Abstract
Reverse transcriptases (RTs) are typically assayed in vitro with 5–10 mM Mg2+, whereas the free Mg2+ concentration in cells is much lower. Artificially high Mg2+ concentrations used in vitro can misrepresent different properties of human immunodeficiency virus (HIV) RT, including fidelity, catalysis, pausing, and RNase H activity. Here, we analyzed nucleoside (NRTIs) and non-nucleoside RT inhibitors (NNRTIs) in primer extension assays at different concentrations of free Mg2+. At low concentrations of Mg2+, NRTIs and dideoxynucleotides (AZTTP, ddCTP, ddGTP, and 3TCTP) inhibited HIV-1 and HIV-2 RT synthesis less efficiently than they did with large amounts of Mg2+, whereas inhibition by the “translocation-defective RT inhibitor” EFdA (4′-ethynyl-2-fluoro-2′-deoxyadenosine) was unaffected by Mg2+ concentrations. Steady-state kinetic analyses revealed that the reduced level of inhibition at low Mg2+ concentrations resulted from a 3–9-fold (depending on the particular nucleotide and inhibitor) less efficient incorporation (based on kcat/Km) of these NRTIs under this condition compared to incorporation of natural dNTPs. In contrast, EFdATP was incorporated with an efficiency similar to that of its analogue dATP at low Mg2+ concentrations. Unlike NRTIs, NNRTIs (nevirapine, efavirenz, and rilviripine), were approximately 4-fold (based on IC50 values) more effective at low than at high Mg2+ concentrations. Drug-resistant HIV-1 RT mutants also displayed the Mg2+-dependent difference in susceptibility to NRTIs and NNRTIs. In summary, analyzing the efficiency of inhibitors under more physiologically relevant low-Mg2+ conditions yielded results dramatically different from those from measurements using commonly employed high-Mg2+ in vitro conditions. These results also emphasize differences in Mg2+ sensitivity between the translocation inhibitor EFdATP and other NRTIs.
Graphical abstract
Highly active antiretroviral therapy (ART), since its introduction in 1995, has dramatically reduced the morbidity and mortality associated with acquired immunodeficiency syndrome (AIDS). However, issues concerning drug resistance, side effects of the drugs (for a review, see ref1), availability, cost, and delivery infrastructure remain major concerns, highlighting the need for the development of new drugs. A better understanding of the efficacy, interactions between drug and drug target, and mechanism of action of the existing antiretroviral drugs is pivotal for designing novel drugs. Reverse transcriptase (RT), the DNA polymerase of human immunodeficiency virus (HIV), is one of the main targets for ART.2 Nucleoside reverse transcriptase inhibitors (NRTIs) and non-nucleoside reverse transcriptase inhibitors (NNRTIs) targeting RT are heavily used in ART therapy.3,4
HIV RT is a heterodimer capable of performing RNA-dependent DNA synthesis and DNA-dependent DNA synthesis and possesses RNase H activity.5 Both the polymerase and RNase H activities of RT require divalent cations as essential cofactors.6,7 Magnesium (Mg2+), the most abundant divalent cation in the cell, functions as the physiological cofactor for both activities. The polymerase active site contains two divalent cation binding sites, and models for both one and two cation binding sites have been proposed for the RNase H active site.8–14
HIV RT polymerase and RNase H activities, on homopolymeric templates, were found to be optimal at 3–8 and 4–12 mM Mg2+, respectively;15–18 hence, most in vitro assays use high Mg2+ levels. Studies examining the mechanism of action and resistance against NRTIs and NNRTIs are also usually studied in in vitro reactions with optimal, ~5–8 mM Mg2+.19–24 However, the concentration of free Mg2+ in lymphocytes is only ~0.25–0.50 mM, despite higher concentrations (~10 mM) of total cellular Mg2+.25,26 The concentration of available Mg2+ can have profound effects on the properties of HIV RT. At physiological Mg2+ concentrations, though primer extension is modestly slower, the fidelity of HIV-1 RT is increased several-fold, thereby bringing the in vitro error rate of HIV-1 RT close to the cellular estimates of reverse transcription fidelity.27 RNA-directed ssDNA synthesis reactions performed with HIV-1 RT also led to more efficient ssDNA synthesis with less polymerase pausing and less RNase H cleavage at low Mg2+ levels.28 These results at suboptimal Mg2+ concentrations suggest that although RT’s catalytic activity is modestly decreased, the DNA synthesis efficiency and fidelity are improved.
Previous experiments showed that low Mg2+ concentrations improved the ability of HIV-1 RT to unblock primers with 3′ AZT and decreased the sensitivity of HIV-1 RT to AZTTP and other NRTIs.28 The authors suggested that the observed effects may be due to a combination of diminished RNase H activity at low Mg2+ concentrations and altered interactions with NRTIs, although the exact mechanism was not investigated. The potency of the NNRTIs, which form a very critical component of the ART regime, in physiologically relevant low Mg2+ concentrations is largely unknown. Determining the underlying mechanism for the observed Mg2+ sensitivity of NRTIs and the effect of physiological Mg2+ levels on NNRTIs may lead to a better understanding of how these inhibitors function in cells. Examination of HIV-2 RT could help shed light on this subject as, despite having an amino acid sequence that is significantly homologous to that of HIV-1 RT, this enzyme demonstrates marked differences in inhibition by RT inhibitors. HIV-2 RT is structurally different at the “NNRTI pocket”29 compared to HIV-1 RT and is not inhibited by most NNRTIs.30 This fact, along with the resistance shown by HIV-2 against HIV-1 protease and fusion inhibitors, has led to limited treatment options against HIV-2 infections.31
Here, we show that low-Mg2+ conditions dramatically alter RT’s susceptibility to NRTIs and NNRTIs: HIV-1 and HIV-2 RT discriminate against NRTIs with modified 3′-hydroxyl (-OH) groups better at physiologically relevant low Mg2+ concentrations than at optimized in vitro conditions. In experiments conducted on a DNA template, NRTIs with 3′-azido (AZTTP), 3′-thiol (3TCTP), and dideoxy compounds lacking groups or substituents at positions 2′ and 3′ (ddCTP and ddGTP) showed an ~5-fold decrease in the level of inhibition at a low Mg2+ concentration (0.25 mM) compared to that at an elevated Mg2+ concentration (6 mM). However, a novel class of NRTIs termed translocation-defective RT inhibitors represented by EFdATP (4′-ethynyl-2-fluoro-2′-deoxyadenosine triphosphate), which has a 3′-OH group (albeit with other significant alterations compared to dATP), inhibited both HIV-1 and HIV-2 RT with similar efficiency at both low and high Mg2+ concentrations. Steady-state kinetic analyses revealed that a lower kcat as well as a higher Km for the 3′-OH-modified NRTIs resulted in decreased potency of these inhibitors against HIV-1 and HIV-2 RT in low-Mg2+ versus high-Mg2+ conditions. In contrast, NNRTIs inhibited HIV-1 RT ~4-fold better at low Mg2+ concentrations than at high Mg2+ concentrations, suggesting that Mg2+ also affects the interactions between NNRTI and the “NNRTI pocket” of HIV-1 RT. Several drug-resistant HIV-1 RT mutants were also examined and showed similar trends. This work, in addition to highlighting the altered potency of RT inhibitors at physiologically relevant Mg2+ concentrations, also provides insight into “rational drug design” strategies by implicating interactions between the dNTP 3′-OH group and RT in stabilizing binding interactions. Future inhibitors that either retain or structurally and chemically mimic this group may be more effective and more difficult to overcome through the development of resistance.
EXPERIMENTAL PROCEDURES
Materials
Exonuclease-free Klenow fragment of Escherichia coli DNA polymerase I, terminal transferase (TdT), and T4 polynucleotide kinase (PNK) were from New England Biolabs. DNase (deoxyribonuclease)-free RNase (ribonuclease), ribonucleotides, and deoxyribonucleotides were obtained from Roche. RNase-free DNase I was from United States Biochemical. The phiX174 Hinf I digest DNA ladder was from Promega. Radiolabeled compounds were from PerkinElmer. DNA oligonucleotides were from Integrated DNA Technologies. G-25 spin columns were from Harvard Apparatus. AZTTP was obtained from PerkinElmer, and ddCTP and ddGTP were from United States Biologicals. Nevirapine (NVP), rilpivirine (RPV), and efavirenz (EFV) were obtained from the National Institutes of Health AIDS Research and Reference Reagent Program. All other chemicals were obtained from Fisher Scientific, VWR, or Sigma.
Preparation of Enzymes
The clones (pRT66 and pRT51) for HIV-1 RT were a generous gift from Professor Emeritus Michael Parniak (University of Pittsburgh, Pittsburgh, PA).32 All the wild-type and mutant proteins of HIV-1 RT were derived from the HXB2 strain. Wild-type HIV-1 RT was prepared as described previously.33 This enzyme is a nontagged heterodimer consisting of equal proportions of p66 and p51 subunits. After induction with isopropyl β-D-1-thiogalactopyranoside (IPTG), the two plasmids express the p66 and p51 subunits. Bacterial cells individually expressing the p66 and p51 subunits were harvested by centrifugation at 4000 rpm for 30 min. The cell pellet was weighed and stored at −80 °C. Cell pellets of both the subunits were mixed and resuspended in buffer A [50 mM Tris-HCl (pH 7.9), 60 mM NaCl, 10% glycerol, and 1 mM 2-mercaptoethanol]. The cell suspension was sonicated, and the debris was removed by ultra-centrifugation. The clarified lysate was purified through a Q-Sepharose column followed by a RESOURCE S column. Nontagged recombinant forms of HIV-1 reverse transcriptases with the mutations D67N, K70R, T215F, K219Q, and K65R were provided generously by Dr. Michael Parniak.32,34
The plasmid clones for the HIV-1 mutant RT with the K103N mutation and HIV-2 RT were provided as a gift from Dr. Stephen Hughes (National Cancer Institute, Rockville, MD). HIV-2 RT was derived from the strain ROD (GenBank accession number HIV2ROD). After induction with IPTG, these plasmids express the His-tagged version of the respective RT along with the protease (PR). Approximately half of the RT in the bacteria is converted into the small subunit by PR.35 Both the HIV-1 K103N and HIV-2 RTs were purified in the same manner, as described in ref35, but with a small modification. The order of the purification columns was reversed. The bacterial cell lysates were first purified through a Q-Sepharose column. RTs, bound to the Q-Sepharose column, were eluted and then purified through a nickel-nitrilotriacetic acid (Ni-NTA) metal affinity column. Aliquots of HIV RT were stored frozen at −80 °C, and fresh aliquots were used for each experiment. An active site titration was performed on purified HIV-1 and HIV-2 RTs at high (6 mM) and low (0.25 mM) Mg2+ concentrations (Figure S1). Both enzymes showed modestly higher active site concentrations with higher Mg2+ concentrations [percent active sites based on the physical amount of RT added to reaction mixtures, 40 ± 2% and 64 ± 6% at 0.25 and 6 mM free Mg2+, respectively, for HIV-1 RT and 37 ± 9% and 50 ± 5% at 0.25 and 6 mM free Mg2+, respectively, for HIV-2 RT (average or three experiments ± the standard deviation)].
5′ End Labeling of Primers
Reactions for labeling various primers were performed in a 50 μL volume of reaction buffer [70 mM Tris-HCl (pH 7.6), 10 mM MgCl2, and 5 mM dithiothreitol (DTT)] containing 50 pmol of the oligo, 5 μL of [γ-32P]ATP (3000 Ci/mmol, 10 mCi/mL), and 10 units of PNK. The reaction mixture was incubated for 60 min at 37 °C, and then the PNK was heat inactivated for 15 min at 70 °C. The material was then run through a Sephadex G-25 spin column.
