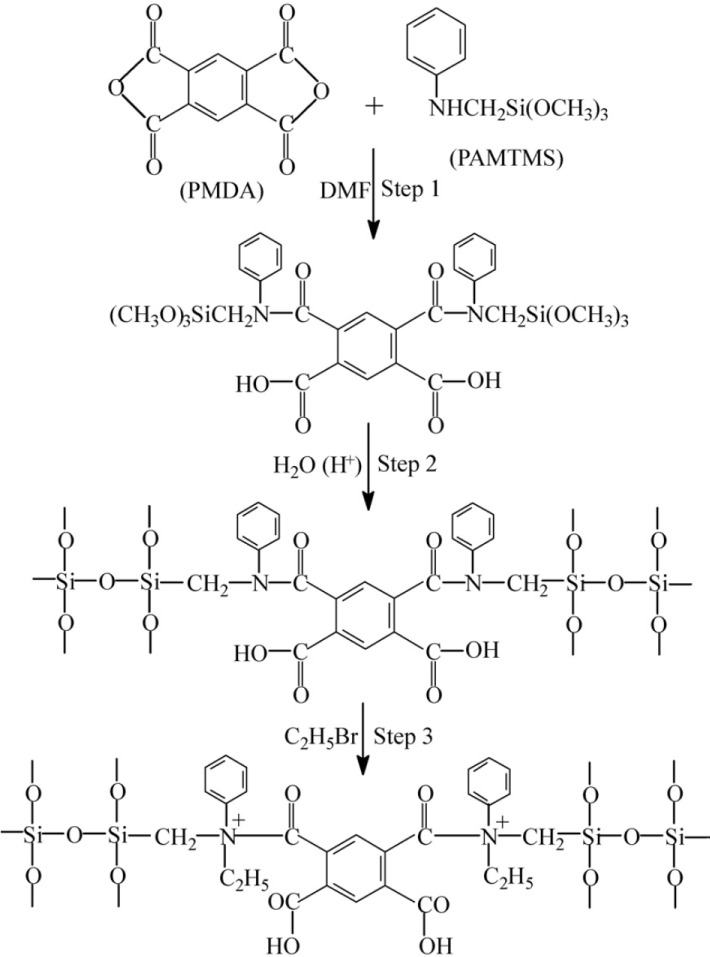
The preparation steps of zwitterionic hybrid polymers. Reprinted with permission from [142]. Copyright 2010 Elsevier.
Ionic groups of opposite charges on the polymer chain (quaternary amines and carboxylic groups) allowed their electrostatic interactions with heavy metals. TGA analysis showed a higher PDMA content of prepared samples, increased their thermal degradation temperature [143].
Kinetics experiments showed about 80% of Pb2+ and Cu2+ ions were adsorbed after approximately 3 h. Kinetics and thermodynamics measurements of Cu2+ and Pb2+ were carried out at pH = 4 and 5, respectively in which these metal ions exist mainly as their free forms.
Some researchers prepared imprinted ionic polymers (IIPs) for adsorption of metal ions [144–147]. IIPs were prepared by either the functionalization of a support surface (e.g., silica gel) using an organic chelating group and subsequently imprinted with metal ions as a template or by the co-condensation of monomers containing functional groups (in the presence of metal ions) and then co-condensation of them by sol-gel processes and finally releasing metal ion from the prepared compound. For example, thiocyanato- [144] and amino-functionalized [145] monomers were used to synthesize Cd2+-imprinted polymers. These compounds showed high adsorption capacity and high selectivity toward templating ions.
SEM images of Cd2+-imprinted and non-imprinted amino-functionalized hybrid polymers show that the former exhibits the porous feature [145]. The amino-functionalized Cd2+-imprinted adsorbent [145] had rough surface and smaller size and thus larger surface area (152 m2/g), compared to its non-imprinted form (101 m2/g). Optimum pHs for the adsorption of Cd2+ were in the range of 4–8 where adsorption sites were deporotonated and Cd2+ ions were not hydroxilated. Kinetics experiments showed that the saturation of these sites was after about 20 min. The qmax (maximum adsorption capacity) value for the adsorption of Cd2+ on adsorbent was 77.2 mg/g. An IIP synthesized using mercapto-functionalized monomers [146] was applied for the separation of Cd2+ from Ni2+, Cu2+ and Zn2+ that have similar ionic radii. The relative selective factor values of Cd2+/Ni2+, Cd2+/Cu2+ and Cd2+/Zn2+ (imprinted relative to non-imprinted) were 41.687, 65.617 and 66.937, respectively. The qmax values for the adsorption of Cd2+ on Cd2+-imprinted and non-imprinted adsorbents were 83.89 and 35.91mg/g, respectively which were due to larger surface area of Cd2+-imprinted adsorbent and its selectivity toward Cd2+.
For a Fe3+-imprinted adsorbent [147] prepared by grafting silica gel with cyanato functional groups, the qmax, optimum pH, and the time for the equilibrium binding of Fe3+ on the Fe3+-imprinted adsorbent were 35.6 mg/g, 3 and 20 min, respectively. The selective coefficient values of Fe3+/Co2+, Fe3+/Pb2+, Fe3+/Cd2+ and Fe3+/Ni2+ of the Fe3+-imprinted adsorbent were 11.95, 50.71, 16.96 and 8.56 times greater than those of a non-imprinted adsorbent, respectively. Due to the high affinity of these functional groups for Fe3+ and Cd2+, the adsorption rate in these kinds of adsorbents is rapid. As reported by a number of authors [144−147], they washed the used imprinted adsorbents with 3 M HCl and reused the regenerated adsorbent repeatedly without significant loss of adsorption capacity.
Some researchers are interested in synthesizing silicon and metal containing compounds [148,149] which one of them is poly-GPTS/Ti(O)OH [148]. Poly-GPTS is prepared from the hydrolysis of 3-glycidyloxypropyltrimethoxysilane (GPTS) and poly-GPTS/Ti(O)OH from the hydrolysis of poly-GPTS and titanium isopropoxide, respecyively (Scheme 5).
Scheme 5.
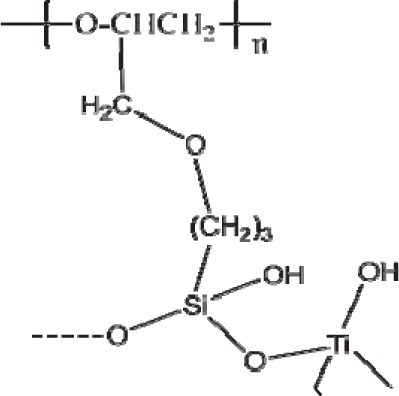
Silicon and titanium-contained adsorbent. Reprinted with permission from [148]. Copyright 2013 Taylor and Francis.
According to the kinetics experiments, the adsorption equilibrium was attained after about 10 min. Their experiments showed that at pH ≈ 5.5, Pb2+, Cu2+ and Cd2+ interact with oxygen atoms of –OH groups of these adsorbents. Authors believed that due to stronger interactions between metal ions and –OH groups of poly-GPTS/Ti(O)OH, the qmax values for the adsorption of Pb2+, Cu2+ and Cd2+ on poly-GPTS/Ti(O)OH (199, 42.79 and 39.41 mg/g, respectively) were bigger than those of poly-GPTS.
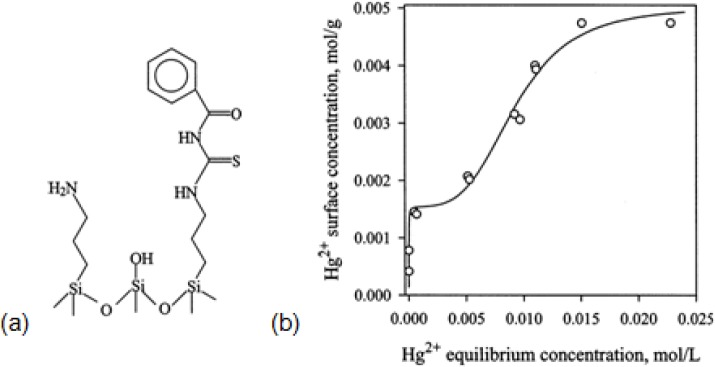
Another example of metal containing compounds is a series of macroporous thiol-functionalized titania and zirconia frameworks with propyl-siloxane, ethyl-sulfonate and propyl-sulfonate linkages [149] (Figure 9). The qmax values for the adsorption of Pb2+ and Hg2+ were in the ranges of 0.27–0.82 and 0.33–1.41 mmol/g, respectively.
Figure 9.

(a) Scheme of macroporous thiol-titania material with propyl-siloxane linkages and (b) its SEM image. Reproduced with permission from [149]. Copyright 2002 The Royal Society of Chemistry.
The ratio of adsorbed moles of Hg2+ and Pb2+ per mole of –SH groups (or their adsorption efficiency) were in the ranges of 0.19–0.82 and 0.21–0.72, respectively. This showed that the inner surface of these compounds were not completely accessible for these metal ions and had a smaller qmax value for Pb2+ compared to that of Poly-GPTS.
Xerogels [150,151] and aerogels [152] have been used for the adsorption of heavy metals [150,151]. Lima et al. [150] used mesoporous 7-amine-4-azahepthylsilica and 10-amine-4-azadecylsilica xerogels (abbreviated as AAH–Si and AAD–Si, respectively) for the adsorption of Pb2+ on which silica gels were functionalized with –(CH2)3–NH–(CH2)3–NH2 and –(CH2)3–NH–(CH2)6–NH2 groups, respectively. AAH–Si and AAD–Si interacted with Pb2+ through their amine groups and qmax values for the adsorption of Pb2+ on AAH–Si and AAD–Si were 36.64 and 30.27 mg/g, respectively and the equilibrium time of process was about 120 min. The higher adsorption capacity of AAH–Si over AAD–Si was due to the ability of AAH–Si to form a stable six-member ring with Pb2+ whereas AAD–Si was expected to form a nine-member ring that shows a transannular strain. In another work, an aniline-functionalized silica xerogel [151] was used for adsorption of Ni2+ and Mn2+ at pH 4–5. The adsorption equilibrium was achieved after about 20 and 30 min for Ni2+ and Mn2+, respectively. The enthalpies of adsorption, obtained from calorimetric measurements, showed that weak interactions occur between xerogel and metal ions. It implied that the steric hindrance of phenyl group limits the accessibility of amine groups towards metal ions. Similar to above-mentioned xerogels, an amino propyl-modified aerogel used for adsorption of Pb2+ and Cd2+ [152]. However, in spite of xerogels, the equilibrium time was 24 h.
Some efforts were done for the separation of metal ions from ethanol [153,154]. Qu et al. [153] investigated it through functionalization of silica gel using a series of amino-terminated dendrimer-like polyamidoamine (PAMAM) polymers. This process was endothermic and the Cu2+ adsorption capacities of these compounds with different amino contents were in the range of 9.8–78.7 mg/g. Kinetic experiments showed its adsorption equilibrium time was about 10 h. Pissetti et al. [154] synthesized an ethylenediamine-modified poly(dimethylsiloxane) elastomeric network (Pen) for the adsorption of Cu2+, Fe3+ and Ni2+ from ethanol. In these compounds, the ethylenediamine functional groups were located at the nodes of the networks constituted by silsesquioxane clusters. The nodes of the polymeric network were constituted of clusters rich in CSi(OSi)3 units. The interacting groups of this polymer were amine groups too (Supporting information Figure S3). Also, characteristics of a number of adsorbents [155–167] prepared by sol-gel method, have been given in Table 1.
3.2. Adsorption of Heavy Metals on Organically-Functionalized Mesoporous Compounds
In this section, adsorption capacities and operation conditions of silica-based mesopores are studied. As referred in Section 2.2, these compounds are synthesized through grafting, co-condensation or in situ grafting of mesopores and by synthesis of periodic mesoporous silicas. Details of a number of examples [168–198] are given in Table 2. One characteristic of mesoporous compounds is their high surface area. For example, it was shown that [168] when under similar conditions SBA–16 mesoporous compound was used or preparation of organic-inorganic hybrid compounds, its qmax values for adsorption of Cu2+ and Co2+ were higher than those of adsorbent prepared by silica gel.
Table 2.
Adsorbed heavy metals, maximum adsorption capacity, pH and temperature for adsorption of heavy metals by mesoporous compounds.
| Adsorbent (interacting group) | Heavy metal (qmax, pH and t (°C)) | Isotherm | Reference |
|---|---|---|---|
| SBA-16 modified with n-propyl-salicylaldimine (–NH2) | Cu2+ (58 mg/g, 4, room) | Experimental | [168] |
| Co2+ (16 mg/g, 4, room) | Experimental | ||
| SBA-16 modified with n-propyl-salicylaldimine and salicylaldehyde (–N=) | Cu2+ (15.2 mg/g, 4, room) | Experimental | – |
| Co2+ (4.5 mg/g, 4, room) | Experimental | ||
| SiO2 modified with n-propyl-salicylaldimine (–NH2) | Cu2+ (45.2 mg/g, 4, room) | Experimental | – |
| Co2+ (11.2 mg/g, 4, room) | Experimental | ||
| SiO2 modified with n-propyl-salicylaldimine and salicylaldehyde (–N=) | Cu2+ (10.2 mg/g, 4, room) | Experimental | – |
| Co2+ (3.6 mg/g, 4, room) | Experimental | ||
| 3-Aminopropyl-functionalized MCM-41 (ammonium group) | Arsenate (64.4 mg/g, 3–4,room) | Experimental | [169] |
| Chromate (52.9 mg/g, 7–8,room) | Experimental | ||
| 3-Aminopropyl-functionalized SBA-1 (ammonium group) | Arsenate (94.2 mg/g, 3–4,room) | Experimental | – |
| Chromate (132.7 mg/g, 7–8,room) | Experimental | ||
| H2N–(CH2)2–NH–(CH2)3–functionalized SBA-15 (–NH2 and –NH–) | Cu2+ (0.83 mmol/g, neutral, 25) | Langmuir | [170] |
| 3-Aminopropyl-functionalized SBA–15–N–C–H (–NH2) | Cu2+ (0.39 mmol/g, neutral, 25) | Langmuir | – |
| 3-Aminopropyl-functionalized SBA–15–N–E (–NH2) | Cu2+ (0.35 mmol/g, neutral, 25) | Langmuir | – |
| 3-Aminopropyl-functionalized SBA–15–N–C (–NH2) | Cu2+ (0.24 mmol/g, neutral, 25) | Langmuir | – |
| 3-(2-Aminoethylamino) propyltrimethoxysilane modified ordered mesoporous silica | As(V) (10.3 mg/g, 7, room) | Experimental | [171] |
| N-propyl aniline-functionalized MCM-41 (–NH–) | Arsenate (0.85 mmol/g, 4.2, room) | Experimental | [172] |
| Hg2+ (0.92 mmol/g, 3.5, room) | Experimental | ||
| Pb2+ (0.78 mmol/g, 5.8, room) | Experimental | ||
| H2N–functionalized SBA-15 (–NH2) | Cu2+ (1.28 mg/g, neutral, room) | Experimental | [173] |
| Pb2+ (1.31 mg/g, neutral, room) | Experimental | ||
| Cd2+ (1.35 mg/g, neutral, room) | Experimental | ||
| Imidazole-functionalized SBA-15 (N atoms of imidazole) | Pd2+ (0.091 mmol/g, 4, room) | Experimental | [174] |
| Pt2+ (0.091 mmol/g, 4, room) | Experimental | ||
| SBA-15 modified with 3-aminopropyl-triethoxysilane and salicylaldehyde (–N= and –O− groups) | Cu2+ (46 mg/g, 4.8, room) | Experimental | [175] |
| Ni2+ (22 mg/g, 4.8, room) | Experimental | ||
| Co2+ (19 mg/g, 4.8, room) | Experimental | ||
| Zn2+ (26 mg/g, 4.8, room) | Experimental | ||
| Amino-functionalized mesoporous silica (–NH2 and –OH) | Ni2+ (2.48 mmol/g, 8.5, 25) | Sips | [176] |
| 2-Mercaptopyrimidine-functionalized SBA-15 (N and S atoms) | Cd2+ (0.99 mmol/g, 6, 25) | Experimental | [177] |
| 3-Aminopropyl-functionalized MCM-41 (–NH2) | Au3+ (0.4 mmol/g, 2.5, room) | Experimental | [178] |
| NH(propyl)-functionalized MCM-41 (–NH–) | Au3+ (0.33 mmol/g, 2.5, room) | Experimental | – |
| N(propyl)2-functionalized MCM-41 (N atom of amine group) | Au3+ (0.2 mmol/g, 2.5, room) | Experimental | – |
| Chemically modified MCM-41 (–NH– and –NH2) | Hg2+ (0.7 mmol/g, 5, 25) | Langmuir | [179] |
| 3-Aminopropyl-functionalized MCM-41 (–NH2) | Ag+ (0.62 mmol/g, 5, 22) | Experimental | [180] |
| Cu2+ (0.84 mmol/g, 5, 22) | Experimental | ||
| 3-Mercaptopropyl-functionalized MCM-41 (–SH) | Ag+ (0.97 mmol/g, 5, 22) | Experimental | – |
| Cu2+ (0.02 mmol/g, 5, 22) | Experimental | ||
| 3-Mercaptopropyl-functionalized SBA-16 (–SH) | Cu2+ (36.42 mg/g, 5.5, 25) | Langmuir | [181] |
| 3-Aminopropyl-modified SBA-15 (–NH2) | Cu2+ (73.5 mg/g, 6.3, 25) | Langmuir | [182] |
| SBB or N-((trimethoxysilyl)propyl)-N,N,N-tri-n-butylammonium chloride-functionalized SBA-15 (quaternary ammonium) | ReO4− (1.85 mmol/g, 6.4, room) | Experimental | [183] |
| 3-Aminopropyl and 3-mercaptopropyl bi-functionalized mesoporous silica (–SH) | Hg2+ (1.51 mmol/g, neutral, room) | Experimental | [184] |
| Meso-structured silica modified with 3-mercaptopropyltrimethoxy silane and 9-(chloromethyl)anthracene (–SH) | Pb2+ (13.96 mg/g, neutral, 25) | Experimental | [185] |
| Cu2+ (12.56 mg/g, neutral, 25) | Experimental | ||
| Hg2+ (12.09 mg/g, neutral, 25) | Experimental | ||
| Zn2+ (3.69 mg/g, neutral, 25) | Experimental | ||
| 3-Mercaptopropyl-functionalized SBA-15 (–SH) | Hg2+ (2.88 mmol/g, 4.5, 20) | Langmuir | [186] |
| Ordered mesoporous silica modified with 2,5-dimercapto-1,3,4-thiadiazole (–SH) | Hg2+ (1.7 g/g, neutral, room) | Experimental | [187] |
| 1-Benzoyl-3-propylthiourea-functionalized MCM-41 (=N, =O, –NH– and –NH2 groups) | Hg2+ (1 g/g, neutral, room) | Experimental | [188] |
| Disulfide-bridged periodical mesoporous organosilica (–S–S–) | Hg2+ (716 mg/g, 2, room) | Experimental | [189] |
| 3-Mercaptopropyl-functionalized MCM-41 (–SH) | Hg2+ (0.59 mmol/g, neutral, room) | Experimental | [36] |
| 3-Mercaptopropyl-functionalized HMS (–SH) | Hg2+ (1.5 mmol/g, neutral, room) | Experimental | – |
| Mesoporous thioether-functionalized polyvinylpyrrolidone (PVP)/SiO2 composite (–S–) | Hg2+ (4.26 mmol/g, 2, room) | Experimental | [190] |
| Disulfide-functionalized SBA-1 (–SH) | Hg2+ (849 mg/g, 2, room) | Experimental | [191] |
| 3-(((5-ethoxybenzenethiol)imino)methyl)-salicylic acid immobilized onto mesoporous silica (–N= and –SH groups) | Pd2+ (164.2 mg/g, 3, room) | Langmuir | [192] |
| 3-(3-(Methoxycarbonyl)benzylidene) hydrazinyl)benzoic acid immobilized onto mesoporous silica (C=O and –N=NH–) | Cu2+ (145.98 mg/g, 7, room) | Langmuir | [193] |
| 3-(2-Aminoethylamino)propyl-functionalized mesoporous silica (–NH– and –NH2) | Cu2+ (0.107 mmol/g, 3, room) | Experimental | [194] |
| 3-Aminopropyl-functionalized MCM-41 (–NH2) | Cd2+ (0.71 mmol/g, 5, 22) | Experimental | [195] |
| Ni2+ (0.69 mmol/g, 5, 22) | Experimental | ||
| Diamino-functionalized MCM-41 | Co2+ (0.69 mmol/g, neutral, 25) | Experimental | [196] |
| Ni2+ (0.52 mmol/g, neutral, 25) | Experimental | ||
| Diamino-functionalized MCM-48 | Co2+ (1 mmol/g, neutral, 25) | Experimental | – |
| Ni2+ (0.86 mmol/g, neutral, 25) | Experimental | ||
| Aminopropyl grafted SBA-15 modified with EDTA (–NH– and –COO−) | Pb2+ (273.2 mg/g, 5, 25) | Langmuir | [197] |
| CONH2-functionalized SBA-15 (CONH2 group) | Cu2+ (1.4 mmol/g, 5, 298) | Langmuir | [198] |
Another factor affecting the adsorption capacity of an adsorbent is the density of its functional groups [169,170]. Yoshitale et al. [169] studied and compared effects of functional group density on the adsorption of chromate and arsenate oxyanions and other heavy metals. The H2N–(CH2)3–, H2N–(CH2)2–NH–(CH2)3– and H2N–(CH2)2–NH–(CH2)2–NH–(CH2)3– groups were grafted on the surface of MCM–41and SBA–1 to prepare their mono-, di- and triamino-functionalized derivatives, respectively [169]. These amino groups are abbreviated as N–, NN– and NNN–, respectively. They showed that the pore size and the surface area of these compounds decrease with the increase of their amine density and similar results have been reported for mono-, di- and triamino-functionalized SBA–15 [170]. Experimental results implied that the interaction between the amine groups and oxyanions was ionic and all amine groups can involve in the adsorption process. Thus, qmax values for the adsorption of CrO4− and HAsO42− increase from mono- to triamino-functionalized derivatives of both MCM–41 and SBA–1 (Figure 10) [169]. As reported [169,171,172], amino groups under acidic conditions had much higher qmax for the adsorption of arsenate. At the adsorption saturation, the stoichiometries of oxyanions to N atoms were 0.5 for all adsorbents derived from SBA–1, whereas they were 0.25–0.35 for MCM–41 derivatives [169]. This was due to, on SBA–1, all amino heads were randomly distributed and acted as adsorption sites but, on MCM–41, they formed domains on MCM–41.
