Abstract
Objectives
This paper describes a novel approach (same-patient processing, or SPP) aimed at improving left ventricular segmentation accuracy in patients with multiple SPECT studies, and evaluates its performance compared to conventional processing in a large population of 962 patients undergoing rest and stress electrocardiography-gated SPECT MPI, for a total of 5,772 image datasets (6 per patient).
Methods
Each dataset was independently processed using a standard algorithm, and a shape quality control score (SQC) was produced for every segmentation. Datasets with a SQC score higher than a specific threshold, suggesting algorithmic failure, were automatically reprocessed with the SPP-modified algorithm, which incorporates knowledge of the segmentation mask location in the other datasets belonging to the same patient. Experienced operators blinded as to whether datasets had been processed based on the standard or SPP approach assessed segmentation success/failure for each dataset.
Results
The SPP approach reduced segmentation failures from 219/5772 (3.8%) to 42/5772 (0.7%) overall, with particular improvements in attenuation-corrected (AC) datasets with high extracardiac activity (from 100/962 (10.4%) to 12/962 (1.4%) for rest AC, and from 41/962 (4.3%) to 9/962 (0.9%) for stress AC). The number of patients who had at least one of their 6 datasets affected by segmentation failure decreased from 141/962 (14.7%) to 14/962 (1.7%) using the SPP approach.
Conclusion
Whenever multiple image datasets for the same patient exist and need to be processed, it is possible to deal with the images as a group rather than individually. The same-patient processing approach can be implemented automatically, and may substantially reduce the need for manual reprocessing due to cardiac segmentation failure.
INTRODUCTION
The essential prerequisite for automated quantification of cardiac SPECT and PET images is that the software employed be able to correctly identify and isolate (segment) the heart, or portion of the heart to which the quantification algorithm is to be applied. Segmentation failures must be recognized and corrected, either by “masking” the image before reprocessing it with the same algorithm, or through full manual override.
Cardiac quantification is generally based on the premise that each image dataset is processed separately, with the notable exception of the registration of image pairs to a common template for the purpose of measuring perfusion stress-rest “change” 1. Recent developments in nuclear cardiology have led to the proliferation of new cameras, hardware, patient acquisition stances, doses, protocols and post-acquisition processing approaches 2. Serial studies over years are now common, such that a patient may have been imaged multiple times, often on different cameras, using different radiopharmaceuticals and protocols. Doses may be significantly different between acquisitions, due to the increasing awareness of radiation dose considerations. 3
Whenever multiple image datasets for the same patient exist and need to be processed and analyzed, there is an opportunity to take advantage of the fact that the heart involved is the same, and to deal with the images as a group rather than individually. If images with better count characteristics or fewer artifacts could be used to help in the processing or quantification of lesser quality images of the same patient, it’s reasonable to assume that better overall results could be achieved. The aim of this paper is to describe the application of the same-patient processing (SPP) approach to improving left ventricular (LV) segmentation accuracy and to evaluate its performance compared to conventional processing in a large population of 962 patients. A companion paper will investigate its application to improving the repeatability of quantification for parameters of myocardial perfusion and cardiac function.
MATERIALS AND METHODS
Patient population
Consecutive subjects who were referred to the Nuclear Medicine Department of Sacred Heart Medical Center, Eugene, Oregon, from March 1, 2003 to December 31, 2006 for single-photon emission computed tomography myocardial perfusion imaging (SPECT MPI) were selected, excluding all patients with a prior history of coronary artery disease (CAD), significant valve disease, left bundle branch block and paced rhythm. Patient characteristics are listed in Table 1. Each of the 962 patients underwent rest and stress electrocardiography-gated SPECT MPI, with the summed rest and stress images being additionally attenuation corrected (AC), resulting in 6 image datasets per patient (gated rest, gated stress, rest, stress, AC-rest, and AC-stress), for a total of 5,772 image datasets.
Table 1.