Nucleic Acid Hybridization
Primer–template hybrids were prepared by mixing the template and end-labeled DNA primer at the indicated ratio in buffer containing 50 mM Tris-HCl (pH 8), 80 mM KCl, and 1 mM DTT. The mixtures were heated to 65 °C for 5 min and then cooled slowly to room temperature.
Polyacrylamide Gel Electrophoresis
Denaturing polyacrylamide gels [8, 12, and 16% (w/v)] were prepared and run as described previously.36
Primer Extension Reactions
Radiolabeled primer extension reactions were performed to study the inhibition of extension by NRTIs and NNRTIs. For each reaction, 15 nM radiolabeled primer (5′-TTGTTGTCTCTTCCCCAAAC-3′) was hybridized with 22.5 nM template (5′-TGGCCTTCCCACAAGGGAAGGCCAGGGAATTTTCTTCAGAGCAGACCAGAGCCAACAGCCCCACCAGAAGAGAGCTTCAGGTTTGGGGAAGAGACAACAA-3′) at a ratio of 1:1.5. Hybrids were preincubated for 3 min at 37 °C in 8.5 μL of reaction buffer [50 mM Tris-HCl (pH 8), 1 mM DTT, and 80 mM KCl] and between 0.25 and 6 mM free Mg2+, 5 μM dNTPs, and various concentrations of the NRTIs (as noted in the figure legends). Extension was initiated by adding 4 μL of recombinant RTs (final concentration of 100 nM at similar activities of primer extension). The final pH of the reactions was 7.7 unless otherwise indicated. After extension for 30 min, the reactions were terminated by adding 12.5 μL of 2× gel loading buffer [90% formamide, 10 mM EDTA (pH 8.0), and 0.025% bromophenol blue and xylene cyanol]. NNRTIs were dissolved in 90% dimethyl sulfoxide (DMSO). For primer extension reactions with NNRTIs, RTs were preincubated with the NNRTI in 50 mM Tris-HCl (pH 8), 1 mM DTT, 80 mM KCl, and 10% DMSO at room temperature for 10 min before extension of the hybrids was initiated. Extension reaction mixtures also contained a final concentration of 10% DMSO. Reactions were terminated with 12.5 μL of 2× gel loading buffer as described above, and samples were then resolved on an 8% denaturing urea gel, dried, and imaged using a Fujifilm FLA-5100 or FLA-7000 camera. Because dNTPs and the NRTIs are the major chelators of the divalent cations in these reactions, the amount of free Mg2+ in each reaction mixture was adjusted according to the total dNTP and the inhibitor concentration. A theoretical estimate of the approximate concentration of free Mg2+ present in the reactions was calculated using the formula
where Et, Dt, and [ED] represent the concentrations of total Mg2+, total dNTP, and Mg2+ bound to the dNTPs, respectively. The equilibrium dissociation constant (Kd) for dNTP with Mg2+ was assumed to be the same as that of ATP with Mg2+ (Kd = 89.1 × 10−6 M).28 This assumption leads to an approximate value for the free concentration of Mg2+ in reactions.
Primer Rescue Assay
Primer rescue experiments were conducted according to previously published results.24 The unlabeled AZTMP-terminated primer was first prepared by incubating 200 pmol of the primer (5′-CTACTAGTTTTCTCCATCTAGACGATACCAGA-3′) with 100 μM AZTTP and 45 units of TdT. The AZTMP-terminated primer was then labeled at the 5′ end with32P as described above. The labeled primer was then run on a 16% denaturing gel to isolate the primers with the AZTMP at the 3′ end. The gel-purified primer (5 nM) was annealed with 20 nM template (5′-GAGTGCTGAGGTCTTCATTCTGGTATCGTCTAGATGGAGAAAACTAGTAG-3′). The hybrid was incubated with 200 nM HIV-1 RT or AZT-resistant RT (at similar activities of primer extension), 0.25 or 6 mM free Mg2+ (Mg2+ concentration adjusted according to the concentration of ATP), and increasing concentrations of ATP (as indicated in Figure 4) in 10 μL of buffer containing 50 mM Tris-HCl, 1 mM DTT, and 80 mM KCl for 30 min at 37 °C. The final pH of the reactions was 7.7. The reaction was terminated when the mixture was heated at 90 °C for 5 min. The total free Mg2+ concentration in 0.25 mM Mg2+ reaction mixtures was adjusted to 6 mM before proceeding to the next step. The samples were placed on ice for 5 min, followed by addition of 3.5 μL of the same reaction buffer containing exonuclease-free Klenow fragment of E. coli DNA polymerase I (0.1 unit/μL) and 100 μM dNTPs. Addition of exonuclease-free enzyme allows selective extension of primers that have had AZTMP removed from the 3′ end by RT, while primers retaining AZTMP will not be extended. The samples were incubated at 37 °C for 30 min. The reaction was terminated by addition of 2× gel loading buffer, and the samples were separated by electrophoresis on a 12% denaturing gel.
Figure 4.
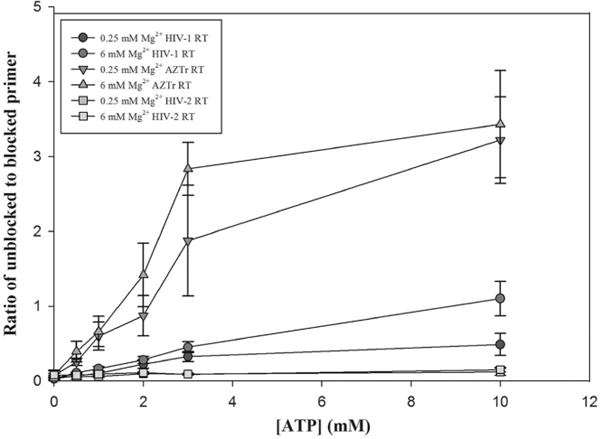
ATP-dependent unblocking of the chain-terminated primer by different RTs was not affected by Mg2+ concentrations. Primer unblocking experiments were performed using the primer rescue assay described in Experimental Procedures. The p unblocking activity by HIV-1, HIV-2, and AZTr RT was similar in both 0.25 and 6 mM Mg2+. As expected, the unblocking efficiency of different RTs decreased in the following order: HIV-1 AZTr > HIV-1 > HIV-2. Error bars are standard deviations from three independent experiments.
Single-Nucleotide Incorporation Assay
These assays were performed as described in ref37. Four different 19-mer DNA templates containing sequence variations (N) at the 5′ end nucleotide (5′-NTGGCGCCCGAACAGGGAC-3′) and a 5′ end-labeled 18-mer DNA primer (5′-GTCCCTGTTCGGGCGCCA-3′) were used for the assay. The nucleotide at the 5′ end of the primer determined the dNTP or the NRTI to be measured. Primer–template hybrids were generated by mixing the radiolabeled primer and template at a 1:1 ratio, as described above in reaction buffer [50 mM Tris-HCl, 1 mM DTT, 80 mM KCl, and 0.1 mg/mL BSA (pH 7.7)]. The primer–template hybrids (10 nM) were preincubated in 8 μL of reaction buffer along with 5 μM (dT)20, various concentrations of dNTP or NRTI, and MgCl2 at 37 °C for 3 min. Reactions were initiated by adding 2 μL of RT protein in the same buffer (final concentration listed in Tables 1 and 2) and mixtures were incubated at 37 °C for the indicated time (see below). Enzyme concentrations and time points were chosen to ensure that <60% of the primers were extended at the highest nucleotide concentration. The final concentration of free Mg2+ (adjusted according to the concentration of dNTP or NRTI) in the reaction mixtures was 0.25 or 6 mM. The final pH of the reactions was 7.7 unless otherwise indicated. HIV-1 RT reactions were performed for the following times: dATP and EFdATP reactions for 2 min (6 mM Mg2+) and 4 min (0.25 mM Mg2+), dCTP and ddCTP reactions for 2 and 4 min, respectively (6 mM Mg2+), and 4 and 30 min, respectively (0.25 mM Mg2+), and dTTP and AZTTP reactions for 4 min (6 mM Mg2+) and 10 and 30 min, respectively (0.25 mM Mg2+). HIV-2 RT reactions were performed for the following times: dATP and EFdATP reactions for 2 min (6 mM Mg2+) and 4 min (0.25 mM Mg2+), dCTP and ddCTP reactions for 4 min (6 mM Mg2+) and 10 and 30 min, respectively (0.25 mM Mg2+), and dTTP and AZTTP reactions for 2 min (6 mM Mg2+) and 10 and 30 min, respectively (0.25 mM Mg2+). Reactions measuring dTTP and AZTTP incorporation by AZTr RT (D67N, K70R, T215F, and K219Q in an HXB2 backbone38) were performed for 4 and 10 min at 0.25 mM Mg2+ and 2 min at 6 mM Mg2+. dCTP and ddCTP reactions with K65R RT were performed for 2 and 4 min at 6 mM Mg2+ and 10 and 30 min at 0.25 mM Mg2+. Reactions were terminated by addition of 10 μL of 2× gel loading buffer. Samples were run on a 16% denaturing gel and visualized with a phosphoimager. The proportion of primer extension in each reaction was calculated by determining the ratio of extended versus total (extended and unextended) primers. This was converted to a reaction velocity (nanomolar per minute) based on the concentration of the primer–template hybrid and the reaction time. The reaction velocity was plotted against the concentration of nucleotide or inhibitor, and the data were fit to a curve using the hyperbola single-rectangle two-parameter curve fit [equation y = ax/(b + x)] in SigmaPlot 10. Steady-state constants Vmax (a in the equation) and Km were then calculated from the curve. kcat was calculated by dividing Vmax by the total enzyme concentration. The numbers listed in Tables 1 and 2 are averages from three or more measurements ± standard deviations (SDs).
Table 1.
Vmax, Km, and kcat Values for Incorporations of TTP and AZTTP, dCTP and ddCP, or dATP and EFdATP by HIV-1 RT at 6 and 0.25 mM Mg2+
| conditiona | [RT] (nM) | Vmax (nM/min) | kcatb (min−1) | Km (μM) | kcat/Kmc (x-fold decrease) | p valued |
|---|---|---|---|---|---|---|
| 6 mM Mg2+ dTTP | 0.8 | 1.1 ± 0.1 | 1.3 ± 0.09 | 2.3 ± 0.2 | 0.57 | – |
| 0.25 mM Mg2+ dTTP | 0.8 | 0.65 ± 0.07 | 0.81 ± 0.09 | 5.7 ± 0.7 | 0.14 (4.1) | <0.001 |
| 6 mM Mg2+ AZTTP | 0.8 | 0.95 ± 0.08 | 1.2 ± 0.09 | 2.3 ± 0.4 | 0.52 | – |
| 0.25 mM Mg2+ AZTTP | 0.8 | 0.26 ± 0.01 | 0.32 ± 0.01 | 12 ± 1 | 0.027 (19) | <0.001 |
| 6 mM Mg2+ dCTP | 2 | 2.1 ± 0.1 | 1.0 ± 0.1 | 1.3 ± 0.4 | 0.77 | – |
| 0.25 mM Mg2+ dCTP | 2 | 1.4 ± 0.1 | 0.68 ± 0.03 | 3.9 ± 1.7 | 0.17 (4.5) | 0.05 |
| 6 mM Mg2+ ddCTP | 0.5 | 1.4 ± 0.2 | 0.72 ± 0.08 | 5.0 ± 0.3 | 0.14 | – |
| 0.25 mM Mg2+ ddCTP | 0.5 | 0.23 ± 0.05 | 0.12 ± 0.03 | 12.5 ± 3.5 | 0.01 (18) | <0.001 |
| 6 mM Mg2+ dATP | 0.8 | 2.1 ± 0.2 | 2.6 ± 0.3 | 2.2 ± 0.8 | 1.2 | – |
| 0.25 mM Mg2+ dATP | 0.8 | 0.72 ± 0.08 | 0.90 ± 0.09 | 2.5 ± 1.1 | 0.36 (3.3) | 0.1 |
| 6 mM Mg2+ EFdATP | 0.8 | 0.72 ± 0.02 | 0.90 ± 0.01 | 1.1 ± 0.1 | 0.82 | – |
| 0.25 mM Mg2+ EFdATP | 0.8 | 0.53 ± 0.27 | 0.66 ± 0.35 | 3.5 ± 2.2 | 0.19 (4.3) | 0.1 |
Assays were conducted with a 20-nucleotide template and a 19-nucleotide 5′ end-labeled primer as described previously.37 The single template-directed nucleotide was a T opposite an A, a C opposite a G, or an A opposite a T for AZT, ddCTP, or EFdATP, respectively. All assays used 0.8 nM RT. Results are averages of three experiments ± SD.
kcat was calculated by dividing Vmax by the enzyme concentration (0.8 nM).
