Abstract
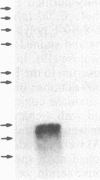
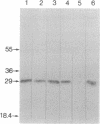
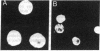
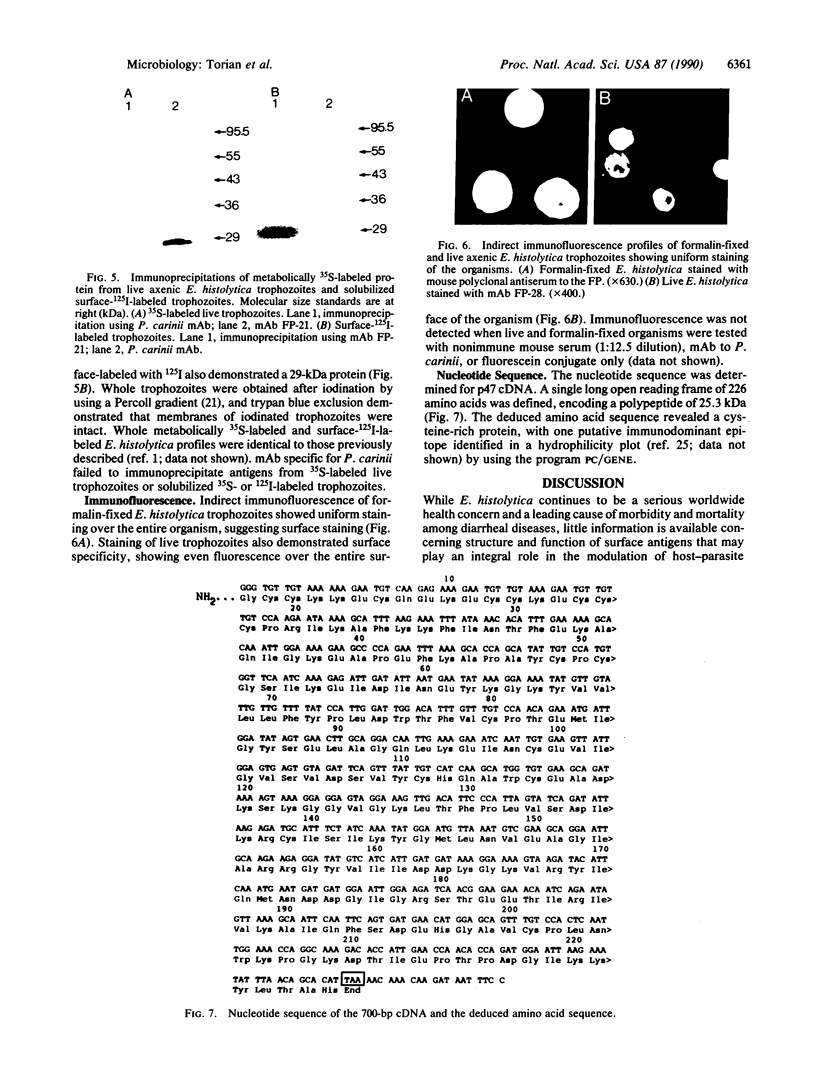
A gamma gt11 cDNA library was constructed from poly(U)-Sepharose-selected Entamoeba histolytica trophozoite RNA in order to clone and identify surface antigens. The library was screened with rabbit polyclonal anti-E. histolytica serum. A 700-base-pair cDNA insert was isolated and the nucleotide sequence was determined. The deduced amino acid sequence of the cDNA revealed a cysteine-rich protein. DNA hybridizations showed that the gene was specific to E. histolytica since the cDNA probe reacted with DNA from four axenic strains of E. histolytica but did not react with DNA from Entamoeba invadens, Acanthamoeba castellanii, or Trichomonas vaginalis. The insert was subcloned into the expression vector pGEX-1 and the protein was expressed as a fusion with the C terminus of glutathione S-transferase. Purified fusion protein was used to generate 22 monoclonal antibodies (mAbs) and a mouse polyclonal antiserum specific for the E. histolytica portion of the fusion protein. A 29-kDa protein was identified as a surface antigen when mAbs were used to immunoprecipitate the antigen from metabolically 35S-labeled live trophozoites. The surface location of the antigen was corroborated by mAb immunoprecipitation of a 29-kDa protein from surface-125I-labeled whole trophozoites as well as by the reaction of mAbs with live trophozoites in an indirect immunofluorescence assay performed at 4 degrees C. Immunoblotting with mAbs demonstrated that the antigen was present on four axenic isolates tested. mAbs recognized epitopes on the 29-kDa native antigen on some but not all clinical isolates tested.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg J. M. Potential metal-binding domains in nucleic acid binding proteins. Science. 1986 Apr 25;232(4749):485–487. doi: 10.1126/science.2421409. [DOI] [PubMed] [Google Scholar]
- Calderón J., Avila E. E. Antibody-induced caps in Entamoeba histolytica: isolation and electrophoretic analysis. J Infect Dis. 1986 May;153(5):927–932. doi: 10.1093/infdis/153.5.927. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Degen J. L., Neubauer M. G., Degen S. J., Seyfried C. E., Morris D. R. Regulation of protein synthesis in mitogen-activated bovine lymphocytes. Analysis of actin-specific and total mRNA accumulation and utilization. J Biol Chem. 1983 Oct 25;258(20):12153–12162. [PubMed] [Google Scholar]
- Diamond L. S., Harlow D. R., Cunnick C. C. A new medium for the axenic cultivation of Entamoeba histolytica and other Entamoeba. Trans R Soc Trop Med Hyg. 1978;72(4):431–432. doi: 10.1016/0035-9203(78)90144-x. [DOI] [PubMed] [Google Scholar]
- Edman U., Meza I., Agabian N. Genomic and cDNA actin sequences from a virulent strain of Entamoeba histolytica. Proc Natl Acad Sci U S A. 1987 May;84(9):3024–3028. doi: 10.1073/pnas.84.9.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elleman T. C. The amino acid sequence of protein SCMK-B2A from the high-sulphur fraction of wool keratin. Biochem J. 1972 Dec;130(3):833–845. doi: 10.1042/bj1300833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freyd G., Kim S. K., Horvitz H. R. Novel cysteine-rich motif and homeodomain in the product of the Caenorhabditis elegans cell lineage gene lin-11. Nature. 1990 Apr 26;344(6269):876–879. doi: 10.1038/344876a0. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hagen F. S., Gray C. L., Kuijper J. L. Assaying the quality of cDNA libraries. Biotechniques. 1988 Apr;6(4):340–345. [PubMed] [Google Scholar]
