Abstract
Ovarian cancer is one of the most common types of reproductive cancer, and has the highest mortality rate amongst gynecological cancer subtypes. The majority of ovarian cancers are diagnosed at an advanced stage, resulting in a five-year survival rate of ~30%. Early diagnosis of ovarian cancer has improved the five-year survival rate to ≥90%, thus the current imperative requirement is to identify biomarkers that would allow the early detection, diagnosis and monitoring of the progression of the disease, or of novel targets for therapy. In the present study, secreted proteins from purified ovarian control, benign and cancer cells were investigated by mass spectrometry, in order to identify novel specific markers that are easy to quantify in patients sera. A total of nine proteins revealed significant differential secretion from control and benign cells, in comparison with ovarian cancer cells. The mRNA expression levels of three of these proteins (Dickkopf protein 3, heat shock protein 10 kDa and gelsolin) were subsequently evaluated by reverse transcription-quantitative polymerase chain reaction. Combined with the protein level in serum, the present study identified that gelsolin may be a useful marker of ovarian cancer.
Keywords: ovarian cancer, gelsolin, secretome, marker, target
Introduction
Ovarian cancer is the fifth leading cause of cancer-associated mortality in the Western world, the second most common cause of gynecological cancer and the leading cause of mortality due to gynecological malignancies (1). Epithelial ovarian cancer (EOC), which accounts for 85–90% of ovarian types of cancer, does not follow a traditional metastatic route via the bloodstream (2). The metastatic process occurs by two alternate pathways as follows: By direct extension to adjacent organs (for example extra-ovarian pelvic organs, colon, bladder and liver), or by exfoliation of EOC cells from the primary tumor (2). The latter pathway leads to aggregation into multicellular spheroids carried by the peritoneal tumor fluid, ascites, to the surrounding organs in the peritoneal cavity (2).
Largely asymptomatic, ≥70% of patients with ovarian cancer have reached an advanced stage of the disease by the time of initial diagnosis, and the overall five-year survival rate for these patients is <30% (3). The current regimen of chemotherapy for ovarian cancer consists of a taxane and platinum based therapy (4). This strategy for the treatment of advanced-stage ovarian cancer results in an initial remission for ≤80% of patients (4). However, following a short remission period (usually 6–22 months), recurrence occurs in almost all patients (3). The lack of effective diagnostic and prognostic tools for ovarian cancer renders this particular type of cancer challenging to manage, and the majority of patients develop chemotherapy resistant tumors and relapse (3).
The carbohydrate antigen 125 (CA125) is the most widely used tumor marker in ovarian cancer (5,6). However, the sensitivity and specificity of CA125 are disadvantages as its expression level is increased in ~80% of all EOC cells and in 30% of stage I EOC cells (5,6). Furthermore, positive values may also be identified in numerous physiological phenomena and benign diseases, including benign pelvic tumors or pelvic inflammatory diseases. CA125 is useful for the follow-up of high-risk patients (including breast cancer 1 positive patients) and for assessing the response to chemotherapy. However, CA125 alone is not a sufficient marker for the diagnosis of ovarian cancer (5,6). Human epididymis protein 4 (HE4), a relatively novel marker for ovarian carcinoma, may provide an improved correlation with the presence of ovarian and endometrial cancer, compared with CA125 (7). Ultimately, the combination of markers including CA125 and HE4, may be the best way to improve the diagnosis of EOC (7). The data suggest that markers with increased effectiveness remain to be identified.
During previous decades, proteomics has become an extensively developed technique used for biomarker identification in various human diseases, including ovarian cancer (8). Proteomics may also improve the understanding of ovarian cancer at the molecular level (8). It may provide an image of a proteome, that may allow identification of the mechanisms underlying the initiation of ovarian cancer, and reveal novel directions for the treatment of the disease (8).
Certain previous studies have analyzed the proteomic profiles of ovarian tumor tissues, cell lines, urine, ascites fluid and blood samples (8–14). Analysis of the ovarian cancer cell line secretome has also provided information on potential markers (11,15). However, these studies were based on cancer cell line comparison analyses, and the in vivo physiological and pathological relevance was questionable. In order to overcome these difficulties, a recent study used primary ovarian cells instead of ovarian cell lines (16). From this study, three proteins were selected and validated by immunoassay in blood samples as potent markers for ovarian cancer. However, for proteomic and immunoassay investigations, no patients with benign disease of the ovary were included. This group of patients is useful for validating the specificity of ovarian cancer markers. Furthermore, ovarian cancer samples from various histological types were pooled into a single ovarian cancer group, whereas those samples led to alternate pathologies based on their origin. The present study investigated the proteome of secreted proteins from the ovarian control (CTRL), benign ovarian lesion (BOL) and high-grade serous ovarian carcinoma (HGSOC) cells in order to identify novel specific markers easily measurable in patients' sera.
Materials and methods
Patient characteristics
The present study was approved be Ethics Committee of Maternity and Pediatrics, University Hospital of Geneva (Geneva, Switzerland). Written informed consent was obtained from all patients prior to enrollment in the present study (between 2009 and 2011). All tissue samples were separated into three alternate groups of epithelial ovarian cells. For the CTRL group (n=4), the tissue extraction and the purification of cells was performed on ovaries without significant histological modification. BOL cells were obtained from patients with BOL pathology (n=3). For the final group, cancer cells were isolated from ascites (n=8) in patients with HGSOC, International Federation of Gynecology and Obstetrics stage IIIC. Furthermore, no significant differences were identified in mean age between the CTRL (64.25±7.59), BOL (52.33±11.5) and HGSOC (55.38±12.49) groups.
Purification of cancer cells
Ascites were centrifuged at 600 × g for 8 min at room temperature (RT). The cell pellets were washed in Hanks' balanced salt solution (HBSS; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) substituted with 25 mM HEPES (Gibco; Thermo Fisher Scientific, Inc.) and 0.05 mg/ml gentamicin (Invitrogen; Thermo Fisher Scientific, Inc.), filtered through a 100 µm mesh (BD Biosciences, San Jose, CA, USA) and centrifuged again at 600 × g for 8 min at RT. The resulting pellets were resuspended in Dulbecco's modified Eagle's medium (DMEM; Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) substituted with 10% fetal bovine serum (FBS; Biochrom, Ltd., Cambridge, UK) and 25 µg/ml plasmocin (InvivoGen; San Diego, CA, USA). This cell suspension was loaded onto a Percoll® (GE Healthcare Life Sciences, Chalfont, UK) gradient (4 layers of Percoll® diluted in 10, 30, 40 and 70% HBSS) and centrifuged at 1,200 × g for 20 min at RT. Subsequently, the cellular ring between Percoll® layers 40 and 20% were collected, diluted in DMEM and centrifuged at 600 × g for 8 min at RT. The pellets were resuspended and cells were seeded at a density of 5×105 in a 3-cm petri-dish.
