Abstract
Alzheimer’s disease (AD) is characterized neuropathologically by neuronal cell loss, extracellular neuritic plaques composed of β-amyloid (Aβ), and intracellular neurofibrillary tangles composed of hyperphosphorylated tau protein. Aβ is generated by proteolytic processing of the β-amyloid precursor protein (APP). Most individuals with Down syndrome (DS) have three copies of APP, leading to elevated APP expression, increased Aβ deposition, and characteristic AD neuropathology. Sequencing of APP in familial early-onset AD identified missense mutations that cause AD, while a recently discovered coding variant, APP A673T, reduces the risk for AD. Cellular and animal studies show that risk-associated mutations increase total Aβ levels, Aβ42 levels, or Aβ fibrillogenesis, while protective alleles reduce Aβ levels. Together, these studies provide compelling evidence for the Aβ hypothesis and suggest that therapeutics that reduces Aβ levels or Aβ fibrillogenesis should lower the risk for or prevent AD.
β-Amyloid precursor protein (APP) is cleaved to form β-amyloid (Aβ), which aggregates in the brain in Alzheimer’s disease. Changes in the APP gene sequence or its expression may influence Aβ levels and disease risk.
Alzheimer’s disease (AD) exists as two genetically distinct forms: familial AD (fAD), which is usually characterized by the clinical onset before 60 years of age and Mendelian inheritance, and late-onset or sporadic AD (sAD) (Sadowski et al. 1999), which usually has a clinical onset after 60 years of age and exhibits no consistent pattern of inheritance (Bertram and Tanzi 2005). Early-onset fAD, which represents <1% of AD cases, is caused by rare and fully penetrant mutations in three different genes encoding β-amyloid precursor protein (APP) on chromosome 21, presenilin-1 (PSEN1) on chromosome 14, and presenilin-2 (PSEN2) on chromosome 1. (For additional details on the presenilin complexes, see Johnson et al. 2016.) In contrast, the most common form of the disease, late-onset or sAD, probably reflects the cumulative effects of both common and rare genetic risk factors and the environment. The ɛ4 allele of the apolipoprotein E (APOE) gene is the most common risk factor for AD and is associated with a dose-dependent increase in the risk of developing late-onset AD (a threefold increase for one copy of APOE4 and a 10-fold increase for two copies of APOE4) and decrease in age at onset.
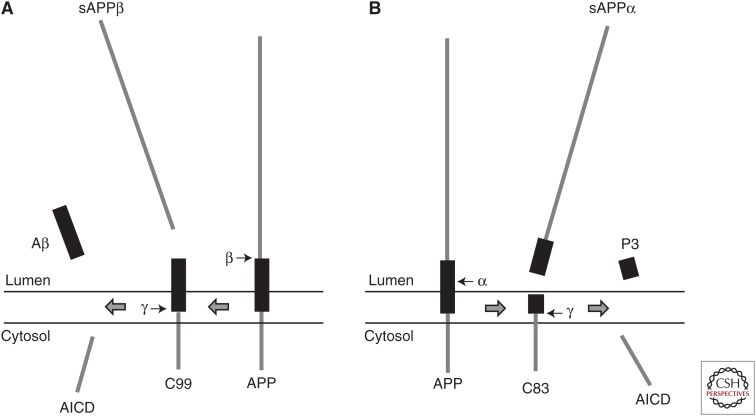
The human APP gene was first identified in 1987 using partial protein sequence information from purified β-amyloid (Aβ) to identify the corresponding cDNA (Kang et al. 1987). The gene was mapped to chromosome 21 (21q21.2-3) (Goldgaber et al. 1987; Tanzi et al. 1987). APP is a type I membrane protein with a large extracellular domain and a short cytoplasmic region. Two cleavage events, one in the extracellular domain (β-secretase cleavage) and one in the transmembrane region (γ-secretase cleavage), are necessary to release Aβ from APP (Fig. 1A). Several different APP proteins can be derived by alternative splicing from this single gene (695–770 amino acids). The major splice form in neurons is APP695 (Sandbrink et al. 1996).
Figure 1.
Processing of β-amyloid precursor protein (APP) by the secretases: (A) amyloidogenic processing and (B) nonamyloidogenic processing.
