Abstract
In 2015, 232,670 women will be diagnosed with breast cancer and most will undergo surgery. Postoperative nausea and vomiting (PONV) and post discharge nausea and vomiting (PDNV) continue to be common and disturbing complications experienced after surgery, particularly in women and especially in women undergoing breast cancer surgery. The purpose of this study was to assess the incidence and risk factors associated with PONV and PDNV from pre-operative through 48 hours postoperatively in 97 women scheduled for breast cancer surgery. Twenty-nine (29.8%) women experienced nausea and nine (9%) experienced nausea and vomiting while in the PACU despite close attention to the need for prophylactic antiemetic medications. Women who experienced PONV had higher levels of pain and received more opioids than those women who did not experienced PONV. Women who received IV acetaminophen did not experience less PONV in this study. PDNV occurred more frequently than PONV, with 34 women (35%) reporting occurrence after discharge. 13 women who did not experience PONV while in the PACU subsequently experienced PDNV after leaving the hospital, evidence for the importance of patient discharge teaching regarding these symptoms. While clinical guidelines are necessary, our observation is that nurses in the PACU setting continuously challenge themselves to individualize the combination of medications and activities for each patient to reduce PONV following surgery.
Postoperative nausea and vomiting (PONV) continues to be one of the most common and disturbing complications patients experience after surgery, particularly for women undergoing cancer surgery.1-3 The incidence of PONV following breast cancer surgery has been reported to be as high as 80%.4-6 Since 232,670 women are expected to be diagnosed with breast cancer in 2015 and most will undergo surgery as part of their curative treatment, PONV in this population is a highly significant clinical problem.7 PONV has a profound impact on the health and well-being of women with breast cancer and is related to significant morbidity (dehydration, wound dehiscence, pain, immobility),8 delayed discharge from the post anesthesia care unit (PACU), increased length of hospital stay, increased hospital costs,9-11 and poor patient satisfaction.12 PONV is also the most common reason for unplanned overnight stays following breast cancer surgery.9 In addition, with the emergence of same-day surgery as the standard for most breast cancer resections, these women also experience post-discharge nausea and vomiting (PDNV) at home without nursing support.13,14 Patients have reported that they experience their highest level of nausea on the day of discharge following ambulatory surgery.15
American Society of Clinical Oncology current guidelines indicate that the goal for treatment-induced nausea and vomiting should be complete control.16 However, this goal has remained elusive. Recent research has shown that nearly a third of all women continue to experience PONV and PDNV following surgery for breast cancer, despite adherence to appropriate antiemetic guidelines.17 Well-established risk factors have been identified that increase the risk of PONV and PDNV. They include the use of opioids for postoperative pain,18 female gender, a negative smoking history and previous history of PONV or motion sickness.19-22 In addition, some studies have found preoperative psychological factors, including anxiety and distress, to increase the severity of PONV for women with breast cancer.23
Purpose
The purpose of this prospective study was to assess the incidence and risk factors associated with PONV and PDNV from pre-operative through 48 hours postoperatively in women scheduled for breast cancer surgery.
Methods
Design
This study, which was approved by the University of Pittsburgh Institutional Review Board, employed a prospective comparative design to compare women with breast cancer who experienced nausea and vomiting following surgery with women with breast cancer who did not. Women who were diagnosed with breast cancer and were scheduled for surgery were evaluated for incidence of vomiting, as well as the presence and severity of nausea.
To be eligible for the study, women were diagnosed with early stage breast cancer (Stage I. II and IIIA) and scheduled for surgical resection of their cancer under general anesthesia, or combined general and regional anesthesia Only women with an American Society of Anesthesiologists (ASA) physical status of I, II or III were included, and because the study design included follow-up telephone calls after discharge, they also had to have access to a telephone. Exclusion criteria included having breast reconstruction surgery (due to length of anesthesia time), or any previous history of neurologic conditions such as stroke, head injury, spinal cord injury and intracerebral hemorrhage that could also be the cause of nausea. Given the planned objectives of the study, a sample of 100 was estimated to yield parameter estimation with margin of error (in terms of the half-width of the confidence interval) of at most 0.1015 for the proportions (or 10.15 for percentages) (conservatively assuming a base proportion (or percentage) of .50 (or 50%) and 0.198σ for means (the population standard deviation for the continuous type variable of interest). Additionally when exploring for risk factors of PONV and PDNV and assuming rates of occurrence of 25% for PONV and PDNV, odds ratios as small as 3.242 for categorical risk factors and 1.910 for continuous risk factors may be detected with 80% power at a level of significance set at .05 for two-sided hypothesis testing when using univariate binary logistic regression.