Characteristics of patients
| Characteristic | Value |
|---|---|
| Total (n) | 962 |
| Males | 486 (51%) |
| Females | 476 (49%) |
| BMI <30 kg/m2 | 551 (57%) |
| BMI ≥30 kg/m2 | 411 (43%) |
| Diabetes | 172 (18%) |
| Hypertension | 529 (55%) |
| Hypercholesteremia | 455 (47%) |
| Smoking | 195 (20%) |
| Family history of CAD | 440 (46%) |
| Typical Angina | 200 (21%) |
| Atypical angina/no angina | 762 (79%) |
| Dyspnea | 86 (9%) |
| Average | |
| Age (yrs) | 60±12.7 |
| BMI | 30±6.3 |
Image acquisition and reconstruction protocol
The details of image acquisition and tomographic reconstruction have been previously described 4. In brief, 8-frame gated acquisitions were performed by using standard 99mTc-sestamibi rest/stress protocols. All subjects were imaged at 60 min after the administration of Tc-99m sestamibi at rest, followed by stress imaging performed 15–45 min after radiopharmaceutical injection, either during treadmill exercise (543/962 or 56%) or pharmacologic stress (adenosine infusion with low-level exercise, 419/962 or 44%). Dual-detector scintillation cameras with low energy high-resolution collimators (Vertex™, Philips Medical Systems) and the Vantage Pro™ attenuation correction hardware and software, based on two gadolinium-153 scanning line sources, were used for all SPECT MPI acquisitions. Tomographic reconstruction was performed using previously described automated software 5, with the gated and summed emission images automatically corrected for non-uniformity, radioactive decay, center of rotation and motion, then reconstructed using filtered backprojection. The summed emission images were also used, together with the attenuation maps, to reconstruct the AC images using an iterative maximum likelihood expectation maximization algorithm, which in the Vantage Pro™ implementation includes scatter correction and non-stationary, depth-dependent resolution compensation.
Conventional processing
Each of the 5772 datasets was processed independently using a standard, widely available version of the Cedars-Sinai algorithm (Cedars Cardiac Suite 2008), resulting in a segmentation mask for the LV based on local activity maxima as well as on expected size/shape/location characteristics, as previously described 5–6.
For each dataset, a “shape quality control” (SQC) score was also calculated as previously described 7, as a means of objectively analyzing the appropriateness of automatic myocardial segmentation. Briefly, eight unique shape parameters related to LV orientation, volume, area, eccentricity and count intensity were identified and related to the average values of the same parameters from the correctly segmented LVs of very large patient population (8793 sequential stress gated and ungated datasets from the Cedars-Sinai database7, unrelated to the current study), with the overall SQC score being larger the more the dataset’s parameters were different from the average ones.
Each of the 5772 processed images was also assessed by an experienced operator (OP1) with respect to whether the automatically-derived contours correctly followed the LV myocardial contours. Instances in which the standard algorithm was judged to have failed were tabulated as a whole, and also broken down by type of acquisition (stress vs rest, gated vs. ungated), as well as based on whether attenuation correction had been applied.
Receiver-operating characteristic (ROC) curves were used to evaluate the SQC scores’ ability to detect LV segmentation failure as defined by the expert operator’s standard, as previously described 7.
Same-patient processing
Each independently processed dataset with a SQC score higher than a specific threshold SQCthr (as defined in the Results section), suggesting algorithmic failure, was automatically reprocessed with a version of the standard algorithm modified based on knowledge of the LV’s mask location in the other datasets belonging to the same patient. Specifically, the sub-volume (defined by the LV epicardial wall) of the dataset with the best (lowest) SQC score was used as a template to scan the problematic dataset(s), and the location of the optimal match was used as a segmentation seed point in reprocessing. If necessary, the best shape information from the best SQC LV was also applied. If all the datasets in the “independently processed” sextet had SQC scores suggesting failure, no reprocessing was performed.
The full set of 5772 automatically processed datasets (comprised of “worse SQC” images reprocessed using the modified algorithm and “better SQC” images, not reprocessed) were evaluated by a second experienced operator (OP2), blinded to the results of the first operator’s assessment and without knowledge of which images, if any, had been reprocessed. Specifically, the second operator did not know whether he was evaluating datasets processed using the standard or the modified algorithm. Again, segmentation failures were tabulated as a whole, as well as broken down by dataset type. Intra-operator reproducibility was measured by comparing the two operators’ assessment in the datasets that had not undergone reprocessing.