The precursor proteins are proteolytically cleaved by two distinct pathways. In the nonamyloidogenic processing pathway, APP is cleaved within the Aβ domain by α-secretase, with the formation of a large soluble ectodomain (sAPPα) and an 83-residue membrane-associated C-terminal fragment (C83) (Fig. 1B). Several members of the ADAM family of proteases have α-secretase activity. Subsequent cleavage of C83 by γ-secretase leads to the formation of P3 and the APP intracellular domain (AICD) (Fig. 1B). In the amyloidogenic pathway, APP is cleaved at the N-terminus of the Aβ domain by β-secretase or BACE, a membrane-tethered protease, resulting in the generation of a soluble ectodomain (sAPPβ) and a 99-residue, membrane-retained C-terminal fragment (C99) (Fig. 1A). Subsequently, γ-secretase, a membrane-embedded complex with presenilin as the catalytic component, cleaves C99 to release Aβ peptides and AICD (Fig. 1A) (Haass 2004). Because the site of γ-secretase cleavage is promiscuous, it generates Aβ peptides with different C termini, including Aβ1–40 (Aβ40), Aβ1–42 (Aβ42), and other minor species (Ling et al. 2003; Kaminsky et al. 2010). Under normal physiological conditions, Aβ40 is the most abundant species generated in the amyloidogenic pathway, with Aβ42 representing only 10% of total Aβ (Wiltfang et al. 2002). However, Aβ42 is considered the harmful peptide because it is more prone to fibril formation and promotes Aβ aggregates, which are the key effectors of neurotoxicity (Wolfe and Guenette 2007; see also Prusiner 2016; Tycko 2016).
Although mutations in the APP gene explain only a small proportion of AD cases, these mutations, including duplication of APP and missense mutations, directly implicate Aβ generation as a causal factor in AD pathology. The mechanistic link between AD and APP has been further strengthened by studying Down syndrome (DS).
DOWN SYNDROME: TRISOMY OF CHROMOSOME 21
DS is the most common human aneuploidy, caused by trisomy of all or part of human chromosome 21 (HSA21) (Patterson 2009). This additional genetic material alters brain development and causes lifelong intellectual disability. Interestingly, AD pathology occurs at a high frequency in DS patients and progresses in an age-dependent manner. All individuals with DS caused by complete trisomy of HSA21 develop a neuropathology indistinguishable from AD by the age of 30–40 years (Burger and Vogel 1973; Oyama et al. 1994), and 67% develop an AD-type dementia by the age of 72 (Wisniewski et al. 1985; Mann and Esiri 1989; Zigman 2013). Multiple brain regions in individuals with DS undergo significant atrophy and loss of neurons with increasing age. The brains of older adults with DS show more than 40% of total volume loss and a 90% reduction in neuronal density in the entorhinal cortex. Additionally, like individuals with AD, individuals with DS display an age-dependent Aβ deposition, progression of neuroinflammation, neurofibrillary tangles, hyperphosphorylation of the microtubule-associated protein tau, and degeneration of basal forebrain cholinergic neurons (Hof et al. 1995; Sadowski et al. 1999). Aβ deposits begin to appear in individuals with DS as young as 10 years of age but are consistently found in the brains of DS individuals over 40 (Rumble et al. 1989). The abnormal accumulation of Aβ in the brains of both AD and DS patients induces cognitive decline through neural dysfunction. Because the APP gene is located on HSA21, it is present in three copies in DS individuals, leading to overexpression of APP and increased generation of Aβ. Even in the brains of fetuses with DS, the excess gene dosage of APP leads to early elevation of Aβ levels (Teller et al. 1996). It has been hypothesized that the triplication of APP in DS leads to AD symptoms early in life through overexpression of APP (Rumble et al. 1989), followed by deposition of Aβ and neurodegeneration (Wisniewski et al. 1985). Studies of DS, therefore, strongly support APP as a candidate causal gene in AD. However, a caveat to this is that many other genes are encoded by chromosome 21 and are overexpressed in DS (Hattori et al. 2000). Studies of the small percentage of DS cases that are caused by partial trisomy of HSA21 have helped to address this issue. Fine mapping in these individuals has shown that the so-called DS critical region (21q22.3), which is sufficient to produce the characteristic facies and developmental delay associated with DS, does not include the APP gene (Korenberg et al. 1990). However, AD neuropathology was not among the cardinal features considered for the DS phenotypes in this study. Other supportive evidence comes from the postmortem examination of a 78-yr-old woman with DS features because of a partial trisomy involving the distal 21q region. Despite her advanced age, she had no neuropathological evidence of AD (Prasher et al. 1998). Although a segment of HSA21 was triplicated, this region did not include the APP gene. Thus, the study of partial trisomies of HSA21 supports the hypothesis that triplication of APP is necessary for AD pathology in DS cases. These data also strongly suggest that DS and AD share pathogenic mechanisms and that the early onset of AD pathology in DS is in part a result of overexpression of the APP gene by gene–dosage imbalance (Salehi et al. 2006).