After receiving Institutional Review Board approval, recruitment and initial data collection took place in the preoperative holding area of a large women's hospital which houses a Comprehensive Breast Care Program. The study team conferred with the attending surgeon and or members of the surgical team each morning to review the operating room (OR) schedule and determine if patients who met the study criteria were scheduled for surgery. Potential participants were approached by a member of the clinical team to assess interest in the study. The PI provided patients, who indicated interest, a description of the study and if they agreed to participate, informed consent was obtained.
Study Variables and Data Collection
Preoperative data collection
Individual characteristics, including age, race, smoking history, history of PONV and motion sickness, were collected from the patient in the preoperative holding area after informed consent was obtained. Preoperative distress was measured using the tension-anxiety subscale of the short form Profile of Mood States (POMS SF).24,25 The tension-anxiety subscale has six items (mood words), which are rated on a Likert-type scale ranging from 0 (not at all) to 4 (extremely). The score is the sum of the responses for the six items. This scale has demonstrated validity and reliability in women with breast cancer,26 with excellent internal consistency (Cronbach's alpha=0.91).23
Postoperative data collection
The outcome variables for this study were PONV and PDVN, or more specifically postoperative and post discharge vomiting, as well as postoperative and post discharge nausea. Vomiting was assessed both as a nominally scaled, binary variable (Yes/No) and as a continuous variable to measure the number of separate emetic events. Nausea was measured on a verbal numeric, 11-point numeric nausea scale (NNS), with 0 being the absence of nausea and 10 being the highest level of nausea ever experienced. PONV data were collected continuously until completion of both phases of recovery. In our previous work we found that patients in the PACU were not able to record their nausea on a visual analog scale, but were able to respond verbally to the NNS. Cork27 reported equivalence in reliability and validity when comparing a verbal response to a Visual Analog Scale.
Pain was measured on an analogous verbal numeric, 11-point pain scale. The amount and type of antiemetic and/or pain medications administered were abstracted from patient records. In addition the type of surgery, surgical time, anesthetic agents and medications administered in the OR were collected.
48 hours after surgery
Participants were telephoned at home to assess for reoccurring or new PDNV 48 hours after surgery, using the same measures as employed for the postoperative evaluation. Subjects who were still hospitalized were assessed in person. Discharged patients were asked to keep track of the amount and type of antiemetic and pain medications taken, and those data were collected via the phone call in addition to the assessment of PDNV and pain.
Data analysis
Data were analyzed using IBM® SPSS® Statistics 23.0 (IBM Corporation, Armonk, NY). Given of the level of measurement of the descriptor and its observed data distribution, appropriate descriptive statistics were computed to describe the total sample and groups based on PONV and PDNV status. In particular, the percentage of women having experienced PONV and PDNV was estimated and reported with 95% confidence intervals. In addition, univariate binary logistic regression was used to identify risk factors for PONV and PDNV and to compute crude (unadjusted) odds ratios (ORs) with 95% confidence intervals. The level of statistical significance for two-sided hypothesis testing was set at .05.
Findings
In total the sample consisted of 97 women with early stage breast cancer who were recruited prior to surgery. The sample characteristics are presented in Tables 1 and 2. Eighty-eight women (92%) were white, 84 (88%) were non-smokers and 48 (50%) had a positive history of PONV. The type of surgery for the majority of women was a modified partial or segmental mastectomy (55%), with 21 women (22%) having full mastectomies. All patients were administered ondansetron and all but five were also administered dexamethasone while still in the operating room as a prophylaxis for PONV. In addition, 13 patients (14%) had a scopolamine patch placed, and seven patients were administered an oral substance-p antagonist prior to the operating room while still in the preoperative holding area.