The x-fold decrease in enzyme efficiency (as judged by kcat/Km) compared to the 6 mM result (number above) with the same nucleotide or inhibitor.
p values were calculated using a standard Student’s t test for kcat/Km values between 0.25 and 6 mM Mg2+.
Table 2.
Vmax, Km, and kcat Values for Incorporation of TTP and AZTTP, dCTP and ddCP, or dATP and EFdATP by HIV-2 RT at 6 and 0.25 mM Mg2+
| conditiona | [RT] (nM) | Vmax (nM/min) | kcatb (min−1) | Km (μM) | kcat/Kmc (x-fold decrease) | p valued |
|---|---|---|---|---|---|---|
| 6 mM Mg2+ dTTP | 0.4 | 0.48 ± 0.07 | 1.2 ± 0.2 | 3.0 ± 1.0 | 0.40 | – |
| 0.25 mM Mg2+ dTTP | 0.8 | 0.25 ± 0.09 | 0.31 ± 0.11 | 6.1 ± 1.1 | 0.05 (8) | 0.001 |
| 6 mM Mg2+ AZTTP | 0.4 | 0.82 ± 0.45 | 1.5 ± 0.4 | 4.4 ± 1.8 | 0.34 | – |
| 0.25 mM Mg2+ AZTTP | 0.8 | 0.05 ± 0.03 | 0.07 ± 0.04 | 7.2 ± 1.5 | 0.01 (34) | 0.001 |
| 6 mM Mg2+ dCTP | 1.6 | 1.2 ± 0.2 | 0.78 ± 0.15 | 6.9 ± 2.0 | 0.11 | – |
| 0.25 mM Mg2+ dCTP | 1.6 | 0.42 ± 0.02 | 0.26 ± 0.01 | 5.3 ± 2.4 | 0.05 (2.2) | 0.01 |
| 6 mM Mg2+ ddCTP | 1.6 | 0.99 ± 0.63 | 0.62 ± 0.39 | 5.1 ± 2.3 | 0.12 | – |
| 0.25 mM Mg2+ ddCTP | 1.6 | 0.09 ± 0.07 | 0.06 ± 0.01 | 9.7 ± 2.3 | 0.006 (20) | 0.01 |
| 6 mM Mg2+ dATP | 0.8 | 1.6 ± 0.3 | 2.0 ± 0.4 | 3.5 ± 0.4 | 0.57 | – |
| 0.25 mM Mg2+ dATP | 0.8 | 0.45 ± 0.19 | 0.56 ± 0.24 | 5.3 ± 3.1 | 0.11 (5.2) | 0.005 |
| 6 mM Mg2+ EFdATP | 1.6 | 1.2 ± 0.4 | 0.77 ± 0.18 | 0.97 ± 0.41 | 0.79 | – |
| 0.25 mM Mg2+ EFdATP | 1.6 | 0.29 ± 0.02 | 0.18 ± 0.01 | 1.4 ± 0.4 | 0.13 (6.1) | 0.03 |
Assays were conducted with a 20-nucleotide template and 19-nucleotide 5′ end-labeled primer as described previously.37 The single template-directed nucleotide was a T opposite an A, a C opposite a G, or an A opposite a T for AZTTP, ddCTP, or EFdATP, respectively. All assays used one of the following RT concentrations: 0.4, 0.8, or 1.6 nM. Results are averages of three experiments ± SD.
kcat was calculated by dividing Vmax by the enzyme concentration.
The x-fold decrease in enzyme efficiency (as judged by kcat/Km) compared to the 6 mM result (number above) with the same nucleotide or inhibitor.
p values were calculated using a standard Student’s t test for kcat/Km values between 0.25 and 6 mM Mg2+.
Assays for Determining the Half-Maximal Inhibitory Concentration (IC50)
The template that was extended by HIV-1 RT with maximal efficiency (5′ T) (Table 1) in the previous experiment was chosen for this assay. These assays were performed as described in ref39. The levels of catalysis by recombinant HIV-1 and HIV-1 K103N RT enzymes were determined by measuring the percentage of extension of the labeled hybrid in the presence of different amounts of NNRTIs. The NNRTI compound to be evaluated (NVP) was serially diluted in 50% DMSO. The reaction mixtures containing 150 nM labeled primer–template hybrid, 100 μM dATP, 0.25 or 6 mM free Mg2+ (adjusted according to the concentration of dATP), and 5% DMSO were preincubated at 37 °C for 3 min in a total volume of 8.5 μL of reaction buffer (see above). Twenty-five nM HIV-1 or K103N RT (at similar activities of primer extension) was preincubated with different dilutions of NVP (as noted in the figure legends) for 10 min at room temperature. Reaction was then initiated by adding the NVP/RT mix to the primer–template hybrid. The final pH of the reactions was 7.7. After extension for 3 min, the reactions were stopped by adding 12.5 μL of 2× gel loading buffer and the samples were resolved in a 16% denaturing polyacrylamide–7 M urea gel. The percentage of primer extension at each concentration of NVP was determined. The IC50 value was determined by plotting the percentage of primer extension relative to the logarithm of the inhibitor concentration. A four-parameter logistic equation was used for curve fitting with SigmaPlot 10.0 to obtain the IC50 for NVP.
RESULTS
Low-Mg2+ Conditions Decrease the Inhibitory Effect of NRTIs with the Exception of That of EFdATP
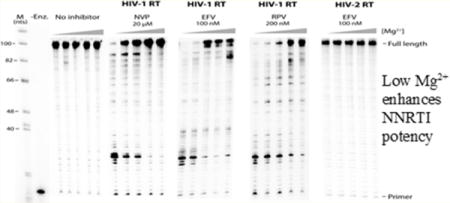
We first tested the influence of the free Mg2+ concentration on NRTI inhibition of DNA-dependent DNA synthesis by HIV-1 and HIV-2 RT. A 100-nucleotide region from the HIV gag gene hybridized to a 32P-labeled 20-nucleotide primer (Figure 1) was used for ssDNA synthesis at different Mg2+ concentrations in the presence of the following triphosphorylated NRTIs (all of which lack a 3′-OH group): zidovudine (AZTTP), zalcitabine (ddCTP), ddGTP, and lamivudine (3TCTP). These NRTIs compete with cellular dNTPs as substrates for HIV RT and induce chain termination after being incorporated into the elongating DNA.40 Inhibition of HIV-1 RT DNA synthesis by these NRTIs was highly dependent on Mg2+. In the experiment shown in Figure 2, the amount of total dNTPs was constant (5 μM each) and the amount of NRTI inhibitor was chosen such that little or no full-length DNA synthesis product was observed at the highest Mg2+ concentration used (6 mM free Mg2+). A pronounced decrease in the level of inhibition of DNA synthesis was observed as the concentration of Mg2+ was decreased. This was evident from an increase in the amount of fully extended products and a decrease in the intensity of NRTI-terminated products. The presence of weak (hydrogen for ddCTP and ddGTP), moderate (thiol for 3TCTP), or stronger (azido for AZTTP) electronegative groups at the 3′ position of the analogue did not affect the general trend of the Mg2+-dependent decrease in the level of inhibition. The common theme of the inhibitors tested above is the lack of a 3′-OH group. Another type of NRTI termed a “translocation-defective RT inhibitor”41,42 was also tested. This new class of drugs (represented by EFdATP) has been shown to be highly effective in inhibiting HIV replication at low concentrations with very low toxicity and has been shown to be highly effective in protecting humanized mice from HIV infections.43 Translocation inhibitors, despite retaining the 3′-OH group, inhibit RT by multiple mechanisms.42 EFdA stabilizes the enzyme in the pretranslocation site, preventing the enzyme from translocating to the next site on the template,44 primarily acting as a chain terminator by blocking RT translocation. In some cases, EFdA functions as a delayed chain terminator, allowing incorporation of an additional dNTP before blocking DNA synthesis. EFdATP can also be more efficiently misincorporated than dATP by RT, leading to mismatched primers that are extremely hard to extend and are protected from excision.42 Unlike the other NRTIs, chain termination by EFdATP was not strongly affected by Mg2+ concentration (Figure 2). Both total full-length products and the intensities of the EFdA-terminated products were similar at all tested Mg2+ concentrations, with a modest increase in the level of termination products and a decrease in the level of full-length products at the lowest Mg2+ concentrations. Although EFdATP possesses a 3′-OH group, other modifications to the base and sugar groups preclude an unequivocal determination of why it was not affected by Mg2+ concentrations like other NRTIs (see Discussion). However, our findings may provide insight into the high efficacy of translocation inhibitors in cell culture. EFdA inhibited HIV-1 replication in activated peripheral blood mononuclear cells with an EC50 of 0.05 nM.44
Figure 1.
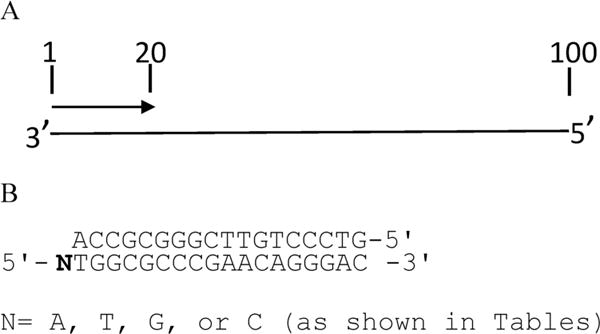
(A) Schematic representation of the primer–template hybrid used for primer extension assays. Numbers indicate size in nucleotides. Primers labeled at 5′ end were hybridized to the 100-nucleotide DNA from the HIV gag region of the genome (nucleotides 1647–1746 of HXB2), and reactions were performed with 5 μM dNTPs and the indicated amount of NRTIs or NNRTIs with increasing concentrations of MgCl2. Reactions were stopped after 30 min and mixtures electrophoresed on an 8% denaturing gel. B) Primer–template constructs used in the steady-state assays. The sequence of the DNA constructs used in the steady-state assay is shown. Different templates with a different nucleotide at the 5′ end (N) and a common primer were used in these assays. Reactions were performed with different nucleotides or the corresponding analogues for the periods of time indicated in the table footnote.
Figure 2.