APP LOCUS DUPLICATION FAMILIES
Another feature of the mammalian genome that induces variation in gene expression is the presence of copy number variations (CNVs), which include both gene duplications and deletions. CNVs are regions of DNA, which can be variable in size, the copy number of which varies between individuals. Both common and rare de novo CNVs have been reported in the human genome (Zarrei et al. 2015). Much effort has been expended to identify and map CNVs in normal individuals and in disease (Henrichsen et al. 2009). Duplication of a region of HSA21 containing the APP gene has been reported to cause AD in several families with an autosomal-dominant form of the disease (Rovelet-Lecrux et al. 2006; Sleegers et al. 2006). Genomic duplications of small regions of HSA21, including the APP locus, have been reported in nine families of different ethnic origins—French, Dutch, Japanese, and Swedish (Rovelet-Lecrux et al. 2006; Sleegers et al. 2006; Guyant-Marechal et al. 2008; Kasuga et al. 2009; Thonberg et al. 2011). These families have different overlapping duplications that each includes the APP locus. None of the families exhibited any clinical features suggestive of DS, other than progressive dementia of the AD type. Neuropathological examination of these brains showed abundant Aβ deposits and neurofibrillary tangles in the parenchyma and the induction of Aβ-related cerebral amyloid angiopathy (CAA) in the cerebral vasculature (Ellis et al. 1996; Pfeifer et al. 2002; Guyant-Marechal et al. 2008).
To address how far the duplications extend into the flanking chromosomal regions and investigate whether any neighboring genes were duplicated in addition to APP, copy-number assays targeting the genes around APP were analyzed in these families. Further fine mapping of the chromosomal region by array-comparative genome hybridization confirmed the presence of a genomic duplication of the APP gene and evaluated the size of the duplicated region. The measured size of duplicated segments, including the APP locus from five French families, ranged from 0.58 to 6.37 Mb and contained from five to 12 annotated genes, centromeric to the DS critical region (Rahmani et al. 1989). Compared to the French families, the duplicated genomic region in early-onset AD Dutch patients was much smaller (0.7 Mb) and included no other genes but APP (Sleegers et al. 2006). A real-time quantitative polymerase chain reaction of the APP promoter confirmed that the genomic duplication included APP and its promoter region but none of the adjacent genes. This suggests that a genomic duplication of APP is sufficient to cause the mixed phenotype of AD and CAA, without contribution from any of the adjacent genes. The size of the duplicated region in the Swedish cases was also small, 1.01–1.09 Mb, and included no flanking genes.
In the French families, a genomic duplication in the APP locus was observed in five out of 65 families (nearly 8%) with early-onset AD (Rovelet-Lecrux et al. 2006). In the Dutch population-based sample, APP duplications were detected at a frequency of one in 10 (10%) of the early-onset AD cases (Sleegers et al. 2006). While in the Swedish sample, one in 22 individuals (4.5%) diagnosed with clinical early-onset AD carried a duplication on HSA21, including the APP locus (Thonberg et al. 2011). Together with the data from partial trisomies of HSA21, these families provide compelling evidence that duplication of APP, resulting in overexpression of APP and elevated Aβ levels, is sufficient to cause AD pathology and CAA.