Purification of BOL and CTRL cells
Ovarian tissue was digested using 4 mg/ml dispase (Gibco; Thermo Fisher Scientific, Inc.) in HBSS-HEPES (filtered on 0.22 µm) substituted with 1 µg/ml deoxyribonuclease (Roche Diagnostics GmbH, Mannheim, Germany) for 30 min at 37°C. Ovarian tissues and supernatants were plated into a 10-cm petri-dish, and the tissue was scraped using a scalpel. Subsequently, the supernatant was collected, neutralized with 5% FBS and centrifuged at 600 × g for 8 min at RT. The resulting pellet was resuspended in DMEM with 10% FBS-0.05 mg/ml gentamicin-25 µg/ml plasmocin.
Cell culture
Cells from the CTRL, BOL and HGSOC groups were cultured in DMEM substituted with 10% FBS, 0.05 mg/ml gentamicin and 25 µg/ml plasmocin for 24 h, followed by 48 h in culture medium without FBS at 37°C (5% CO2). Subsequently, the supernatants were collected, centrifuged at 600 × g for 8 min at RT and stored at −20°C until they were prepared for analysis.
Proteomic analysis
Supernatant concentration
Cell culture supernatants were concentrated using Vivaspin® 500 3 kDa (GE Healthcare Life Sciences), according to the manufacturer's protocol. The protein concentrations were evaluated using the Bio-Rad Protein assay (Bio-Rad Laboratories, Inc. Hercules, CA, USA), according to the manufacturer's protocol.
Liquid digestion of proteins
For each sample, 10 µg total proteins were dissolved in 100 µl 6 M urea and 50 mM ammonium bicarbonate (AB), and the mixture was incubated at 37°C for 30 min. Subsequently, 2 µl of 50 mM dithioerythritol (diluted in distilled water) was added, and the reduction was carried out at 37°C for 1 h. Alkylation was performed by adding 2 µl of 400 mM iodoacetamide (in distilled water), prior to incubation for 1 h at RT in the dark whilst being agitated. The samples were subsequently diluted 3 times in 50 mM AB and 5 µl 200 ng/µl trypsin porcine solution (sequence grade modified; Promega Corporation, Madison, WI, USA) and the digestion was performed overnight at 37°C. Finally, the samples were desalted using a C18 microspin column (Harvard Apparatus, Holliston, MA, USA), dried and dissolved in 5% CH3CN/0.1% formic acid (FA) prior to liquid chromatography (LC)-electrospray ionization (ESI)-mass spectrometry (MS)/MS analysis.
Peptide fragmentation sequencing
LC-ESI-MS/MS was performed using a linear trap quadrupole (LTQ) Orbitrap Velos (Thermo Fisher Scientific, Inc.) equipped with a NanoAcquity system (Waters Corporation, Milford, MA, USA). Peptides were trapped on a 5 µm 200 Å Magic C18 AQ (Bruker-Michrom, Auburn, CA, USA) 0.1×20 mm pre-column, and were separated using a commercial 0.075×150 mm analytical nanocolumn (C18, 5 µm, 100 Å; Nikkyo Technos Co., Ltd., Tokyo, Japan). The analytical separation was performed for 65 min using a gradient of H2O/FA 99.9%/0.1% (solvent A) and CH3CN/FA 99.9%/0.1% (solvent B). The gradient was performed as follows: 0–1 min 95% A and 5% B, subsequently 65% A and 35% B for 55 min and finally 20% A and 80% B for 65 min, at a flow rate of 220 nl/min. ESI was performed at atmospheric pressure in a positive ionization mode, without nebulizing gas. For MS analysis, the orbitrap resolution was set at 60,000 and the ion population was set at 5×105 with an m/z window of 400–2,000. For protein identification, ≤8 precursor ions were selected for collision-induced dissociation (CID) in the LTQ. The ion population was set at 1×104 (isolation width of 2 m/z), whereas for MS/MS detection in the orbitrap, it was set at 1×105 with an isolation width of 2 m/z units. The normalized collision energies were set to 35% for CID.
Protein identification
Peak lists (MGF file format) were generated from raw orbitrap data using the EasyProtConv conversion tool (version 1.6) from the EasyProt software platform (17). The peak list files were searched against the SwissProt database (release 15.10 of 21-Sept-2011) using Mascot (version 2.2.0; Matrix Science, Ltd., London, UK). Human taxonomy (20,323 sequences) was specified for database searching. The parent ion tolerance was set to 10 ppm. Variable amino acid modifications were oxidized using methionine and carbamidomethyl cysteine. Trypsin was selected as the enzyme, with one potential missed cleavage, and the normal cleavage mode was used. The Mascot search was validated using Scaffold 3.6.5 (Proteome Software, Portland, OR, USA). Proteins matching two alternate peptides with a minimum probability score of 95% were selected for further analysis.
RT-qPCR
Cells from CTRL, BOL and HGSOC groups were cultured for 72 h, prior to total RNA extraction using a PureLink® RNA Mini kit (Ambion; Thermo Fisher Scientific, Inc.), according to the manufacturer's instructions. Reverse transcription was performed with 1 µg total RNA in a final volume of 20 µl, using the High Capacity cDNA Reverse Transcription kit (Applied Biosystems; Thermo Fisher Scientific, Inc.). The quantitative detection of the PCR product was performed using a KAPA SYBR® FAST Universal qPCR kit (KAPA Biosystems, Inc., Wilmington, MA, USA). The relative expression levels of gelsolin, Dickkopf protein 3 (DKK3), heat shock protein 10 kDa (HSP-10) and mRNA were normalized to the housekeeping gene, hypoxanthine phosphoribosyltransferase 1. The primer sequences are presented in Table I.
Table I.