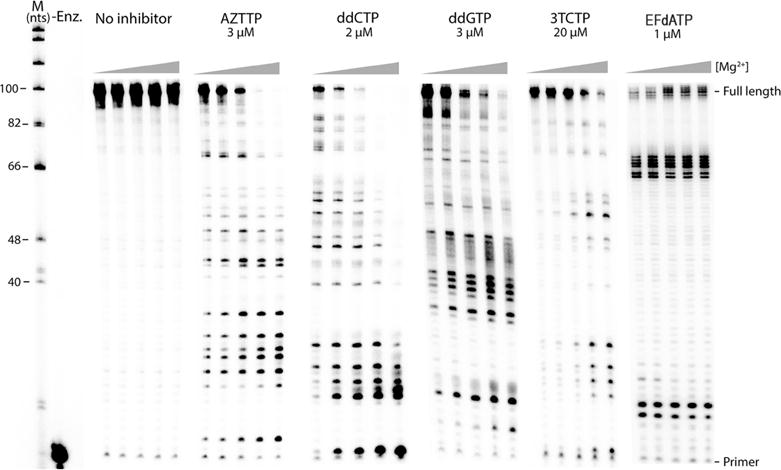
HIV-1 RT shows a lower level of inhibition by NRTIs lacking a 3′-OH group at lower Mg2+ concentrations. Extension with 100 nM wild type HIV-1 RT was monitored with increasing concentrations of Mg2+ (0.25, 0.5, 1, 3, and 6 mM from left to right, respectively), and 5 μM dNTP for 30 min along with the indicated concentrations of the NRTI. M represents the size of the marker in nucleotides. -Enz. represents reactions without the enzyme. A decrease in the total amount of fully extended products along with a corresponding increase in the intensity of premature termination products was observed for AZTTP, ddCTP, ddGTP, and 3TCTP, when the Mg2+ concentration was increased. EFdATP inhibition was, however, not significantly altered by Mg2+ levels. Experiments were repeated a minimum of three times.
We then examined if inhibition of HIV-2 RT by NRTIs varies depending on the concentration of Mg2+. Though HIV-1 and HIV-2 RTs have similar polymerase activity,45,46 there are notable differences with some biochemical properties. HIV-2 RT is less processive than HIV-1 RT,35,46,47 and this effect is enhanced under reduced dNTP pools.35 The RNase H activity of HIV-2 RT is ~10-fold lower than that of HIV-1 RT.45 There are also some reported differences in the susceptibility to zidovudine,48 and HIV-2 RT is not inhibited by first-generation NNRTIs.49 However, inhibition of HIV-2 RT DNA synthesis by the NRTIs tested above showed a very similar trend in comparison to that of HIV-1 RT with lower Mg2+ concentrations (Figure 3). The levels of the various NRTIs required to observe little or no inhibition at the highest Mg2+ concentration were also similar, although HIV-2 RT was less sensitive than HIV-1 RT to 3TCTP. EFdATP displayed potent inhibition of HIV-2 RT, suggesting that it may also be effective in treating HIV-2 infections. Thus, the level of inhibition of HIV-1 and HIV-2 RT DNA synthesis by NRTIs with the exception of EFdATP is reduced at more physiological Mg2+ levels, suggesting that both RTs discriminate against these analogues with better efficiency under these conditions.
Figure 3.

HIV-2 RT also shows a lower level of inhibition by NRTIs lacking a 3′-OH group at lower Mg2+ concentrations. Primer extension reactions were performed with 100 nM wild-type HIV-2 RT, increasing concentrations of Mg2+ (0.25, 0.5, 1, 3, and 6 mM from left to right, respectively), 5 μM dNTPs, and the indicated concentrations of the NRTIs for 30 min. -Enz. represents the reaction without the enzyme. An inhibition profile similar to that of HIV-1 RT was observed for AZTTP, ddCTP, ddGTP, 3TCTP, and EFdATP. Experiments were repeated a minimum of three times.
Steady-State Kinetic Analyses Reveal a Lower Affinity as Well as Slower Kinetics of NRTIs Lacking a 3′-OH Group for HIV-1 and HIV-2 RT
To further examine the mechanism for decreased NRTI sensitivity with low Mg2+ concentrations, the Vmax, Km, and kcat for incorporation of AZTTP, ddCTP, and EFdATP by HIV-1 RT were determined using a steady-state assay that examines incorporation of a single nucleotide on a short template (Figure 1 and Table 1; see Figure S2 for an example of an experiment with EFdATP and HIV-1 and HIV-2 RTs).37 The efficiency of incorporation (as judged by kcat/Km) of both the natural substrates (dTTP, dCTP, and dATP) and nucleotide analogue inhibitors (AZTTP, ddCTP, and EFdATP) was calculated at 6 and 0.25 mM free Mg2+. The efficiency of incorporation of the natural substrates decreased when using the lower-Mg2+ condition [e.g., 4.1-fold for dATP (Table 1)], which is consistent with the decreased extension rate observed under the low-Mg2+ condition.27 However, the efficiency of nucleotide analogue inhibitors lacking a 3′-OH group decreased to a much greater extent with 0.25 mM Mg2+ (e.g., 19-fold for AZTTP). The results with dCTP and ddCTP at 6 and 0.25 mM Mg2+ also displayed a similar pattern (Table 1). This results in AZTTP and ddCTP being less competitive versus the natural nucleotide at low Mg2+ concentrations. The lower affinity for AZTTP and ddCTP (higher Km values compared to that of the natural substrate at 0.25 mM Mg2+) as well as intrinsically slower kinetics for AZTTP and ddCTP (lower kcat values at 0.25 mM Mg2+) contributed to the observed results. In contrast, a comparable decrease in efficiency was observed at 0.25 mM Mg2+ for both dATP and EFdATP (3.4- and 4.2-fold, respectively), consistent with the finding that EFdATP inhibition is not significantly affected by the Mg2+ concentration (Figure 2).
HIV-2 RT, indeed, showed a very similar decrease in efficiency of incorporation of nucleotide analogue inhibitors without a 3′-OH group under low-Mg2+ conditions (Figure 3). The decrease in efficiency of the natural substrates at 0.25 mM Mg2+ (8- and 2.2-fold for dTTP and dCTP, respectively) was completely overshadowed by a more dramatic decrease in the efficiency of AZTTP (34-fold) and ddCTP (20-fold). With HIV-2 RT, slower kinetics (i.e., a lower kcat) for AZTTP and ddCTP incorporation was most responsible for the observed results (Table 2). The decreases in efficiency observed at 0.25 and 6 mM Mg2+ for dATP and EFdATP were comparable (5.2-and 6.1-fold, respectively). These results are consistent with EFdATP maintaining potent inhibition against both HIV-1 and HIV-2 RTs at low Mg2+ concentrations (Figures 2 and 3).
Mg2+ Conditions Do Not Affect the ATP-Dependent Rescue Efficiency of AZT-Terminated Primers
HIV-1 RT develops resistance against NRTIs primarily by two mechanisms: an increased level of exclusion by discriminating against the NRTIs and ATP-dependent primer unblocking by excision of the incorporated NRTI. NRTI drugs such as Zalcitabine, Lamivudine (3TC), dideoxyinosine, and Stavudine (d4T) select for mutations in RT which confers resistance through the exclusion mechanism via the K65R mutation and other mutations.50–53 Resistance to the thymidine analogue class of NRTIs such as 3′-azido-3′-deoxythymidine (AZT) can be conferred by facilitating excision of the incorporated AZTMP from the growing chain of DNA through thymidine analogue mutations (TAM) such as the K70R and T215F mutations.38,54 Next, we examined if the concentration of Mg2+ impacts the ability of HIV-1 and HIV-2 RT, and a version of HIV-1 RT that has gained resistance to AZT through acquisition of TAMs [AZTr (see Experimental Procedures)], to excise AZTMP-terminated primers by reversing the polymerization reaction using a pyrophosphate donor.55,56 HIV-1 RT in vitro can use inorganic phosphate, pyrophosphate, or ATP as a pyrophosphate donor; however, physiologically ATP is used,24,38,57,58 and the most common form of HIV-1 resistance to AZT is enhancing the ATP-mediated excision activity.38 The primer rescue assay24 was used to measure ATP-dependent removal of AZT-terminated primers. Previous experiments on a RNA template indicated that low Mg2+ concentrations led to an improvement in the ability to unblock primers with 3′ AZT.28 The authors suggested that the reduced RNase H activity at low Mg2+ concentrations would allow more time for unblocking before RNase H activity degraded the RNA to the point where the primer dissociated from the template. Our experiments on a DNA template showed that the unblocking activity of HIV-1 and HIV-2 RT was not strongly affected (although HIV-1 RT appears to be slightly less active at low Mg2+ concentrations) by the concentration of Mg2+ (Figure 4), supporting the idea that diminished RNase H activity may be the reason for the previously observed results.28 HIV-2 RT was much less able to unblock AZT-terminated primers than HIV-1 RT was, at both high and low Mg2+ concentrations (Figure 4), as has been shown previously.23 In contrast to HIV-1 RT, HIV-2 RT primarily develops resistance against AZT by selecting for the Q151M mutation,59 thereby enhancing the ability of HIV-2 RT to discriminate against the analogue.23 As expected, AZTr showed unblocking activity much greater than that of either wild-type RT.
NNRTIs Are More Effective against HIV-1 RT under Low-Mg2+ Conditions
Non-nucleoside reverse transcriptase inhibitors are noncompetitive inhibitors, which bind at a hydrophobic site in the p66 subunit of the heterodimer.60 NNRTIs bind very close to the polymerase active site, ~10 Å from the active site,61 and inhibit the enzyme by slowing the rate of catalysis.62 In the experiment monitoring inhibition of RT synthesis by NNRTIs at different Mg2+ concentrations, the amount of NNRTI inhibitor was chosen such that little or no full-length DNA synthesis product was observed at the lowest rather than highest (as in the NRTI experiments) Mg2+ concentration used (0.25 mM free Mg2+). Interestingly, first-generation [nevirapine (NVP) and efavirenz (EFV)] and second-generation [rilpivirine (RPV)] NNRTIs were more potent in inhibiting HIV-1 RT at low Mg2+ concentrations than at high Mg2+ concentrations (Figure 5). A decrease in the amount of fully extended products and an increase in the intensity of premature termination products were observed with decreasing Mg2+ levels. The half-maximal inhibitory values (IC50) of NVP at 6 and 0.25 mM Mg2+ were also measured (Table 3). Consistent with the results presented above, there was an ~4-fold decrease in the IC50 value of NVP at 0.25 mM Mg2+, confirming the increased potency of NVP at the lower-Mg2+ condition. These results suggest that NNRTIs may be more potent in cells than would be predicted from commonly used in vitro RT assays and that Mg2+ affects the interactions between NNRTI and the NNRTI binding pocket of the enzyme in an inverse manner compared to that of NRTIs. Consistent with the published data, HIV-2 RT was not inhibited by these first-generation NNRTIs in any of the tested Mg2+ conditions (Figure 5). HIV-2 RT, with positional changes of conserved residues and significant side-chain differences compared to HIV-1 RT,29 does not bind these NNRTIs.
Figure 5.

HIV-1 RT is inhibited by NNRTIs better at lower Mg2+ concentrations. Primer extension reactions were performed with 100 nM wild-type HIV-1 RT or HIV-2 RT, 5 μM dNTPs, the indicated concentrations of the NNRTIs, and increasing concentrations of Mg2+ (0.25, 0.5, 1, 3, and 6 mM from left to right, respectively) for 30 min. M represents the size marker in nucleotides. -Enz. represents reactions without the enzyme. An increase in the total amount of fully extended products by HIV-1 RT was observed for NVP, EFV, and RPV, when the Mg2+ concentration was increased. HIV-2 RT was not inhibited by NNRTIs; a lack of inhibition by EFV is shown. Experiments were repeated a minimum of three times.
Table 3.