- Helfman D. M., Feramisco J. R., Fiddes J. C., Thomas G. P., Hughes S. H. Identification of clones that encode chicken tropomyosin by direct immunological screening of a cDNA expression library. Proc Natl Acad Sci U S A. 1983 Jan;80(1):31–35. doi: 10.1073/pnas.80.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafatos F. C., Jones C. W., Efstratiadis A. Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acids Res. 1979 Nov 24;7(6):1541–1552. doi: 10.1093/nar/7.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson O., Thor S., Norberg T., Ohlsson H., Edlund T. Insulin gene enhancer binding protein Isl-1 is a member of a novel class of proteins containing both a homeo- and a Cys-His domain. Nature. 1990 Apr 26;344(6269):879–882. doi: 10.1038/344879a0. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lostrom M. E., Stone M. R., Tam M., Burnette W. N., Pinter A., Nowinski R. C. Monoclonal antibodies against murine leukemia viruses: identification of six antigenic determinants on the p 15(E) and gp70 envelope proteins. Virology. 1979 Oct 30;98(2):336–350. doi: 10.1016/0042-6822(79)90557-9. [DOI] [PubMed] [Google Scholar]
- Rasschaert D., Laude H. The predicted primary structure of the peplomer protein E2 of the porcine coronavirus transmissible gastroenteritis virus. J Gen Virol. 1987 Jul;68(Pt 7):1883–1890. doi: 10.1099/0022-1317-68-7-1883. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Torian B. E., Connelly R. J., Stephens R. S., Stibbs H. H. Specific and common antigens of Trichomonas vaginalis detected by monoclonal antibodies. Infect Immun. 1984 Jan;43(1):270–275. doi: 10.1128/iai.43.1.270-275.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torian B. E., Lukehart S. A., Stamm W. E. Use of monoclonal antibodies to identify, characterize, and purify a 96,000-dalton surface antigen of pathogenic Entamoeba histolytica. J Infect Dis. 1987 Aug;156(2):334–343. doi: 10.1093/infdis/156.2.334. [DOI] [PubMed] [Google Scholar]
- Torian B. E., Reed S. L., Flores B. M., Creely C. M., Coward J. E., Vial K., Stamm W. E. The 96-kilodalton antigen as an integral membrane protein in pathogenic Entamoeba histolytica: potential differences in pathogenic and nonpathogenic isolates. Infect Immun. 1990 Mar;58(3):753–760. doi: 10.1128/iai.58.3.753-760.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torian B. E., Reed S. L., Flores B. M., Plorde J., Stamm W. E. Serologic response to the 96,000-Da surface antigen of pathogenic Entamoeba histolytica. J Infect Dis. 1989 Apr;159(4):794–797. doi: 10.1093/infdis/159.4.794. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschachler E., Buchow H., Gallo R. C., Reitz M. S., Jr Functional contribution of cysteine residues to the human immunodeficiency virus type 1 envelope. J Virol. 1990 May;64(5):2250–2259. doi: 10.1128/jvi.64.5.2250-2259.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]