Figure 10.
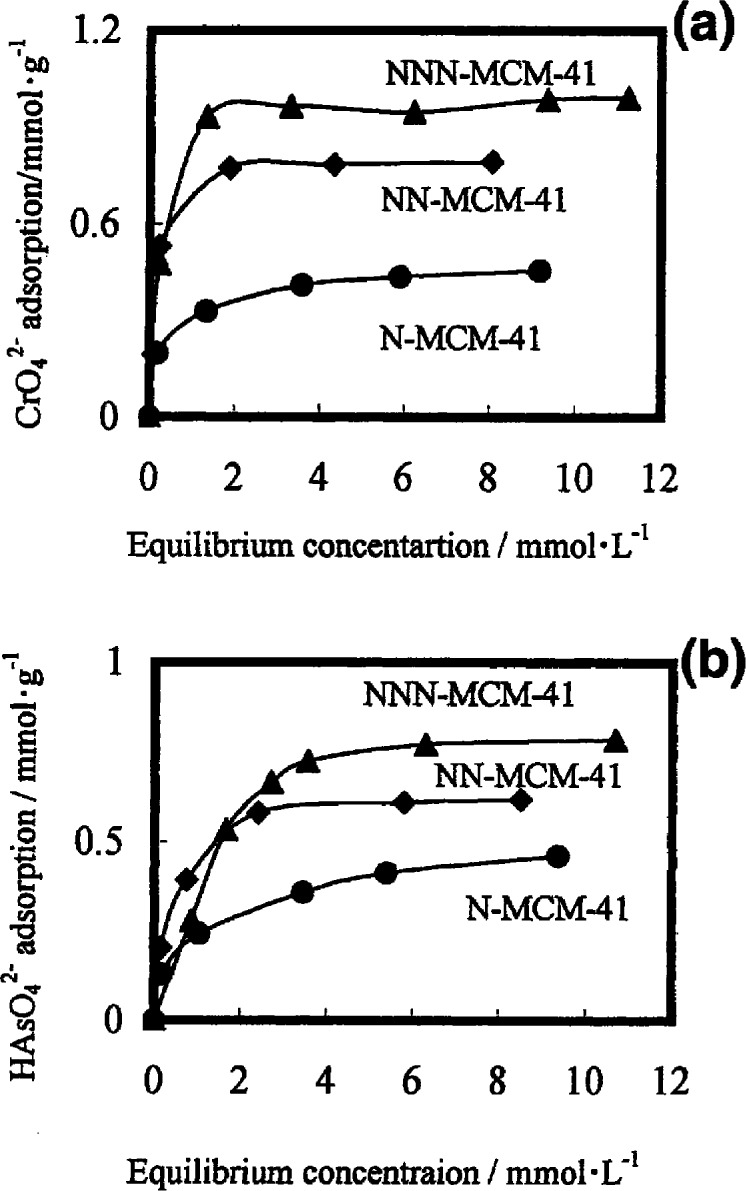
Adsorption isotherms of (a) arsenate and (b) chromate on mono-, di- and triamino-functionalized MCM–41 which are shown with N–MCM–41, NN–MCM–41 and NNN–MCM–41, respectively. Reprinted with permission from [169]. Copyright 2002 American Chemical Society.
Arencibia et al. [170] compared effect of synthesis condition on the adsorption of a number of metallic ions by monoamino-functionalized SBA–15 prepared by calcination, extraction and further hydration of calcined silica. They showed that qmax values of these adsorbents for Ni2+, Cu2+, Zn2+, Cd2+ and Pb2+ were different from each other and there was no relation between the pore size and surface area of mesopores with their qmax values for the adsorption of metallic ions from solutions. Also, different functional groups on SBA–15 can adsorb different metal cations [173–177].
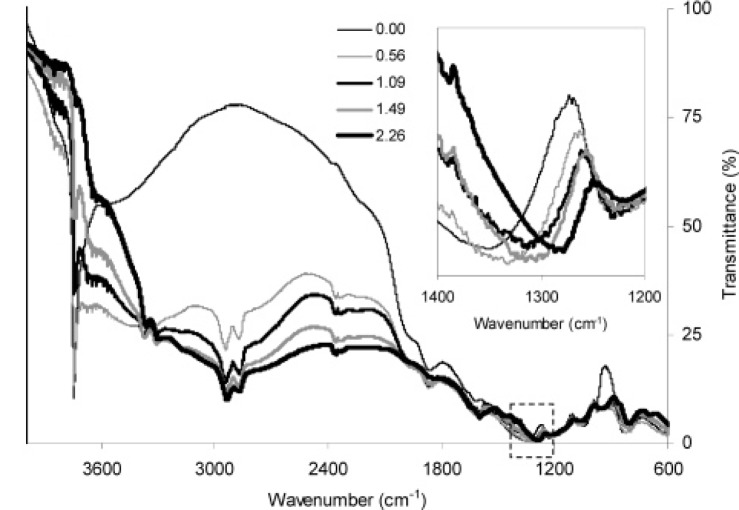
In some works, amino-functionalized MCM–41 mesopores were used for adsorption of heavy metal cations [178–180]. Yeoung et al. [178] studied the steric hindrance effect on the adsorption of Au3+, Cu2+ and Ni2+ on NH2CH2CH2CH2–, CH3CH2CH2NHCH2CH2CH2– and (CH3CH2CH2)2NCH2CH2CH2–functionalized MCM–41 which their abbreviated names are NH2–MCM–41, NHR–MCM–41 and NR2–MCM–41, respectively. The results showed that only Au3+ (from the mixture of these ions) was selectively adsorbed on these compounds, resulted from interactions of Au3+ ions with lone pair electrons of N atom of amine groups. They observed that the adsorption capacity of Au3+ decreased from NH2–MCM–41 to NR2–MCM–41 which was due to the increase in steric hindrance of these spacious groups on the lone-pair electrons of N atoms. Authors showed that Au3+ was adsorbed on NH2–MCM–41 with the amine loading ratios higher than 0.6 mmol/g. This was because, at low amine loading ratios (<0.6), strong H-bonding between silanol and amine groups prevented interactions of amine groups with Au3+ ions whereas at the amine loading ratio higher than 0.6 amine groups formed patches of amino-derived areas and this destroys H-bonding between silanol and amine groups which was revealed at the peak shifting of around 1300 cm−1 in the FT-IR spectra (Figure 11). The used NH2–MCM–41 was washed by 5 M HCl and all the adsorbed gold ions were recovered and the regenerated adsorbent retained its adsorption capacity after repeating usages.
Figure 11.
FTIR spectrum of NH2–MCM–41 with different NH2 loadings. The insert figure displays the peak shift caused by the interaction between the amino and surface silanol groups. Reprinted with permission from [178]. Copyright 2006 American Chemical Society.
In another work, researchers changed contents of functional groups and used different ratios of 3-mercaptopropyl triethoxysilane (TMMPS) and TEOS to synthesize thiol-functionalized SBA–16 (SH–SBA–16) samples [181]. With the increase in the sulfur content of samples, –SH groups on the surface partially blocked the adsorption of nitrogen molecules and decrease their surface areas and pore sizes and the adsorption capacity of SH–SBA–16 samples increases. The used SH–SBA–16 sample for the adsorption of Cu2+ was recovered by 1 M HCl and after the seventh stripping cycle, its adsorption capacity was 90.5% of its initial value.
The adsorption of Cu2+ on aminopropyl-substituted SBA–15 (SBA–15–N) and a temperature-aged sample (SBA–15–N–T) was studied by Soler–Illia et al. [182]. It was observed that upon the addition of functional groups, the surface area of SBA–15–N and SBA–15–N–T decrease compared to SBA–15. Experiments showed that the qmax value for the adsorption of Cu2+ on SBA–15–N was bigger than that of SBA–15–N–T which was due to the rearrangement of inorganic network in SBA–15–N–T. Due to the thermal treatment of SBA–15–N intensive H-bond interactions between its amino groups and the surface silanols disrupted and this improved the accessibility of amino groups on the pore surface.
Dai et al. [183] studied effect of hydrophobicity of functional groups on their adsorption capacity. HMS, SBA–15 and PME (PMO material bridged with ethylene groups) functionalized with N-((trimethoxysilyl)propyl)-N,N,N-tri-n-butylammonium (TSPBC) and N-((trimethoxysilyl)propyl)-N,N,N-trimethylammonium (TSPMC) ions were used for the adsorption of hydrophobic ReO4− ions [183]. The results showed that TSPBC-functionalized HMS, SBA–15 and PME had bigger qmax values for the adsorption of ReO4− than those functionalized with TSPMC. This was due to the higher hydrophobic character of butyl groups compared to methyl groups that enhanced the adsorption of ReO4− ions. When a cationic surfactant (here CTAC) was used for synthesizing mesopores (called as MEB sample), its chloride ions attached to the protonated silanol groups and anion-exchange sites and hindered the adsorption of ReO4− ions. To avoid high chloride content in these mesopores, authors used TSPBC and a neutral surfactant (rather than CTAC) to synthesize mesoporous compound (called as NMEB) that increased greatly its qmax for the adsorption of ReO4− ions compared to MEB. In another work, a bifunctional HMS, prepared by mercaptopropyl and aminopropyl groups, was used for the adsorption of Hg2+, Cu2+ and Cd2+ [184]. Heavy Metal ions were adsorbed by –SH heads of mercaptopropyl groups. Due to higher affinity between –SH group and different mechanisms of its adsorption, the Hg2+ adsorption and its adsorption selectivity increased greatly compared to Cu2+ and Cd2+ (Figure 12). Hg2+ can be adsorbed on the inner surface of pores whereas Cu2+ and Cd2+ were adsorbed on the outer surface of adsorbent or near the pore openings. As reported [36,180,185–187], −SH groups of organically-functionalized mesopores have a great qmax for adsorption of Hg2+. An example of a compound with two functional groups is 1-benzoyl-3-propylthiourea-functionalized MCM–41 (MCM–41 BTU) which was synthesized through a two-step modification process [188] to better control removal of Hg2+ and to prevent unnecessary pore blocking. In the first step they prepared 3-aminopropyl-MCM–41 (MCM–41 NH2) and in the second step, they prepared MCM–41 BTU using MCM–41 NH2. Their surface area and pore size were decreased as in order as MCM–41 > MCM–41 NH2 > MCM–41 BTU.
Figure 12.

Difference between adsorption mechanism of Hg2+ and other metallic ions. Reprinted with permission from [184]. Copyright 2001 Elsevier.
The concentration of 1-benzoyl-3-propylthiourea and (unreacted) aminopropyl groups on the surface of MCM–41 BTU were 1.5 and 0.65 mmol/g, respectively (Figure 13). The adsorption isotherm of Hg2+ on MCM–41 BTU had two regions (Figure 13). In the first region, Hg2+ strongly were adsorbed on 3-aminopropyl residuals (K1 = 1.41 × 105 M−1 and qmax = 1.55 mmol/g) and then in the second region the ions were adsorbed on the 1-benzoyl-3-propylthiourea residuals (K2 = 1.08 × 102 M−1 and qmax = 3.48 mmol/g).
Figure 13.
(a) Functional groups of MCM–41BTU and (b) adsorption isotherm for adsorption of Hg2+ on MCM–41 BTU. The initial sharp part of adsorption isotherm is related to adsorption by 3-aminopropyl residuals. Reprinted with permission from [188]. Copyright 2003 Springer Science and Business Media.
Zhao et al. [189] synthesized a number of two-dimensional disulfide-bridged periodical mesoporous organosilicas (PMO) with high sulfide contents for the adsorption of Hg2+. They used bis(triethoxysilylpropyl) disulfide (BTSPDS) and TEOS as precursors Zn(NO3)2 in a number of syntheses. XRD patterns demonstrate that in the presence of Zn2+ highly ordered hexagonal disulfide-bridged PMO composites were obtained. Eventually, Zn2+ ions were etched off in a strong acid. XRD patterns of the Zn2+ treated compounds showed their high hydrothermal stability which was enhanced with the increase in their BTSPDS/TEOS ratios. Hg2+ ions interacted with sulfur atoms of –S–S– bridges of adsorbents. Adsorption capacities of these compounds for Hg2+, similar to those of tetrasulfide-functionalized polyvinylpyrrolidone (PVP)/SiO2 composite mesopores [190] or disulfide-functionalized SBA–1 [191], were high and increased in their BTSPDS content [189]. This shows that double sulfide functioalized mesopores are emerging candidates for adsorption of Hg2+.
Also, other kinds of mesoporous compounds with various functional groups were used for adsorption of heavy metals [192–198]. Yeung et al. [195] increased selectivity of an aminopropyl-grafted MCM–41 (NH2–MCM–41) for the adsorption of Ni2+ and Cd2+ through chelation of these ions. The isoelectric point of NH2–MCM–41 was 3.2. Below this pH, the adsorbent did not adsorb Ni2+ and Cd2+ and at pH = 5, its qmax values of Cd2+ and Ni2+ were 0.71 and 0.69 mmol/g, respectively. In binary mixtures of Ni2+ and Cd2+ at pH > 3.2, the qmax values of NH2–MCM–41 for Ni2+ and Cd2+ were 0.60 and 0.16 mmol/g, respectively. The authors tuned the selectivity of adsorbent by an appropriate chelate, EDTA. EDTA selectively bound to Ni2+ (as NiEDTA2− complex) and changed its adsorption selectivity toward Cd2+. Authors tried to separate these ions by adding EDTA and adjusting the pH of solution. At pH = 2, only Ni2+ ions (as NiEDTA2−) were adsorbed by –NH3+ headgroups in mesopores and, at pH = 5, only Cd2+ ions were adsorbed by –NH2 groups of adsorbent (Supporting information Figure S4).
The H2N(CH2)2NH(CH2)3–functionalized MCM–41 and MCM–48 (abbreviated as NN–MCM–41 and NN–MCM–48) [196] were used for the adsorption of Fe3+, Cu2+, Co2+ and Ni2+. Then, the authors applied the used mesopores for the adsorption of arsenate. The Fe3+, Cu2+ and Co2+ ions bound to ethylenediamine (en) headgroups of mesoperes as Fe(en)2, Cu(en)2 and Co(en) whereas Ni2+ interacted with surface silanol groups. The adsorption capacities of NN–MCM–48 for these metal ions were much larger than those of NN–MCM–41. Also, it was shown that Cl− and SO42− anions inhibited arsenate adsorption on these kinds of adsorbents by different extents.
3.3. Adsorption of Heavy Metals on Composites of Layered Compounds with Organic Polymers
As pointed in Section 2.3.3, these kinds of compounds are prepared as phase separated, intercalated and exfoliated structures [118]. Here, we study a number of works carried out regarding their abilities for the adsorption of heavy metals. Some examples [199–232] are summarized in Table 3.
Table 3.