Vmax, Km, and kcat Values for Incorporation of TTP and AZTTP by AZTr RT and of dCTP and ddCTP by RTK65R at 6 and 0.25 mM Mg2+
| conditiona | [RT] (nM) | Vmax(nM/min) | kcatb (min−1) | Km (μM) | kcat/Kmc (x-fold decrease) | p valued | |
|---|---|---|---|---|---|---|---|
| AZTr | 6 mM Mg2+ dTTP | 1 | 1.6 ± 0.2 | 1.6 ± 0.2 | 4.8 ± 0.5 | 0.33 | – |
| 0.25 mM Mg2+ dTTP | 1 | 0.55 ± 0.22 | 0.55 ± 0.22 | 2.2 ± 1 | 0.25 (1.3) | 0.05 | |
| 6 mM Mg2+ AZTTP | 1 | 1.3 ± 0.1 | 1.3 ± 0.1 | 5.2 ± 1.0 | 0.25 | – | |
| 0.25 mM Mg2+ AZTTP | 1 | 0.32 ± 0.05 | 0.32 ± 0.05 | 5.5 ± 1.8 | 0.06 (4.2) | 0.009 | |
| K65R | 6 mM Mg2+ dCTP | 2 | 1.5 ± 0.4 | 0.75 ± 0.18 | 1.6 ± 0.5 | 0.59 | – |
| 0.25 mM Mg2+ dCTP | 2 | 0.24 ± 0.02 | 0.12 ± 0.01 | 1.0 ± 0.4 | 0.15 (5.2) | 0.007 | |
| 6 mM Mg2+ ddCTP | 2 | 1.1 ± 0.1 | 0.53 ± 0.04 | 6.2 ± 0.3 | 0.08 | – | |
| 0.25 mM Mg2+ ddCTP | 2 | 0.07 ± 0.04 | 0.03 ± 0.02 | 9.1 ± 0.5 | 0.003 (27) | <0.001 |
Assays were conducted with a 20-nucleotide template and 19-nucleotide 5′ end-labeled primer as described previously.37 The single template-directed nucleotide was a T opposite an A and a C opposite a G for AZT and ddCTP, respectively. All assays used 0.8 or 1.6 nM RT. Results were averages of three experiments ± SD.
kcat was calculated by dividing Vmax by the enzyme concentration.
The x-fold decrease in enzyme efficiency (as judged by kcat/Km) compared to the 6 mM result (number above) with the same nucleotide or inhibitor.
p values were calculated using a standard Student’s t test for kcat/Km values between 0.25 and 6 mM Mg2+.
Drug-Resistant HIV-1 RTs Are Also Sensitive to Changes in Mg2+ Concentration
Acquisition of drug resistance typically alters amino acids that interact with the nucleic acid, dNTPs, or divalent cation. These amino acids could be involved in the Mg2+ sensitivity observed above for NRTIs and NNRTIs. One possible mechanism for gaining resistance would be to further exacerbate the differential sensitivity of NRTIs to low physiological Mg2+ concentrations. To examine this further, HIV-1 RTs with a K65R mutation (RTK65R) and AZTr RT (see above for a description of these enzymes) were used to check the impact of the Mg2+ concentration on the exclusion of ddCTP and AZT, respectively. Inhibition of RTK65R by ddCTP showed a Mg2+-dependent pattern similar to that of wild-type HIV-1 RT. An increase in fully extended products and a decrease in the intensity of NRTI-terminated products were observed with a decreasing Mg2+ concentration. As expected, a higher concentration of ddCTP was required to observe a level of inhibition comparable to that of wild-type HIV-1 with RTK65R (Figure 6). When the assay was performed with the same level of ddCTP that was used with wild-type RT, although the effects of Mg2+ were still evident, the level of incorporation of ddCTP (judging from the presence and intensity of premature termination sites) was greatly reduced. Steady-state kinetic analyses were consistent with RTK65R excluding ddCTP more effectively under low-Mg2+ conditions (Table 3). Overall, the results were consistent with the K65R mutation conferring greater resistance to ddCTP but retaining sensitivity to Mg2+ to approximately the same extent as wild-type RT. In addition, they suggest that RTK65R is more effective under the low-Mg2+ conditions present in cells (see the introductory section) compared to the high-Mg2+ conditions used in typical in vitro drug assays.
Figure 6.

HIV-1 drug-resistant mutant RTs also show Mg2+-dependent sensitivity to NRTIs and NNRTIs. Primer extension reactions, performed using the same conditions described in the legend of Figure 2, with the indicated drug-resistant RT at 100 nM are shown. Increasing concentrations of Mg2+ were 0.25, 0.5, 1, 3, and 6 mM from left to right, respectively. M represents the size marker in nucleotides. -Enz. represents reactions without the enzyme. The inhibition profile for AZTr RT was very similar to that of HIV-1 RT. K65R and K103N RTs also showed Mg2+-dependent inhibition similar to that of HIV-1 RT, but only at higher concentrations of the inhibitor. Experiments were repeated a minimum of three times.
Similar to RTK65R, AZTr RT also remained sensitive to changes in Mg2+, showing stronger exclusion of AZTTP as the Mg2+ concentration was decreased (Figure 6). Profiles observed with AZTr were similar to those of the wild type, as both enzymes had similar efficiency for incorporation of AZTTP (Figure 6 and Table 3). As stated above, AZTr RT primarily confers resistance against AZT through the excision pathway. As was the case with HIV-1 and HIV-2 wild-type RTs, Mg2+ did not have a significant effect on the unblocking activity of AZTr RT, although this activity was higher with AZTr RT than with wild-type enzymes as expected (Figure 4).
HIV-1 RT resistance against NNRTIs develops through mutations in the NNRTI binding pocket of the enzyme. The Lys103Asn (K103N) mutant is 10–100-fold more resistant than wild-type HIV-1 to most NNRTIs, including nevirapine, efavirenz, and TIBO.63–65 K103N RT was evaluated to see if Mg2+ affects the mutated NNRTI binding pocket in a manner similar to that of the wild-type enzyme. K103N, as expected, was more resistant than wild-type RT to NVP and showed similar patterns of increased sensitivity to NVP at low Mg2+ concentrations and high Mg2+ concentrations (Figure 6). Consistent with these results, IC50 values with K103N decreased ~4-fold under low-Mg2+ conditions compared to that under high-Mg2+ conditions (Table 4).
Table 4.
IC50 Values for NVP with WT HIV-1 and HIV-1 K103N RT at 0.25 and 6 mM Mg2+
| conditiona | IC50b (μM) | x-fold decreasec (p value) | |
|---|---|---|---|
| HIV-1 RT | 6 mM Mg2+ | 16 ± 0.5 | – |
| 0.25 mM Mg2+ | 4.3 ± 0.7 | 3.7 (<0.00001) | |
| K103N RT | 6 mM Mg2+ | 49 ± 5 | – |
| 0.25 mM Mg2+ | 14 ± 3 | 3.5 (0.0003) |
Assays were as described in Experimental Procedures. The single template-directed nucleotide used in this assay was a dATP opposite T (5′ T template). All assays used 25 nM RT.
Half-maximal inhibitory values (IC50) were calculated, as described in Experimental Procedures, from the relationship between the concentration of nevirapine and the percent of inhibition achieved at the particular inhibitor concentration. Results are averages of three experiments ± SD.
The x-fold decrease in IC50 values compared to the 6 mM result (number above) with the same RT. A lower IC50 value at 0.25 mM Mg2+ confirms better inhibition with the lower Mg2+ concentration. Values were calculated using a standard Student’s t test.
The Influence of Mg2+ on NRTIs and NNRTIs Is Not Altered by KCl Concentrations, while the Potency of NRTIs Other Than EFdATP Is Affected by pH
In vitro reactions have also been optimized for other factors (e.g., salt and pH) that may affect reactions with inhibitors, and some of these were examined. Changing the KCl concentration in reactions to either 10 or 120 mM did not significantly change the influence of Mg2+ on NRTIs in primer extension reactions. There was some change in the overall profile of DNA synthesis at the higher KCl concentrations, probably resulting from stabilization of structures in the template and suboptimal RT activity (see Figure S3 for an example with ddCTP). In contrast, the potency of NRTIs with the exception of EFdATP was decreased at lower pH (7.2 vs 7.7 used for the experiments presented above). The trend for decreased potency at lower Mg2+ concentrations was still evident at pH 7.2 but not as pronounced as it was at pH 7.7 (Figures S4 and S5). In contrast, NNRTIs were not affected by pH, but the potency of the drugs was proportional to the KCl concentration with the largest amount of inhibition observed at the highest KCl concentration (120 mM). The trend of increased potency at lower Mg2+ concentrations persisted under all conditions with NNRTIs (Figure S6).
DISCUSSION
In this report, we show that elevated Mg2+ concentrations in in vitro reactions alter the potency of RT inhibitors. When studied at lower, more physiological Mg2+ concentrations, NRTIs show less inhibition, whereas NNRTIs showed stronger inhibition than under high-Mg2+ conditions typically used in vitro (see Tables 1 and 2 and Figures 2 and 3). The results presented for EFdATP indicate that the potency of this “translocation inhibitor” is not significantly altered at lowered Mg2+ concentrations. Of course, inhibition of reverse transcription by NRTIs and NNRTIs under cellular conditions might be affected by cellular and/or viral proteins involved in the reverse transcription complex, as well as other cell factors not included in our studies.
Steady-state analyses at high (6 mM) and low (0.25 mM) Mg2+ concentrations revealed that both dNTP and NRTI incorporation was less efficient (as judged by kcat/Km) at low Mg2+ concentrations (Tables 1 and 2). This is consistent with RT showing higher catalytic activity at high Mg2+ concentrations.27,28 The magnitude of the decrease in efficiency, however, was typically several-fold greater for NRTIs without 3′-OH groups (e.g., dCTP vs ddCTP and TTP vs AZTTP), resulting in less efficient incorporation of these NRTIs in primer extension reactions as the concentration of Mg2+ was decreased. The decreased efficiency of ddCTP versus that of dCTP (and ddGTP vs dGTP, although kinetic constants were not determined for these nucleotides) under low-Mg2+ conditions represents the clearest example of the influence of the 3′-OH group on incorporation efficiency as replacement of the 3′-OH group with a H group is the only difference between ddCTP and dCTP. However, discretion is advised when using steady-state kinetic analyses to quantify the inhibition of HIV-1 or HIV-2 RTs by NRTIs. The efficiency of incorporating nucleotides and NRTIs is sequence-dependent,66 and the kinetic assays presented here using a specific sequence and incorporation at the end of the template may not fully reflect the efficiency of RT in incorporating the nucleoside analogue and dNTPs at other sequences or template locations. However, the kinetic assays were in strong agreement with the assays that measure incorporation over several different sites (like those in Figures 2 and 3), which provide a better qualitative representation of viral genome replication.