RESULTS
Overall, the LV was deemed by OP1 to have been incorrectly segmented in 219 of the 5772 conventionally (individually) processed datasets, or 3.8% (Table 2, left). As shown in Figure 1, the most frequent cause of segmentation failure was high extra-cardiac activity with distribution resembling that expected for a typical LV. The confounding activity was virtually always below the actual LV, in the intestinal, splenic or hepatic regions, and its effect was exacerbated by the use of attenuation correction, which usually “amplifies” counts in the LV’s inferior wall as well as adjacent regions 4,8. Indeed, the rate of failure was highest in AC-rest SPECT (where the extracardiac activity is greatest) at 10.4% (100/962), and the second highest failure rate was 4.3% (41/962) in AC-stress SPECT, with rates in non-AC gated and ungated SPECT varying from 1.1% to 3.0% (Table 2, left). Of note, with the exception of one AC-stress dataset, all failures in the conventionally processed 2886 stress datasets were associated with pharmacologic stress.
Table 2.
LV segmentation failures in the 5772 image datasets processed using (left) the conventional algorithm and (right) the “same-patient processing” approach, broken down by acquisition protocol type. With the exception of one conventionally processed AC-stress dataset, all failures in the 2886 stress datasets (whether processed conventionally or with the same-patient approach) were associated with pharmacologic stress.
| Failures (conventional processing) |
Failures (“same-patient” approach) |
|
|---|---|---|
| AC-rest | 100/962 (10.4%) | 12/962 (1.2%) |
| AC-stress | 41/962 (4.3%) | 9/962 (0.9%) |
| Rest | 26/962 (2.7%) | 6/962 (0.6%) |
| Stress | 12/962 (1.2%) | 6/962 (0.6%) |
| Gated rest | 29/962 (3.0%) | 5/962 (0.5%) |
| Gated stress | 11/962 (1.1%) | 4/962 (0.4%) |
| TOTAL | 219/5772 (3.8%) | 42/5772 (0.7%) |
Fig.1.
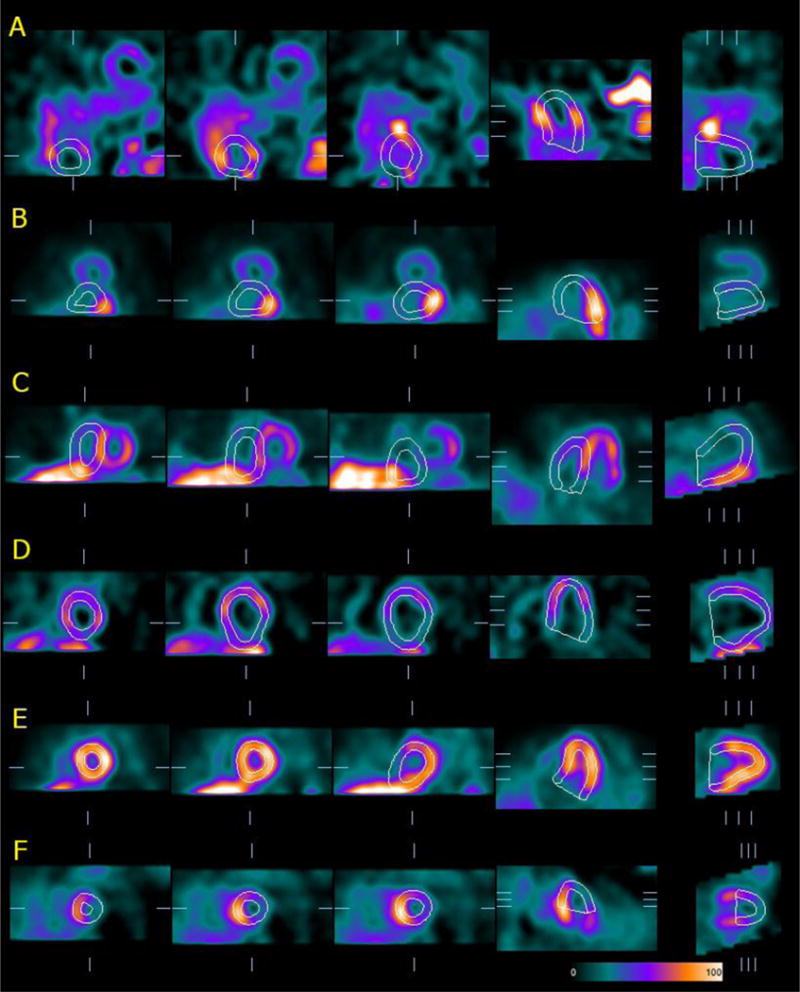