Adsorbed heavy metals, maximum adsorption capacity, pH and temperature for adsorption of heavy metals by organic polymer/layered compound hybrids.
| Adsorbent (interacting group) | Heavy metal (qmax, pH and t (°C)) | Isotherm | Reference |
|---|---|---|---|
| Chitosan/cloisite 10 A (protonated –NH2) | Cr(VI) (357.14 mg/g, 3, 35) | Langmuir | [199] |
| Chitosan/bentonite (ion exchange property of bentonite and –OH and –NH2 groups of chitosan) | Pb2+ (15 mg/g, 4, 25) | Langmuir | [200] |
| Cu2+ (12.6 mg/g, 4, 25) | Langmuir | ||
| Ni2+ (6.1 mg/g, 4, 25) | Langmuir | ||
| Chitosan/perlite (protonated –NH2) | Cr(VI) (153.8 mg/g, 4, 25) | Experimental | [201] |
| Chitosan/perlite (–NH2 and –OH) | Cu2+ (196.08 mg/g, 5, room) | Langmuir | [202] |
| Ni2+ (114.95 mg/g, 5, room) | Langmuir | ||
| Chitosan/perlite (–NH2) | Cd2+ (178.6 mg/g, 6, 25) | Experimental | [203] |
| Chitosan/perlite (–NH2 and –OH) | Cu2+ (1.59 mmol/g, 4.5, 25) | Langmuir | [204] |
| Chitosan/clinoptilolite (–NH2) | Cu2+ (11.32 mmol/g, 5, 25) | Langmuir | [205] |
| Co2+ (7.94 mmol/g, 5, 25) | Langmuir | ||
| Ni2+ (4.21 mmol/g, 5, 25) | Langmuir | ||
| Chitosan/alumina (protonated amino groups) | Cr(VI) (9.71 mg/g, 4, 40) | Langmuir | [206] |
| Chitosan/alumina (protonated –NH2) | Cr(VI) (153.85 mg/g, 4, 25) | Langmuir | [207] |
| Chitosan/montmorillonite (protonated –NH2) | Cr(VI) (40.65 mg/g, 4, 35) | Langmuir | [208] |
| Chitosan/montmorillonite (protonated –NH2 group of chitosan) | Selenate (18.4 mg/g, 4, room) | Langmuir | [209] |
| Chitosan/calcium alginate (–NH2 and –OH) | Ni2+ (222.2 mg/g, 5, room) | Langmuir | [210] |
| Cellulose/hydroxyapatite (ion exchange property of hydroxyapatite) | Pb2+ (16.32 mg/g, neutral, 25) | Langmuir | [211] |
| Chitosan/bentonite (–NH2) | Cu2+ (9.85 mg/g, 4, 25) | Langmuir | [212] |
| Epichlorohydrin-crosslinked chitosan/bentonite (–NH2) | Cu2+ (11.75 mg/g, 4, 25) | Langmuir | – |
| Ethylene glycol diglycidyl ether-crosslinked chitosan/bentonite (–NH2) | Cu2+ (10.52 mg/g, 4, 25) | Langmuir | – |
| Glutaraldehyde-crosslinked chitosan/bentonite (–NH2) | Cu2+ (4.17 mg/g, 4, 25) | Langmuir | – |
| Chitosan-g-poly(acrylic acid)/attapulgite/sodium humate (–COO−, –COOH, –NH2 and –O−) | Pb2+ (809.5 mg/g, 5.5, 35) | Langmuir | [213] |
| Chitosan-g-poly(acrylic acid)/attapulgite (–COO−) | Cu2+ (303.03 mg/g, 5.5, 30) | Langmuir | [214] |
| Chitosan-g-poly(acrylic acid)/attapulgite (–COOH, –NH2 and –OH) | Hg2+ (785.2 mg/g, 5, 30) | Langmuir | [215] |
| Poly(methacrylic acid) grafted chitosan/bentonite (–COOH) | U(VI) (117.2 mg/g, 5.5, 30) | Langmuir | [216] |
| Poly-methacrylic acid grafted chitosan/bentonite (amino and hydroxyl groups of chitosan) | Hg2+ (125 mg/g, 6, room) | Langmuir | [217] |
| Pb2+ (111 mg/g, 6, room) | Langmuir | ||
| Cd2+ (83 mg/g, 6, room) | Langmuir | ||
| Chitosan-g-poly(acrylic acid)/vermiculite (–COOH, –NH2 and –OH) | Pb2+ (3.08 mmol/g, neutral, 30) | Langmuir | [218] |
| Cd2+ (2.94 mmol/g, neutral, 30) | Langmuir | ||
| Humic acid-immobilized-amine modified polyacrylamide/bentonite (–COOH) | Cu2+ (106.2 mg/g, 5, 30) | Langmuir | [219] |
| Zn2+ (96.1 mg/g, 9, 30) | Langmuir | ||
| Co2+ (52.9 mg/g, 8, 30) | Langmuir | ||
| Polyethylenimine800/magnetite-montmorillonite (protonated –NH2) | Cr(VI) (8.77 mg/g, 3, 25) | Langmuir | [220] |
| Polyacrylic acid crossliked by N,N′-methylenebisacrylamide/montmorillonite (–CONH– and –COOH) | Ni2+ (270.3 mg/g, neutral, 25) | Langmuir | [221] |
| Pb2+ (1666.7 mg/g, neutral, 25) | Langmuir | ||
| Poly[N-(4-vinylbenzyl)-N-methyl-D-glucamine]/montmorillonite (protonated tertiary amine) | As(V) (72.26 mg/g, 6, 30) | Langmuir | [222] |
| As(V) (72.99 mg/g, 6, 40) | Langmuir | ||
| As(V) (82.64 mg/g, 6, 50) | Langmuir | ||
| Polyacrylamide/bentonite (–NH2) | Cu2+ (32.81 mg/g, 6.2, 20) | Langmuir | [223] |
| Polyacrylamide-bentonite composite (negatively charged sites of bentonite) | Pb2+ (0.16 mmol/g, 4.5–5, room) | Langmuir | [224] |
| Phytic acid-modified polyacrylamide-bentonite composite (negatively charged sites of bentonite) | Pb2+ (0.18 mmol/g, 4.5–5, room) | Langmuir | – |
| Polyaniline/attapulgite (–NH– and –N=) | Hg2+ (909.1 mg/g, 6, 25) | Langmuir | [225] |
| Polyacrylamide/attapulgite (–NH2) | Hg2+ (192.5 mg/g, 4.4, 30) | Langmuir | [226] |
| Polyvinyl alcohol/attapulgite (–OH) | Pb2+ (169.5 mg/g, 5, 30) | Langmuir | [227] |
| Acrylamide-2-acrylamido-sodium 2-methylpropane sulfonate copolymer/clay (–SO3−) | Cu2+ (1.07 mmol/g, 4.5, 25) | Experimental | [228] |
| Cd2+ (1.28 mmol/g, 4.5, 25) | Experimental | ||
| Pb2+ (1.03 mmol/g, 4.5, 25) | Experimental | ||
| Poly(methoxyethyl)acrylamide/clay (–NH–) | Pb2+ (0.385 mmol/g, 5, 30) | Langmuir | [229] |
| 3-Aminopropyltriethoxysilane/sepiolite (–NH2) | Fe2+ (0.44 mmol/g, 3, 25) | Langmuir | [230] |
| Cu2+ (0.14 mmol/g, 4, 25) | Langmuir | ||
| Triethoxy-3-(2-imidazolin-1-yl)propylsilane/sepiolite (negatively charged sites of sepiolite and N atoms of imidazolin) | Mn2+ (0.085 mmol/g, 4, 25) | Langmuir | [231] |
| Cu2+ (0.13 mmol/g, 4, 25) | Langmuir | ||
| Fe3+ (0.05 mmol/g, 2.5, 25) | Langmuir | ||
| Zn2+ (0.035 mmol/g, 4, 25) | Langmuir | ||
| Co2+ (0.28 mmol/g, 4, 25) | Langmuir | ||
| Cd2+ (0.09 mmol/g, 4, 25) | Langmuir | ||
| Iodine-modified chitosan/bentonite (I2 and I−) | Gas-phase Hg0 at 110 °C | Experimental | [232] |
Chitosan is a natural polycationic biopolymer that can chelate with heavy metals by its amino and hydroxyl groups and its nanocomposites with clays and zeolites are used for adsorption of heavy metals. However, it has a low surface area, with weak chemical and mechanical properties. Thus, physical and chemical modifications are necessary to overcome these limitations. On the other hand, the clay has a lamellar structure with negatively charged surface that interacts with polycationic chitosan. There is high possibility that one or both surfaces of the clay layers can be modified by chitosan. Chitosan-functionalized closite 10A [199], bentonite [200], perlite [201–204], clinoptilolite [205], alumina [206,207], montmerilonite [208,209] and calciul alginate [210] were used for the adsorption of heavy metals from water. In these compounds, functional groups of chitosan (–NH2 and –OH) interact with heavy metals. In spite of cellulose/hydyoxyapatite in which negative sites of hydroxylapatite are interacting sites of nanocomposite [211]. XRD showed the interlayer spacing of chitosan-functionalized bilayer compounds increases, which showed a complete [199] or partial exfoliated [209] surface morphology. Electrostatic interaction between chitosan and negatively-charged surface of layer compounds such as closite A [199], perlite [203,204] and, alumina [206,207] resulted in the formation of nanocomposites. As an example for the adsorption of heavy metals by these kinds of compounds, authors studied the adsorption of Cr(VI) polyanions by closite 10 A/chitosan nanocomposite (CCN) (Figure 14) [199]. The TEM image demonstrated the formation of exfoliated surface morphology. The zeta potential of CCN surface at pH below eight was positive because the amine groups of chitosan were protonated at pH below eight and Cr(VI) ions existed as Cr2O72−, CrO42− and HCrO4− polyanions at pH range of 2–6. Thus, The optimum pH for the adsorption process was found to be 3. Similarly, nanocomposites of chitosan with perlite [201], alumina [206,207], montmorillonite [208], with Cr(VI) oxyanion or chitosan-clay with selenate [209] occurs via their protonated amino groups. As reported [200,202–205,210] chitosan-layered nanocomposites interaction with metal cations occurs through –OH and –NH2 groups of chitosan. The qmax values of these adsorbents completely depend on the used layered compounds, Table 3. The used CCN was regenerated by washing with 0.01 N H2SO4 and, after four cycles, its adsorption capacity was 78.47% of its initial value [199].
Figure 14.
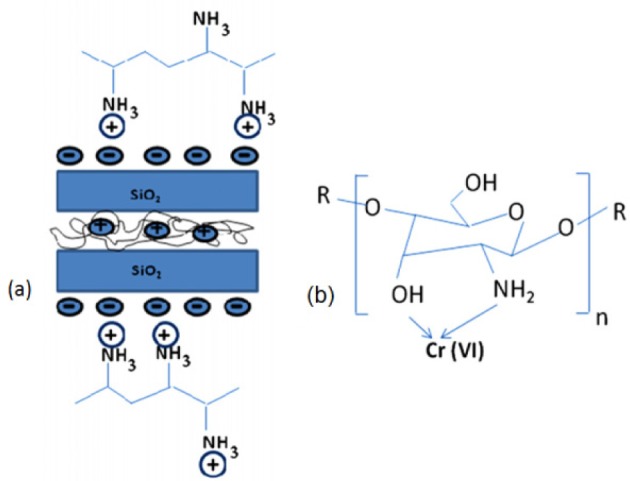
(a) Schematic structure of chitosan and clay hybrid and (b) mechanism of interaction of closite 10 A/chitosan nanocomposite (CCN) with Cr(VI). Reprinted with permission from [199]. Copyright 2011 Elsevier.
In another work, Wan et al. [212] used chitosan/bentonite and crosslinked chitosan immobilized on bentonite as adsorbent for Cu2+. Crosslinking agents are used to amend mechanical and chemical properties of the adsorbents.
Some researchers prepared nanocomposites using a natural carbohydrate (e.g., chitosan), a polymer and a layered compound [213–219]. Wang et al. [213] increased the number of compounds for synthesizing hybrid polymer and prepared chitosan-g-poly(acrylic acid)/attapulgite/sodium humate composite hydrogels for the adsorption of Pb2+. In this adsorbent, –NH2 groups of chitosan (CSA), –COOH and –COO− groups of polyacrylic acid (PAA), –COO− and Ph–CO− groups of sodium humate (SH) and Si–OH groups of attapulgite (APT) adsorbed Pb2+ ions. Depending on the used quantity of the above constituents, the adsorption capacity of hybrid polymer for Pb2+ was in the range of 702.35 to 843.86 mg/g.
Chitosan-g-poly(acrylic acid)/attapulgite (CTS–g–PAA/APT) composites were used for the fast adsorption of Cu2+ [214]. Results showed that attapulgite (APT) really reacted with the CTS–g–PAA polymer (Supporting information Figure S5). Adsorption experiments were carried out at pH = 5.5. It was fast and more than 90% of Cu2+ was adsorbed after about 15 min. FTIR spectra of CTS–g–PAA/APT compound before and after the adsorption of Cu2+ showed that –NH2, –OH groups of chitosan and –COOH groups of PAA in the composites interacted with Cu2+. This compound had a high qmax (785.2 mg/g) for the adsorption of Hg2+ [215], as well.
Poly(methacrylic acid)-grafted chitosan/bentonite (CTS–g–PMAA/Bent) composite (Supporting information Figure S6) was synthesized for the adsorption of U(VI) [216]. They used N,N’-methylenebisacrylamide as a crosslinking agent. XRD patterns showed that bentonite was exfoliated during the formation of composite. The adsorption process was carried out at pH = 5.5 in which the CTS–g–PMAA/Bent surface charge was negative and UO2(OH)+ was the predominant species. XPS spectra showed that –COO− groups of composite interacted with UO22+. Similarly, methacrylic acid grafted chitosan/bentonite [217], Chitosan-g-poly(acrylic acid)/vermiculite [218] and humic acid-immobilized-amine modified polyacrylamide immobilized on bentonite [219] were used for the adsorbtion of a number of heavy metal cations.
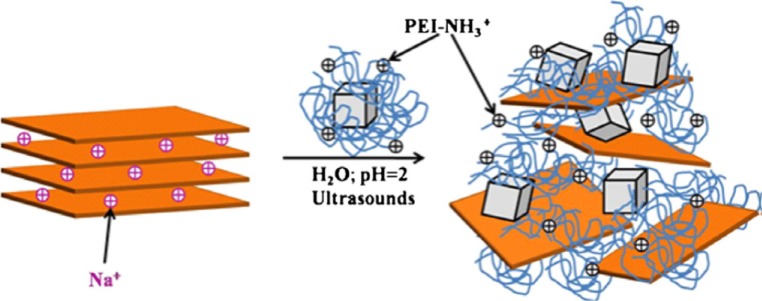
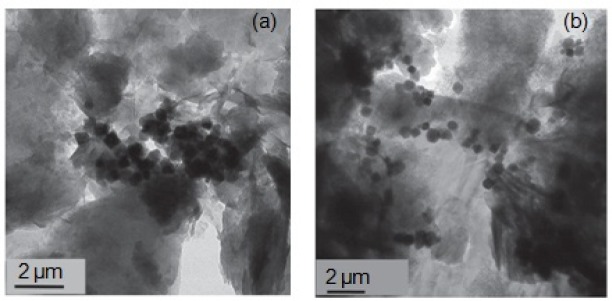
In another groups of organically-functioalized layered compounds, synthetic polymers were used as organic moiety. Fe3O4 nanoparticles coated with polyethylenimine (PEI) polymer were intercalated between sodium rich montmorillonite (MMT) layers [220] under acidic conditions (pH = 2) and used it as a magnetic sorbent for the adsorption of Cr(VI). At pH = 2, amine groups of PEI were protonated and intercalated between MMT platelets by cationic exchange (Scheme 6). Two different molecular weights of PEI were investigated (x = 800 or 25,000 g/mol). The TEM images of Fe3O4–PEIx–MMT compound showed MMT existed as individual exfoliated platelets and intercalation tactoids composed by a few sheets (Figure 15).
Scheme 6.
Schematic representation of Fe3O4–PEIx–MMT formation. Reprinted with permission from [220]. Copyright 2012 Elsevier.
Figure 15.
TEM images of (a) Fe3O4–PEI800–MMT and (b) Fe3O4–PEI2500–MMT hybrid materials. Reprinted with permission from [220]. Copyright 2012 Elsevier.
A better dispersion of magnetites was obtained in Fe3O4–PEI2500–MMT. The pH of zero point of charge of magnetite was 8.3 and the amine groups of PEI were protonated at pH below 10.4 and adsorbed Cr(VI) polyanions (CrO42−, HCrO4− and Cr2O72−) through electrostatic interactions. In pH lower than 2, Cr(VI) ions were mainly as H2CrO4 and the adsorption decreased. Experiments showed that they can be used for a wide pH range and the optimum pH for the adsorption process was 6. The qmax values of Fe3O4–PEI800–MMT and Fe3O4–PEI25000–MMT compounds were 8.77 and 7.69 mg/g, respectively.
A novel superabsorbent composite was synthesized by copolymerization reaction of partially neutralized acrylic acid (AA) on bentonite micropowder using N,N′-methylenebisacrylamide as a crosslinker [221]. The superabsorbent composite (SAC) was characterized by Fourier transform infrared spectroscopy (FTIR), thermogravimetric analysis (TGA) and scanning electron microscopy (SEM). The qmax values for adsorption of Pb2+, Ni2+, Cd2+ were 1666.67, 270.27, 416.67 and 222.22 mg/g respectively, Scheme 7.
Scheme 7.
Adsorption isotherms of Pb2+, Ni2+, Cd2+ and Cu2+. Reprinted with permission from [221]. Copyright 2009 Elsevier.
Organic-modified montmorillonite coated by N-(4-vinylbenzyl)-N-methyl-D-glucamine polymer was studied in the presence of a crosslinking reagent for the adsorption of arsenate [222]. XRD patterns showed that the intercalation process was not complete. Also, TEM images confirmed the dispersion of montmorillonite within matrix. Adsorption experiments were carried out at pH = 6 and As(V) polyanions (as H2AsO4−) interacted with ammonium groups of adsorbent and its qmax was 72.99 mg/g.
Bentonite (BENT) embedded in polyacrylamide (PAAm) composite was used [223] for the removal of Cu2+ from water. XRD patterns of BENT and BENT–PAAm (Figure 16) shows that acrylamide polymerization destroyed BENT structure and resulted in the crystal confusion. Results showed that the optimum pH for the adsorption of Cu2+ was 6.2. Increasing pH, increased the negatively charged or deprotonated amines and SiOH groups of BENT–PAAm and also increased the hydrolysis of Cu(II).
Figure 16.
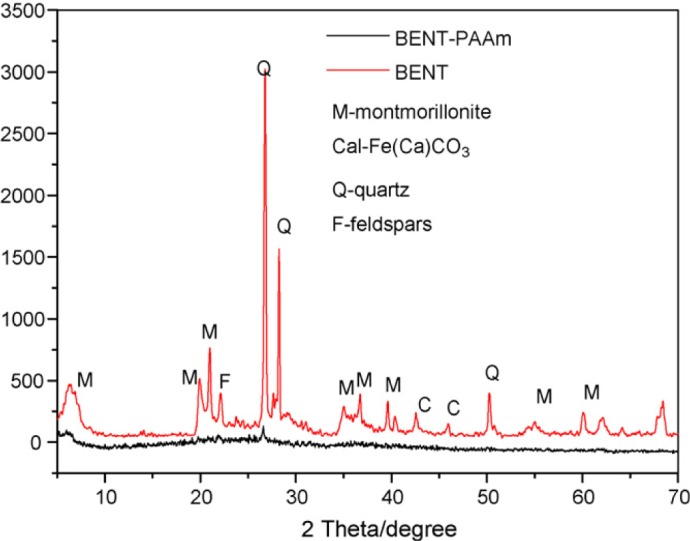
XRD patterns of Bentonite (BENT) and BENT–PAAm. Reprinted with permission from [223]. Copyright 2010 Elsevier.
Below pH = 6.5, Cu(II) is mainly as Cu2+ (Supporting information Figure S7). In pHs of 5 and 6.2, qmax values of BENT–PAAm were bigger than those of BENT which was due to the presence of amine groups of PAAm. On the other hand, it was reported that negatively charged sites of bentonite in BENT–PAAm composite adsorbed Pb2+ [224]. In this compound, polyacrylamide chains just increase thermal and mechanical stabilities of the nanocomposite and its qmax for the adsorption of Pb2+ is less than that of pure bentonite.