Primer sequences.
| Primer | Sequence |
|---|---|
| hHPRT1-F | 5′-ATG ACC AGT CAA CAG GGG AC-3′ |
| hHPRT1-R | 5′-TGC CTG ACC AAG GAA AGC AA-3′ |
| hDKK3-F | 5′-CTG TGT GTC TGG GGT CAC TG-3′ |
| hDKK3-R | 5′-GCT CTA GCT CCC AGG TGA TG-3′ |
| hHSP-10-F | 5′-TTG GAT CGG GTT CTA AAG GA-3′ |
| hHSP-10-R | 5′-TGC CTC CAT ATT CTG GGA GA-3′ |
| hgelsolin-F | 5′-CCC TCA AAA CAG CCT CTG AC-3′ |
| hgelsolin-R | 5′-TCT GCT TGG GGT AGT CCA TC-3′ |
F, forward; R, reverse.
ELISA
The expression levels of gelsolin, DKK3 and HSP-10 in serum from the CTRL (n=19) or HGSOC patients (n=18) were quantified by ELISA. ELISA's for gelsolin (Aviscera Bioscience, Santa Clara, CA, USA), DKK3 (Sigma-Aldrich; Merck Millipore) and HSP-10 (Uscn Life Sciences, Inc., Wuhan, China) were performed according to the manufacturer's instructions.
Statistical analysis
Data are expressed as the mean ± standard error of the mean of various samples. For RT-qPCR, the experiments were performed in triplicate. P-values were calculated using the Student's t-test (Microsoft Office Excel 2007). P<0.05 was considered to indicate a statistically significant difference.
Potential clinical value of the nine significant secreted proteins identified between CTRL/BOL and HGSOC cells using two datasets
The PROGgene website (watson.compbio.iupui.edu/chirayu/proggene/database) was used to investigate the correlation between the mRNA expression levels of the nine significant secreted proteins identified in CTRL/BOL and HGSOC cells, and the survival rate in ovarian cancer, in the TCGA-OVAD and GSE13876 datasets.
Results
Global overview
By LC-ESI-MS/MS analysis, a total of 589 proteins were identified, as presented in Fig. 1. The Venn diagrams revealed that 159 proteins were identified to be mutually expressed in the three groups (CTRL, BOL and HGSOC). A total of 271 proteins were exclusively secreted by HGSOC cells, 36 by CTRL cells and 10 by BOL cells. These assays allowed the identification of proteins exclusively secreted by CTRL/BOL cells, compared with HGSOC cells. Finally, 13 proteins common to the CTRL and BOL groups are not secreted by the HGSOC group.
Figure 1.
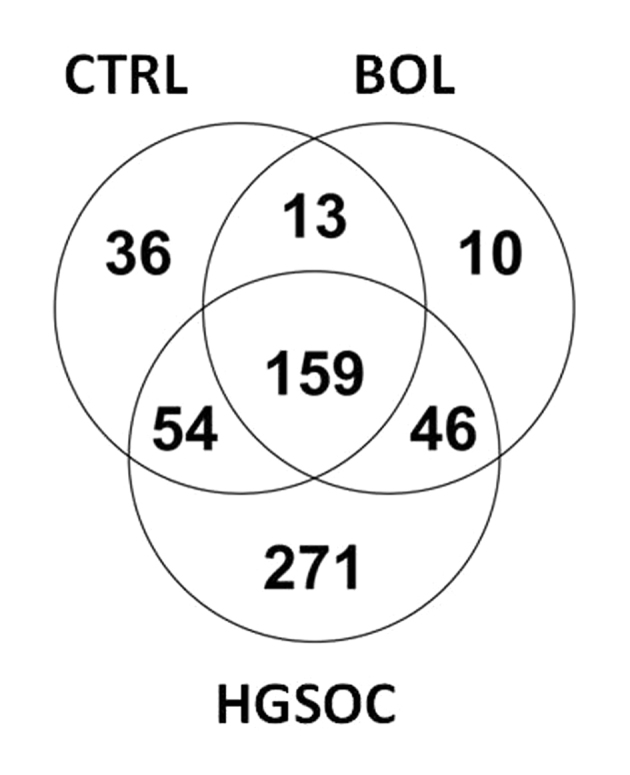
Protein Venn diagram. Culture supernatants of CTRL (n=4), BOL (n=3) and HGSOC (n=8) cells were analyzed by liquid chromatography-electrospray ionization-mass spectrometry/mass spectrometry. The number of identified proteins is indicated for each category. CTRL, ovarian control; BOL, benign ovarian lesion; HGSOC, high-grade serous ovarian carcinoma.
Identification of the proteins significantly and differentially secreted by HGSOC cells, compared with CTRL cells and BOL cells
Among the 589 proteins identified, 64 were revealed to be significantly and differentially secreted in CTRL cells compared with HGSOC cells (P<0.05). The 32 most relevant proteins are presented in Table II. As presented in Table III, 21 proteins were revealed to be significantly and differentially secreted from HGSOC cells compared with BOL cells. The majority were not present in the list of proteins differentially secreted by CTRL cells in comparison with HGSOC cells.
Table II.
Proteins significantly and differentially secreted in CTRL and HGSOC cells.