Table 1. Patient Characteristics According to Postoperative Nausea and Vomiting (PONV) Status.
| Variable | No PONV (n= 68) Mean ± SD or Number (%) | PONV (n= 29) Mean ± SD or Number (%) | Odds Ratio | 95% Confidence Intervals |
|---|---|---|---|---|
| Age (years) | 58.8 ± 9.5 | 57.8 ± 8.4 | 0.988 | 0.94 - 1.03 |
| Race | 0.369 | .042 - 3.21 | ||
| White | 61 (89.7%) | 27 (93.1%) | ||
| African American | 6 (8.8%) | 1 (3.4%) | ||
| Non-smokers | 58 (85.3%) | 26 (89.7%) | 0.622 | 0.17 – 2.82 |
| History of PONV* | 25 (36.8%) | 23 (79.3%) | 7.028 | 2.51 – 19.62 |
| Anxiety (POMS) | 9.8 ± 5.9 | 11.2 ± 4.8 | 1.047 | 0.97 – 1.13 |
| Pain in PACU* | 3.34 ± 3.05 | 5.31 ± 2.69 | 1.258 | 1.07 – 1.48 |
| Total Opioids in PACU (MME)* | 4.9 ± 6.0 | 8.9 ± 6.1 | 1.131 | 1.04 – 1.28 |
| Scopalamine Patch | 7 (10.3%) | 6 (20.7%) | 0.177 | 0.69 – 7.48 |
| IV Tylenol Given | 29 (42.6%) | 12 (41.4%) | 0.949 | 0.39 – 2.29 |
P<0.01
Table 2.
Comparison of patient characteristics based on presence of PDNV.
| Variable | No PDNV (n= 63) Mean ± SD or Number (%) | PDNV (n= 34) Mean ± SD or Number (%) | Odds Ratio | 95% Confidence Intervals |
|---|---|---|---|---|
| Age (years) | 58.16±9.3 | 59.0±8.8 | 1.01 | 0.96 -1.06 |
| Race | ||||
| White | 56 (89%) | 34 (100%) | ||
| African American | 7 (11%) | 0 (0%) | ||
| Non-smokers | 58 (85.3%) | 26 (89.7%) | 0.897 | 0.25 - 3.34 |
| History of PONV* | 25 (36.8%) | 23 (79.3%) | 3.035 | 1.27 – 7.21 |
| Anxiety (POMS) | 9.8±5.9 | 10.8±4.8 | 1.030 | 0.96 – 1.10 |
| Pain at 48 hours postoperative (VPS)* | 3.34±3.05 | 5.31±2.69 | 1.301 | 1.03 – 1.64 |
| Total Opioids in 48 hours (MME)* | 7.09±7.9 | 12.53±13.5 | 1.052 | 1.00 – 1.10 |
| Scopalamine Patch | 7 (10.3%) | 6 (20.7%) | 1.626 | 0.50 – 5.29 |
| IV Tylenol Given | 28 (45%) | 13 (37.4% | 0.718 | 0.31 – 1.67 |
p<.03
PONV
Twenty-nine (29.8%) women experienced nausea and nine (9%) experienced nausea and vomiting while in the PACU. The mean nausea intensity score for those women who reported nausea in the PACU was 6.34 ± 2.65, with a range from 2 to 10. The study sample characteristics based on PONV status are presented in Table 1. Using two-sample t-tests, we compared women who experienced PONV with those who did not, and found that women with PONV had significantly higher verbal pain scores (p=.003) and higher received a greater amount of total amount opioids (p=.001) than those women who did not experienced PONV. Using the chi-square test of independence a history of previous PONV was found to be significantly more common (p=.001) in the group with PONV compared to those with no PONV; smoking levels were similar between groups based on PONV status. Additionally, a trend was found for women with PONV reporting higher anxiety scores as measured by the POMS-SF (p=.08).