MISSENSE MUTATIONS IN THE APP GENE AND ITS NEUROPATHOLOGICAL PROFILE
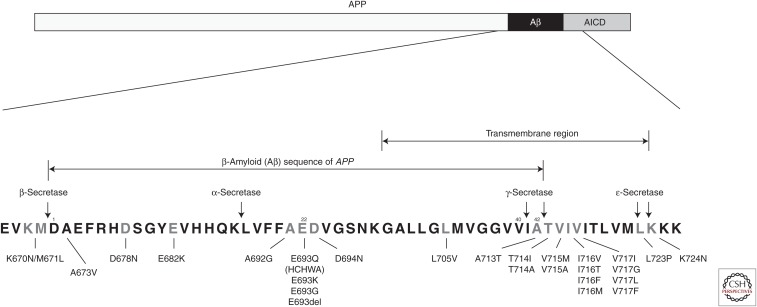
Like the duplications in APP, most missense variants in APP are associated with autosomal-dominant inheritance of AD, usually with complete penetrance by age 60 (www.molgen.ua.ac.be/ADMutations). An autosomal-recessive mutation at codon 673 of APP was recently discovered and reported to be associated with AD (Giaccone et al. 2010). The APP gene is encoded by 18 exons that are alternatively spliced to produce proteins ranging in size from 695 to 770 amino acids. The Aβ peptide is encoded by parts of exons 16 and 17 (Yoshikai et al. 1990). To date, 26 pathogenic missense mutations have been reported within the APP gene (Table 1). These mutations are located within or immediately flanking the Aβ sequence. Aβ is generated from APP by the sequential cleavage of two enzymes, β-secretase and γ-secretase (Figs. 1A and 2). We will discuss further the mechanisms by which these mutations act and display their neuropathological hallmarks.
Table 1.
Number of pathogenic mutations in APP gene in each type and domain
| Type | Domain | Number of mutations |
|---|---|---|
| APP duplication | Entire sequence | 26 (50.0%) |
| APP missense mutation | N terminal Aβ sequence C terminal |
1 (1.9%) 11 (21.2%) 14 (26.9%) |
| Total | 52 |
Figure 2.
Scheme depicting the structure of the APP sequence (top) and the location of pathogenic substitutions in APP (bottom).
Autosomal-Dominant Mutations in APP and Their Associated Neuropathological Profiles
Mutations in the N-Terminal Aβ Domain and Their Effects on Aβ Formation
A double mutation in exon 16 of APP at codons 670 and 671 (using the numbering associated with the longest transcript of APP, APP770) was identified in a Swedish fAD pedigree (Mullan et al. 1992). These mutations result in a lysine-to-asparagine substitution at codon 670 and a methionine-to-leucine substitution at codon 671 (K670N/M671L) in the APP protein (Fig. 2; Table 1). These mutations are located at the N terminus of Aβ at the β-secretase cleavage site within the extracellular domain of APP. The K670N/M671L mutation appears to augment the production of total Aβ, resulting in higher levels of both Aβ40 and Aβ42 in vitro (Busciglio et al. 1993; Citron et al. 1994). In addition, a significant threefold increase in Aβ release is detected in peripheral fibroblasts from individuals carrying the Swedish mutation.
Mutations within the Aβ Domain and Their Effects on Aβ Formation
Ten pathogenic mutations have been reported within the Aβ sequence: D678N, E682K (Leuven mutation), A692G (Flemish mutation), E693Q (Dutch mutation, HCHWA-D), E693K (Italian mutation), E693G (Arctic mutation), E693del, D694N (Iowa mutation), L705V, and A713T (Fig. 2; Table 1). The first pathogenic mutation to be reported in the APP gene was APP E693Q, reported in a Dutch family with an inherited cerebral hemorrhage with amyloidosis (HCHWA-D) in which the amyloidosis is caused by Aβ (Levy et al. 1990). In vitro analysis of the full-length Aβ peptide or fragments containing the E693Q mutation has revealed that the mutated peptide aggregates and forms amyloid-like fibrils much faster than the wild-type (WT) Aβ sequence (van Duinen et al. 1987; Wisniewski et al. 1991).