| Quantification value | ||||
|---|---|---|---|---|
| Proteins | Accession no. | P-value | CTRL | HGSOC |
| Fibulin-5a | Q9UBX5 | <0.001 | 4.25±-0.5 | 0.3±0.3 |
| Insulin-like growth factor-binding protein 2b | P18065 | <0.001 | 2.3±2.3 | 15.25±0.9 |
| 72 kDa type IV collagenasea | P08253 | <0.001 | 52.5±7 | 11.4±2.9 |
| IgG superfamily containing leucine-rich repeat proteina | O14498 | <0.001 | 8.5±2 | 0.0 |
| Fibulin-1a | P23142 | <0.001 | 11.8±2.5 | 0.5±0.5 |
| Latent-transforming growth factor β-binding protein 2a | Q14767 | <0.001 | 13±2 | 1±1 |
| Procollagen-lysine, 2-oxoglutarate 5-dioxygenase 1a | Q02809 | <0.001 | 5.8±0.8 | 0.8±0.5 |
| Nidogen-1a | P14543 | <0.001 | 12.5±3.5 | 0.0 |
| Collagen α-3 (VI) chaina | P12111 | <0.001 | 40.3±12.6 | 0.0 |
| Thrombospondin-1a | P07996 | 0.001 | 18.3±3.3 | 3.1±1.7 |
| Ezrinb | P15311 | 0.001 | 2.5±0.5 | 14.5±1.8 |
| Collagen alpha-2 (VI) chaina | P12110 | 0.001 | 5.3±0.8 | 0.9±0.6 |
| Connective tissue growth factora | P29279 | 0.001 | 4±1.4 | 0.0 |
| Keratin, type II cytoskeletal 8b | P05787 | 0.001 | 0.0 | 24.5±3.8 |
| Stromelysin-3a | P24347 | 0.001 | 7±2.4 | 0.0 |
| Procollagen C-endopeptidase enhancer 1a | Q15113 | 0.002 | 13.5±4.9 | 0.0 |
| Brain acid soluble protein 1b | P80723 | 0.002 | 0.0 | 2.5±0.4 |
| Vimentin OS=Homo sapiensa | P08670 | 0.002 | 36.3±5.7 | 12.8±3 |
| Extracellular matrix protein 1a | Q16610 | 0.003 | 7.3±2.7 | 0.0 |
| EMILIN-1a | Q9Y6C2 | 0.003 | 3.5±1.3 | 0.0 |
| Endosialina | Q9HCU0 | 0.003 | 2.3±0.9 | 0.0 |
| Stanniocalcin-1a | P52823 | 0.003 | 2.3±0.9 | 0.0 |
| Malate dehydrogenase, mitochondrialb | P40926 | 0.003 | 1.5±0.5 | 7.9±1.1 |
| Keratin, type I cytoskeletal 19b | P08727 | 0.003 | 0.5±05 | 17.4±3 |
| Complement C3b | P01024 | 0.003 | 7.3±3.3 | 77.1±12.5 |
| Macrophage colony-stimulating factor 1b | P09603 | 0.004 | 0.5±0.5 | 2.6±0.3 |
| Pigment epithelium-derived factora | P36955 | 0.004 | 3±1.2 | 0.0 |
| Stromelysin-1a | P08254 | 0.005 | 27.3±11.1 | 0.3±0.3 |
| Keratin, type II cytoskeletal 7b | P08729 | 0.005 | 0.0 | 20.5±3.9 |
| Collagen α-1 (I) chaina | P02452 | 0.005 | 58.8±13.8 | 18.3±4.6 |
| Gelsolina | P06396 | 0.006 | 8.5±1.7 | 2.9±1.7 |
| Cathepsin Ka | P43235 | 0.008 | 2.8±1.3 | 0.0 |
Proteins with a significantly decreased level of secretion by HGSOC cells vs. CTRL cells.
Proteins with a significantly increased level of secretion by HGSOC cells vs. CTRL cells. CTRL, control; HGSOC, high-grade serous ovarian carcinoma.
Table III.
Proteins significantly and differentially secreted in BOL and HGSOC cells.
| Quantification value | |||||
|---|---|---|---|---|---|
| Proteins | Accession no. | MW, kDa | P-value | BOL | HGSOC |
| Insulin-like growth factor-binding protein 2a | P18065 | 35 | <0.001 | 5.3±2 | 15.3±0.9 |
| Insulin-like growth factor-binding protein 7b | Q16270 | 29 | <0.001 | 28±2.9 | 15±1.3 |
| Macrophage colony-stimulating factor 1a | P09603 | 60 | <0.001 | 0 | 2.6±0.3 |
| Connective tissue growth factorb | P29279 | 38 | 0.006 | 4±2 | 0 |
| Neuroblastoma suppressor of tumorigenicity 1b | P41271 | 19 | 0.006 | 1.3±0.7 | 0 |
| Dickkopf-related protein 3b | Q9UBP4 | 38 | 0.010 | 11.7±2.2 | 3.8±1.3 |
| Keratin, type II cytoskeletal 1a | P04264 | 66 | 0.013 | 4.7±0.3 | 10.4±1.1 |
| Monocyte differentiation antigen CD14b | P08571 | 40 | 0.021 | 6.3±3.5 | 0.6±0.4 |
| SPARCb | P09486 | 35 | 0.021 | 53±8.2 | 32.6±3.4 |
| Tenascin-Xb | P22105 | 464 | 0.023 | 36.6±20.3 | 4.6±1.9 |
| Inter-α-trypsin inhibitor heavy chain H5b | Q86UX2 | 105 | 0.023 | 2.7±1.8 | 0 |
| Tenascinb | P24821 | 241 | 0.023 | 5.3±3.5 | 0 |
| Fibronectinb | P02751 | 263 | 0.024 | 162.7±65.5 | 59.1±6.9 |
| Gelsolinb | P06396 | 86 | 0.027 | 6.3±0.3 | 2.9±0.8 |
| 10 kDa heat shock protein, mitochondrialb | P61604 | 11 | 0.030 | 0 | 1.6±0.4 |
| Malate dehydrogenase, mitochondriala | P40926 | 36 | 0.031 | 2.7±1.5 | 7.9±1.1 |
| α-2-HS-glycoproteina | P02765 | 39 | 0.036 | 1.7±0.9 | 3.3±0.3 |
| Vimentinb | P08670 | 54 | 0.037 | 26.7±4.8 | 12.8±3 |
| Prostaglandin-H2 D-isomerasea | P41222 | 21 | 0.038 | 1±1 | 4±0.7 |
| Peroxiredoxin-2a | P32119 | 22 | 0.043 | 0 | 3.9±1 |
| Sulfhydryl oxidase 1a | O00391 | 83 | 0.048 | 6.7±1.9 | 15.8±2.3 |
Proteins with a significantly increased level of secretion by HGSOC cells vs. BOL cells.
Proteins with a significantly decreased level of secretion by HGSOC cells vs. BOL cells. BOL, benign ovarian lesion; HGSOC, high-grade serous ovarian carcinoma; CD14, cluster of differentiation; MW, molecular weight.