Polyaniline/attapulgite (PANI/ATP) composite was used achieving a high adsorption of mercury [225]. X-ray photoelectron spectroscopy showed that after the adsorption of Hg2+ on PANI/ATP, its amine (–NH–) content reduced from 6.97 to 4.55 atom% and its imine (–NH+–) content also diminished from 4.54 to 0.96 atom%. This showed that Hg2+ was adsorbed on both amine and imine functional groups. The optimum pH for the adsorption process was 6 and its qmax values at ionic strengths of 0.01, 0.1 and 1M were 909.1, 813.1 and 781.3 mg/g, respectively. As reported before [226], polyacrylamide/attapulgite adsorbed Hg2+ via its –NH2 functional groups and its qmax value was 192.5 mg/g. It was observed that Cl− decreased drastically its adsorption capacity of Hg2+ via forming HgCl42− compound. The adsorption capacity of PANI/ATP was preserved at 93% by the fifth cycle. Also, as seen in Table 3, –OH, –SO3− and –NH– groups of organic moiety in polyvinyl alcohol/attapulgite [227], acrylamide-2-acrylamido-sodium 2-methylpropane sulfonate/clay [228] and poly(methoxyethyl)acrylamide/clay [229], respectively had a relatively high affinity for adsorption of Pb2+.
3.4. Adsorption of Heavy Metals on Organic-Inorganic Core-Shell Nanocomposites (CSNs)
CSNs have different applications and were studied briefly in Section 2.3.4. Here, we discuss the role of these compounds in the adsorption of metal ions. A number of examples [233–252] have been given in Table 4.
Table 4.
Adsorbed heavy metals, maximum adsorption capacity, pH and temperature for adsorption of heavy metals by organic-inorganic core/shell and hierarchically structured nanocomposites.
| Adsorbent (interacting group) | Heavy metal (qmax, pH and t (°C)) | Isotherm | Reference |
|---|---|---|---|
| Core/shell nanocomposites
| |||
| SiO2/salicyclic acid functionalized polystyrene (O atom of –COOH and N atom of –N=N–) | Cu2+ (1.29 mmol/g, 5, room) | Langmuir | [233] |
| Ag+ (1.85 mmol/g, 5, room) | Langmuir | ||
| Au3+ (1.61 mmol/g, 2.7, room) | Langmuir | ||
| SiO2/amino functionalized polystyrene (–NH2) | Cu2+ (0.17 mmol/g, neutral, room) | Experimental | [234] |
| Ag+ (0.47 mmol/g, neutral, room) | Experimental | ||
| Au3+ (0.59 mmol/g, neutral, room) | Experimental | ||
| SiO2/imidazole-functionalized polystyrene (–N=N– and imidazole) | Au3+ (1.7 mmol/g, 2.7, room) | Langmuir | [235] |
| SiO2/aniline formaldehyde condensate (–NH2) | Cu2+ (76.33 mg/g, 5.4–5.7, room) | Langmuir | [236] |
| SiO2/polyacrylamide (–NH2) | Hg2+ (26.5 mg/g, acidic, 40) | Experimental | [237] |
| SiO2/Chetoceros sp microalgae | Pb2+ (0.19 mmol/g, 5, 27) | Langmuir | [238] |
| SiO2/chitosan imprinted by sucrose (amino groups) | Cu2+ (3.2 mg/g, 6, 25) | Langmuir | [239] |
| SiO2/chitosan imprinted by polyethylene glycol 4000 (amino groups) | Cu2+ (9.1 mg/g, 6, 25) | Langmuir | – |
| SiO2/chitosan imprinted by sucrose and polyethylene glycol 4000 (amino groups) | Cu2+ (10.5 mg/g, 6, 25) | Langmuir | – |
| SiO2/Cd2+-imprinted chitosan (amino groups) | Cd2+ (1.14 mmol/g, 6, room) | Experimental | [240] |
| SiO2/chitosan (amino groups) | Cd2+ (0.58 mmol/g, 6, room) | – | – |
| SiO2/chitosan (–NH2 and –OH) | Cu2+ (0.2 mmol/g, 5.5, 25) | Experimental | [241] |
| SiO2/chitosan (amino groups) | Ni2+ (182 mg/g, 7, 25) | Langmuir | [242] |
| SiO2(CO2H)/chitosan (amino groups) | Ni2+ (210 mg/g, 7, 25) | Langmuir | – |
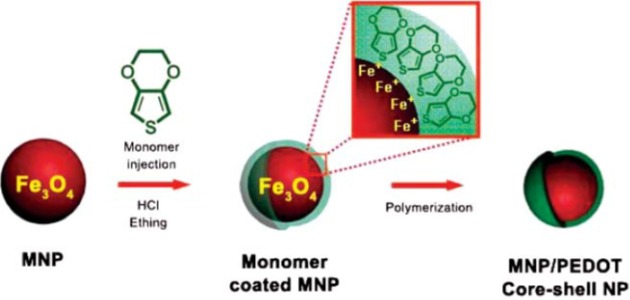
| Fe3O4/poly(3,4-ethylenedioxythiophene) (–O–) | Ag+ (27.96 mmol/g, neutral, room) | Experimental | [243] |
| Hg2+ (16.02 mmol/g, neutral, room) | Experimental | ||
| Pb2+ (14.99 mmol/g, neutral, room) | Experimental | ||
| γ-Fe2O3/polyrhodanine (oxygen, nitrogen and sulfur atoms of polyrhodanine) | Hg2+ (179 mg/g, 4, 25) | Langmuir | [244] |
| Fe3O4 nanoparticle/chitosan (amino groups) | Cu2+ (21.5 mg/g, 5, 27) | Langmuir | [245] |
| Fe3O4 nanoparticle/chitosan (amino groups) | Au3+ (59.52 mg/g, 2, 25) | Langmuir | [246] |
| Fe3O4 nanoparticle/thiol-functionalized mesoporous microsphere (–SH) | Hg2+ (185.19 mg/g, 5, 20) | Langmuir | [247] |
| Pb2+ (114.7 mg/g, 5, 20) | Langmuir | ||
| Chitosan/zerovalent iron nanoparticles (complexation between Fe and Arsenic) | As3+ (94 mg/g, 7, 25) | Langmuir | [248] |
| Arsenate (119 mg/g, 7, 25) | Langmuir | ||
| Polystyrene/nano-Fe3O4 (Fe3O4) | Arsenate (139.3 mg/g, 6, 25) | Langmuir | [249] |
| Nanosized hydrous MnO2/porous polystyrene cation exchanger resin (–SO3−, –Mn(OH) and –Mn(OH)2 groups) | Cd2+ (1.96 mmol/g, 4.7, 25) | Langmuir | [250] |
| Zn2+ (1.67 mmol/g, 4.7, 25) | Langmuir | ||
| Fe3O4/SiO2/poly(1,2-diaminobenzene) (–NH2, –NH– and –N=) | As3+ (84.5 mg/g, 6, 25) | Langmuir | [251] |
| Cr3+ (77 mg/g, 5.3, 25) | Langmuir | ||
| Cu2+ (65 mg/g, 6, 25) | Langmuir | ||
| SiO2/Fe3O4/ion-imprinted polymer (–NH –CH2 –CH2 –NH2) | Pb2+ (19.61 mg/g, 7.5, room) | Langmuir | [252] |
| SiO2/Fe3O4/non-imprinted polymer (–NH –CH2 –CH2 –NH2) | Pb2+ (6.57 mg/g, 7.5, room) | Experimental | – |
| Polyaniline nanorods on graphene oxide nanosheets (amine group) | Cr(VI) (1149.4 mg/g, 3, 25) | Langmuir | [253] |
| Silica/polystyrene (–SH) | Cu2+ (11.33 mg/g, 5, 15) | Langmuir | [254] |
| CaCO3-pepsin (CaCO3 and adsorption occurs through formation of PbCO3 and CuCO3) | Pb2+ (1167 mg/g, neutral, room) | Experimental | [255] |
| Cu2+ (611 mg/g, neutral, room) | Experimental | ||
| CaCO3-maltose (CaCO3 and adsorption occurs through formation of PbCO3, CuCO3, NiCO3 and CdCO3) | Pb2+ (3242.48 mg/g, 7, 25) | Langmuir | [256] |
| Cd2+ (487.8 mg/g, 7, 25) | Langmuir | ||
| Cu2+ (628.93 mg/g, 7, 25) | Langmuir | ||
| Ni2+ (769.23 mg/g, 7, 25) | Langmuir | ||
Silica gel is a commonly used supporting material in inorganic-organic hybrid materials due to its environmental and economic factors and high thermal and mechanical stabilities [233–241]. Polystyrene diazo-coupled with salicyclic acid [233] (SG–PS–azo–SA) and encapsulated silica gel by polystyrene containing amino groups (SG–PS–NH2) [234] (Scheme 8) were used for the removal of a number of heavy metals.
Scheme 8.
Structures of (a) SG–PS–NH2 and (b) SG–PS–azo–SA. Reprinted with permission from [233]. Copyright 2010 Springer Science and Business Media.
Thermogravimetric analysis of SG–PS–NH2 and SG–PS–azo–SA showed that the respective organic layer had a high thermal stability. Also, the values of BET surface area, pore volume and pore size decreased greatly from silica gel to SG–PS–NH2 to SG–PS–azo–SA. SEM images of silica gel, SG–PS–NH2 and SG–PS–azo–SA indicated that the spherical shape and sizes of these three samples were similar (Supporting information Figure S8), proving that the microspheres of silica gel had good mechanical stability during the process of reaction. The qmax values of SG–PS–NH2 for Cu2+, Ag+ and Au3+ were 0.17, 0.47 and 0.59 mmol/g [234] and those of SG–PS–azo–SA were 1.29, 1.85 and 1.61 mmol/g, respectively [233]. Amine groups of SG–PS–NH2 and –N=N– and salicyclic acid groups of SG–PS–azo–SA are their interacting groups.
On the other hand, adsorption capacities of organic functional groups change considerably depending on the nature of inorganic moiety. For example, qmax value of polyacrylamide in SiO2/polyacrylamide [237] for Hg2+ was much less than that of polyacrylamide/attapulgite [226].
Some authors have used natural carbohydrate and biomass as shell of their adsorbents. Immobilization of biomass (Chetoceros sp microalgae) on a SiO2 core resulted in an adsorbent for adsorption of Pb2+ [238]. Chitosan-grafted silica gel imprinted by sucrose and polyethylene glycol 4000 (PEG 4000) was used for the adsorption of Cu2+ [239]. This method has been used for direct preparation of porous sorbent with low mass transfer resistance, available functional ligand and excellent mechanical resistance [240]. Sucrose and PEG 4000 interacted with chitosan via hydrogen bonds and extraction of them, by breakage of H-bonds, resulted in a porous structure. The optimum pH for the adsorption of Cu2+ on this adsorbent was 6. At this pH, copper was mostly as Cu2+ form and most of amine groups of chitosan (its interacting group) were as –NH2. TG/DSC analyses showed that the thermal stability of grafted chitosan was higher than pure chitosan (Supporting information Figure S9). The XRD patterns show no peak for crystallization regions of chitosan in non-supported hybrid material compared to those of pure chitosan which might be ascribed to the demolition of its crystallinity (Figure 17). The qmax values of Cu2+ adsorption for the adsorbent imprinted with sucrose and PEG 4000, PEG 4000, sucrose and non-imprinted adsorbent [241] were 10.5, 9.1, 3.2 and 0.2 mmol/g, respectively.
Figure 17.
XRD patterns of (a) pure chitosan and (b) chitosan in non-supported hybrid material. Reprinted with permission from [239]. Copyright 2007 Elsevier.
The used adsorbent was washed by 0.1 M HCl and the capacity of the regenerated sorbent through five cycles was detected to be 94% of the fresh one and equilibration time in the case of sucrose and PEG 4000 was 25 min. Also, qmax value of SiO2(CO2H)/chitosan for adsorption of Ni2+ was higher than that of SiO2/chitosan [241] which shows effect of support on the adsorption property of adsorbent [242]. Another material that was used frequently in core-shell nanocomposites was Fe3O4 magnetic nanoparticle [243–248]. These kinds of compounds can be separated with the help of an external magnetic force. In a number of works, organic polymers were used as shell for Fe3O4 core [243,244]. Jang et al. [243] encapsulated Fe3O4 by poly(3,4-ethylenedioxythiophene) (PEDOT) and used the produced compound (Fe3O4–PEDOT) for the removal of metal ions (Scheme 9). PEDOT has an excellent environmental stability and can interact with positively charged metal ions through its sulfur atom. The PEDOT shell has a lower surface energy than the core of magnetic nanoparticles, thus Fe3O4–PEDOT nanoparticles show amended stability and dispersibility in aqueous solutions, compared to pristine Fe3O4 nanoparticles. Adsorption experiments showed that adsorption capacities of Fe3O4–PEDOT nanoparticles were: Ag+ > Hg2+ > Pb2+. It was regenerated by acid treatment and the recovered adsorbent by ten cycles showed no loss in its adsorption capacity.
Scheme 9.
Synthesis steps of Fe3O4–PEDOT nanoparticles by seeded polymerization mediated with acidic etching. Reproduced with permission from [243]. Copyright 2007 The Royal Society of Chemistry.
Chitosan-bound Fe3O4 magnetic nanoparticle has been used for the adsorption of Cu2+ and Au(III) ions [245,246]. TEM images showed that Fe3O4 and chitosan-bound Fe3O4 nanoparticles had a similar particle size of 13.5 nm and were monodisperse [245]. This revealed the reaction between Fe3O4 and chitosan occured only on the surface of Fe3O4. Also, XRD patterns showed that the binding of chitosan to the surface of Fe3O4 did not result in the phase change of Fe3O4. Experiments showed that the pH of isoelectric point of chitosan-bound Fe3O4 nanoparticles was 5.9 and the optimum pH for the adsorption of Cu2+ ions was in the range of 2–5 [245] and that of Au(III) negatively-charged complex was 2 [246]. The qmax values for Cu2+ and Au(III) were 21.5 [245] and 59.52 mg/g [246], respectively. In a different study, Tao et al. [247] used thiol-functionalized magnetic mesoporous microsphere (TMMM) for adsorption of Hg2+ and Pb2+. The Fe3O4 nanoparticles were used as a core and coated with two silica layers, a tightly crossed silica thin layer and a mesoporous silica shell. The thiol moiety had a strong affinity towards these metal ions. The porous structure of the silica shell brought large surface area and provided the opportunity to graft organic moiety for adsorption of metal ions. qmax value of Hg2+ was 185.19 mg/g at 20 °C and decreased in the presence of Ca2+, Mg2+ and Na+ ions.
In some works, organic moieties (chitosan [248] and polystyrene [249]) were used as core. Polystyrene-supported Fe3O4 nanoparticles (PS–Fe3O4 NPs) was used for the adsorption of arsenate oxyanion [249]. Zeta potential measurements showed the polystyrene (PS) latex was negatively charged in a wide pH range and Fe3O4 nanoparticles were positively charged in acidic or neutral solutions. This was the driving force for the acervation of Fe3O4 nanoparticles on PS and the formation of PS-Fe3O4 NPs. At pH of 6, As(V) was as HAsO42− and PS–Fe3O4 NPs had the maximum adsorption capacity for its adsorption. TEM images showed that the diameter of fresh Fe3O4 spherical beads (due to aggregation) were in the range of 350–400 nm, but there was not observed coalescence between PS–Fe3O4 NPs. Due to these, the qmax value for the adsorption of arsenate on PS–Fe3O4 was 139.3 mg/g whereas that of Fe3O4 was 78.4 mg/g. The used PS-Fe3O4 NP was recovered by 0.1 M NaOH and its recycling efficiency after the sixth cycle was 89.6%.
Lv et al. [250] synthesized nanosized hydrous MnO2 encapsulated within porous polystyrene cation exchanger as adsorbent for heavy metals. The –SO3− groups of polymeric matrix and –MnOH and –Mn(OH)2 groups of MnO2 interacted with Zn2+ and Cd2+. In the presence of Ca2+, due to its electrostatic interaction with –SO3− groups, qmax values of nanocomposite for adsorption with Zn2+ and Cd2+ were decreased greatly.
Some researchers have used two inorganic compounds (e.g., SiO2 and Fe3O4) as the core [251,252]. Firstly, they prepared Fe3O4 on which a layer of SiO2 was synthesized, and then carried out the polymerization on the surface of SiO2. Song et al. [251] synthesized Fe3O4–SiO2–poly(1,2-diaminobenzene) sub-micron particle (FSP) for the removal of Cr(III), As(III) and Cu(II). Zhang et al. [252] used 3-(2-aminoethylamino)propyltrimethoxysilane (AAPTS) as the functional monomer and Pb2+ as the template to prepare an ion-imprinted polymer (Fe3O4@SiO2@IIP) for the separation of Pb2+ ion from water (Scheme 10).
Scheme 10.
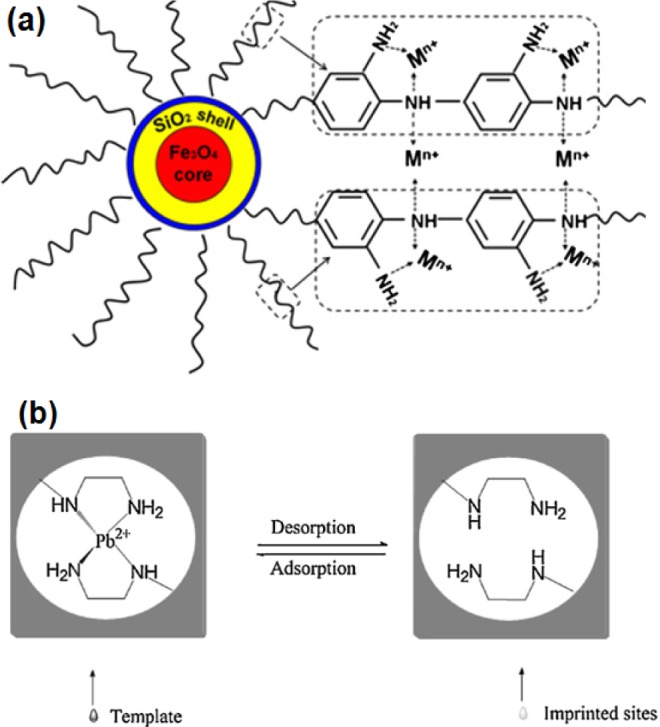
Schematic structures of (a) FSP (Reprinted with Permission from [251]. Copyright 2012 Elsevier) and (b) template in Fe3O4@SiO2@IIP (Reprinted with permission from [252]. Copyright 2011 Elsevier).
XRD patterns showed that peaks of Fe3O4 of the core-shell compounds were similar to those of naked Fe3O4 nanoparticles and the surface modification of Fe3O4 did not result in its phase change. In both adsorbents, amine groups interacted with metal ions. Experiments using Fe3O4@SiO2@IIP at pH = 7.5 showed that in the presence of Cu2+, Zn2+, Cd2+ and Hg2+, the relative selectivity factor values of Pb2+/Cu2+, Pb2+/Zn2+, Pb2+/Cd2+ and Pb2+/Hg2+ were 7.41, 6.76, 3.75 and 6.39, respectively [252]. However, when authors used a non-imprinted polymer to synthesize this core-shell polymer (Fe3O4@SiO2@NIP), values of the above-mentioned relative selectivity factor were about 1 and this adsorbent was not selective toward Pb2+.
As we saw in this section, synthesing core-shell nanocomposites does not result in the phase change of its inorganic moiety. In some cases, more than one inorganic compound was used as the core. Functional groups of the shell have a major role in the adsorption of heavy metal ions and core-shell nanocomposites had good mechanical stability and organic moiety of these compounds had higher thermal stability than that of its pure form.