Examples of algorithmic failure in identifying the LV myocardium in reconstructed, tomographic SPECT image volumes. A) the segmentation mask chooses an area with high activity and size/shape similar to the actual LV, which in turn has much lower uptake and is located in an unusual portion of the image volume; B–C) the contours comprise the inferior wall or septum of the actual LV, but also high hepatic or intestinal uptake (frequently associated with attenuation correction); D–E) the LV is correctly identified, but extra-cardiac activity adjacent to the inferior wall “pulls” the contours, in some cases also affecting the detected valve plane location; F) incorrect valve plane detection causes “cutting-off” of the LV.
When the analysis was conducted by patient rather than by individual study (Table 3), 14.7% (141/962) of the patients had at least one dataset of the six associated with them affected by LV segmentation failure, with the overwhelming majority represented by failure in a single dataset (10.2%, 98/962) or two datasets (2.2%, 21/962).
Table 3.
LV segmentation failures in the 962 patients whose image datasets were processed using (left) the conventional algorithm and (right) the “same-patient processing” approach, broken down by how many of the six datasets associated with each patient were affected by failure.
| Failures (conventional processing) |
Failures (“same-patient” approach) |
|
|---|---|---|
| 1 dataset | 98/962 (10.2%) | 5/962 (0.5%) |
| 2 datasets | 21/962 (2.2%) | 3/962 (0.3%) |
| 3 datasets | 13/962 (1.3%) | 5/962 (0.5%) |
| 4 datasets | 7/962 (0.7%) | 1/962 (0.1%) |
| 5 datasets | 0/962 (0%) | 0/962 (0%) |
| 6 datasets (all) | 2/962 (0.2%) | 2/962 (0.2%) |
| At least 1 dataset | 141/962 (14.7%) | 16/962 (1.7%) |
The ROC curve representing the ability of the SQC score to detect LV segmentation failure in the 5772 conventionally processed datasets is shown in Figure 2. The area under the ROC curve was found to be very close to unity, specifically 0.995 (with 95% confidence interval of 0.993 to 0.996, standard error of 0.0008). Because our objective was to reprocess every dataset where segmentation had failed, we chose an SQCthr value corresponding to a sensitivity of 100% and a specificity of 98% - this resulted in 341 datasets having to be reprocessed, including the 219 in which segmentation failure had occurred.
Fig.2.
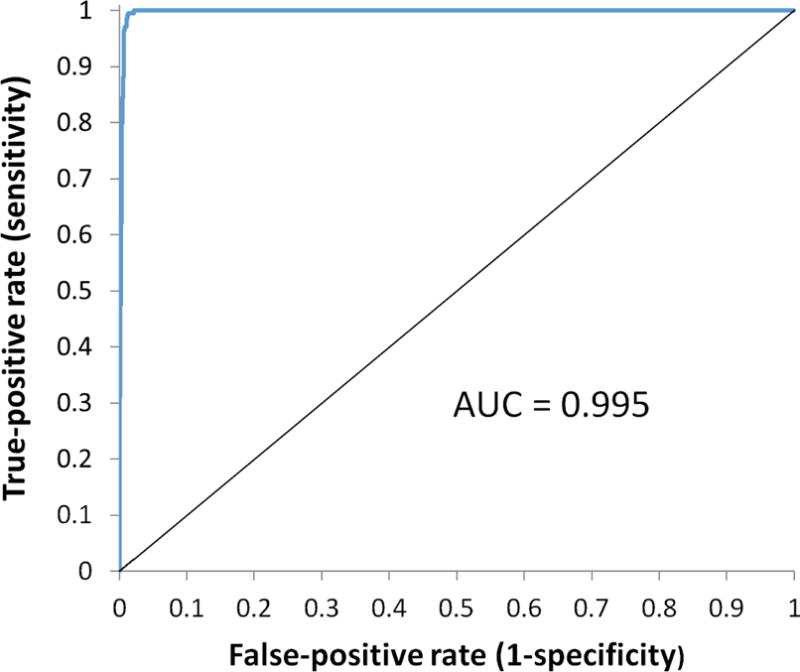
ROC curve for the detection of LV segmentation failure by the SQC score in 5772 image datasets processed with the conventional algorithm. AUC = Area under the curve.