Mutations within the Aβ domain can have complicated effects on APP processing, including impaired α-secretase cleavage and increased hydrophobicity of secreted Aβ species, thereby enhancing aggregation of Aβ into amyloid fibrils (Wisniewski et al. 1991; Haass et al. 1994). For example, E693G enhances Aβ protofibril formation (Nilsberth et al. 2001). In contrast to other fAD cases with predominantly Aβ42 plaques in the brain, it has been shown that AD patients carrying the A692G mutation predominantly deposit Aβ40, particularly in the vascular walls (Hendriks et al. 1992). Mutations associated with elevated Aβ40 species are associated with significant CAA and hemorrhagic stroke.
Mutations in the C-Terminal Aβ Domain and Their Effects on Aβ Formation
The first mutation to be associated with fAD was V717l in exon 17 of APP in a family of British origin with an age at onset in the mid-50s (Goate et al. 1991). Since this report, many other families have been reported carrying this same mutation as well as several other mutations at the same amino acid (V717G, V717L, and V717F). Early-onset AD mutations close to the γ-secretase cleavage site within the transmembrane domain of APP are located at APP714–717 and near the ɛ-secretase cleavage site at APP723–724. Fourteen mutations have been reported in the C-terminal Aβ domain, including T714l/A, V715M/A, V716V/T/F/M, and V717l/G/L/F at codons 714–717, and L723P and K724N at codons 723–724 (Fig. 2; Table 1). These mutations influence the activity of their respective secretases, resulting in aberrant processing of APP. Indeed, these APP mutations near the C-terminal region of the Aβ sequence lead to a selective increase in the production of longer Aβ peptides, especially those ending at residue 42 (Aβ42), which is prone to more rapid aggregation (Hardy 1997).
Neuropathological Profile of Autosomal-Dominant Mutations
Autosomal-dominant APP mutations close to the sites of β- or γ-secretase cleavage and flanking the Aβ sequence cause either elevated levels of total Aβ production or a specific increase in Aβ42 peptides, which are more hydrophobic and thus more prone to fibrillogenesis (Suzuki et al. 1994; Tamaoka et al. 1994; Younkin 1994). However, those that alter amino acids within the Aβ domain facilitate rapid aggregation and fibrillization (Levy et al. 2006). Mutations that cause an increase in Aβ42 peptides are associated with the classical clinical and neuropathological features of AD, including progressive dementia and senile plaques, neurofibrillary tangles, and neuronal cell loss (Rocchi et al. 2003). In contrast, mutations that are associated with an increase in total Aβ also have prominent Aβ deposition in cerebral vessels (CAA) and can be clinically associated with cerebral hemorrhages and stroke (Kumar-Singh et al. 2002; Castellani et al. 2004). Although both CAA and senile plaques are observed in the brains of patients with DS, HCHWA-D, and AD, mutations within the Aβ sequence are predominantly vasculotropic and lack neurofibrillary tangles (Obici et al. 2005; Bugiani et al. 2010). These mutations are also associated with more severe CAA, which is widely distributed throughout the brain (van Duinen et al. 1987; Hendriks et al. 1992; Rossi et al. 2004; Rovelet-Lecrux et al. 2006).
Autosomal-Recessive Mutation and Its Neuropathological Profile
The A673V Mutation and Its Effects on Aβ Formation
A novel APP mutation, an alanine-to-valine substitution at codon 673 (A673V), has been observed as a homozygous recessive mutation in a single Italian pedigree (Fig. 2) (Giaccone et al. 2010). Patients with A673V have early-onset AD with behavioral abnormalities at the onset and neurological deficits in later stages. On the basis of the formal neuropsychological assessment, individuals heterozygous for this mutation (aged between 21 and 88 years) had no signs of cognitive decline even at an advanced age.
The A673V APP variant shifts APP processing toward the amyloidogenic pathway, with increased production of Aβ peptides, and markedly enhances the aggregation and fibrillogenic properties of both Aβ40 and Aβ42 (Giaccone et al. 2010). Aβ40 is markedly increased in the insoluble fraction and is predominant over Aβ42, suggesting that this mutation strongly enhances the formation of Aβ40 aggregates (Di Fede et al. 2012). However, in vitro studies with synthetic peptides revealed that co-incubation of Aβ containing the A673V variant and WT Aβ species resulted in reduced amyloidogenesis and neurotoxicity of Aβ, consistent with the observation that heterozygous carriers do not develop the disease. These opposite effects of the A673V mutation on amyloidogenesis, in the homozygous and heterozygous states, likely account for the autosomal-recessive pattern of inheritance.