Common proteins differentially secreted by CTRL and BOL cells, compared with HGSOC cells
A total of nine proteins were significantly and differentially secreted in CTRL and BOL cells, in comparison with HGSOC cells (Table IV). These proteins were evaluated for their potential clinical value by analyzing the association between their expression level and survival, using publically available datasets (Table V). The connective tissue growth factor (CTGF) mRNA expression level was revealed to be associated with the survival rate of patients with ovarian cancer in the TCGA-OVAD database but not in the GSE13876 database. In the GSE13876 database, insulin like growth factor binding protein 2 (IGFBP2) and vimentin mRNA expression levels demonstrated an association with the rate of survival. Furthermore, all the evaluated genes [gelsolin, DKK3, CTGF, IGFBP2, IGFBP7, colony stimulating factor 1 (CSF1), malate dehydrogenase 2 (MDH2) and vimentin] demonstrated a significant association with the rate of survival using this database. Subsequently, the present study focused on three of these proteins: Gelsolin, DKK3 and HSP-10. Gelsolin and DKK3 demonstrated significantly lower secretion in HGSOC cells, in comparison with CTRL and BOL cells (Tables II and III). Conversely, HGSOC cells secreted greater levels of HSP-10 proteins, compared with CTRL or BOL cells (Tables II and III).
Table IV.
Common proteins significantly and differentially secreted in CTRL and BOL cells, vs. HGSOC cells.
| Proteins | Accession no. | MW, kDa |
|---|---|---|
| 10 kDa heat shock protein, mitochondrial | P61604 | 11 |
| Connective tissue growth factor | P29279 | 38 |
| Dickkopf-related protein 3 | Q9UBP4 | 38 |
| Gelsolin | P06396 | 86 |
| Insulin-like growth factor-binding protein 2 | P18065 | 35 |
| Insulin-like growth factor-binding protein 7 | Q16270 | 29 |
| Macrophage colony-stimulating factor 1 | P09603 | 60 |
| Malate dehydrogenase, mitochondrial | P40926 | 36 |
| Vimentin | P08670 | 54 |
CTRL, ovarian control; BOL, benign ovarian lesion; HGSOC, high-grade serous ovarian carcinoma; MW, molecular weight.
Table V.
The nine significantly secreted proteins identified in control/benign and ovarian cancer cells were evaluated for their potential clinical value by analyzing the association between their mRNA expression levels and patient mortality, using two alternate publically available datasets.
| Genes | TCGA-OVAD database P-value | GSE13876 database P-value |
|---|---|---|
| Gelsolin | 0.434 | 0.589 |
| Dickkopf-related protein 3 | 0.083 | 0.241 |
| Connective tissue growth factor | 0.008a | 0.962 |
| Insulin-like growth factor-binding protein 2 | 0.221 | 0.013a |
| Insulin-like growth factor-binding protein 7 | 0.871 | 0.568 |
| Macrophage colony-stimulating factor 1 | 0.356 | ND |
| Malate dehydrogenase, mitochondrial | 0.496 | 0.193 |
| Vimentin | 0.305 | 0.018a |
| 10 kDa heat shock protein | ND | ND |
| Sum of these genes | 0.18 | 0.048a |
P<0.05 ND, not determined
Gelsolin, DKK3 and HSP-10 mRNA expression levels in HGSOC and CTRL cells
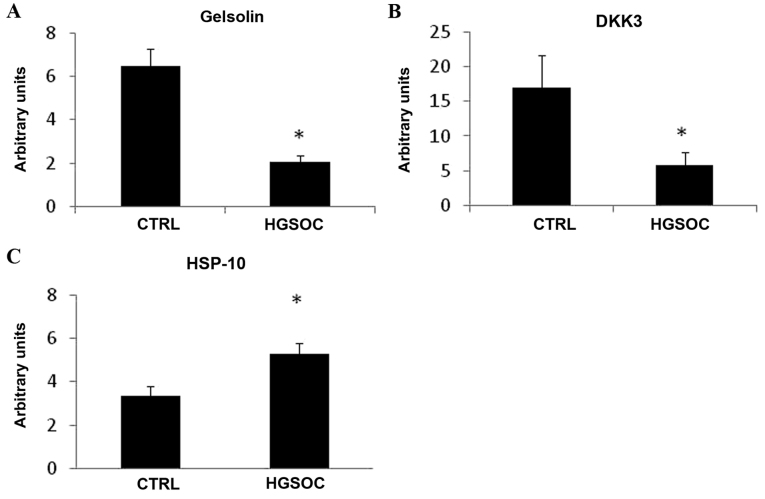
In order to determine if gelsolin, DKK3 and HSP-10 expression levels are modified in cancer cells, the present study analyzed their mRNA expression levels in HGSOC and CTRL cells. Gelsolin and DKK3 mRNA demonstrated significantly lower expression levels in HGSOC cells, compared with in CTRL cells (Fig. 2A and B). However, HGSOC cells expressed higher levels of HSP-10 mRNA, compared with CTRL cells (Fig. 2C).
Figure 2.
mRNA expression levels of gelsolin, DKK3 and HSP-10. CTRL (n=4) and HGSOC (n=8) cells were cultured for 72 h prior to RNA extraction and reverse transcription-quantitative polymerase chain reaction was performed for (A) gelsolin, (B) DKK3 and (C) HSP-10. Expression levels were normalized to the housekeeping gene hypoxanthine phosphoribosyltransferase 1. *P<0.05 vs. CTRL. DKK3, Dickkopf protein 3; HSP-10, heat-shock protein 10 kDa; CTRL, control; HGSOC, high-grade serous ovarian carcinoma.