3.5. Adsorption of Heavy Metals on Organic-Inorganic Hierarchically Structured Materials
Hierarchically structured compounds were explained briefly in Section 2.3.5. Li et al. [253] synthesized a hierarchical nanocomposite by the polymerization of aniline arrays on the surface of grapheme oxide nanosheets and used it as a superadsorbent for the adsorption of Cr(VI) oxyanion from water (Figure 18 and Table 4). Aniline monomers (ANI) were adsorbed on the surface of graphene oxide (GO) through electrostatic interactions with its reactive oxygen-containing functional groups and further the polymerization of ANIs resulted in the formation of aligned polyaniline (PANI) nanorods on the surface of GO (PANI/GO).
Figure 18.
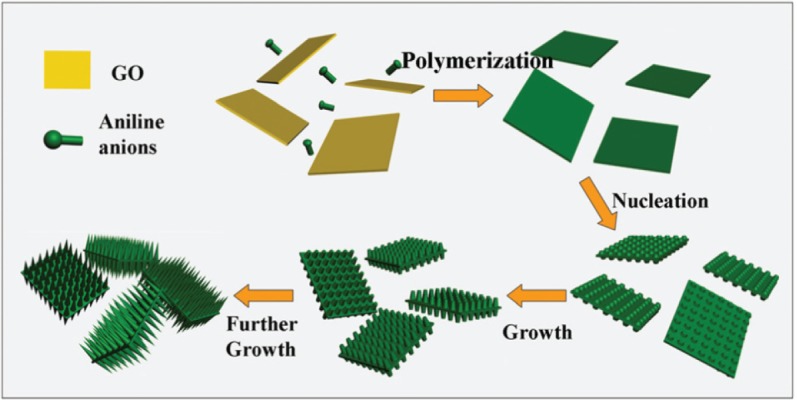
Mechanism of formation of polyaniline (PANI) nanorods on the surface of graphene oxide (GO) (PANI/GO) hierarchical nanocomposites. Reproduced with permission from [253]. Copyright 2013 The Royal Society of Chemistry.
XRD patterns showed that the interplanar distance of GO nanosheets was 0.88 nm (as agglomerated) and PANI/GO demonstrated characteristic peaks of PANI (Supporting information Figure S10). These evidence proved that GO and PANI/GO were as agglomerated and separated nanosheets, respectively. Adsorption experiments were carried out at pH = 2 in which Cr(VI) adapting the form of HCrO4− interacted with the protonated nitrogen atoms of PANI in pure PANI and PANI/GO. Pure PANI was simply aggregated and its surface area was smaller than PANI/GO and the qmax values of PANI and PANI/GO were 490.2 and 1149.4 mg/g, respectively.
In a novel work, Li et al. [254] fabricated polystyrene (PS) nanofibrous mats by an electrospinning method and used as a skeleton for thiol-functionalized mesoporous silica compound (Figure 19 and Table 4). In this hierarchical structure the PS nanofibers had macroporous structures (diameters of 3–10 μm) and were randomly distributed in the membrane (Supporting information Figure S11). The optimum pH for the adsorption of Cu2+ was about 5 and its qmax value was 11.33 mg/g. Cu2+ ions were adsorbed by –SH groups within mesopores. The amount of thiol groups in the adsorbent was 0.32 mmol/g and the adsorption efficiency (Cu2+/SH molar ratio) of adsorbent was about 80%. After the recovery of adsorbent by 0.5 M HCl, its adsorption capacity of Cu2+ was basically maintained after five cycles.
Figure 19.
Schematic illustration of preparing thiol-functionalized membranes using electrospun nanofibrous mats as the skeleton. Reproduced with permission from [254]. Copyright 2012 The Royal Society of Chemistry.
In another different works with hierarchical structure compounds, Ma et al. synthesized CaCO3–pepsin [255] and CaCO3–maltose [256] and used them as adsorbents. Here, structure and adsorption characteristics of CaCO3–pepsin [255] are studied (Figure 20). Pepsin is an enzyme which is made up of 327 amino acids. SEM images showed that CaCO3–pepsin consisted of a large number of tetrahedral micro-aggregates that were composed of smaller tetrahedral building nano-blocks (Supporting information Figure S12).
Figure 20.
Mechanism of the formation of hierarchical structures of CaCO3–pepsin from amorpuus precursors. Reprinted with permission from [255]. Copyright 2012 Elsevier.
XRD, SEM and IR results showed that CaCO3 and pepsin interacted through both coordination of Ca2+ and pepsin and interactions between the surface of CaCO3 nanocrystals and –OH and –CO groups of pepsin.
Adsorption experiments were carried out in neutral pH and the adsorption capacity of CaCO3–pepsin for Pb2+ and Cu2+ were 1167 and 611 mg/g, respectively and the removal of Pb2+ was very rapid (about 20 min). Solubility product of PbCO3 and CuCO3 are less than CaCO3 and thus Cu2+ and Pb2+ precipitate as their carbonates.
On the other hand, adsorption capacities of CaCO3–maltose [256], another hierarchical structure superadsorbent, for Pb2+, Ni2+, Cu2+ and Cd2+ (as metal carbonate) were 3242.48, 769.23, 628.93 and 487.8 mg/g, respectively whereas adsorption capacity of CaCO3 for Pb2+ is 62.5 mg/g and this showed clearly synergic effects between organic and inorganic moieties in these compounds. As we saw, the organic-inorganic hierarchically structured compounds are superadsorbent.
3.6. Characteristics of Organic-Inorganic Hybrid Polymers
To study Sections 3.1–3.5, the characteristics of different kinds of organic-inorganic hybrid polymers were as follows: (1) Some functional groups of these adsorbents are –SH, –COOH, amines and –S–S–, –OH, –SO3− and ion exchange sites of hybrid polymer; (2) adsorption processes mostly obey the Langmuir isotherm that shows the adsorption is monolayer and there is a uniform distribution of sites on the surface of adsorbents; (3) the adsorption capacity of each certain organic functional group changes depending on its content [181,214], the used organic compound, inorganic support [166,195], crosslinking agent [212], aggregation of nanoparticles [253] and the used synthesis method [182,189]; (4) in some cases, organic compounds are used only for increasing thermal and mechanical stability of adsorbents and without adsorbing heavy metal ions [224]; (5) mesoprous compounds, due to their porous structures have a rather higher adsorption capacities compared to those of organically-functionalized layered, core-shell compounds and products of sol-gel method; (6) heavy metals as cations and oxyanions are adsorbed via electrostatic interactions and the increase in the hydrophobicity of functional groups of adsorbent (e.g., –N(C2H5)2 [156] or from –NH2 to –NH(propyl) to –N(propyl)2 [178]) decreases their adsorption capacity for metal cations and increases their qmax values (e.g., from trimethylammonium to tri-n-butylammonium functional groups) for the adsorption of some hydrophobic oxyanions [183]; (7) In the most cases, for example [141–147,216,221,225,241,243,249,252], the used adsorbents are recovered by acid treatment and in the cases that acid dissolves the adsorbent [199], they have used another washing solutions; (8) some ions may react with metallic ions, e.g., Cl− with Hg2+ [226], and inhibit their adsorption; (9) some ions, e.g., Cl− [183], SO42− [196] or metallic cations [252], may react with functional groups of hybrid adsorbents and decrease their adsorption capacity for heavy metal ions; (10) steric hindrance on functional group decreases its adsorption capacity [151]; (11) in most cases, adsorption capacities of organic-inorganic hybrid polymers are bigger than those of their organic or inorganic constituents; (12) most of heavy metals were adsorbed in the pH range 4–7. With the increase in alkalinity of solution, heavy metal ions convert to metal hydroxides and this decreases their affinity for interaction with binding sites of adsorbents [224] and in highly acidic solutions, H+ ions compete with metal ions for adsorption on the adsorbent surface [212]; (13) increasing the content of functional groups increases the adsorption capacity of these kinds of hybrid polymers although it decreases their pore size and surface area [153,169,170]; and (14) adsorption capacities of hierarchically structure compounds are much greater than the other kinds of discussed organic-inorganic hybrid polymers and in the future they can be used as superadsorbents for the adsorption of heavy metals.
4. Conclusions and Outlook
Organic-inorganic hybrid polymers are obtained through sol-gel processes, self-assembly processes, assembling or dispersion of nanobuilding blocks, hierarchical structures and interpenetrating networks. In these compounds, the functional variation of organic materials combines with the benefits of a sturdy and thermally stable inorganic substrate. These materials have strong binding affinities toward selected metal ions (as cations or oxyanions) and relatively high metal ion adsorption capacities and can be used for wastewater treatment and solid-state separation of heavy metals. They are modified by suitable functional groups for high efficiency adsorption of heavy metal ions under the used experimental conditions. Techniques such as XRD and SEM show that in hybrid polymers, the structure of organic and inorganic moieties change and synergy effects between them increase their adsorption capacities, compared to their individual pristine organic or inorganic moieties. Heavy metal selective adsorbents can be prepared by methods such as cation-IIP. Although adsorbents similar to activated carbon are used routinely for their low cost and high adsorption capacity, different combinations of organic and inorganic moieties provide us a large number of selections of hybrid adsorbents that can be used under different experimental conditions, are reusable and can be prepared for selective adsorption of heavy metals and in some cases act as superadsorbents. For synthesis of these compounds, environmentally friendly compounds such as chitosan, humic acid, cellulose and bentonite are used and their partial leaching during adsorption processes has no negative impact on the environment.
Supporting Information
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Bohn H.L., McNeal B.L., O’Connor G.A. Soil Chemistry. John Wiely; New York, NY, USA: 1985. [Google Scholar]
- 2.Bradl H.B. Heavy Metals in the Environment: Origin, Interaction and Remediation. Elsevier; Amsterdam, The Netherlands: 2005. [Google Scholar]
- 3.Fergusson J.E. The Heavy Elements: Chemistry, Environmental Impact and Health Effects. Pergamon Press; Exeter, UK: 1990. [Google Scholar]
- 4.Jha M.K., Lee J.-C., Kim M.-S., Jeong J., Kim B.-S., Kumar V. Hydrometallurgical recovery/recycling of platinum by the leaching of spent catalysts: A review. Hydrometallurgy. 2013;133:23–32. [Google Scholar]
- 5.Syed S. Recovery of gold from secondary sources—A review. Hydrometallurgy. 2012;115–116:30–51. [Google Scholar]
- 6.Esalah O.J., Weber M.E., Vera J.H. Removal of lead, cadmium and zinc from aqueous solutions by precipitation with sodium di-(n-octyl) phosphinate. Can. J. Chem. Eng. 2000;78:948–954. [Google Scholar]
- 7.Lertlapwasin R., Bhawawet N., Imyim A., Fuangswasdi S. Ionic liquid extraction of heavy metal ions by 2-aminothiophenol in 1-butyl-3-methylimidazolium hexafluorophosphate and their association constants. Sep. Purif. Technol. 2010;72:70–76. [Google Scholar]
- 8.Emamjomeh M.M., Sivakumar M. Review of pollutants removed by electrocoagulation and electrocoagulation/flotation processes. J. Environ. Manag. 2009;90:1663–1679. doi: 10.1016/j.jenvman.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 9.Mahmoud A., Hoadley A.F. An evaluation of a hybrid ion exchange electrodialysis process in the recovery of heavy metals from simulated dilute industrial wastewater. Water Res. 2012;46:3364–3376. doi: 10.1016/j.watres.2012.03.039. [DOI] [PubMed] [Google Scholar]
- 10.Yurloval L., Kryvoruchko A., Kornilovich B. Removal of Ni (II) ions from wastewater by micellar-enhanced ultrafiltration. Desalination. 2002;144:255–260. [Google Scholar]
- 11.Greenlee L.F., Lawler D.F., Freeman B.D., Marrot B., Moulin P. Reverse osmosis desalination: Water sources, technology, and today’s challenges. Water Res. 2009;43:2317–2348. doi: 10.1016/j.watres.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 12.Benit Y., Ruiz M.L. Reverse osmosis applied to metal finishing wastewater. Desalination. 2002;142:229–234. [Google Scholar]
- 13.Rubio J., Souza M.L., Smith R.W. Overview of flotation as a wastewater treatment technique. Miner. Eng. 2002;15:139–155. [Google Scholar]
- 14.Ghurye G., Clifford D., Tripp A. Iron coagulation and direct microfiltration to remove arsenic from groundwater. AWWA. 2004;96:143–152. [Google Scholar]
- 15.Barakat M.A. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011;4:361–377. [Google Scholar]
- 16.Pan B.J., Pan B.C., Zhang M.W., Lv L., Zhang Q.X., Zheng S.R. Development of polymeric and polymer-based hybrid adsorbents for pollutants removal from waters. Chem. Eng. J. 2009;151:19–29. [Google Scholar]
- 17.Mishra S.P., Singh V.K., Tiwari D. Radiotracer technique in adsorption study. 14. Efficient removal of mercury from aqueous solutions by hydrous zirconium oxide. Appl. Radiat. Isot. 1996;47:15–21. [Google Scholar]
- 18.Uzun I., Güzel F. Adsorption of some heavy metal ions from aqueous solution by activated carbon and comparison of percent adsorption results of activated carbon with those of some other adsorbents. Turk. J. Chem. 2000;24:291–297. [Google Scholar]
- 19.Biskup B., Subotic B. Kinetic analysis of the exchange processes between sodium ions from zeolite A and cadmium, copper and nickel ions from solutions. Sep. Purif. Technol. 2004;37:17–31. [Google Scholar]
- 20.Cincotti A., Mameli A., Locci A.M., Orru R., Cao G. Heavy metals uptake by Sardinian natural zeolites: Experiment and modeling. Ind. Eng. Chem. Res. 2006;45:1074–1084. [Google Scholar]
- 21.Gier S., Johns W.D. Heavy metal-adsorption on micas and clay minerals studied by X–ray photoelectron spectroscopy. Appl. Clay Sci. 2000;16:289–299. [Google Scholar]
- 22.Koppelman M.H., Dillard J.G. A study of the adsorption of Ni(II) and Cu(II) by clay minerals. Clays Clay Miner. 1977;25:457–462. [Google Scholar]
- 23.O’Connell D.W., Birkinshaw C., O’Dwyer T.F. Heavy metal adsorbents prepared from the modification of cellulose: A review. Bioresour. Technol. 2008;99:6709–6724. doi: 10.1016/j.biortech.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 24.Dang V.B.H., Doan H.D., Dang-Vuc T., Lohi A. Equilibrium and kinetics of biosorption of cadmium(II) and copper(II) ions by wheat straw. Bioresour. Technol. 2009;100:211–219. doi: 10.1016/j.biortech.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 25.Zewail T.M., El-Garf S.A.M. Preparation of agriculture residue based adsorbents for heavy metal removal. Desalin. Water Treat. 2010;22:363–370. [Google Scholar]
- 26.Nagarale R.K., Gohil G.S., Shahi V.K. Recent developments on ion-exchange membranes and electro-membrane processes. Adv. Colloid Interface Sci. 2006;119:97–130. doi: 10.1016/j.cis.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 27.Wang L., Wu X.L., Xu W.-H., Huang X.-J., Liu J.-H., Xu A.-W. Stable organic-inorganic hybrid of polyaniline/α-zirconium phosphate for efficient removal of organic pollutants in water environment. Appl. Mater. Interfaces. 2012;4:2686–2692. doi: 10.1021/am300335e. [DOI] [PubMed] [Google Scholar]
- 28.Gao B., Gao Y., Li Y. Preparation and chelation adsorption property of composite chelating material poly(amidoxime)/SiO2 towards heavy metal ions. Chem. Eng. J. 2010;158:542–549. [Google Scholar]
- 29.Zaitseva N., Zaitsev V., Walcarius A. Chromium(VI) removal via reduction–sorption on bi-functional silica adsorbents. J. Hazard. Mater. 2013;250–251:454–461. doi: 10.1016/j.jhazmat.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 30.Simsek E.B., Duranoglu D., Beker U. Heavy metal adsorption by magnetic hybrid-sorbent: An experimental and theoretical approach. Sep. Sci. Technol. 2012;47:1334–1340. [Google Scholar]
- 31.Suchithra P.S., Vazhayal L., Mohamed A.P., Ananthakumar S. Mesoporous organic-inorganic hybrid aerogels through ultrasonic assisted sol-gel intercalation of silica-PEG in bentonite for effective removal of dyes, volatile organic pollutants and petroleum products from aqueous solution. Chem. Eng. J. 2012;200–202:589–600. [Google Scholar]
- 32.Repo E., Warchoł J.K., Bhatnagar A., Sillanpää M. Heavy metals adsorption by novel EDTA-modified chitosan-silica hybrid materials. J. Colloid Interface Sci. 2011;358:261–267. doi: 10.1016/j.jcis.2011.02.059. [DOI] [PubMed] [Google Scholar]
- 33.Ge P., Li F., Zhang B. Synthesis of modified mesoporous materials and comparative studies of removal of heavy metal from aqueous solutions. Pol. J. Environ. Stud. 2010;19:301–308. [Google Scholar]
- 34.Pang Y., Zeng G., Tang L., Zhang Y., Liu Y., Lei X., Li Z., Zhang J., Xie G. PEI-grafted magnetic porous powder for highly effective adsorption of heavy metal ions. Desalination. 2011;281:278–284. [Google Scholar]
- 35.Wang L., Zhang J., Wang A. Fast removal of methylene blue from aqueous solution by adsorption onto chitosan-g-poly (acrylic acid)/attapulgite composite. Desalination. 2011;266:33–39. [Google Scholar]
- 36.Mercier L., Pinnavaia T.J. Heavy metal ion adsorbents formed by the grafting of a thiol functionality to mesoporous silica molecular sieves: Factors affecting Hg(II) uptake. Environ. Sci. Technol. 1998;32:2749–2754. [Google Scholar]
- 37.Sanchez C., Julián B., Belleville P., Popall M. Applications of hybrid organic-inorganic nanocomposites. J. Mater. Chem. 2005;15:3559–3592. [Google Scholar]
- 38.Walcarius A. Electrochemical applications of silica-based organic-inorganic hybrid materials. Chem. Mater. 2001;13:3351–3372. [Google Scholar]
- 39.Kim D.-G., Kang H., Han S., Lee J.-C. Dual effective organic/inorganic hybrid star-shaped polymer coatings on ultrafiltration membrane for bio- and oil-fouling resistance. Appl. Mater. Interfaces. 2012;4:5898–5906. doi: 10.1021/am301538h. [DOI] [PubMed] [Google Scholar]
- 40.Paun G., Neagu E., Tache A., Radu G.L., Parvulescu V. Application of the nanofiltration process for concentration of polyphenolic compounds from geranium robertianum and salvia officinalis extracts. Chem. Biochem. Eng. Quart. 2011;25:453–460. [Google Scholar]
- 41.Wen X.F., Wang K., Pi P.-H., Yang J.-X., Cai Z.-Q., Zhang L.-J., Qian Y., Yang Z.-R., Zheng D.-F., Cheng J. Organic-inorganic hybrid superhydrophobic surfaces using methyltriethoxysilane and tetraethoxysilane sol-gel derived materials in emulsion. Appl. Surf. Sci. 2011;258:991–998. [Google Scholar]
- 42.Sparks B.J., Hoff E.F., Xiong L., Goetz J.T., Patton D.L. Superhydrophobic hybrid inorganic-organic thiol-ene surfaces fabricated via spray-deposition and photopolymerization. Appl. Mater. Interfaces. 2013;5:1811–1817. doi: 10.1021/am303165e. [DOI] [PubMed] [Google Scholar]
- 43.Yu Y.-Y., Chen W.-C. Transparent organic-inorganic hybrid thin films prepared from acrylic polymer and aqueous monodispersed colloidal silica. Mater. Chem. Phys. 2003;82:388–395. [Google Scholar]
- 44.Nagappan S., Choi M.-C., Sung G., Park S.S., Moorthy M.S., Chu S.-W., Lee W.-K., Ha C.-S. Highly transparent, hydrophobic fluorinated polymethylsiloxane/silica organic-inorganic hybrids for anti-stain coating. Macromol. Res. 2013;21:669–680. [Google Scholar]
- 45.Rottman C., Grader G., de Hazan Y., Melchior S., Avnir D. Surfactant-induced modification of dopants reactivity in sol-gel matrixes. J. Am. Chem. Soc. 1999;121:8533–8543. [Google Scholar]
- 46.Date J.D., Moet L., Koster J.A., de Boer B., Blom P.W.M. Hybrid polymer solar cells from highly reactive diethylzinc: MDMO-PPV versus P3HT. Chem. Mater. 2007;19:5856–5861. [Google Scholar]
- 47.He M., Qiu F., Lin Z. Toward high-performance organic-inorganic hybrid solar cells: Bringing conjugated polymers and inorganic nanocrystals in close contact. J. Phys. Chem. Lett. 2013;4:1788–1796. doi: 10.1021/jz400381x. [DOI] [PubMed] [Google Scholar]
- 48.Souza F.L., Bueno P.R., Longo E., Leite E.R. Sol-gel nonhydrolytic synthesis of a hybrid organic-inorganic electrolyte for application in lithium-ion devices. Solid State Ion. 2004;66:83–88. [Google Scholar]
- 49.Lee C.-F., Leigh D.A., Pritchard R.G., Schultz D., Teat S.J., Timco G.A., Winpenny R.E.P. Hybrid organic-inorganic rotaxanes and molecular shuttles. Nature. 2009;458:314–318. doi: 10.1038/nature07847. [DOI] [PubMed] [Google Scholar]
- 50.Singh A., Singh N.P., Singh R.A. Biomimetic synthesis and characterization of semiconducting hybrid organic-inorganic composite materials based on polyaniline-polyethylene glycol-CdS system. Bull. Mater. Sci. 2011;34:1017–1026. [Google Scholar]
- 51.Collins D.J., Zhou H.-C. Hydrogen storage in metal-organic frameworks. J. Mater. Chem. 2007;17:3154–3160. [Google Scholar]
- 52.Rowsell J.L.C., Millward A.R., Park K.S., Yaghi O.M. Hydrogen sorption in functionalized metal-organic frameworks. J. Am. Chem. Soc. 2004;126:5666–5667. doi: 10.1021/ja049408c. [DOI] [PubMed] [Google Scholar]
- 53.Raj G., Swalus C., Guillet A., Devillers M., Nysten B., Gaigneaux E.M. Supramolecular organization in organic-inorganic heterogeneous hybrid catalysts formed from polyoxometalate and poly(ampholyte) polymer. Langmuir. 2013;29:4388–4395. doi: 10.1021/la400055t. [DOI] [PubMed] [Google Scholar]
- 54.Fu G., Yue X., Dai Z. Glucose biosensor based on covalent immobilization of enzyme in sol-gel composite film combined with Prussian blue/carbon nanotubes hybrid. Biosens. Bioelectron. 2011;26:3973–3976. doi: 10.1016/j.bios.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 55.Gwak G.H., Paek S.M., Oh J.M. Electrophoretic preparation of an organic-inorganic hybrid of layered metal hydroxide and hydrogel for a potential drug-delivery system. Eur. J. Inorg. Chem. 2012;2012:5269–5275. [Google Scholar]
- 56.Chou T.P., Chandrasekaran C., Cao G.Z. Sol-gel-derived hybrid coatings for corrosion protection. JSST. 2003;26:321–327. [Google Scholar]
- 57.Bourbigot S., Duquesne S. Fire retardant polymers: Recent developments and opportunities. J. Mater. Chem. 2007;17:2283–2300. [Google Scholar]
- 58.Kim S.S., Ahn K.M., Park M.S., Lee J.H., Choi C.Y., Kim B.S. A poly(lactic-coglycolide)/hydroxyapatite composite scaffold with enhanced osteoconductivity. J. Biomed. Mater. Res. A. 2007;80A:206–215. doi: 10.1002/jbm.a.30836. [DOI] [PubMed] [Google Scholar]
- 59.Kwon Y.K., Han J.K., Lee J.M., Ko Y.S., Oh J.H., Leeb H.-S., Lee E.-H. Organic-inorganic hybrid materials for flexible optical waveguide applications. J. Mater. Chem. 2008;18:579–585. [Google Scholar]
- 60.Frisch H.L., Mark J.E. Nanocomposites prepared by threading polymer chains through zeolites, mesoporous silica, or silicananotubes. Chem. Mater. 1996;8:1735–1738. [Google Scholar]
- 61.Hench L.L., West J.K. The sol-gel process. Chem. Rev. 1990;90:33–72. [Google Scholar]
- 62.Sol-Gel. [(acessed on 10 December 2013)]. Available online: http://en.wikipedia.org/wiki/sol-gel.