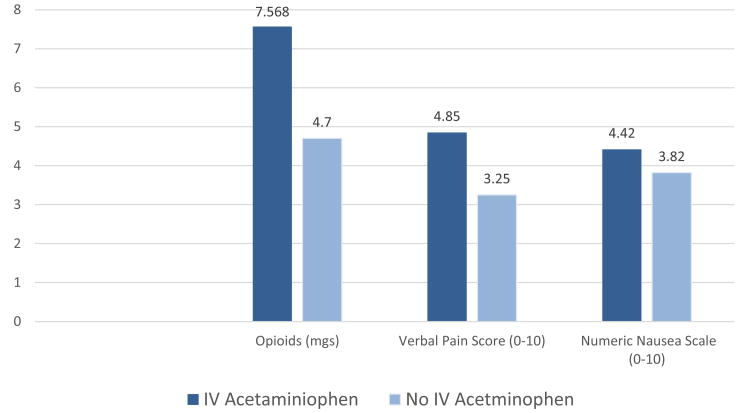
Forty-one women (40%) in this study received IV acetaminophen (Figure 1). There was no significant difference in the incidence of PONV between women who received IV acetaminophen and those who did not receive IV acetaminophen. The average pain score for the women who received IV acetaminophen was significantly higher (p=.01), as was the amount of opioids received in the PACU (p=.022).
Figure 1.
Comparison of verbal post anesthesia care unit (PACU) pain scores, verbal nausea scores for those women who experienced nausea, and amount of opioids in morphine equivalents, given in the PACU for those women who received IV acetaminophen and those who did not following their surgery for breast cancer.
PDNV
Findings for PDNV were slightly different than for PONV (Table 2). Thirty-four women (35.1%, 95%CI=[25.56%, 44.55%]) reported having experienced nausea, while eight (8.2%, 95%CI= [2.77%, 13.72%]) experienced vomiting in the 48 hours postoperatively. There were 13 (13%) women who did not experience PONV but who reported nausea and/or vomiting after discharge, and eight (8%) women who experienced PONV that did not report PDNV. The mean numeric nausea score 48 hours after discharge was 5.13 ± 2.92. Pain and amount of opioids taken were significantly higher in the group with PDNV; no other characteristics were significantly different. Though a very small number, of the seven (7%) women self-identifying as African American, no one experienced PDNV and only one African American subject experienced postoperative nausea.
Discussion
The primary goal for this prospective study was to describe the variability of nausea and vomiting from pre-surgery through 48 hours post-discharge from the PACU, in women undergoing surgery for early stage breast cancer. Consistent with most PONV studies, we found a history of PONV to be a highly significant predictor of PONV.28 Smoking is also a well-established risk factor for PONV 29, but was not significantly different between women with and without PONV in this study, perhaps due to the low number of smokers in the sample. There were only 12 current smokers in this sample, and though 8 of the 12 did not experience nausea or vomiting, the numbers were not high enough to influence the results.
It is not surprising that pain scores, and thus the increased need for postoperative opioids were also significantly higher in the women who experienced PONV; both are established risk factors that have been well-documented. 3,28,30 Also consistent with previous research is the fact that this study found that nausea was more prevalent than vomiting.31
The addition of pre-operative antiemetic medication as recommended for high risk patients,32 either an oral neurokinin-1 or a scopolamine patch were effective half of the time that they were used. Certainly the clinical guidelines32 suggest we would find that the addition of another antiemetic to the standing order of ondansetron/dexamethasone in high risk patients should decrease the risk for PONV.28 Our findings regarding the 41 women who received IV acetaminophen group after their surgery are not consistent with other studies,33 as IV acetaminophen has been reported to decrease postoperative pain and postoperative opioids, and then consequently
PONV
The trend (p=0.08) toward women with PONV to report higher levels of preoperative anxiety is consistent with the findings of Montgomery and colleagues.34 In that study however, anxiety was assessed at the physician's office prior to surgery, which may be a more predictive time than immediately prior to surgery. It will be of interest in future research to explore the possibility that anxiety levels on the day of surgery may be less of a problem because at that time clinical decisions are already made, and the women have had a chance to live with the diagnosis for some time.