Studies comparing different analogues of EFdA also emphasized the contribution of the 3′-OH group for the inhibition potency of EFdA.44 EFdA inhibited HIV-1 replication in phytohemagglutinin-activated PBMCs with an EC50 value of 0.05 nM, whereas an analogue of EFdA lacking the 3′-OH group had an EC50 value of 570 nM.44 EFdATP, after incorporation into the growing DNA strand, locks the enzyme in the pretranslocation site (N site), thereby preventing the binding of the next correct nucleotide. HIV-1 RT is able to unblock the EFdA-terminated primers locked at the N site efficiently; however, facile reincorporation of EFdATP due to the 3′-OH and the 4′-ethynyl groups maintains the potency of inhibition.44 Although it is tempting to suggest that the presence of a 3′-OH on EFdATP was responsible for its better incorporation in comparison to other NRTIs under low-Mg2+ conditions, other alterations compared to dATP such as the fluorine at position 2 of the adenine base and an ethynyl group at position 4′ of the deoxyribose sugar could have played a role. Previous reports have shown that the 4′-ethynyl group enhances binding, resulting in an ~9-fold lower Km and a 5-fold higher kcat in comparison to those of dATP.41 Crystal structures containing RT, the primer–template hybrid, and EFdA mono- and triphosphates corroborate this finding as the ethynyl group is able to access a previously unexploited conserved hydrophobic pocket in the polymerase active site that may lead to stabilization of binding.67 The major effect of the fluorine group was enhancing resistance to adenosine deaminase.41 It is possible that these unique structural or chemical properties rather than the presence of a 3′-OH were responsible for EFdATP’s ability to associate more strongly with RT under low-Mg2+ conditions. A dideoxy version of EFdATP would be useful in differentiating these possibilities; however, it is not currently available.
Our results agree with the mechanism proposed by Goldschmidt et al. to explain the increased level of discrimination against NRTIs at the low Mg2+ concentration.28 Crystal structures of RT with the primer–template complex68 show that three networks of interactions maintain the deoxyribose and phosphate parts of the dNTP: (i) the side chains of D113, Y115, F116, and Q151 (Q151 has also been directly implicated in binding to the 3′-OH by biochemical analysis69) and the main chains of D113 and Y115 with the 3′-OH group of the dNTP, (ii) the side chains of R72 (with the β-phosphate) and K65 (with the γ-phosphate) with the convex face of the triphosphate group,68 and (iii) a nonbridging oxygen, on the concave face, from each phosphate of the triphosphate with a Mg2+ ion. The α-phosphate also interacts with the second Mg2+ ion. The authors suggested that RT can function with reasonable efficiency when one of the three interaction sets is perturbed, but simultaneous perturbations of two networks result in a more pronounced reduction in efficiency. The results herein show that under low-Mg2+ conditions (presumably destabilizing the Mg2+ network) destabilizing the 3′-OH network by deleting this group results in poor incorporation efficiency for NRTIs without 3′-OH, whereas destabilizing either the Mg2+ network alone (EFdATP at low Mg2+ concentrations) or the 3′-OH network alone (AZT and ddCTP at high Mg2+ concentrations) results in a less severe decrease in efficiency. In addition, our kinetic analyses suggest that the altered conformation affects mostly the ddNTP catalysis step (as judged by a decrease in kcat), although binding (as judged by an increase in Km) is also affected to a lesser extent.
It is also possible that the departure from the conformation of the natural substrate, in the case of NRTIs, may result in slightly different binding to the metal ions. The presence of a 3′-azido in AZT, the lack of a 3′-OH in ddC, or the significantly different geometry of 3TCTP compared to that of the natural dNTPs may alter the chelation properties of Mg2+ at the polymerase (pol) active site. As a result, higher concentrations of the metal ions would be required to overcome the minor structural differences. Interestingly, the efficiency of EFdATP, which binds at the pol site even better than the canonical dATP substrate,42,44,70–72 remains equally potent at low and high metal ion concentrations.
The results with NNRTIs are consistent with experiments showing that the binding of some NNRTIs (O-TIBO and Cl-TIBO) to HIV-1 RT is better with no Mg2+ than with 10 mM Mg2+.62 Increased binding affinity may explain the observed increase in potency of NNRTI under the low-Mg2+ condition (Figure 5 and Table 3). The authors hypothesized that interactions of Mg2+ with the active site aspartate residues may impede the binding of NNRTIs to the “NNRTI pocket” of RT. NNRTIs bind HIV-1 RT near the polymerase active site42 containing the conserved aspartate residues (D110, D185, and D186), which interact with two Mg2+ ions during catalysis.68 In fact, the structural mechanism for the inhibition of HIV-1 RT by NNRTIs is through distortion of the active site aspartates.73 When possible interplay between the catalytic active site and the NNRTI site was examined, the binding affinities of the NNRTIs in the presence of Mg2+ decreased.62 Equilibrium dissociation constant values (KD) of O-TIBO and Cl-TIBO, in the presence of 10 mM Mg2+, increased 3.5- and 6-fold compared, respectively, to the KD values in the absence of Mg2+. It is plausible that interactions between Mg2+ ions and the aspartate residues hinder the binding of NNRTIs, while once the inhibitor is bound, the dissociation rate is governed by its intrinsic affinity for RT.62 The authors could not detect a similar Mg2+-dependent change in the KD of NVP, but our results suggest that NVP and EFV may be similarly affected by the high-Mg2+ conditions.
Crystal structures of HIV-1 RT in complex with NNRTI, the template–primer hybrid (TP), dNTP, and divalent cations (at the pol active site) have not been reported. However, several structures of NNRTI-bound, TP-bound, and TP-, dNTP-, and Mg2+-bound HIV-1 RT have been reported.68,74–77 These crystal structures were compared to obtain a possible structural explanation for the Mg2+ concentration-dependent susceptibility of NNRTIs. Binding of NNRTI to HIV-1 RT causes significant structural changes when compared to the structure of an E/TP/dNTP ternary complex. Specifically, the NNRTI binding to HIV-1 RT (i) restricts the flexibility of the thumb subdomain78–80 and (ii) alters the position of the primer grip76,77 and the position of the primer terminus relative to the catalytic site.76,77 More importantly, the NNRTIs restrict the conformational change of the YMDD loop that is needed for the metal binding conformation of catalytic aspartates (Figure 7). The conformational restraint on the YMDD loop upon NNRTI binding supports the hypothesis that NNRTIs may affect metal coordination with catalytic aspartates. This is consistent with our experimental observations, which show that NNRTI susceptibility varies with Mg2+ concentration (Figure 5). At low Mg2+ concentrations, the YMDD loop, in the presence of NNRTIs, may be in a conformation that does not support metal coordination with the catalytic residues (as seen in the crystal structures). However, high Mg2+ concentrations may help the catalytic aspartates to access Mg2+, leading to a conformation of the YMDD loop that is competent for catalysis, even in the presence of NNRTIs. This, in turn, may facilitate the polymerase reaction. Further structural and biochemical analysis will be required to determine if this model or others can explain the observed Mg2+ sensitivity of NNRTIs.
Figure 7.
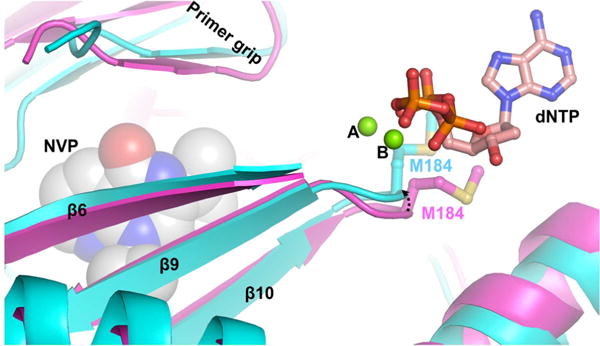
Conformational restriction of the YMDD loop upon NNRTI binding. This figure shows the difference in the YMDD loop conformation between NNRTI-bound (cyan, Protein Data Bank entry 4PUO) and template–primer (TP)- and dNTP-bound structures (magenta, Protein Data Bank entry 4PQU) of HIV-1 RT.77 The arrow shows the shift in the position of the YMDD loop upon binding of NVP to RT compared to that of the RT/TP/dNTP ternary complex. The metal ions are depicted as green spheres. The dNTP is shown in ball-and-stick mode with atoms colored by atom type (carbon, pink; nitrogen, blue; oxygen, red; phosphorus, orange). For the sake of clarity, only M184 of YMDD in two complexes is shown (ball-and-stick mode) with carbon atoms colored cyan and magenta in RT/NVP and RT/TP/dNTP complexes, respectively. Nevirapine is shown with space-filling atoms.
The results presented here demonstrate that interactions between NRTIs and NNRTIs are highly dependent on Mg2+ conditions. For NRTIs, the lack of a 3′-OH appears to play an important role, while novel NRTIs with 3′-OH groups (represented by EFdATP) may be more potent at physiological Mg2+ concentrations, although the exact role of the 3′-OH versus other alterations requires further testing. The results also emphasize the need to include experiments that use physiological amounts of free Mg2+ when studying future RT inhibitors.
Supplementary Material
Acknowledgments
Funding
We acknowledge funding for this work from National Institutes of Health (NIH) grants P50 GM103368 and GM116645.
ABBREVIATIONS
- ART
antiretroviral therapy
- HIV
human immunodeficiency virus
- RT
reverse transcriptase
- RNase H
ribonuclease H
- PNK
T4 polynucleotide kinase
- TdT
terminal transferase
- NRTI
nucleoside reverse transcriptase inhibitor
- NNRTI
non-nucleoside reverse transcriptase inhibitor
- EFV
efavirenz
- NVP
nevirapine
- RPV
rilpivirine
- AZT
zidovudine
- ddCTP
zalcitabine
- 3TCTP
lamivudine
- EfdA
4′-ethynyl-2-fluoro-2′-deoxyadenosine
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.biochem.6b00943.
Additional data (PDF)
ORCID
Jeffrey J. DeStefano: 0000-0002-8710-7622
Notes
The authors declare no competing financial interest.