Analysis of the selected secreted proteins in the sera of patients with HGSOC and the CTRL cohort
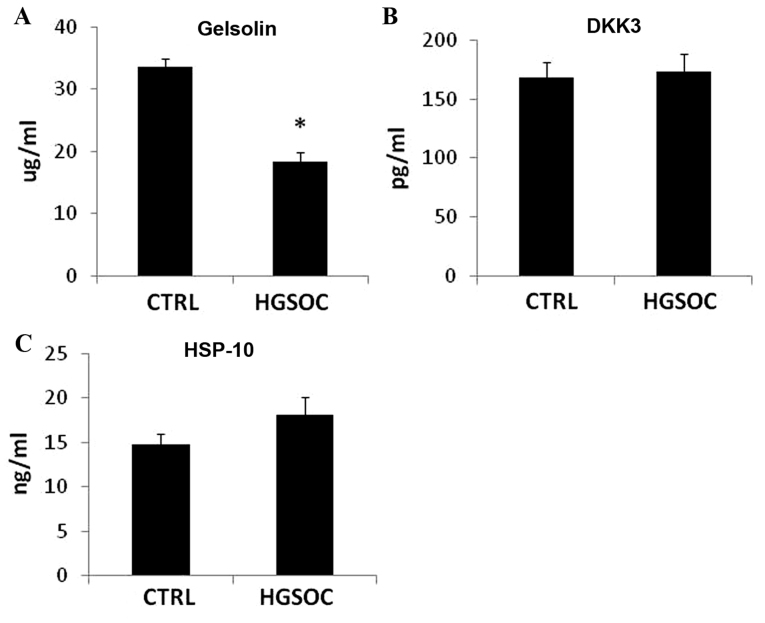
The expression levels of gelsolin, DKK3 and HSP-10 were evaluated in the serum of the CTRL group and patients with HGSOC. As demonstrated in Fig. 3, circulating DKK3 does not alternate between the two groups. The expression level of HSP-10 was observed to be higher in the serum of the HGSOC group compared with CTRL patients; however, this increase was not statistically significant (Fig. 3). The serum expression level of gelsolin was significantly different in the healthy cohort vs. the HGSOC cohort of patients (P<0.001), with decreased expression levels observed in the patients with HGSOC (Fig. 3).
Figure 3.
Expression levels of (A) gelsolin, (B) DKK3 and (C) HSP-10 in the serum of HGSOC patients and healthy volunteers (CTRL) determined by ELISA. *P<0.01. DKK3, Dickkopf protein 3; HSP-10, heat shock protein 10 kDa; HGSOC, high-grade serous ovarian carcinoma; CTRL, control.
Discussion
Secreted proteins are circulating proteins detectable in blood samples using highly sensitive techniques, including immunological methods (ELISA) (18). Although in vitro culture conditions may not fully reflect the in vivo conditions, identification of ovarian proteins differentially secreted by malignant cells may be a first step towards the elucidation of ovarian cancer markers in blood samples. In this context, secretome analysis of ovarian cancer cell lines has contributed to the establishment of a list of potent biomarkers (11,15,16). However, the pathological relevance of the cell line secretome is debatable. Previously, Zhang et al (16) analyzed the conditioned media of cells purified from normal ovarian tissues and tumor tissues, and established an ovarian cancer-associated conditioned media protein database. Certain proteins differentially secreted by ovarian cancer cells in comparison with normal cells, may be secreted as a general response to an acute condition and they must not be considered as tumor specific. In order to avoid this bias, the present study performed a proteomic analysis of secreted proteins from normal and BOL cells, compared with serous cancer cells purified from ovarian tissues and ascites. A total of 589 proteins secreted in the supernatant of cultured ovarian cells were identified as a result. Spectral counts, used as an evaluation of abundance, coupled with comparison of proteins identified in the supernatant of cells purified from the ovarian tissues of each group of patients, allowed the identification of proteins differentially secreted by cancer cells. Among these 589 proteins, 64 were significantly and differentially secreted by ovarian cancer cells, in comparison with normal cells. Certain proteins, including metallopeptidase inhibitor 2 (TIMP-2) and nidogen 1, had previously been identified by analysis of secreted proteins from normal and cancer cells, and validated in the serum of healthy volunteers and patients with ovarian cancer (16). However, despite nidogen 1 and TIMP-2 exhibiting higher expression levels in the serum of ovarian cancer patients in comparison with healthy patients, the present study's proteomic analysis on conditioned media revealed the opposite. Furthermore, these proteins were not identified to be differentially secreted from HGSOC cells compared with BOL cells, suggesting that they may be secreted as a general response to an acute condition and they should not be considered as tumor specific.
A total of 21 proteins were revealed to be significantly and differentially secreted by HGSOC cells compared with BOL cells. However, only nine of the proteins identified matched those in the list of the 64 proteins differentially secreted by cancer cells in comparison with normal cells. The present study evaluated the potential of these nine proteins by analyzing the association between their mRNA expression levels and the survival rate, using publically available datasets. The evaluated genes were determined to have a significant clinical value using the GSE13876 database. However, analysis of certain genes revealed contrary results depending on the database used, thus limiting the use of these databases for the evaluation of gene clinical potential. In particular, the mRNA expression level may not be associated with the protein expression level and/or secretion.
Among the nine significantly secreted proteins identified between CTRL/BOL and HGSOC cells, four were revealed to have an increased rate of secretion from HGSOC cells compared with BOL and CTRL cells, including, HSP-10, IGFBP-2, macrophage CSF-1 and malate dehydrogenase. These proteins have previously been studied in ovarian cancer cells (19–23). CSF-1 may serve an important role in ovarian carcinogenesis, as it has the capacity to increase the invasive ability of ovarian cancer cells (24) and to promote metastasis (25). Furthermore, high expression levels of CSF-1 are associated with poor patient outcome, suggesting that this protein may be a potential biomarker and prognostic factor (20,21). IGFBP-2 also serves a significant role in EOC pathogenesis and may represent an additional serum biomarker with utility in the detection and monitoring of EOC (23,26). HSP-10 is known to serve a role in the mechanism underlying mitochondrial protein-folding. HSP-10 is also present in additional subcellular compartments, following stress induction, and may be identified in the extracellular space and in the bloodstream, where it may be a critical factor in the suppression of T cell activation (27). HSP-10 was present in the serum of patients with ovarian cancer, whereas it was not detected in the serum of healthy patients, suggesting that it may be secreted by ovarian cancer cells (19). In the present study, it was demonstrated that the HSP-10 mRNA expression level was significantly lower in healthy ovarian cells, compared with in malignant cells. HSP-10 was also revealed to have a higher expression level in the serum of patients with malignant cancer, compared with in the sera of healthy patients, but this was not significant.
Subsequently, the present study investigated two additional proteins, gelsolin and DKK3, as potential markers of ovarian cancer as they exhibited a significantly lower level of secretion from ovarian cancer cells, compared with the normal and BOL cells. The Dickkopf family consists of four main secreted proteins, which are known to be antagonists of the Wnt signaling pathway (28). However, the functional role of DKK3 in the Wnt/β-catenin signaling pathway remains unknown (28). DKK3 was previously described as a pro-angiogenic factor implicated in neovascularization during tumor development (28). There are currently few studies on the expression of DKK3 in ovarian cancer (29). A previous study by Jiang et al (30) suggested that this protein may be used as biomarker of ovarian cancer. Although the present study demonstrated that DKK3 is under-expressed and under-secreted by HGSOC cells compared with CTRL cells, this marker was not validated in the serum of patients.