Figure 3 shows an example of the same-patient processing approach applied to the dataset previously shown in Figure 1A (now presented as A1), in which segmentation had failed. Using as a template the dataset with the best SQC score (A3 in this case) it was possible to successfully segment the LV in A1. Overall, the LV was deemed by OP2 to have been incorrectly segmented in 42 of the 5772 datasets processed with the same-patient processing approach, or 0.7%, compared to 3.8% with conventional processing. The rate of failure was still highest in AC-rest SPECT, but substantially reduced from 10.4% to 1.2% (12/962), with other rates varying from 0.4% to 0.9% (Table 2, right). Of note, all failures in the 2886 stress datasets processed using the same-patient approach were associated with pharmacologic stress. Conducting the analysis by patient rather than by individual study (Table 3, right), only 1.7% (14/962) of the patients had at least one dataset of the six associated with them affected by LV segmentation failure, again a substantial improvement compared to the 14.7% with conventional processing. As expected, results in the two patients in which all datasets were incorrectly segmented by conventional processing could not be improved by the new approach.
Fig.3.
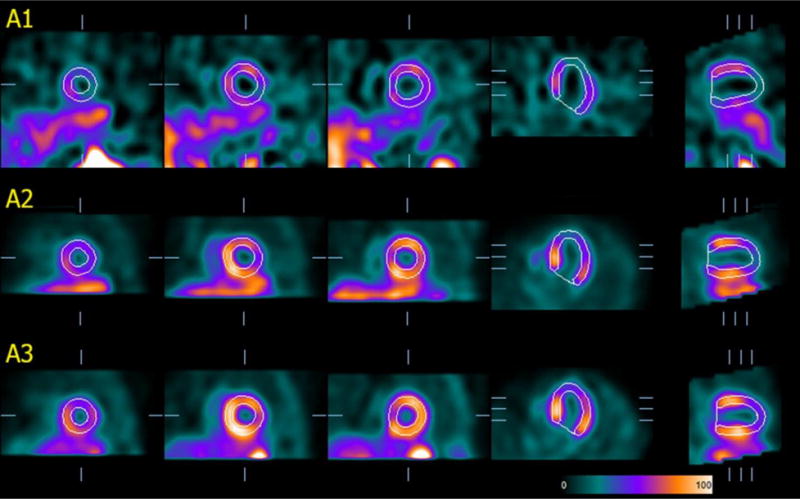
Effect of the “same-patient processing” technique applied to the patient dataset previously displayed in Fig. 1-A. The image volume in A1 is actually the same as in Fig. 1-A, but this may not be immediately apparent, since counts are normalized relative to the LV maximum and representative slices to display automatically chosen based on the contours. For simplicity of illustration, only 3 of the 6 datasets in this patient’s folder are shown (A1=stress gated, A2 and A3 = stress and rest attenuation-corrected, respectively).
Of note, the 5431 datasets that had not undergone reprocessing (based on their SQC score) were judged by both operators to have been properly segmented, with perfect inter-observer reproducibility.
DISCUSSION
While software and algorithms for the processing of cardiac SPECT and PET studies are widespread and have reached high levels of automation 9–11, their ability to correctly segment the LV is still not perfect, and LV segmentation failures do occur and need to be recognized and corrected by the technologist or physician performing or interpreting the study.