- 63.Warrick E. Forty Years of Firsts a Recollection of Dow Corning Pioneer. McGraw-Hill; New York, NY, USA: 1990. [Google Scholar]
- 64.Hay J.N., Raval H.M. Synthesis of organic-inorganic hybrids via the non-hydrolytic sol-gel process. Chem. Mater. 2001;13:3396–3403. [Google Scholar]
- 65.Wen J., Wilkes G.L. Organic/inorganic hybrid network materials by the sol-gel approach. Chem. Mater. 1996;8:1667–1681. [Google Scholar]
- 66.Chong M.N., Jin B. Sol-gel synthesis of inorganic mesostructured composite photocatalyst for water purification: An insight into the synthesis fundamentals, reaction, and binding mechanisms. Synth. React. Inorg. Met. Org. Chem. 2012;42:68–75. [Google Scholar]
- 67.Arkles B. Comercial applications of sol-gel-derived hybrid materials. MRS Bull. 2001;26:402–408. [Google Scholar]
- 68.Sol-Gel. [(accessed on 10 December 2013)]. Avaliable on line: http://en.wikipedia.org/wiki/Sol-gel.
- 69.Kessler V.G., Spijksma G.I., Seisenbaeva G.A., Hakansson S., Blank D.H.A., Bouwmeester H.J.M. New insight in the role of modifying ligands in the sol-gel processing of metal alkoxide precursors: A possibility to approach new classes of materials. J. Sol-Gel Sci. Technol. 2006;40:163–179. [Google Scholar]
- 70.Xiao Y., Shen J., Xie Z., Zhou B., Wu G. Microstructure control of nanoporous silica thin film prepared by sol-gel process. J. Mater. Sci. Technol. 2007;23:504–508. [Google Scholar]
- 71.Ogoshi T., Chujo Y. Organic-inorganic polymer hybrids prepared by the sol-gel method. Compos. Interfaces. 2005;11:539–566. [Google Scholar]
- 72.Loy D.A. Sol-Gel Processing of Hybrid Organic-Inorganic Materials Based on Polysilsesquioxanes. In: Kickelbick G., editor. Hybrid Materials: Synthesis, Characterization, and Applications. Wiley-VCH; Weinheim, Germany: 2006. [Google Scholar]
- 73.Andreyev M.K., Zubkov O., editors. Zeolites: Synthesis, Chemistry, and Applications. Nova Science Publishers; New York, NY, USA: 2012. [Google Scholar]
- 74.Wright P.A. Microporous Framework Solids. RSC Publishing; Cambridge, UK: 2008. p. 186. [Google Scholar]
- 75.Bellussi G., Millini R., Montanari E., Carati A., Rizzo C., Parker W.O., Jr., Cruciani G., de Angelis A., Bonoldi L., Zanardi S. A highly crystalline microporous hybrid organic-inorganic aluminosilicate resembling the AFI-type zeolite. Chem. Commun. 2012;48:7356–7358. doi: 10.1039/c2cc33417h. [DOI] [PubMed] [Google Scholar]
- 76.Maeda K., Kiyozumi Y., Mizukami F. Synthesis of the first microporous aluminum phosphonate with organic groups covalently bonded to the skeleton. Angew. Chem. Int. Ed. 1994;33:2335–2337. [Google Scholar]
- 77.Zhou D., Lu X., Xu J., Yu A., Li J., Deng F., Xia Q. Dry gel conversion method for the synthesis of organic-inorganic hybrid MOR zeolites with modifiable catalytic activities. Chem. Mater. 2012;24:4160–4165. [Google Scholar]
- 78.Jones C.W., Tsuji K., Davis M.E. Organic-functionalized molecular sieves as shape-selective catalysts. Nature. 1998;393:52–54. [Google Scholar]
- 79.Inagaki S., Yokoi T., Kubota Y., Tatsumi T. Unique adsorption properties of organic-inorganic hybrid zeolite IEZ-1 with dimethylsilylene moieties. Chem. Commun. 2007;48:5188–5190. doi: 10.1039/b713466e. [DOI] [PubMed] [Google Scholar]
- 80.Kresge C.T., Leonowicz M.E., Roth W.J., Vartuli J.C., Beck J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature. 1992;359:710–712. [Google Scholar]
- 81.Beck J.S., Vartuli J.C., Roth W.J., Leonowicz M.E., Kresge C.T., Schmitt K.D., Chu C.T.-W., Olson D.H., Sheppard E.W., McCullen S.B., et al. A new family of mesoporous molecular sieves prepared with liquid crystal templates. J. Am. Chem. Soc. 1992;114:10834–10843. [Google Scholar]
- 82.Ying J.Y., Mehnert C.P., Wong M.S. Synthesis and applications of supramolecular-templated mesoporous materials. Angew. Chem. Int. Ed. 1999;38:56–77. [Google Scholar]
- 83.Nohair B., MacQuarrie S., Crudden C.M., Kaliaguine S. Functionalized mesostructured silicates as supports for palladium complexes: Synthesis and catalytic activity for the Suzuki-Miyaura coupling reaction. J. Phys. Chem. C. 2008;112:6065–6072. [Google Scholar]
- 84.Serrano D.P., Calleja G., Botas J.A., Gutierrez F.J. Adsorption and hydrophobic properties of mesostructured MCM–41 and SBA–15 materials for volatile organic compound removal. Ind. Eng. Chem. Res. 2004;43:7010–7018. [Google Scholar]
- 85.Zhao D., Huo Q., Feng J., Chmelka B.F., Stucky G.D. Nonionic triblock and star diblock copolymer and oligomeric surfactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures. J. Am. Chem. Soc. 1998;120:6024–6036. [Google Scholar]
- 86.Chen D., Li Z., Wan Y., Tu X., Shi Y., Chen Z., Shen W., Yu C., Tu B., Zhao D. Anionic surfactant induced mesophase transformation to synthesize highly ordered large-pore mesoporous silica structures. J. Mater. Chem. 2006;16:1511–1519. [Google Scholar]
- 87.Da’na E., Sayari A. Adsorption of heavy metals on amine-functionalised SBA-15 prepared by co-condensation: Applications to real water samples. Desalination. 2012;285:62–67. [Google Scholar]
- 88.Brown J., Richer R., Mercier L. One-step synthesis of high capacity mesoporous Hg2+ adsorbents by non-ionic surfactant assembly. Microporous Mesoporous Mater. 2000;37:41–48. [Google Scholar]
- 89.Antochshuk V., Jaroniec M. Adsorption, thermogravimetric, and NMR studies of FSM–16 material functionalized with alkylmonochlorosilanes. J. Phys. Chem. B. 1999;103:6252–6261. [Google Scholar]
- 90.Ariga K., Vinu A., Yamauchi Y., Ji Q., Hill J.P. Nanoarchitectonics for mesoporous materials. Bull. Chem. Soc. Jpn. 2012;85:1–32. [Google Scholar]
- 91.Soler-Illia G.J., Sanchez C., Lebeau B., Patarin J. Chemical strategies to design textured materials: From microporous and mesoporous oxides to nanonetworks and hierarchical structures. Chem. Rev. 2002;102:4093–4138. doi: 10.1021/cr0200062. [DOI] [PubMed] [Google Scholar]
- 92.Huo Q., Margolese D.I., Ciesla U., Demuth D.G., Feng P., Gier T.E., Sieger P., Firouzi A., Chmelka B.F., Schüth F., et al. Organization of organic molecules with inorganic molecular species into nanocomposite biphase arrays. Chem. Mater. 1994;6:1176–1191. [Google Scholar]
- 93.Hoffmann F., Cornelius M., Morell J., Fröba M. Silica-based mesoporous organic-inorganic hybrid materials. Angew. Chem. Int. Ed. 2006;45:3216–3251. doi: 10.1002/anie.200503075. [DOI] [PubMed] [Google Scholar]
- 94.Muto S., Imai H. Relationship between mesostructures and pH conditions for the formation of silica-cationic surfactant complexes. Microporous Mesoporous Mater. 2006;95:200–205. [Google Scholar]
- 95.Wirnsberger G., Yang P., Huang H.C., Scott B., Deng T., Whitesides G.M., Chmelka B.F., Stucky G.D. Patterned block-copolymer-silica mesostructures as host media for the laser dye rhodamine 6G. J. Phys. Chem. B. 2001;105:6307–6313. [Google Scholar]
- 96.Zhang Q., Liu F., Nguyen K.T., Ma X., Wang X., Xing B., Zhao Y. Multifunctional mesoporous silica nanoparticles for cancer-targeted and controlled drug delivery. Adv. Funct. Mater. 2012;22:5144–5156. [Google Scholar]
- 97.Wight A.P., Davis M.E. Design and preparation of organic-inorganic hybrid catalysts. Chem. Rev. 2002;102:3589–3614. doi: 10.1021/cr010334m. [DOI] [PubMed] [Google Scholar]
- 98.Pal N., Bhaumik A. Soft templating strategies for the synthesis of mesoporous materials: Inorganic, organic-inorganic hybrid and purely organic solids. Adv. Colloid Interface Sci. 2013;189–190:21–41. doi: 10.1016/j.cis.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 99.Kickelbick G. Concepts for the incorporation of inorganic building blocks into organic polymers on a nanoscale. Prog. Polym. Sci. 2003;28:83–114. [Google Scholar]
- 100.Schubert U. Cluster-based inorganic-organic hybrid materials. Chem. Soc. Rev. 2011;40:575–582. doi: 10.1039/c0cs00009d. [DOI] [PubMed] [Google Scholar]
- 101.Agustin D., Dallery J., Coelho C., Proust A., Thouvenot R. Synthesis, characterization and study of the chromogenic properties of the hybrid polyoxometalates [PW11O39(SiR)2O]3−. J. Organomet. Chem. 2007;692:746–754. [Google Scholar]
- 102.Li H., Eddaoudi M., O’Keeffe M., Yaghi O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature. 1999;402:276–279. [Google Scholar]
- 103.Férey G. Some suggested perspectives for multifunctional hybrid porous solids. Dalton Trans. 2009;2009:4400–4415. doi: 10.1039/b817360p. [DOI] [PubMed] [Google Scholar]
- 104.Guillerm V., Gross S., Serre C., Devic T., Bauer M., Férey G. A zirconium methacrylate oxocluster as precursor for the low-temperature synthesis of porous zirconium (IV) dicarboxylates. Chem. Commun. 2010;46:767–769. doi: 10.1039/b914919h. [DOI] [PubMed] [Google Scholar]
- 105.Proust A., Matt B., Villanneau R., Guillemot G., Gouzerh P., Izzet G. Functionalization and post-functionalization: A step towards polyoxometalate-based materialsw. Chem. Soc. Rev. 2012;41:7605–7622. doi: 10.1039/c2cs35119f. [DOI] [PubMed] [Google Scholar]
- 106.Niederberger M., Garnweitner G., Krumeich F., Nesper R., Cölfen H., Antonietti M. Tailoring the surface and solubility properties of nanocrystalline titania by a nonaqueous in-situ functionalization process. Chem. Mater. 2004;16:1202–1208. [Google Scholar]
- 107.Dimitrijevic N.M., Saponjic Z.V., Rabatic B.M., Rajh T. Assembly and charge transfer in hybrid TiO2 architectures using biotin-avidin as a connector. J. Am. Chem. Soc. 2005;127:1344–1345. doi: 10.1021/ja0458118. [DOI] [PubMed] [Google Scholar]
- 108.Potapova I., Mruk R., Prehl S., Zentel R., Basche T., Mews A. Semiconductor nanocrystals with multifunctional polymer ligands. J. Am. Chem. Soc. 2003;125:320–321. doi: 10.1021/ja028641c. [DOI] [PubMed] [Google Scholar]
- 109.Vitale F., Fratoddi I., Battocchio C., Piscopiello E., Tapfer L., Russo M.V., Polzonetti G., Giannini C. Mono- and bi-functional arenethiols as surfactants for gold nanoparticles: Synthesis and characterization. NRL. 2011;6 doi: 10.1186/1556-276X-6-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schroden R.C., Blanford C.F., Melde B.J., Johnson B.J.S., Stein A. Direct synthesis of ordered macroporous silica materials functionalized with polyoxometalate clusters. Chem. Mater. 2001;13:1074–1081. [Google Scholar]
- 111.Mittal V. Polymer layered silicate nanocomposites: A review. Materials. 2009;2:992–1057. [Google Scholar]
- 112.Soundararajah Q.Y., Karunaratne B.S.B., Rajapakse R.M.G. Montmorillonite polyaniline nanocomposites: Preparation, characterization and investigation of mechanical properties. Mater. Chem. Phys. 2009;113:850–855. [Google Scholar]
- 113.Lira-Cantu M., Gomez-Romero P. Synthesis and characterization of intercalate phases in the organic–inorganic polyaniline/V2O5 system. J. Solid State Chem. 1999;147:601–608. [Google Scholar]
- 114.Huang S., Yang Z., Zhu H., Ren L., Tjiu W.W., Liu T. Poly(vinly alcohol)/nano-sized layered double hydroxides nanocomposite hydrogels prepared by cyclic freezing and thawing. Macromol. Res. 2012;20:568–577. [Google Scholar]
- 115.Santos C.M., Mangadlao J., Ahmed F., Leon A., Advincula R.C., Rodrigues D.F. Graphene nanocomposite for biomedical applications: Fabrication, antimicrobial and cytotoxic investigations. Nanotechnology. 2012;23:395101. doi: 10.1088/0957-4484/23/39/395101. [DOI] [PubMed] [Google Scholar]
- 116.Alexandre M., Dubois P. Polymer layered silicate nanocomposites: Preparation, properties and uses of a new class of materials. Mater. Sci. Eng. R Rep. 2000;28:1–63. [Google Scholar]
- 117.Kameda T., Takeuchi H., Yoshioka T. Kinetics of uptake of Cu2+ and Cd2+ by Mg-Al layered double hydroxides intercalated with citrate, malate, and tartrate. Colloids Surf. A Physicochem. Eng. Asp. 2010;355:172–177. [Google Scholar]
- 118.Rojas R., Perez M.R., Erro E.M., Ortiz P.I., Ulibarri M.A., Giacomelli C.E. EDTA modified LDHs as Cu2+ scavengers: Removal kinetics and sorbent stability. J. Colloid Interface Sci. 2009;331:425–431. doi: 10.1016/j.jcis.2008.11.045. [DOI] [PubMed] [Google Scholar]
- 119.Gangopadhyay R., De A. Conducting polymer nanocomposites: A brief overview. Chem. Mater. 2000;12:608–622. [Google Scholar]
- 120.Daigle J.-C., Claverie J.P. A. Simple method for forming hybrid core-shell nanoparticles suspended in water. J. Nanomater. 2008;2008:609184:1–609184:8. [Google Scholar]
- 121.Wei S., Wang Q., Zhu J., Sun L., Lin H., Guo Z. Multifunctional composite core-shell nanoparticles. Nanoscale. 2011;3:4474–4502. doi: 10.1039/c1nr11000d. [DOI] [PubMed] [Google Scholar]
- 122.Wang Y., Teng X., Wang J.-S., Yang H. Solvent-free atom transfer radical polymerization in the synthesis of Fe2O3@polystyrene core-shell nanoparticles. Nano Lett. 2003;3:789–793. [Google Scholar]
- 123.Huang C.L., Matijevic E. Coating of uniform inorganic particles with polymers. 3. Polypyrrole on different metal oxides. J. Mater. Res. 1995;10:1327–1336. [Google Scholar]
- 124.Payne R., Fritz G., Narmann H. Investigation into the interactions of metals and metal ions in polymer matrixes. Angew. Makromol. Chem. 1986;144:51–72. [Google Scholar]
- 125.Liu S., Zhang N., Tang Z.-R., Xu Y.-J. Synthesis of one-dimensional CdS@TiO2 core-shell nanocomposites photocatalyst for selective redox: The dual role of TiO2 shell. ACS Appl. Mater. Interfaces. 2012;4:6378–6385. doi: 10.1021/am302074p. [DOI] [PubMed] [Google Scholar]
- 126.Ozin G.A., Ho K., Lotsch B.V., Cademartiri L., Puzzo D.P., Scotognella F., Ghadimi A., Thomson J. Nanofabrication by self- assembly. Mater. Today. 2009;12:12–23. [Google Scholar]
- 127.Nikolov S., Petrov M., Lymperakis L., Friák M., Sachs C., Fabritius H.-O., Raabe D., Neugebauer J. Revealing the design principles of high-performance biological composites using Ab initio and multiscale simulations: The example of lobster cuticle. Adv. Mater. 2010;22:519–526. doi: 10.1002/adma.200902019. [DOI] [PubMed] [Google Scholar]
- 128.Fratzel P. Collagen: Structure and Mechanics. Springer; New York, NY, USA: 2008. [Google Scholar]
- 129.Kharisov B.I. A. Review for synthesis of nanoflowers. Recent Pat. Nanotechnol. 2008;2:190–200. doi: 10.2174/187221008786369651. [DOI] [PubMed] [Google Scholar]
- 130.Soten I., Ozin G.A. Porous hydroxyapatite- dodecylphosphate composite film on titania-titanium substrate. J. Mater. Chem. 1999;9:703–710. [Google Scholar]
- 131.Dag Ö., Verma A., Ozin G.A., Kresge C.T. Salted mesostructures: Salt-liquid crystal templating of lithium triflate-oligo(ethylene oxide) surfactant-mesoporous silica nanocomposite films and monoliths. J. Mater. Chem. 1999;9:1475–1482. [Google Scholar]