More women experienced PDNV than PONV, though the mean score for the severity of nausea was higher while women were in the hospital. As for women with PONV, opioids taken over 48 hours and average post discharge pain scores were significantly higher for the women with PDNV than for the women without PDNV. Our finding that 13 women who did not have PONV while in the PACU subsequently experienced PDNV after leaving the hospital underscores the importance of patient discharge teaching regarding these symptoms. One situation that was described multiple times by patients during the follow up phone conversation was the fact that prior to discharge, women received an oral opioid for pain, then got in the car to drive home, basically on an empty stomach and in one patient's words “threw-up the whole way home.” Also interesting was the number of women who did not report PDNV at the time of the 48-hour postoperative phone call. Yet, when questioned about pain, eleven women reported that they did not fill their pain medication prescriptions after discharge due to previous postoperative experience of nausea, choosing in one patient's words, “to deal with the pain instead of having to deal with that awful nausea.” This preference was not uncommon, with 13 women who did not experience PDNV reporting that they would choose pain over nausea. This information was not solicited with a question, but was a fairly frequent response during the follow up phone call when patients were asked about opioids they might have taken for pain.
Race has not been found in most studies to be a significant predictor of nausea and vomiting.28 However, our finding that none of the women of African-American descent in our study experienced nausea or vomiting at home is consistent with two published papers.35,36 One report found that white children had more side effects from smaller amount of opioids than their African-American counterparts, while another report from South Africa suggested the need for a modified risk score due to decreased frequency of PONV in Black African patients.35,36 Certainly more research is needed to understand racial and ethnic differences in these symptoms.
Limitations
We conducted this study in a single center, and practices in regard to administration of pre and postoperative medications may have influenced the occurrence of PONV and PDNV. In addition, because of the small number of subjects who were not Caucasian our findings cannot be generalized to other racial/ethnic groups. A larger study is planned to increase the ethnic and racial diversity of subjects included to better describe the experience of PONV and PDNV in all women following breast cancer surgery.
Conclusion
Though PONV and PDNV are recognized as continuing to be problems in need of clinical management, our ability to prevent these troublesome symptoms, especially nausea appears to be limited. A recent article noted that “we have currently reached the point of knowing a lot, but doing too little for our patients and that implementation of evidence- based guidelines should be the major goal regarding PONV for the next decade”.37 While clinical guidelines may be helpful, our observation is that nurses in the PACU setting are already continuously challenging themselves to individualize the combination of medications and activities for each patient to reduce PONV following surgery for breast cancer. Future research to better control PONV and PDNV should be directed toward new prevention and treatment strategies grounded in a better understanding of the underlying biology of nausea and vomiting in the surgical setting.
Acknowledgments
This study was funded by the Oncology Nursing Society Foundation and the NINR T32 Training Grant: NR011972A –Training of Nurse Scientists in Cancer Survivor Research
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Apfel C, Kranke P, Eberhart L, Roos A, Roewer N. Comparison of predictor models for postoperative nausea and vomiting. Br J Anaesth. 2002;88:234–240. doi: 10.1093/bja/88.2.234. [DOI] [PubMed] [Google Scholar]
- 2.Ruiz JR, Kee SS, Frenzel JC, et al. The Effect of an anatomically classified procedure on antiemetic administration in the postanesthesia care unit. Anesth Analg. 2010;110(2):403–409. doi: 10.1213/ane.0b013e3181a9d076. [DOI] [PubMed] [Google Scholar]
- 3.Le TP, Gan TJ. Update on the management of postoperative nausea and vomiting and postdischarge nausea and vomiting in ambulatory surgery. Anesth Clin. 2010;28(2):225–249. doi: 10.1016/j.anclin.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Gan TJ, Meyer T, Apfel C, et al. Society for Ambulatory Anesthesia guidelines for the management of PONV. Ambulatory Anesthesia. 2007;105(6):1615–1627. doi: 10.1213/01.ane.0000295230.55439.f4. [DOI] [PubMed] [Google Scholar]
- 5.Fujii Y. Prophylaxis of postoperative nausea and vomiting in patients scheduled for breast surgery. Clin Drug Investigation. 2006;26(8):427–437. doi: 10.2165/00044011-200626080-00001. [DOI] [PubMed] [Google Scholar]
- 6.Bakshi SG, Jibhkate B, Sareen R, Badwe R. Nausea and vomiting after breast cancer surgery, and relationship with tumor receptor status. J of Anesthesia. 2012;26(2):187–195. doi: 10.1007/s00540-011-1274-5. [DOI] [PubMed] [Google Scholar]
- 7.American Cancer Society. Breast Cancer Facts and Figures. 2015 http://www.cancer.org/Cancer/BreastCancer/DetailedGuide/breast-cancer-key-statistics.