With respect to the Cedars-Sinai software approach 9, success rates for automated LV segmentation have been reported to vary from 96.3% in projection images 5 to 98.5% in transaxial SPECT images 12 and 100% in a smaller sample of reoriented gated SPECT images 13. Of note, those results were based on relatively small patient populations and/or a limited range of acquisition protocols, specifically never including AC images. A patient population consistent with the one in the current study, comprising rest and stress gated, ungated and AC images, has been previously investigated by our group in the context of quality control assessment 7, using the same conventional processing software as in the current report. In that case, it was found that major segmentation failure rates (defined as LV mask failure due to incorrect ellipsoid determination, and not including incorrect positioning of the LV’s valve plane) ranged from 3% to 8% in 318 patients who had undergone SPECT MPI 7. The current study performed a similar analysis on a substantially larger population of 962 patients, with analogous results (mask + major valve plane failure rates ranging from 1.1% to 10.4%, depending on acquisition protocol type). In both studies, it was found that attenuation-corrected images were more likely to be associated with segmentation failures.
Conventional processing is predicated on dealing with every image dataset individually, but when several datasets are available for the same patient, it makes sense to process all images together, giving higher weight to the better quality ones. This, in essence, is the foundation of the “same-patient processing” approach. In our current analysis, focused on LV segmentation, since failures were more frequent in the AC datasets, results from the non-AC images were used to “guide” the segmentation process in all. The novel aspect of the approach is that a deterministic assessment of the “best quality” dataset in each patient is performed automatically, based on a previously described and validated algorithm; thus, no a priori assumption of which class of images is more reliable is needed, and only datasets failing objective quality control criteria are reprocessed. Although not specifically tested in this investigation, it is presumable that the same-patient processing technique would not be limited to AC images, but would also apply to prone images, low-count images, or images having undergone a wide range of compensations or corrections, as long as at least one “good quality” dataset exists for that patient. Of course, no improvement in LV segmentation success rates should be expected in cases where conventional processing has failed in all datasets, as was the case for two patients in our study – in that event, no reprocessing would occur, and the SQC score-generating application would issue a quality control alert.
Since the “same-patient processing” concept represents a general approach, its implementation (and the tools employed to apply it) can be tailored to the specific goals to be achieved, or type of images available. For example, it would be possible to reconstruct different projection image datasets belonging to a same patient as a common set, rather than individually - to some extent, this is already done for gated and ungated datasets, particularly when the latter are directly derived from the former through summation. However, protocol-specific compensations and corrections increasingly associated with modern nuclear cardiology imaging are commonly implemented during reconstruction (which in turn has led to the popularity of iterative reconstruction techniques), and as a result image differences leading to inconsistent segmentation performance are more likely found in the reconstructed datasets. This is why we decided to focus our analysis on three-dimensional, tomographic image volumes, considering also that projection datasets may not be available for some or most acquisitions, particularly if studies were acquired at different locations or at different times.
CONCLUSION
Improving LV segmentation success rates through the same-patient processing approach is feasible, can be performed in automated fashion through incorporation in the processing algorithm, and represents a relatively straightforward task to evaluate, as “success” is a binary variable that can be readily and reproducibly assessed by experienced operators. Our next objective, utilizing grouped processing of image datasets belonging to a same patient to improve the repeatability of image quantification, will require a different algorithm, and will be the subject of a separate paper.
NEW KNOWLEDGE GAINED.
The concept that processing different image datasets of a same patient as a group can result in better LV segmentation was described and tested in a large patient population (962 patients, 5,772 datasets). We learned that this “same-patient processing” approach did reduce segmentation failure from 3.8% to 0.7% overall, with particular improvements in attenuation-corrected images. This new knowledge can easily be incorporated in existing automated image processing algorithms.
Acknowledgments
This research was supported in part by Grant R01HL089765 from the National Heart, Lung, and Blood Institute/National Institutes of Health (NHLBI/NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NHLBI/NIH.