- 132.Du J., Lai X., Yang N., Zhai J., Kisailus D., Su F., Wang D., Jiang L. Hierarchically ordered macro-mesoporous TiO2-graphene composite films: Improved mass transfer, reduced charge recombination, and their enhanced photocatalytic activities. ACS Nano. 2011;5:590–596. doi: 10.1021/nn102767d. [DOI] [PubMed] [Google Scholar]
- 133.Pope E.J.A., Asami M., Mackenzie J.D. Transparent silica gel-PMMA composites. J. Mater. Res. 1989;4:1018, 1026. [Google Scholar]
- 134.Deng Q., Moore R.B., Mauritz K.A. Novel Nafion/ORMOSIL hybrids via in situ sol-gel reactions. 1. Probe of ORMOSIL phase nanostructures by infrared spectroscopy. Chem. Mater. 1995;7:2259–2268. [Google Scholar]
- 135.Hajji P., David L., Gerard J.F., Pascault J.P., Vigier G. Synthesis, structure, and morphology of polymer-silica hybrid nanocomposites based on hydroxyethyl methacrylate. J. Polym. Sci. B Polym. Phys. 1999;37:3172–3187. [Google Scholar]
- 136.Frisch H.L., Xue Y.-P., Maaref S., Beaucage G., Pu Z., Mark J. Pseudo interpenetrating polymer networks and interpenetrating polymer networks of zeolite 13 X and polystyrene and poly(ethyl acrylate) Macrmol. Symp. 1996;106:147–166. [Google Scholar]
- 137.Frisch H.L., Chen Z.J., Chen X. Electrical conductivity of iodine doped pseudo interpenetrating polymer networks. Macromol. Symp. 1994;81:181–194. [Google Scholar]
- 138.Abd-El-Aziz A.S., Strohm E.A. Transition metal-containing macromolecules: En route to new functional materials. Polymer. 2012;53:4879–4921. [Google Scholar]
- 139.McCurdie M.P., Belfiore L.A. Solid-state complexes of poly (l-histidine) with metal chlorides from the first row of the d-block. J. Polym. Sci. Part B Polym. Phys. 1999;37:301–309. [Google Scholar]
- 140.Hardy C.G., Ren L., Tamboue T.C., Tang C. Side-chain ferrocene-containing (meth)acrylate polymers: Synthesis and properties. J. Polym. Sci. 2011;49:1409–1420. [Google Scholar]
- 141.Mori H. Design and synthesis of functional silsesquioxane-based hybrids by hydrolytic condensation of bulky triethoxysilanes. Int. J. Polym. Sci. 2012;2012:173624:1–173624:17. [Google Scholar]
- 142.Liu J., Ma Y., Zhang Y., Shao G. Novel negatively charged hybrids. 3. Removal of Pb2+ from aqueous solution using zwitterionic hybrid polymers as adsorbent. J. Hazard. Mater. 2010;173:438–444. doi: 10.1016/j.jhazmat.2009.08.097. [DOI] [PubMed] [Google Scholar]
- 143.Liu J., Ma Y., Xu T., Shao G. Preparation of zwitterionic hybrid polymer and its application for the removal of heavy metal ions from water. J. Hazard. Mater. 2010;178:1021–1029. doi: 10.1016/j.jhazmat.2010.02.041. [DOI] [PubMed] [Google Scholar]
- 144.Wu J.-B., Zang S.-Y., Yi Y.-L. Sol-gel derived ion imprinted thiocyanato-functionalized silica gel as selective adsorbent of cadmium(II) J. Sol-Gel Sci. Technol. 2013;66:434–442. [Google Scholar]
- 145.Wu J.-B., Yi Y.-L. Removal of cadmium from aqueous solution by organic-inorganic hybrid sorbent combining sol-gel processing and imprinting technique. Korean J. Chem. Eng. 2013;30:1111–1118. [Google Scholar]
- 146.Buhani, Narsito, Nuryono, Kunarti E.S. Production of metal ion imprinted polymer from mercapto-silica through sol-gel process as selective adsorbent of cadmium. Desalination. 2010;251:83–89. [Google Scholar]
- 147.Fan H.-T., Sun T. Selective removal of iron from aqueous solution using ion imprinted cyanato-functionalized silica gel sorbents. Sep. Sci. Technol. 2012;47:507–512. [Google Scholar]
- 148.Bozbas S.K., Ay U., Kayan A. Novel inorganic-organic hybrid polymers to remove heavy metals from aqueous solution. Desalin. Water Treat. 2013;51:7208–7215. [Google Scholar]
- 149.Schroden R.C., Al-Daous M., Sokolov S., Melde B.J., Lytle J.C., Stein A., Carbajo M.C., Fernández J.T., Rodríguez E.E. Hybrid macroporous materials for heavy metal ion adsorption. J. Mater. Chem. 2002;12:3261–3267. [Google Scholar]
- 150.Passos C.G., Lima E.C., Arenas L.T., Simon N.M., da Cunha B.M., Brasil J.L., Costa T.M.H., Benvenutti E.V. Use of 7-amine-4-azahepthylsilica and 10-amine-4-azadecylsilica xerogels as adsorbent for Pb(II) kinetic and equilibrium study. Colloids Surf. A. 2008;316:297–306. [Google Scholar]
- 151.Pavan F.A., Lima I.S., Benvenutti E.V., Gushikem Y., Airoldi C. Hybrid aniline/silica xerogel cation adsorption and thermodynamics of interaction. J. Colloid Interface Sci. 2004;275:386–391. doi: 10.1016/j.jcis.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 152.Faghihian H., Nourmoradi H., Shokouhi M. Performance of silica aerogels modifi ed with amino functional groups in Pb(II) and Cd(II) removal from aqueous solutions. PJCHT. 2012;14:50–56. [Google Scholar]
- 153.Qu R., Sun C., Ma F., Cui Z., Zhang Y., Sun X., Ji C., Wang C., Yin P. Adsorption kinetics and equilibrium of copper from ethanol fuel on silica-gel functionalized with amino- terminated dendrimer-like polyamidoamine polymers. Fuel. 2012;92:204–210. [Google Scholar]
- 154.Pissetti F.L., Yoshida I.V.P., Gushikem Y., Kholin Y.V. Metal ions adsorption from ethanol solutions on ethylenediamine-modified poly(dimethylsiloxane) elastomeric network. Colloids Surf. A. 2008;328:21–27. doi: 10.1016/j.jcis.2007.05.040. [DOI] [PubMed] [Google Scholar]
- 155.Fan H.T., Su Z.J., Fan X.L., Guo M.M., Wang J., Gao S., Sun T. Sol-gel derived organic-inorganic hybrid sorbent for removal of Pb2+, Cd2+ and Cu2+ from aqueous solution. J. Sol-Gel Sci. Technol. 2012;64:418–426. [Google Scholar]
- 156.Kim Y.S., In G., Choi J.M. Solid phase extraction of trace Cu(II), Mn(II), Pb(II) and Zn(II) in water samples with pulverized silica-salen(NEt2)2. Bull. Korean Chem. Soc. 2006;27:1557–1561. [Google Scholar]
- 157.Hongjie W., Jin K., Huijuan L., Jiuhui Q. Preparation of organically functionalized silica gel as adsorbent for copper ion adsorption. J. Environ. Sci. 2009;21:1473–1479. doi: 10.1016/s1001-0742(08)62442-0. [DOI] [PubMed] [Google Scholar]
- 158.Qu R., Niu Y., Sun C., Ji C., Wang C., Cheng G. Syntheses, characterization, and adsorption properties for metal ions of silica-gel functionalized by ester- and amino-terminated dendrimer-like polyamidoamine polymer. Microporous Mesoporous Mater. 2006;97:58–65. [Google Scholar]
- 159.Zhang Y., Wang X., Liu J., Wu L. Removal of copper (Cu2+) from water using novel hybrid adsorbents: Kinetics and isotherms. J. Chem. Eng. Data. 2013;58:1141–1150. [Google Scholar]
- 160.Prakash S., Kumar M., Tripathi B.P., Shahi V.K. Sol-gel derived poly(vinylalcohol)-3-(2-aminoethylamino) propyl trimethoxysilane: Cross-linked organic-inorganic hybrid beads for the removal of Pb(II) from aqueous solution. Chem. Eng. J. 2010;162:28–36. [Google Scholar]
- 161.Hao J., Han M.-J., Meng X. Preparation and evaluation of thiol-functionalized activated alumina for arsenite removal from water. J. Hazard. Mater. 2009;167:1215–1221. doi: 10.1016/j.jhazmat.2009.01.124. [DOI] [PubMed] [Google Scholar]
- 162.Barczak M., Dobrowolski R., Dobrzyǹska J., Zięba E., Dąbrowski A. Amorphous and ordered organosilicas functionalized with amine groups as sorbents of platinum (II) ions. Adsorption. 2013;19:733–744. [Google Scholar]
- 163.Chen J., Lin Y.-S. Sol-gel-immobilized recombinant E. coli for biosorption of Cd2+ J. Chin. Inst. Chem. Eng. 2007;38:235–243. [Google Scholar]
- 164.Mehmood R.F., Mehmood F., Akhtar J., Shah S.S., Khosa M.A. Adsorption of Cd(II) by sol-gel silica doped with N (dipropylcarbamothioyl)thiophene-2-carboxamide. J. Dispers. Sci. Technol. 2013;34:153–160. [Google Scholar]
- 165.Prado A.G.S., Arakaki L.N.H., Airoldi C. Adsorption and separation of cations on chemically modified silica gel synthesised via the sol-gel process. J. Chem. Soc. Dalton Trans. 2001:2206–2209. [Google Scholar]
- 166.Najafi M., Yousefi Y., Rafati A.A. Synthesis, characterization and adsorption studies of several heavy metal ions on amino-functionalized silica nano hollow sphere and silica gel. Sep. Purif. Technol. 2012;85:193–205. [Google Scholar]
- 167.Pavan F.A., Costa T.M.H., Benvenutti E.V. Adsorption of CoCl2, ZnCl2 and CdCl2 on aniline/silica hybrid material obtained by sol-gel method. Colloids Surf. A. 2003;226:95–100. [Google Scholar]
- 168.Parvulescu V., Mureseanu M., Reiss A., Ene R., Suh S.-H. Metal-organic hybrids obtained by functionalization of mesoporous silica. Rev. Roum. Chim. 2010;55:1001–1008. [Google Scholar]
- 169.Yoshitake H., Yokoi T., Tatsumi T. Adsorption of chromate and arsenate by amino-functionalized MCM–41 and SBA–1. Chem. Mater. 2002;14:4603–4610. [Google Scholar]
- 170.Aguado J., Arsuaga J.M., Arencibia A., Lindo M., Gascón V. Aqueous heavy metals removal by adsorption on amine-functionalized mesoporous silica. J. Hazard. Mater. 2009;163:213–221. doi: 10.1016/j.jhazmat.2008.06.080. [DOI] [PubMed] [Google Scholar]
- 171.Chen D., Huang C., He M., Hu B. Separation and preconcentration of inorganic arsenic species in natural water samples with 3-(2-aminoethylamino) propyltrimethoxysilane modified ordered mesoporous silica micro-column and their determination by inductively coupled plasma optical emission spectrometry. J. Hazard. Mater. 2009;164:1146–1151. doi: 10.1016/j.jhazmat.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 172.Showkat A.M., Zhang Y.-P., Kim M.S., Gopalan A.I., Reddy K.R., Lee K.-P. Analysis of heavy metal toxic ions by adsorption onto amino-functionalized ordered mesoporous silica. Bull. Korean Chem. Soc. 2007;28:1985–1992. [Google Scholar]
- 173.Zhang D., Li J.H. Ordered SBA-15 mesoporous silica with high amino-functionalization for adsorption of heavy metal ions. Chin. Sci. Bull. 2013;58:879–883. [Google Scholar]
- 174.Kang T., Park Y., Choi K., Sang Leec J., Yi J. Ordered mesoporous silica (SBA–15) derivatized with imidazole-containing functionalities as a selective adsorbent of precious metal ions. J. Mater. Chem. 2004;14:1043–1049. [Google Scholar]
- 175.Mureseanu M., Reiss A., Stefanescu I., David E., Parvulescu V., Renard G., Hulea V. Modified SBA–15 mesoporous silica for heavy metal ions remediation. Chemosphere. 2008;73:1499–1504. doi: 10.1016/j.chemosphere.2008.07.039. [DOI] [PubMed] [Google Scholar]
- 176.Hao S., Hou J. Adsorption of Ni2+ on aminofunctionalized mesoporous silica templated by an anionic surfactant route. J. Mater. Res. 2013;28:2325–2331. [Google Scholar]
- 177.Pérez-Quintanilla D., del Hierro I., Fajardo M., Sierra I. Adsorption of cadmium(II) from aqueous media onto a mesoporous silica chemically modified with 2-mercaptopyrimidine. J. Mater. Chem. 2006;16:1757–1764. [Google Scholar]
- 178.Lam K.F., Yeung K.L., McKay G. An investigation of gold adsorption from a binary mixture with selective mesoporous silica adsorbents. J. Phys. Chem. B. 2006;110:2187–2194. doi: 10.1021/jp055577n. [DOI] [PubMed] [Google Scholar]
- 179.Puanngam M., Unob F. Preparation and use of chemically modified MCM–41 and silica gel as selective adsorbents for Hg(II) ions. J. Hazard. Mater. 2008;154:578–587. doi: 10.1016/j.jhazmat.2007.10.090. [DOI] [PubMed] [Google Scholar]
- 180.Lam K.F., Yeung K.L., McKay G. A rational approach in the design of selective mesoporous adsorbents. Langmuir. 2006;22:9632–9641. doi: 10.1021/la061410p. [DOI] [PubMed] [Google Scholar]
- 181.Xue X., Li F. Removal of Cu(II) from aqueous solution by adsorption onto functionalized SBA–16 mesoporous silica. Microporous Mesoporous Mater. 2008;116:116–122. [Google Scholar]
- 182.Lombardo M.V., Videla M., Calvo A., Requejo F.G., Soler-Illia G.J.A.A. Aminopropyl-modified mesoporous silica SBA–15 as recovery agents of Cu(II)-sulfate solutions: Adsorption efficiency, functional stability and reusability aspects. J. Hazard. Mater. 2012;223–224:53–62. doi: 10.1016/j.jhazmat.2012.04.049. [DOI] [PubMed] [Google Scholar]
- 183.Lee B., Bao L.-L., Im H.-J., Dai S., Hagaman E.W., Lin J.S. Synthesis and characterization of organic-inorganic hybrid mesoporous anion–exchange resins for perrhenate (ReO4−) anion adsorption. Langmuir. 2003;19:4246–4252. [Google Scholar]
- 184.Lee B., Kim Y., Lee H., Yi J. Synthesis of functionalized porous silicas via templating method as heavy metal ion adsorbents: The introduction of surface hydrophilicity onto the surface of adsorbents. Microporous Mesoporous Mater. 2001;50:77–90. [Google Scholar]
- 185.Liu Y.-C., Liu S.-T. A recyclable thiol-functionalized mesoporous silica for detection and removal of Cu(II) ions. JCCS. 2010;57:946–949. [Google Scholar]
- 186.Aguado J., Arsuaga J.M., Arencibia A. Adsorption of aqueous mercury(II) on propylthiol-functionalized mesoporous silica obtained by co-condensation. Ind. Eng. Chem. Res. 2005;44:3665–3671. [Google Scholar]
- 187.Olkhovyk O., Jarioniec M. Ordered mesoporous silicas with 2,5-dimercapto-1,3,4-thiadiazole ligand: High capacity adsorbents for mercury ions. Adsorption. 2005;11:205–214. [Google Scholar]
- 188.Antochshuck V., Olkhovyk O., Jaroniec M., Park I.-S., Ryoo R. Benzoylthiourea-modified mesoporous silica for mercury(II) removal. Langmuir. 2003;19:3031–3034. [Google Scholar]
- 189.Hao N., Han L., Yang Y., Wang H., Webley P.A., Zhao D. A metal-ion-assisted assembly approach to synthesize disulfide-bridged periodical mesoporous organosilicas with high sulfide contents and efficient adsorption. Appl. Surf. Sci. 2010;256:5334–5342. [Google Scholar]
- 190.Teng M., Wang H., Li F., Zhang B. Thioether-functionalized mesoporous fiber membranes: Sol-gel combined electrospun fabrication and their applications for Hg2+ removal. J. Colloid Interface Sci. 2011;355:23–28. doi: 10.1016/j.jcis.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 191.Chiu P.-J., Vetrivel S., Chiang A.S.T., Kao H.-M. Synthesis and characterization of cubic periodic mesoporous organosilicas with a high loading of disulfide groups. New J. Chem. 2011;35:489–494. [Google Scholar]
- 192.Awual M.R., Yaita T., El-Safty S.A., Shiwaku H., Okamoto Y., Suzuki S. Investigation of palladium(II) detection and recovery using ligand modified conjugate adsorbent. Chem. Eng. J. 2013;222:172–179. [Google Scholar]
- 193.Awual M.R., Yaita T., El-Safty S.A., Shiwaku H., Suzuki S., Okamoto Y. Copper(II) ions capturing from water using ligand modified a new type mesoporous adsorbent. Chem. Eng. J. 2013;221:322–330. [Google Scholar]
- 194.Lee H., Yi J. Removal of cooper using functionalizaed mesoporous silica in aqueous solution. Sep. Sci. Technol. 2001;36:2433–2444. [Google Scholar]
- 195.Lam K.F., Yeung K., McKay G. Efficient approach for Cd2+ and Ni2+ removal and recovery using mesoporous mdsorbent with tunable selectivity. Environ. Sci. Technol. 2007;41:3329–3334. doi: 10.1021/es062370e. [DOI] [PubMed] [Google Scholar]
- 196.Yoshitake H., Yokoi T., Tatsumi T. Adsorption behavior of arsenate at transition metal cations captured by amino-functionalized mesoporous silicas. Chem. Mater. 2003;15:1713–1721. [Google Scholar]