Gelsolin is a calcium-activated actin-linked protein, present in the cytoplasm or secreted by cells (31). The secreted form of gelsolin differs from the intracellular structure by a 25-amino acid signaling peptide and the presence of a disulfide bond between cysteine residues at positions 188 and 201 (31). In mutant mice, this protein is associated with a number of pathologies, including inflammation and cancer (32). Gelsolin is involved in cell motility, phagocytosis, apoptosis, platelet formation and activation (31). Loss of gelsolin expression in human ovarian carcinoma was previously demonstrated by Noske et al (33), and it was suggested that it may be associated with tumor grade. This decreased expression level may be mediated by epigenetic modification (33). Despite the relatively small number of tissues and ascites samples investigated in the present study, decreased mRNA expression levels of gelsolin were revealed in HGSOC cells compared with CTRL cells, and a decrease in the level of gelsolin secreted from HGSOC cells, compared with CTRL and BOL cells, in vitro was also demonstrated. It was also revealed that the transfection of gelsolin into HGSOC cells induced a reduction in colony formation, suggesting it may have growth suppressive activity in HGSOC cells. Thus, reconstitution of gelsolin expression levels in HGSOC cells may be a promising therapeutic intervention for ovarian cancer (33). Furthermore, gelsolin expression levels were demonstrated to be significantly reduced in the serum of patients with ovarian cancer, compared with healthy patients, suggesting that gelsolin may be a useful biomarker of ovarian cancer.
In conclusion, the proteomic approach remains a useful tool for the investigation of potential biomarkers by comparison of protein expression patterns between the CTRL and HGSOC samples. Despite the relatively small number of tissue and ascites specimens studied, the majority of proteins identified in the present study as being significantly differentially secreted from malignant and CTRL cell samples had been detected in previous studies (8–16), confirming their potent and important role. To the best of our knowledge, proteins including HSP-10 and DKK3, had not previously been identified by a proteomic approach, but had been suggested as potent markers using alternate approaches (19,30); however the present study was unable to confirm their utility as biomarkers of serous ovarian cancer by the immunological method. Conversely, gelsolin may be a successful potential biomarker of serous ovarian cancer or a therapeutic target.
Acknowledgements
The present study was supported by the Swiss National Science Foundation (grant no. 31003A-127392). The authors thank Mrs. Ginette Rosseel for enrolling patients and collecting ascites, and Mrs. Patrizia Arboit and Mrs. Aurore Britan for the technical assistance provided.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-tieulent J, Jemal A. Global Cancer statistics, 2012. Ca Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Latifi A, Luwor RB, Bilandzic M, Nazaretian S, Stenvers K, Pyman J, Zhu H, Thompson EW, Quinn MA, Findlay JK, Ahmed N. Isolation and characterization of tumor cells from the ascites of ovarian cancer patients: Molecular phenotype of chemoresistant ovarian tumors. PLoS One. 2012;7:e46858. doi: 10.1371/journal.pone.0046858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Su Z, Graybill WS, Zhu Y. Detection and monitoring of ovarian cancer. Clin Chim Acta. 2013;415:341–345. doi: 10.1016/j.cca.2012.10.058. [DOI] [PubMed] [Google Scholar]
- 4.Têtu B, Popa I, Bairati I, L'Esperance S, Bachvarova M, Plante M, Harel F, Bachvarov D. Immunohistochemical analysis of possible chemoresistance markers identified by micro-arrays on serous ovarian carcinomas. Mod Pathol. 2008;21:1002–1010. doi: 10.1038/modpathol.2008.80. [DOI] [PubMed] [Google Scholar]
- 5.Li J, Dowdy S, Tipton T, Podratz K, Lu WG, Xie X, Jiang SW. HE4 as a biomarker for ovarian and endometrial cancer management. Expert Rev Mol Diagn. 2009;9:555–566. doi: 10.1586/erm.09.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rein BJ, Gupta S, Dada R, Safi J, Michener C, Agarwal A. Potential markers for detection and monitoring of ovarian cancer. J Oncol. 2011;2011:475983. doi: 10.1155/2011/475983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nguyen L, Cardenas-Goicoechea SJ, Gordon P, Curtin C, Momeni M, Chuang L, Fishman D. Biomarkers for early detection of ovarian cancer. Womens Health (Lond) 2013;9:171–187. doi: 10.2217/whe.13.2. [DOI] [PubMed] [Google Scholar]
- 8.Toss A, de Matteis E, Rossi E, Casa LD, Iannone A, Federico M, Cortesi L. Ovarian cancer: Can proteomics give new insights for therapy and diagnosis? Int J Mol Sci. 2013;14:8271–8290. doi: 10.3390/ijms14048271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.An HJ, Kim DS, Park YK, Kim SK, Choi YP, Kang S, Ding B, Cho NH. Comparative proteomics of ovarian epithelial tumors. J Proteome Res. 2006;5:1082–1090. doi: 10.1021/pr050461p. [DOI] [PubMed] [Google Scholar]
- 10.Cortesi L, Rossi E, Casa L Della, Barchetti A, Nicoli A, Piana S, Abrate M, La Sala GB, Federico M, Iannone A. Protein expression patterns associated with advanced stage ovarian cancer. Electrophoresis. 2011;32:1992–2003. doi: 10.1002/elps.201000654. [DOI] [PubMed] [Google Scholar]