- 8.Murphy MJ, Hooper VD, Sullivan E, Clifford T, Apfel CC. Identification of risk factors for postoperative nausea and vomiting in the perianesthesia adult patient. Journal of Perianesthesia Nursing. 2006;21(6):377–384. doi: 10.1016/j.jopan.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Marla S, Stallard S. Systematic review of day surgery for breast cancer. Int J of Surgery. 2009;7(4):318–323. doi: 10.1016/j.ijsu.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 10.Habib AS, Chen YT, Taguchi A, Hu XH, Gan TJ. Postoperative nausea and vomiting following surgeries in a teaching hospital: A retrospective database analysis. Curr Med Res Opin. 2006;22(6):1093–1099. doi: 10.1185/030079906X104830. [DOI] [PubMed] [Google Scholar]
- 11.Mace L. An audit of post-operative nausea and vomiting, following cardiac surgery: scope of the problem. Nursing in Critical Care. 2003;5(8):187–196. doi: 10.1046/j.1362-1017.2003.00029.x. [DOI] [PubMed] [Google Scholar]
- 12.Silva A, O'Ryan F, Poor D. Postoperative nausea and vomiting (PONV) after orthognathic surgery: A retrospective study and literature review. Journal Oral and Maxillofacial Surgery. 2006;64(9):1385–1397. doi: 10.1016/j.joms.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 13.Wesmiller SW, Henker RA, Sereika SM, et al. The association of CYP2D6 genotype and postoperative nausea and vomiting in orthopedic trauma patients. Biological Research Nursing. 2013;15(4):382–389. doi: 10.1177/1099800412449181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wesmiller S, Bender C, Sereika, et al. The association of the serotonin transport gene and post discharge nausea and vomiting in women following breast cancer surgery. Oncology Nursing Forum. 2013 doi: 10.1188/14.ONF.195-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Odom-Forren J, Jalota L, Moser DK, et al. Incidence and predictors of postdischarge nausea and vomiting in a 7-day population. Journal Clinical Anesthesia. 2013;25(7):551–559. doi: 10.1016/j.jclinane.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 16.Basch E, Prestrud AA, Hesketh PJ, et al. Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J of Clinical Oncology. 2011;29(31):4189–4198. doi: 10.1200/JCO.2010.34.4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Odom-Forren JHV, Moser D, Hall L, et al. Postdischarge Nausea and Vomiting: Management Strategies and Outcomes over 7 Days. J Perianesth Nurs. 2014;29(4):275–284. doi: 10.1016/j.jopan.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 18.Watcha M, White P. Postoperative nausea and vomiting. It's etiology, treatment and prevention. Anesthesiology. 1992;77(1):162–184. doi: 10.1097/00000542-199207000-00023. [DOI] [PubMed] [Google Scholar]
- 19.Apfel C, Greim C, Habuitz I, et al. A risk score to predict the probability of postoperative vomiting in adults. Acta Anaesthesiologica Scandinavica. 2008;42(5):495–501. doi: 10.1111/j.1399-6576.1998.tb05157.x. [DOI] [PubMed] [Google Scholar]
- 20.White P, O'Hara J, Roberson C, Wender R, Candiotti K. The impact of current anti-emetic practices on patient outcomes: A prospective study on high-risk patients. Anesth Analg. 2008;107(2):452–458. doi: 10.1213/ane.0b013e31817b842c. [DOI] [PubMed] [Google Scholar]