- 197.Huang J., Ye M., Qu Y., Chu L., Chen R., He Q., Xu D. Pb (II) removal from aqueous media by EDTA-modified mesoporous silica SBA-15. J. Colloid Interface Sci. 2012;385:137–146. doi: 10.1016/j.jcis.2012.06.054. [DOI] [PubMed] [Google Scholar]
- 198.Ge P., Li F. Kinetics and thermodynamics of heavy metal Cu(II) adsorption on mesoporous silicates. Pol. J. Environ. Stud. 2011;20:339–344. [Google Scholar]
- 199.Pandey S., Mishra S.B. Organic-inorganic hybrid of chitosan/organoclay bionanocomposites for hexavalent chromium uptake. J. Colloid Interface Sci. 2011;361:509–520. doi: 10.1016/j.jcis.2011.05.031. [DOI] [PubMed] [Google Scholar]
- 200.Futalan C.M., Tsai W.-C., Lin S.-S., Hsien K.-J., Dalida M.L., Wan M.-W. Copper, nickel and lead adsorption from aqueous solution using chitosan-immobilized on bentonite in a ternary system. Sustain. Environ. Res. 2012;22:345–355. [Google Scholar]
- 201.Hasan S., Krishnaiah A., Ghosh T.K., Viswanath D.S., Boddu V.M., Smith E.D. Adsorption of chromium (VI) on chitosan-coated perlite. Sep. Sci. Technol. 2003;38:3775–3793. [Google Scholar]
- 202.Kalyani S., Priya J.A., Rao P.S., Abburi K. Removal of copper and nickel from aqueous solutions using chitosan coated on perlite as biosorbent. Sep. Sci. Technol. 2005;40:1483–1495. [Google Scholar]
- 203.Hasan S., Krishnaiah A., Ghosh T.K., Viswanath D.S., Boddu V.M., Smith E.D. Adsorption of divalent cadmium (Cd(II)) from aqueous solutions onto chitosan-coated perlite beads. Ind. Eng. Chem. Res. 2006;45:5066–5077. [Google Scholar]
- 204.Hasan S., Ghosh T.K., Viswanath D.S., Boddu V.M. Dispersion of chitosan on perlite for enhancement of copper(II) adsorption capacity. J. Hazard. Mater. 2008;152:826–837. doi: 10.1016/j.jhazmat.2007.07.078. [DOI] [PubMed] [Google Scholar]
- 205.Dinu M.V., Dragan E.S. Evaluation of Cu2+, Co2+ and Ni2+ ions removal from aqueous solution using anovel chitosan/clinoptilolite composite: Kinetics and isotherms. Chem. Eng. J. 2010;160:157–163. [Google Scholar]
- 206.Gandhi M.R., Viswanathanb N., Meenakshi S. Preparation and application of alumina/chitosan biocomposite. Int. J. Biol. Macromol. 2010;47:146–154. doi: 10.1016/j.ijbiomac.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 207.Boddu V.M., Abburi K., Talbott J.L., Smith E.D. Removal of hexavalent chromium from wastewater using a new composite chitosan biosorbent. Environ. Sci. Technol. 2003;37:4449–4456. doi: 10.1021/es021013a. [DOI] [PubMed] [Google Scholar]
- 208.Fan D., Zhu X., Xu M., Yan J. Adsorption properties of chromium (VI) by chitosan coated montmorillonite. J. Biol. Sci. 2006;6:941–945. [Google Scholar]
- 209.Bleiman N., Mishael Y.G. Selenium removal from drinking water by adsorption to chitosan-clay composites and oxides: Batch and columns tests. J. Hazard. Mater. 2010;183:590–595. doi: 10.1016/j.jhazmat.2010.07.065. [DOI] [PubMed] [Google Scholar]
- 210.Vijaya Y., Popuri S.R., Boddu V.M., Krishnaiah A. Modified chitosan and calcium alginate biopolymer sorbents for removal of nickel (II) through adsorption. Carbohydr. Polym. 2008;72:261–271. [Google Scholar]
- 211.Choi S., Jeong Y. The removal of heavy metals in aqueous solution by hydroxyapatite/cellulose composite. Fibers Polym. 2008;9:267–270. [Google Scholar]
- 212.Grisdanurak N., Akewaranugulsiri S., Futalan C.M., Tsai W.C., Kan C.C., Hsu C.W., Wan M.W. The study of copper adsorption from aqueous solution using crosslinked chitosan immobilized on bentonite. J. Appl. Polym. Sci. 2012;125:132–142. [Google Scholar]
- 213.Zhang J., Jin Y., Wang A. Rapid removal of Pb(II) from aqueous solution by chitosan-g-poly(acrylic acid)/attapulgite/sodium humate composite hydrogels. Environ. Technol. 2011;32:523–531. doi: 10.1080/09593330.2010.504748. [DOI] [PubMed] [Google Scholar]
- 214.Wang X., Zheng Y., Wang A. Fast removal of copper ions from aqueous solution by chitosan–g–poly(acrylic acid)/attapulgite composites. J. Hazard. Mater. 2009;168:970–977. doi: 10.1016/j.jhazmat.2009.02.120. [DOI] [PubMed] [Google Scholar]
- 215.Wang X., Wang A. Adsorption characteristics of chitosan-g-poly(acrylic acid)/attapulgite hydrogel composite for Hg(II) ions from aqueous solution. Sep. Sci. Technol. 2010;45:2086–2094. [Google Scholar]
- 216.Anirudhan T.S., Rijith S. Synthesis and characterization of carboxyl terminated poly(methacrylic acid) grafted chitosan/bentonite composite and its application for the recovery of uranium(VI) from aqueous media. J. Environ. Radioact. 2012;106:8–19. doi: 10.1016/j.jenvrad.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 217.Abdel Khalek M.A., Mahmoud G.A., El-Kelesh N.A. Synthesis and caracterization of poly-methacrylic acid grafted chitosan-bentonite composite and its application for heavy metals recovery. Chem. Mater. Res. 2012;2:1–12. [Google Scholar]
- 218.Wang X., Wang A. Equilibrium isotherm and mechanism studies of Pb(II) and Cd(II) ions onto hydrogel composite based on vermiculite. Desalin. Water Treat. 2012;48:38–49. [Google Scholar]
- 219.Anirudhan T.S., Suchithra P.S. Heavy metals uptake from aqueous solutions and industrial wastewaters by humic acid-immobilized polymer/bentonite composite: Kinetics and equilibrium modeling. Chem. Eng. J. 2010;156:146–156. [Google Scholar]
- 220.Larraza I., López-Gónzalez M., Corrales T., Marcelo G. Hybrid materials: Magnetite-polyethylenimine-montmorillonite, as magnetic adsorbents for Cr(VI) water treatment. J. Colloid Interface Sci. 2012;385:24–33. doi: 10.1016/j.jcis.2012.06.050. [DOI] [PubMed] [Google Scholar]
- 221.Bulut Y., Akçay G., Elma D., Serhatli I.E. Synthesis of clay-based superabsorbent composite and its sorption capability. J. Hazard. Mater. 2009;171:717–723. doi: 10.1016/j.jhazmat.2009.06.067. [DOI] [PubMed] [Google Scholar]
- 222.Urbano B.F., Rivas B.L., Martinez F., Alexandratos S.D. Equilibrium and kinetic study of arsenic sorption by water-insoluble nanocomposite resin of poly[N-(4-vinylbenzyl)-N-methyl-D-glucamine]-montmorillonite. Chem. Eng. J. 2012;193–194:21–30. [Google Scholar]
- 223.Zhao G., Zhang H., Fan Q., Ren X., Li J., Chen Y., Wang X. Sorption of copper(II) onto super-adsorbent of bentonite-polyacrylamide composites. J. Hazard. Mater. 2010;173:661–668. doi: 10.1016/j.jhazmat.2009.08.135. [DOI] [PubMed] [Google Scholar]
- 224.Ulusoy U., Şimşek S. Lead removal by polyacrylamide-bentonite and zeolite composites: Effect of phytic acid immobilization. J. Hazard. Mater. 2005;127:163–171. doi: 10.1016/j.jhazmat.2005.06.036. [DOI] [PubMed] [Google Scholar]
- 225.Cui H., Qian Y., Li Q., Zhang Q., Zhai J. Adsorption of aqueous Hg(II) by a polyaniline/attapulgite composite. Chem. Eng. J. 2012;211–212:216–223. [Google Scholar]
- 226.Zhao Y., Chen Y., Li M., Zhou S., Xue A., Xing W. Adsorption of Hg2+ from aqueous solution onto polyacrylamide/attapulgite. J. Hazard. Mater. 2009;171:640–646. doi: 10.1016/j.jhazmat.2009.06.048. [DOI] [PubMed] [Google Scholar]
- 227.Yang L., Li Y., Hu H., Jin X., Yeb Z., Ma Y., Zhang S. Preparation of novel spherical PVA/ATP composites with macroreticular structure and their adsorption behavior for methylene blue and lead in aqueous solution. Chem. Eng. J. 2011;173:446–455. [Google Scholar]
- 228.Kaşgöz H., Durmus A., Kaşgöz A. Enhanced swelling and adsorption properties of AAm–AMPSNa/clay hydrogel nanocomposites for heavy metal ion removal. Polym. Adv. Technol. 2008;19:213–220. [Google Scholar]
- 229.Şölener M., Tunali S., Özcan A.S., Özcan A., Gedikbey T. Adsorption characteristics of lead(II) ions onto the clay/poly(methoxyethyl)acrylamide (PMEA) composite from aqueous solutions. Desalination. 2008;223:308–322. [Google Scholar]
- 230.Demirbaş O., Alkan M., Dođan M., Turhan Y., Namli H., Turan P. Electrokinetic and adsorption properties of sepiolite modified by 3-aminopropyltriethoxysilane. J. Hazard. Mater. 2007;149:650–656. doi: 10.1016/j.jhazmat.2007.04.036. [DOI] [PubMed] [Google Scholar]
- 231.Turhan Y., Turan P., Dođan M., Alkan M., Namli H., Demirbas Ö. Characterization and adsorption properties of chemically modified sepiolite. Ind. Eng. Chem. Res. 2008;47:1883–1895. [Google Scholar]
- 232.Zhang A.-C., Sun L.-S., Xiang J., Hu S., Fu P., Su S., Zhou Y.-S. Removal of elemental mercury from coal combustion flue gas by bentonite-chitosan and their modifier. J. Fuel Chem. Technol. 2009;37:489–495. [Google Scholar]
- 233.Xu Q., Yin P., Zhao G., Yin G., Qu R. Synthesis and characterization of silica gel microspheres encapsulated by salicyclic acid functionalized polystyrene and its adsorption of transition metal ions from aqueous solutions. Cent. Eur. J. Chem. 2010;8:214–222. [Google Scholar]
- 234.Xu Q., Yin P., Zhao G., Sun Y., Qu R. Adsorption selectivity and dynamic adsorption behaviors of Cu(II), Ag(I), and Au(III) on silica gel encapsulated by amino functionalized polystyrene. J. Appl. Polym. Sci. 2010;117:3645–3650. [Google Scholar]
- 235.Yin P., Xu Q., Qu R., Zhao G., Sun Y. Adsorption of transition metal ions from aqueous solutions onto a novel silica gel matrix inorganic-organic composite material. J. Hazard. Mater. 2010;173:710–716. doi: 10.1016/j.jhazmat.2009.08.143. [DOI] [PubMed] [Google Scholar]
- 236.Kumar G.P., Kumar P.A., Chakraborty S., Ray M. Uptake and desorption of copper ion using functionalized polymer coated silica gel in aqueous environment. Sep. Purif. Technol. 2007;57:47–56. [Google Scholar]
- 237.Zhang K., Wang Q., Meng H., Wang M., Wu W., Chen J. Preparation of polyacrylamide/silica composite capsules by inverse pickering emulsion polymerization. Particuology. 2013 in press. [Google Scholar]
- 238.Buhani, Suharso, Sembiring Z. Immobilization of Chetoceros sp microalgae with silica gel through encapsulation technique as adsorbent of Pb metal from solution. OJC. 2012;28:271–278. [Google Scholar]
- 239.Li F., Du P., Chen W., Zhang S. Preparation of silica-supported porous sorbent for heavy metal ions removal in wastewater treatment by organic-inorganic hybridization combined with sucrose and polyethylene glycol imprinting. Anal. Chim. Acta. 2007;585:211–218. doi: 10.1016/j.aca.2006.12.047. [DOI] [PubMed] [Google Scholar]
- 240.Li F., Jiang H., Zhang S. An ion-imprinted silica-supported organic-inorganic hybrid sorbent prepared by a surface imprinting technique combined with a polysaccharide incorporated sol-gel process for selective separation of cadmium(II) from aqueous solution. Talanta. 2007;71:1487–1493. doi: 10.1016/j.talanta.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 241.Zheng L., Jiang F.-H., Dong P.-J., Zhuang Q.-F., Li F. Preparation of spherical silica-supported biosorbent for copper ions removal in wastewater based on sol-gel reaction and simple treatment with sodium hydroxide. Chem. Res. Chin. Univ. 2010;26:355–359. [Google Scholar]
- 242.Singhon R., Husson J., Knorr M., Lakard B., Euvrard M. Adsorption of Ni(II) ions on colloidal hybrid organic-inorganic silica composites. Colloids Surf. B. 2012;93:1–7. doi: 10.1016/j.colsurfb.2011.12.030. [DOI] [PubMed] [Google Scholar]
- 243.Shin S., Jang J. Thiol containing polymer encapsulated magnetic nanoparticles as reusable and efficiently separable adsorbent for heavy metal ions. Chem. Commun. 2007;2007:4230–4232. doi: 10.1039/b707706h. [DOI] [PubMed] [Google Scholar]
- 244.Song J., Kong H., Jang J. Adsorption of heavy metal ions from aqueous solution by polyrhodanine-encapsulated magnetic nanoparticles. J. Colloid Interface Sci. 2011;359:505–511. doi: 10.1016/j.jcis.2011.04.034. [DOI] [PubMed] [Google Scholar]
- 245.Chang Y.-C., Chen D.-H. Preparation and adsorption properties of monodisperse chitosan-bound Fe3O4 magnetic nanoparticles for removal of Cu(II) ions. J. Colloid Interface Sci. 2005;283:446–451. doi: 10.1016/j.jcis.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 246.Chang Y.-C., Chen D.-H. Recovery of gold(III) ions by a chitosan-coated magnetic nano-adsorbent. Gold Bull. 2006;39:98–102. [Google Scholar]
- 247.Tao S., Wang C., Ma W., Wu S., Meng C. Designed multifunctionalized magnetic mesoporous microsphere for sequential sorption of organic and inorganic pollutants. Microporous Mesoporous Mater. 2012;147:295–301. [Google Scholar]
- 248.Gupta A., Yunus M., Sankararamakrishnan N. Zerovalent iron encapsulated chitosan nanospheres–A novel adsorbent for the removal of total inorganic Arsenic from aqueous systems. Chemosphere. 2012;86:150–155. doi: 10.1016/j.chemosphere.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 249.Jiang W., Chen X.B., Niu Y.J., Pan B.C. Spherical polystyrene-supported nano-Fe3O4 of high capacity and low-field separation for arsenate removal from water. J. Hazard. Mater. 2012;243:319–325. doi: 10.1016/j.jhazmat.2012.10.036. [DOI] [PubMed] [Google Scholar]
- 250.Wan S.L., Zhao X., Lv L., Su Q., Gu H.N., Pan B.C., Zhang W.M., Lin Z.W., Luan J.F. Selective adsorption of Cd(II) and Zn(II) ions by nano-hydrous manganese dioxide (HMO)-encapsulated cation exchanger. Ind. Eng. Chem. Res. 2010;49:7574–7579. [Google Scholar]
- 251.Zhang F., Lan J., Zhao Z., Yang Y., Tan R., Song W. Removal of heavy metal ions from aqueous solution using Fe3O4-SiO2-poly(1,2-diaminobenzene) core-shell sub-micron particles. J. Colloid Interface Sci. 2012;387:205–212. doi: 10.1016/j.jcis.2012.07.066. [DOI] [PubMed] [Google Scholar]
- 252.Zhang M., Zhang Z., Liu Y., Yang X., Luo L., Chen J., Yao S. Preparation of core-shell magnetic ion-imprinted polymer for selective extraction of Pb(II) from environmental samples. Chem. Eng. J. 2011;178:443–450. [Google Scholar]
- 253.Zhang S., Zeng M., Xu W., Li J., Li J., Xu J., Wang X. Polyaniline nanorods dotted on graphene oxide nanosheets as a novel super adsorbent for Cr(VI) Dalton Trans. 2013;42:7854–7858. doi: 10.1039/c3dt50149c. [DOI] [PubMed] [Google Scholar]
- 254.Wu Y.-N., Zhang B., Li F., Zhu W., Xu D., Hannam P., Li G. Electrospun fibrous mats as a skeleton for fabricating hierarchically structured materials as sorbents for Cu2+ J. Mater. Chem. 2012;22:5089–5097. [Google Scholar]
- 255.Ma X., Li L., Yang L., Su C., Wang K., Jiang K. Preparation of hybrid CaCO3–pepsin hemisphere with ordered hierarchical structure and the application for removal of heavy metal ions. J. Cryst. Growth. 2012;338:272–279. [Google Scholar]
- 256.Ma X., Li L., Yang L., Su C., Wang K., Yuan S., Zhou J. Adsorption of heavy metal ions using hierarchical CaCO3–maltose meso/macroporous hybrid materials: Adsorption isotherms and kinetic studies. J. Hazard. Mater. 2012;209–210:467–477. doi: 10.1016/j.jhazmat.2012.01.054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.