References
- 1.da Cunha J, Maselli LM, Stern AC, Spada C, Bydlowski SP. Impact of antiretroviral therapy on lipid metabolism of human immunodeficiency virus-infected patients: Old and new drugs. World Journal of Virology. 2015;4:56–77. doi: 10.5501/wjv.v4.i2.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arts EJ, Hazuda DJ. HIV-1 antiretroviral drug therapy. In: Bushman FD, Nabel GJ, Swanstrom R, editors. HIV: From Biology to Prevention and Treatment. Cold Spring Harbor Laboratory Press; Plainview, NY: 2012. pp. 321–343. [Google Scholar]
- 3.Gotte M. Inhibition of HIV-1 reverse transcription: basic principles of drug action and resistance. Expert Rev Anti-Infect Ther. 2004;2:707–716. doi: 10.1586/14789072.2.5.707. [DOI] [PubMed] [Google Scholar]
- 4.Gallant JE, Gerondelis PZ, Wainberg MA, Shulman NS, Haubrich RH, St Clair M, Lanier ER, Hellmann NS, Richman DD. Nucleoside and nucleotide analogue reverse transcriptase inhibitors: a clinical review of antiretroviral resistance. Antiviral Ther. 2003;8:489–506. [PubMed] [Google Scholar]
- 5.Telesnitsky A, Goff SP. Reverse Transcriptase. Cold Spring Harbor Laboratory Press; Plainview, NY: 1993. [PubMed] [Google Scholar]
- 6.Herschhorn A, Hizi A. Retroviral reverse transcriptases. Cell Mol Life Sci. 2010;67:2717–2747. doi: 10.1007/s00018-010-0346-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goff SP. Retroviral reverse transcriptase: synthesis, structure, and function. J Acquired Immune Defic Syndr. 1990;3:817–831. [PubMed] [Google Scholar]
- 8.Cowan JA, Ohyama T, Howard K, Rausch JW, Cowan SM, Le Grice SF. Metal-ion stoichiometry of the HIV-1 RT ribonuclease H domain: evidence for two mutually exclusive sites leads to new mechanistic insights on metal-mediated hydrolysis in nucleic acid biochemistry. JBIC, J Biol Inorg Chem. 2000;5:67–74. doi: 10.1007/s007750050009. [DOI] [PubMed] [Google Scholar]
- 9.Johnson KA. The kinetic and chemical mechanism of high-fidelity DNA polymerases. Biochim Biophys Acta, Proteins Proteomics. 2010;1804:1041–1048. doi: 10.1016/j.bbapap.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joyce CM, Steitz TA. Function and structure relationships in DNA polymerases. Annu Rev Biochem. 1994;63:777–822. doi: 10.1146/annurev.bi.63.070194.004021. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura H, Katayanagi K, Morikawa K, Ikehara M. Structural models of ribonuclease H domains in reverse transcriptases from retroviruses. Nucleic Acids Res. 1991;19:1817–1823. doi: 10.1093/nar/19.8.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schultz SJ, Champoux JJ. RNase H activity: structure, specificity, and function in reverse transcription. Virus Res. 2008;134:86–103. doi: 10.1016/j.virusres.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang W, Steitz TA. Recombining the structures of HIV integrase, RuvC and RNase H. Structure. 1995;3:131–134. doi: 10.1016/s0969-2126(01)00142-3. [DOI] [PubMed] [Google Scholar]
- 14.Oda Y, Nakamura H, Kanaya S, Ikehara M. Binding of metal ions to E. coli RNase HI observed by 1H-15N heteronuclear 2D NMR. J Biomol NMR. 1991;1:247–255. doi: 10.1007/BF01875518. [DOI] [PubMed] [Google Scholar]
- 15.Wondrak EM, Lower J, Kurth R. Functional purification and enzymic characterization of the RNA-dependent DNA polymerase of human immunodeficiency virus. J Gen Virol. 1986;67(Part 12):2791–2797. doi: 10.1099/0022-1317-67-12-2791. [DOI] [PubMed] [Google Scholar]
- 16.Hoffman AD, Banapour B, Levy JA. Characterization of the AIDS-associated retrovirus reverse transcriptase and optimal conditions for its detection in virions. Virology. 1985;147:326–335. doi: 10.1016/0042-6822(85)90135-7. [DOI] [PubMed] [Google Scholar]
- 17.Rey MA, Spire B, Dormont D, Barre-Sinoussi F, Montagnier L, Chermann JC. Characterization of the RNA dependent DNA polymerase of a new human T-lymphotropic retrovirus (lymphadenopathy associated virus) Biochem Biophys Res Commun. 1984;121:126–133. doi: 10.1016/0006-291x(84)90696-x. [DOI] [PubMed] [Google Scholar]
- 18.Starnes MC, Cheng YC. Human immunodeficiency virus reverse transcriptase-associated RNase H activity. J Biol Chem. 1989;264:7073–7077. [PubMed] [Google Scholar]
- 19.Schauer GD, Huber KD, Leuba SH, Sluis-Cremer N. Mechanism of allosteric inhibition of HIV-1 reverse transcriptase revealed by single-molecule and ensemble fluorescence. Nucleic Acids Res. 2014;42:11687–11696. doi: 10.1093/nar/gku819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Z, Walker M, Xu W, Shim JH, Girardet JL, Hamatake RK, Hong Z. Novel nonnucleoside inhibitors that select nucleoside inhibitor resistance mutations in human immunodeficiency virus type 1 reverse transcriptase. Antimicrob Agents Chemother. 2006;50:2772–2781. doi: 10.1128/AAC.00127-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munshi V, Lu M, Felock P, Barnard RJ, Hazuda DJ, Miller MD, Lai MT. Monitoring the development of non-nucleoside reverse transcriptase inhibitor-associated resistant HIV-1 using an electrochemiluminescence-based reverse transcriptase polymerase assay. Anal Biochem. 2008;374:121–132. doi: 10.1016/j.ab.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Maga G, Amacker M, Ruel N, Hubscher U, Spadari S. Resistance to nevirapine of HIV-1 reverse transcriptase mutants: loss of stabilizing interactions and thermodynamic or steric barriers are induced by different single amino acid substitutions. J Mol Biol. 1997;274:738–747. doi: 10.1006/jmbi.1997.1427. [DOI] [PubMed] [Google Scholar]
- 23.Boyer PL, Sarafianos SG, Clark PK, Arnold E, Hughes SH. Why do HIV-1 and HIV-2 use different pathways to develop AZT resistance? PLoS Pathog. 2006;2:e10. doi: 10.1371/journal.ppat.0020010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meyer PR, Matsuura SE, So AG, Scott WA. Unblocking of chain-terminated primer by HIV-1 reverse transcriptase through a nucleotide-dependent mechanism. Proc Natl Acad Sci U S A. 1998;95:13471–13476. doi: 10.1073/pnas.95.23.13471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delva P, Pastori C, Degan M, Montesi G, Lechi A. Intralymphocyte free magnesium and plasma triglycerides. Life Sci. 1998;62:2231–2240. doi: 10.1016/s0024-3205(98)00201-x. [DOI] [PubMed] [Google Scholar]
- 26.Delva P, Pastori C, Degan M, Montesi G, Lechi A. Catecholamine-induced regulation in vitro and ex vivo of intralymphocyte ionized magnesium. J Membr Biol. 2004;199:163–171. doi: 10.1007/s00232-004-0686-7. [DOI] [PubMed] [Google Scholar]
- 27.Achuthan V, Keith BJ, Connolly BA, DeStefano JJ. Human Immunodeficiency Virus Reverse Transcriptase Displays Dramatically Higher Fidelity under Physiological Magnesium Conditions In Vitro. J Virol. 2014;88:8514–8527. doi: 10.1128/JVI.00752-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldschmidt V, Didierjean J, Ehresmann B, Ehresmann C, Isel C, Marquet R. Mg2+ dependency of HIV-1 reverse transcription, inhibition by nucleoside analogues and resistance. Nucleic Acids Res. 2006;34:42–52. doi: 10.1093/nar/gkj411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ren J, Bird LE, Chamberlain PP, Stewart-Jones GB, Stuart DI, Stammers DK. Structure of HIV-2 reverse transcriptase at 2.35-A resolution and the mechanism of resistance to non-nucleoside inhibitors. Proc Natl Acad Sci U S A. 2002;99:14410–14415. doi: 10.1073/pnas.222366699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beach LB, Rawson JM, Kim B, Patterson SE, Mansky LM. Novel inhibitors of human immunodeficiency virus type 2 infectivity. J Gen Virol. 2014;95:2778–2783. doi: 10.1099/vir.0.069864-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Menendez-Arias L, Alvarez M. Antiretroviral therapy and drug resistance in human immunodeficiency virus type 2 infection. Antiviral Res. 2014;102:70–86. doi: 10.1016/j.antiviral.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 32.Fletcher RS, Holleschak G, Nagy E, Arion D, Borkow G, Gu Z, Wainberg MA, Parniak MA. Single-step purification of recombinant wild-type and mutant HIV-1 reverse transcriptase. Protein Expression Purif. 1996;7:27–32. doi: 10.1006/prep.1996.0004. [DOI] [PubMed] [Google Scholar]
- 33.Hou EW, Prasad R, Beard WA, Wilson SH. High-level expression and purification of untagged and histidine-tagged HIV-1 reverse transcriptase. Protein Expression Purif. 2004;34:75–86. doi: 10.1016/j.pep.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 34.Gu Z, Fletcher RS, Arts EJ, Wainberg MA, Parniak MA. The K65R mutant reverse transcriptase of HIV-1 cross-resistant to 2′, 3′-dideoxycytidine, 2′,3′-dideoxy-3′-thiacytidine, and 2′,3′-dideoxyinosine shows reduced sensitivity to specific dideoxynucleoside triphosphate inhibitors in vitro. J Biol Chem. 1994;269:28118–28122. [PubMed] [Google Scholar]
- 35.Boyer PL, Clark PK, Hughes SH. HIV-1 and HIV-2 reverse transcriptases: different mechanisms of resistance to nucleoside reverse transcriptase inhibitors. J Virol. 2012;86:5885–5894. doi: 10.1128/JVI.06597-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sambrook J, Russell DW. Molecular Cloning: A Laboratory Manual. 3rd. Cold Spring Harbor Laboratory Press; Plainview, NY: 2001. [Google Scholar]
- 37.Diamond TL, Roshal M, Jamburuthugoda VK, Reynolds HM, Merriam AR, Lee KY, Balakrishnan M, Bambara RA, Planelles V, Dewhurst S, Kim B. Macrophage tropism of HIV-1 depends on efficient cellular dNTP utilization by reverse transcriptase. J Biol Chem. 2004;279:51545–51553. doi: 10.1074/jbc.M408573200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arion D, Kaushik N, McCormick S, Borkow G, Parniak MA. Phenotypic mechanism of HIV-1 resistance to 3′-azido-3′-deoxythymidine (AZT): increased polymerization processivity and enhanced sensitivity to pyrophosphate of the mutant viral reverse transcriptase. Biochemistry. 1998;37:15908–15917. doi: 10.1021/bi981200e. [DOI] [PubMed] [Google Scholar]
- 39.Xu HT, Quan Y, Schader SM, Oliveira M, Bar-Magen T, Wainberg MA. The M230L nonnucleoside reverse transcriptase inhibitor resistance mutation in HIV-1 reverse transcriptase impairs enzymatic function and viral replicative capacity. Antimicrob Agents Chemother. 2010;54:2401–2408. doi: 10.1128/AAC.01795-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Clercq E. Anti-HIV drugs: 25 compounds approved within 25 years after the discovery of HIV. Int J Antimicrob Agents. 2009;33:307–320. doi: 10.1016/j.ijantimicag.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 41.Kirby KA, Michailidis E, Fetterly TL, Steinbach MA, Singh K, Marchand B, Leslie MD, Hagedorn AN, Kodama EN, Marquez VE, Hughes SH, Mitsuya H, Parniak MA, Sarafianos SG. Effects of substitutions at the 4′ and 2 positions on the bioactivity of 4′-ethynyl-2-fluoro-2′-deoxyadenosine. Antimicrob Agents Chemother. 2013;57:6254–6264. doi: 10.1128/AAC.01703-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Michailidis E, Huber AD, Ryan EM, Ong YT, Leslie MD, Matzek KB, Singh K, Marchand B, Hagedorn AN, Kirby KA, Rohan LC, Kodama EN, Mitsuya H, Parniak MA, Sarafianos SG. 4′-Ethynyl-2-fluoro-2′-deoxyadenosine (EFdA) inhibits HIV-1 reverse transcriptase with multiple mechanisms. J Biol Chem. 2014;289:24533–24548. doi: 10.1074/jbc.M114.562694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stoddart CA, Galkina SA, Joshi P, Kosikova G, Moreno ME, Rivera JM, Sloan B, Reeve AB, Sarafianos SG, Murphey-Corb M, Parniak MA. Oral administration of the nucleoside EFdA (4′-ethynyl-2-fluoro-2′-deoxyadenosine) provides rapid suppression of HIV viremia in humanized mice and favorable pharmacokinetic properties in mice and the rhesus macaque. Antimicrob Agents Chemother. 2015;59:4190–4198. doi: 10.1128/AAC.05036-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Michailidis E, Marchand B, Kodama EN, Singh K, Matsuoka M, Kirby KA, Ryan EM, Sawani AM, Nagy E, Ashida N, Mitsuya H, Parniak MA, Sarafianos SG. Mechanism of inhibition of HIV-1 reverse transcriptase by 4′-Ethynyl-2-fluoro-2′-deoxyadenosine triphosphate, a translocation-defective reverse transcriptase inhibitor. J Biol Chem. 2009;284:35681–35691. doi: 10.1074/jbc.M109.036616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sevilya Z, Loya S, Hughes SH, Hizi A. The ribonuclease H activity of the reverse transcriptases of human immunodeficiency viruses type 1 and type 2 is affected by the thumb subdomain of the small protein subunits. J Mol Biol. 2001;311:957–971. doi: 10.1006/jmbi.2001.4904. [DOI] [PubMed] [Google Scholar]