- 21.Murphy M, Hooper V, Sullivan E, Clifford T, Apfel C. Identification of risk factors for postoperative nausea and vomiting in the perianesthesia adult patient. J Perianesthesia Nurs. 2006;21(6):377–384. doi: 10.1016/j.jopan.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Meng L, Quinlan J. Assessing risk factors for postoperative nausea and vomiting: A retrospective study in patients undergoing retromastoid craniectomy with microvascular decompression of cranial nerves. J Neurosurgical Anesthesiology. 2006;18(4):235–239. doi: 10.1097/00008506-200610000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Montgomery GH, Schnur JB, Erblich J, Diefenbach MA, Bovbjerg DH. Presurgery Psychological Factors Predict Pain, Nausea, and Fatigue One Week After Breast Cancer Surgery. J of Pain and Symptom Management. 2010;39(6):1043–1052. doi: 10.1016/j.jpainsymman.2009.11.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McNair D, Lorr M, Droppleman L. Manual for the Profile of Mood States. San Diego, CA: Educational and Industrial Testing Service; 1971. [Google Scholar]
- 25.Burgeois A, LeUnes A, Meyers M. Full-scale and short-form of the Profile of Mood States: A factor analytic comparison. J of Sport Behavior. 2010 [Google Scholar]
- 26.Lerman J. Are antiemetics cost-effective for children? Can J Anaesth. 1995;42:263–266. doi: 10.1007/BF03010699. [DOI] [PubMed] [Google Scholar]
- 27.Cork R, Isaac I, Elsharydah A, et al. A Comparison Of The Verbal Rating Scale And The Visual Analog Scale For Pain Assessment. The Internet J of Anesthesiology. 2004;8(1) [Google Scholar]
- 28.Apfel CC, Heidrich FM, Jukar-Rao S, et al. Evidence-based analysis of risk factors for postoperative nausea and vomiting. Br J Anaesth. 2012;109(5):742–753. doi: 10.1093/bja/aes276. [DOI] [PubMed] [Google Scholar]
- 29.Apfel CC, Heidrich FM, Jukar-Rao S, et al. Evidence-based analysis of risk factors for postoperative nausea and vomiting. Br J Anaesth. 2012;109(5):742–753. doi: 10.1093/bja/aes276. [DOI] [PubMed] [Google Scholar]
- 30.Apfel CC, Philip BK, Cakmakkaya OS, et al. Who is at risk for postdischarge nausea and vomiting after ambulatory surgery? Anesthesiology. 2012;117(3):475–486. doi: 10.1097/ALN.0b013e318267ef31. [DOI] [PubMed] [Google Scholar]
- 31.White PF, Sacan O, Nuangchamnong N, Sun T, Eng MR. The relationship between patient risk factors and early versus late postoperative emetic symptoms. Anesth Analg. 2008;107(2):459–463. doi: 10.1213/ane.0b013e31817aa6e4. [DOI] [PubMed] [Google Scholar]
- 32.Gan T, Diemunsch P, Habib A, et al. Consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2014;118:85–113. doi: 10.1213/ANE.0000000000000002. [DOI] [PubMed] [Google Scholar]
- 33.Apfel C, Turan A, Souza, Pergolizzi J, Hornuss C. Intravenous acetaminophen reduces postoperative nausea and vomiting: a systematic review and meta-analysis. Pain. 2103;154(5):677–689. doi: 10.1016/j.pain.2012.12.025. [DOI] [PubMed] [Google Scholar]
- 34.Montgomery GH, Schnur JB, Erblich J, Diefenbach MA, Bovbjerg DH. Presurgery psychological factors predict pain, nausea, and fatigue one week after breast cancer surgery. J of Pain and Symptom Management. 2010;39(6):1043–1052. doi: 10.1016/j.jpainsymman.2009.11.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sadhasivam S, Chidambaran V, Ngamprasertwong P, et al. Race and unequal burden of perioperative pain and opioid related adverse effects in children. Pediatrics. 2012;129(5):832–838. doi: 10.1542/peds.2011-2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodseth RN, Gopalan PD, Cassimjee HM, Goga S. Reduced incidence of postoperative nausea and vomiting in black South Africans and its utility for a modified risk scoring system. Anesth Analg. 2010;110(6):1591–1594. doi: 10.1213/ANE.0b013e3181da9005. [DOI] [PubMed] [Google Scholar]
- 37.Wiesmann T, 1, K P, Eberhart L. Postoperative nausea and vomiting - a narrative review of pathophysiology, pharmacotherapy and clinical management strategies. Expert Opin Pharmaco. 2015;16(7):1069–1077. doi: 10.1517/14656566.2015.1033398. [DOI] [PubMed] [Google Scholar]