- 11.Faça VM, Ventura AP, Fitzgibbon MP, Pereira-Faça SR, Pitteri SJ, Green AE, Ireton RC, Zhang Q, Wang H, O'Briant KC, et al. Proteomic analysis of ovarian cancer cells reveals dynamic processes of protein secretion and shedding of extra-cellular domains. PLoS One. 2008;3:e2425. doi: 10.1371/journal.pone.0002425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackson D, Craven RA, Hutson RC, Graze I, Lueth P, Tonge RP, Hartley JL, Nickson JA, Rayner SJ, Johnston C, et al. Proteomic profiling identifies afamin as a potential biomarker for ovarian cancer. Clin Cancer Res. 2007;13:7370–7379. doi: 10.1158/1078-0432.CCR-07-0747. [DOI] [PubMed] [Google Scholar]
- 13.Li XQ, Zhang SL, Cai Z, Zhou Y, Ye TM, Chiu JF. Proteomic identification of tumor-associated protein in ovarian serous cystadenocarinoma. Cancer Lett. 2009;275:109–116. doi: 10.1016/j.canlet.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 14.Petri AL, Simonsen AH, Yip TT, Hogdall E, Fung ET, Lundvall L, Hogdall C. Three new potential ovarian cancer biomarkers detected in human urine with equalizer bead technology. Acta Obstet Gynecol Scand. 2009;88:18–26. doi: 10.1080/00016340802443830. [DOI] [PubMed] [Google Scholar]
- 15.Gunawardana CG, Kuk C, Smith CR, Batruch I, Soosaipillai A, Diamandis EP. Comprehensive analysis of conditioned media from ovarian cancer cell lines identifies novel candidate markers of epithelial ovarian cancer. J Proteome Res. 2009;8:4705–4713. doi: 10.1021/pr900411g. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Xu B, Liu Y, Yao H, Lu N, Li B, Gao J, Guo S, Han N, Qi J, et al. The ovarian cancer-derived secretory/releasing proteome: A repertoire of tumor markers. Proteomics. 2012;12:1883–1891. doi: 10.1002/pmic.201100654. [DOI] [PubMed] [Google Scholar]
- 17.Gluck F, Hoogland C, Antinori P, Robin X, Nikitin F, Zufferey A, Pasquarello C, Fétaud V, Dayon L, Muller M, et al. EasyProt-an easy-to-use graphical platform for proteomics data analysis. J Proteomics. 2013;79:146–160. doi: 10.1016/j.jprot.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 18.Epiney M, Ribaux P, Arboit P, Irion O, Cohen M. Comparative analysis of secreted proteins from normal and preeclamptic trophoblastic cells using proteomic approaches. J Proteomics. 2012;75:1771–1777. doi: 10.1016/j.jprot.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 19.Akyol S, Gercel-Taylor C, Reynolds LC, Taylor DD. HSP-10 in ovarian cancer: Expression and suppression of T-cell signaling. Gynecol Oncol. 2006;101:481–486. doi: 10.1016/j.ygyno.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 20.Chambers SK. Role of CSF-1 in progression of epithelial ovarian cancer. Future Oncol. 2009;5:1429–1440. doi: 10.2217/fon.09.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chambers SK, Kacinski BM, Ivins CM, Carcangiu ML. Overexpression of epithelial macrophage colony-stimulating factor (CSF-1) and CSF-1 receptor: A poor prognostic factor in epithelial ovarian cancer, contrasted with a protective effect of stromal CSF-1. Clin Cancer Res. 1997;3:999–1007. [PubMed] [Google Scholar]
- 22.Lim HY, Ho QS, Low J, Choolani M, Wong KP. Respiratory competent mitochondria in human ovarian and peritoneal cancer. Mitochondrion. 2011;11:437–443. doi: 10.1016/j.mito.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 23.Wang H, Rosen DG, Wang H, Fuller GN, Zhang W, Liu J. Insulin-like growth factor-binding protein 2 and 5 are differentially regulated in ovarian cancer of different histologic types. Mod Pathol. 2006;19:1149–1156. doi: 10.1038/modpathol.3800637. [DOI] [PubMed] [Google Scholar]
- 24.Chambers SK, Wang Y, Gertz RE, Kacinski BM. Macrophage colony-stimulating factor mediates invasion of ovarian cancer cells through urokinase. Cancer Res. 1995;55:1578–1585. [PubMed] [Google Scholar]
- 25.Toy EP, Azodi M, Folk NL, Zito CM, Zeiss CJ, Chambers SK. Enhanced ovarian cancer tumorigenesis and metastasis by the macrophage colony-stimulating factor. Neoplasia. 2009;11:136–144. doi: 10.1593/neo.81150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lancaster JM, Sayer RA, Blanchette C, Calingaert B, Konidari I, Gray J, Schildkraut J, Schomberg DW, Marks JR, Berchuck A. High expression of insulin-like growth factor binding protein-2 messenger RNA in epithelial ovarian cancers produces elevated preoperative serum levels. Int J Gynecol Cancer. 2006;16:1529–1535. doi: 10.1111/j.1525-1438.2006.00623.x. [DOI] [PubMed] [Google Scholar]
- 27.David S, Bucchieri F, Corrao S, Czarnecka AM, Campanella C, Farina F, Peri G, Tomasello G, Sciumè C, Modica G, et al. Hsp10: Anatomic distribution, functions, and involvement in human disease. Front Biosci. 2013;5:768–778. doi: 10.2741/E657. [DOI] [PubMed] [Google Scholar]
- 28.Niehrs C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene. 2006;25:7469–7481. doi: 10.1038/sj.onc.1210054. [DOI] [PubMed] [Google Scholar]
- 29.Veeck J, Dahl E. Targeting the Wnt pathway in cancer: The emerging role of Dickkopf-3. Biochim Biophys Acta. 2012;1825:18–28. doi: 10.1016/j.bbcan.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 30.Jiang T, Huang L, Wang S, Zhang S. Clinical significance of serum Dkk-3 in patients with gynecological cancer. J Obstet Gynaecol Res. 2010;36:769–773. doi: 10.1111/j.1447-0756.2010.01234.x. [DOI] [PubMed] [Google Scholar]
- 31.Silacci P, Mazzolai L, Gauci C, Stergiopulos N, Yin HL, Hayoz D. Gelsolin superfamily proteins: Key regulators of cellular functions. Cell Mol Life Sci. 2004;61:2614–2623. doi: 10.1007/s00018-004-4225-6. [DOI] [PubMed] [Google Scholar]
- 32.Spinardi L, Witke W. Gelsolin and diseases. Subcell Biochem. 2007;45:55–69. doi: 10.1007/978-1-4020-6191-2_3. [DOI] [PubMed] [Google Scholar]
- 33.Noske A, Denkert C, Schober H, Sers C, Zhumabayeva B, Weichert W, Dietel M, Wiechen K. Loss of Gelsolin expression in human ovarian carcinomas. Eur J Cancer. 2005;41:461–469. doi: 10.1016/j.ejca.2004.10.025. [DOI] [PubMed] [Google Scholar]