- 46.Post K, Guo J, Howard KJ, Powell MD, Miller JT, Hizi A, Le Grice SF, Levin JG. Human immunodeficiency virus type 2 reverse transcriptase activity in model systems that mimic steps in reverse transcription. J Virol. 2003;77:7623–7634. doi: 10.1128/JVI.77.13.7623-7634.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.MacNeil A, Sarr AD, Sankale JL, Meloni ST, Mboup S, Kanki P. Direct evidence of lower viral replication rates in vivo in human immunodeficiency virus type 2 (HIV-2) infection than in HIV-1 infection. J Virol. 2007;81:5325–5330. doi: 10.1128/JVI.02625-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reid P, MacInnes H, Cong ME, Heneine W, Gerardo Garcia-Lerma J. Natural resistance of human immunodeficiency virus type 2 to zidovudine. Virology. 2005;336:251–264. doi: 10.1016/j.virol.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 49.Witvrouw M, Pannecouque C, Switzer WM, Folks TM, De Clercq E, Heneine W. Susceptibility of HIV-2, SIV and SHIV to various anti-HIV-1 compounds: implications for treatment and postexposure prophylaxis. Antiviral Ther. 2004;9:57–65. [PubMed] [Google Scholar]
- 50.Gu Z, Gao Q, Fang H, Salomon H, Parniak MA, Goldberg E, Cameron J, Wainberg MA. Identification of a mutation at codon 65 in the IKKK motif of reverse transcriptase that encodes human immunodeficiency virus resistance to 2′,3′-dideoxycytidine and 2′,3′-dideoxy-3′-thiacytidine. Antimicrob Agents Chemother. 1994;38:275–281. doi: 10.1128/aac.38.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnson VA, Calvez V, Gunthard HF, Paredes R, Pillay D, Shafer R, Wensing AM, Richman DD. 2011 update of the drug resistance mutations in HIV-1. Topics in Antiviral Medicine. 2011;19:156–164. [PMC free article] [PubMed] [Google Scholar]
- 52.Stone C, Ait-Khaled M, Craig C, Griffin P, Tisdale M. Human immunodeficiency virus type 1 reverse transcriptase mutation selection during in vitro exposure to tenofovir alone or combined with abacavir or lamivudine. Antimicrob Agents Chemother. 2004;48:1413–1415. doi: 10.1128/AAC.48.4.1413-1415.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Winters MA, Shafer RW, Jellinger RA, Mamtora G, Gingeras T, Merigan TC. Human immunodeficiency virus type 1 reverse transcriptase genotype and drug susceptibility changes in infected individuals receiving dideoxyinosine monotherapy for 1 to 2 years. Antimicrob Agents Chemother. 1997;41:757–762. doi: 10.1128/aac.41.4.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Larder BA, Kemp SD. Multiple mutations in HIV-1 reverse transcriptase confer high-level resistance to zidovudine (AZT) Science. 1989;246:1155–1158. doi: 10.1126/science.2479983. [DOI] [PubMed] [Google Scholar]
- 55.Boyer PL, Sarafianos SG, Arnold E, Hughes SH. The M184V mutation reduces the selective excision of zidovudine 5′-monophosphate (AZTMP) by the reverse transcriptase of human immunodeficiency virus type 1. J Virol. 2002;76:3248–3256. doi: 10.1128/JVI.76.7.3248-3256.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meyer PR, Matsuura SE, So AG, Scott WA. Unblocking of chain-terminated primer by HIV-1 reverse transcriptase through a nucleotide-dependent mechanism. Proc Natl Acad Sci U S A. 1998;95:13471–13476. doi: 10.1073/pnas.95.23.13471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sarafianos SG, Das K, Hughes SH, Arnold E. Taking aim at a moving target: designing drugs to inhibit drug-resistant HIV-1 reverse transcriptases. Curr Opin Struct Biol. 2004;14:716–730. doi: 10.1016/j.sbi.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 58.Boyer PL, Sarafianos SG, Arnold E, Hughes SH. Selective excision of AZTMP by drug-resistant human immunodeficiency virus reverse transcriptase. J Virol. 2001;75:4832–4842. doi: 10.1128/JVI.75.10.4832-4842.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Descamps D, Damond F, Matheron S, Collin G, Campa P, Delarue S, Pueyo S, Chene G, Brun-Vezinet F. High frequency of selection of K65R and Q151M mutations in HIV-2 infected patients receiving nucleoside reverse transcriptase inhibitors containing regimen. J Med Virol. 2004;74:197–201. doi: 10.1002/jmv.20174. [DOI] [PubMed] [Google Scholar]
- 60.Tambuyzer L, Azijn H, Rimsky LT, Vingerhoets J, Lecocq P, Kraus G, Picchio G, de Bethune MP. Compilation and prevalence of mutations associated with resistance to non-nucleoside reverse transcriptase inhibitors. Antiviral Ther. 2009;14:103–109. [PubMed] [Google Scholar]
- 61.De Clercq E. Non-nucleoside reverse transcriptase inhibitors (NNRTIs): past, present, and future. Chem Biodiversity. 2004;1:44–64. doi: 10.1002/cbdv.200490012. [DOI] [PubMed] [Google Scholar]
- 62.Spence RA, Kati WM, Anderson KS, Johnson KA. Mechanism of inhibition of HIV-1 reverse transcriptase by nonnucleoside inhibitors. Science. 1995;267:988–993. doi: 10.1126/science.7532321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.De Clercq E. The role of non-nucleoside reverse transcriptase inhibitors (NNRTIs) in the therapy of HIV-1 infection. Antiviral Res. 1998;38:153–179. doi: 10.1016/s0166-3542(98)00025-4. [DOI] [PubMed] [Google Scholar]
- 64.Richman DD. Resistance of clinical isolates of human immunodeficiency virus to antiretroviral agents. Antimicrob Agents Chemother. 1993;37:1207–1213. doi: 10.1128/aac.37.6.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rubsamen-Waigmann H, Huguenel E, Shah A, Paessens A, Ruoff HJ, von Briesen H, Immelmann A, Dietrich U, Wainberg MA. Resistance mutations selected in vivo under therapy with anti-HIV drug HBY 097 differ from resistance pattern selected in vitro. Antiviral Res. 1999;42:15–24. doi: 10.1016/s0166-3542(99)00010-8. [DOI] [PubMed] [Google Scholar]
- 66.Huang P, Farquhar D, Plunkett W. Selective action of 3′-azido-3′-deoxythymidine 5′-triphosphate on viral reverse transcriptases and human DNA polymerases. J Biol Chem. 1990;265:11914–11918. [PubMed] [Google Scholar]
- 67.Salie ZL, Kirby KA, Michailidis E, Marchand B, Singh K, Rohan LC, Kodama EN, Mitsuya H, Parniak MA, Sarafianos SG. Structural basis of HIV inhibition by translocation-defective RT inhibitor 4′-ethynyl-2-fluoro-2′-deoxyadenosine (EFdA) Proc Natl Acad Sci U S A. 2016;113:9274–9279. doi: 10.1073/pnas.1605223113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang H, Chopra R, Verdine GL, Harrison SC. Structure of a covalently trapped catalytic complex of HIV-1 reverse transcriptase: implications for drug resistance. Science. 1998;282:1669–1675. doi: 10.1126/science.282.5394.1669. [DOI] [PubMed] [Google Scholar]
- 69.Jamburuthugoda VK, Guo D, Wedekind JE, Kim B. Kinetic evidence for interaction of human immunodeficiency virus type 1 reverse transcriptase with the 3′-OH of the incoming dTTP substrate. Biochemistry. 2005;44:10635–10643. doi: 10.1021/bi050611+. [DOI] [PubMed] [Google Scholar]
- 70.Michailidis E, Ryan EM, Hachiya A, Kirby KA, Marchand B, Leslie MD, Huber AD, Ong YT, Jackson JC, Singh K, Kodama EN, Mitsuya H, Parniak MA, Sarafianos SG. Hypersusceptibility mechanism of Tenofovir-resistant HIV to EFdA. Retrovirology. 2013;10:65. doi: 10.1186/1742-4690-10-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kirby KA, Singh K, Michailidis E, Marchand B, Kodama EN, Ashida N, Mitsuya H, Parniak MA, Sarafianos SG. The sugar ring conformation of 4′-ethynyl-2-fluoro-2′-deoxyadenosine and its recognition by the polymerase active site of HIV reverse transcriptase. Cell Mol Biol (Sarreguemines, Fr, Online) 2011;57:40–46. [PMC free article] [PubMed] [Google Scholar]
- 72.Kirby KA, Michailidis E, Fetterly TL, Steinbach MA, Singh K, Marchand B, Leslie MD, Hagedorn AN, Kodama EN, Marquez VE, Hughes SH, Mitsuya H, Parniak MA, Sarafianos SG. Effects of substitutions at the 4′ and 2 positions on the bioactivity of 4′-ethynyl-2-fluoro-2′-deoxyadenosine. Antimicrob Agents Chemother. 2013;57:6254–6264. doi: 10.1128/AAC.01703-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Esnouf R, Ren J, Ross C, Jones Y, Stammers D, Stuart D. Mechanism of inhibition of HIV-1 reverse transcriptase by non-nucleoside inhibitors. Nat Struct Biol. 1995;2:303–308. doi: 10.1038/nsb0495-303. [DOI] [PubMed] [Google Scholar]
- 74.Ding J, Das K, Hsiou Y, Sarafianos SG, Clark AD, Jr, Jacobo-Molina A, Tantillo C, Hughes SH, Arnold E. Structure and functional implications of the polymerase active site region in a complex of HIV-1 RT with a double-stranded DNA template-primer and an antibody Fab fragment at 2.8 A resolution. J Mol Biol. 1998;284:1095–1111. doi: 10.1006/jmbi.1998.2208. [DOI] [PubMed] [Google Scholar]
- 75.Kohlstaedt LA, Wang J, Friedman JM, Rice PA, Steitz TA. Crystal structure at 3.5 A resolution of HIV-1 reverse transcriptase complexed with an inhibitor. Science. 1992;256:1783–1790. doi: 10.1126/science.1377403. [DOI] [PubMed] [Google Scholar]
- 76.Das K, Martinez SE, Bauman JD, Arnold E. HIV-1 reverse transcriptase complex with DNA and nevirapine reveals non-nucleoside inhibition mechanism. Nat Struct Mol Biol. 2012;19:253–259. doi: 10.1038/nsmb.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Das K, Martinez SE, Bandwar RP, Arnold E. Structures of HIV-1 RT-RNA/DNA ternary complexes with dATP and nevirapine reveal conformational flexibility of RNA/DNA: insights into requirements for RNase H cleavage. Nucleic Acids Res. 2014;42:8125–8137. doi: 10.1093/nar/gku487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sarafianos SG, Marchand B, Das K, Himmel DM, Parniak MA, Hughes SH, Arnold E. Structure and function of HIV-1 reverse transcriptase: molecular mechanisms of polymerization and inhibition. J Mol Biol. 2009;385:693–713. doi: 10.1016/j.jmb.2008.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Singh K, Marchand B, Kirby KA, Michailidis E, Sarafianos SG. Structural Aspects of Drug Resistance and Inhibition of HIV-1 Reverse Transcriptase. Viruses. 2010;2:606–638. doi: 10.3390/v2020606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Menendez-Arias L. Molecular basis of human immunodeficiency virus type 1 drug resistance: overview and recent developments. Antiviral Res. 2013;98:93–120. doi: 10.1016/j.antiviral.2013.01.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