ABBREVIATIONS
- SPP
Same-patient processing
- SPECT
Single photon emission computed tomography
- MPI
Myocardial perfusion imaging
- LV
Left ventricle
- AC
Attenuation corrected
- SQC
Shape quality control
References
- 1.Slomka PJ, Nishina H, Berman DS, Kang XP, Friedman JD, Hayes SW, et al. Automatic quantification of myocardial perfusion stress-rest change: A new measure of ischemia. Journal of Nuclear Medicine. 2004;45:183–191. [PubMed] [Google Scholar]
- 2.Slomka PJ, Pan T, Berman DS, Germano G. Advances in SPECT and PET Hardware. Progress in Cardiovascular Diseases. 2015;57:566–578. doi: 10.1016/j.pcad.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Einstein AJ, Pascual TNB, Mercuri M, Karthikeyan G, Vitola JV, Mahmarian JJ, et al. Current worldwide nuclear cardiology practices and radiation exposure: results from the 65 country IAEA Nuclear Cardiology Protocols Cross-Sectional Study (INCAPS) European Heart Journal. 2015;36:1689–1696. doi: 10.1093/eurheartj/ehv117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slomka PJ, Fish MB, Lorenzo S, Nishina H, Gerlach J, Berman DS, et al. Simplified normal limits and automated quantitative assessment for attenuation-corrected myocardial perfusion SPECT. J Nucl Cardiol. 2006;13:642–651. doi: 10.1016/j.nuclcard.2006.06.131. [DOI] [PubMed] [Google Scholar]
- 5.Germano G, Kavanagh PB, Chen J, Waechter P, Su HT, Kiat H, et al. Operator-less processing of myocardial perfusion SPECT studies. J Nucl Med. 1995;36:2127–2132. [PubMed] [Google Scholar]
- 6.Germano G, Kavanagh PB, Waechter P, Areeda J, Van Kriekinge S, Sharir T, et al. A new algorithm for the quantitation of myocardial perfusion SPECT. I: technical principles and reproducibility. Journal of Nuclear Medicine. 2000;41:712–719. [PubMed] [Google Scholar]
- 7.Xu Y, Kavanagh P, Fish M, Gerlach J, Ramesh A, Lemley M, et al. Automated Quality Control for Segmentation of Myocardial Perfusion SPECT. Journal of Nuclear Medicine. 2009;50:1418–1426. doi: 10.2967/jnumed.108.061333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arsanjani R, Xu Y, Hayes SW, Fish M, Lemley M, Gerlach J, et al. Comparison of Fully Automated Computer Analysis and Visual Scoring for Detection of Coronary Artery Disease from Myocardial Perfusion SPECT in a Large Population. Journal of Nuclear Medicine. 2013;54:221–228. doi: 10.2967/jnumed.112.108969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Germano G, Kavanagh PB, Slomka PJ, Van Kriekinge SD, Pollard G, Berman DS. Quantitation in gated perfusion SPECT imaging: The Cedars-Sinai approach. J Nucl Cardiol. 2007;14:433–454. doi: 10.1016/j.nuclcard.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 10.Garcia EV, Faber TL, Cooke CD, Folks RD, Chen J, Santana C. The increasing role of quantification in clinical nuclear cardiology: The Emory approach. J Nucl Cardiol. 2007;14:420–432. doi: 10.1016/j.nuclcard.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 11.Ficaro EP, Lee BC, Kritzman JN, Corbett JR. Corridor4DM: The Michigan method for quantitative nuclear cardiology. J Nucl Cardiol. 2007;14:455–465. doi: 10.1016/j.nuclcard.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Germano G, Kavanagh PB, Su HT, Mazzanti M, Kiat H, Hachamovitch R, et al. Automatic reorientation of three-dimensional, transaxial myocardial perfusion SPECT images. J Nucl Med. 1995;36:1107–1114. [PubMed] [Google Scholar]
- 13.Germano G, Kiat H, Kavanagh PB, Moriel M, Mazzanti M, Su HT, et al. Automatic quantification of ejection fraction from gated myocardial perfusion SPECT. J Nucl Med. 1995;36:2138–2147. [PubMed] [Google Scholar]