Neuropathological Profile of the A673V Mutation in the Homozygous State
Patients homozygous for the A673V mutation are characterized neuropathologically by the presence of both plaques and tangles but show several distinctive features compared with AD patients carrying dominant mutations (Giaccone et al. 2010). Patients displayed abundant Aβ deposits and CAA in all areas of the cerebral cortex, but the Aβ deposits were unusually large (up to 120 mm in diameter), and the localization of Aβ deposits was consistently perivascular.
PROTECTIVE RECOMBINANT A673T
At the same position in the Aβ peptide, APP673, where the recessive mutation was found (Fig. 2), three homozygous carriers of an alanine-to-threonine substitution (A673T) were discovered by whole-genome sequencing in Icelandic samples (Jonsson et al. 2012). None of these homozygous carriers, whose ages ranged from 67 to 88 years, had a history of dementia. This substitution is adjacent to the β-secretase cleavage site in the APP gene and results in an ∼40% reduction in the formation of Aβ peptides in vitro (Jonsson et al. 2012). This variant was found to be significantly more common in the aged control group than in AD cases (0.62% vs. 0.13%; odds ratio = 5.29; P = 4.78 × 10−7), suggesting that A673T reduces the risk for AD. A673T is a rare variant, with a reported frequency of 0.2%, 0.4%, 0.45%, and 0.5% in the Norwegian, Swedish, Icelandic, and Finnish general populations, respectively, whereas it has not been observed in elderly individuals of Asian descent and is rarely seen in the U.S. population (Jonsson et al. 2012; Kero et al. 2013; Liu et al. 2014).
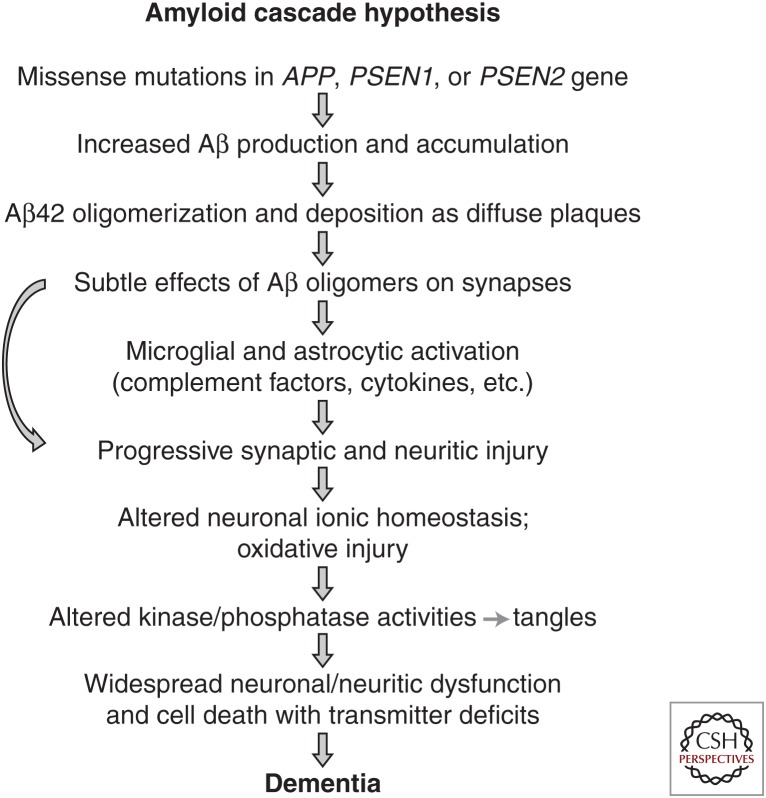
In vitro studies show that A673T results in a reduced production of sAPPβ and ∼40% less Aβ40 and Aβ42 compared with WT APP, suggesting that A673T reduces BACE1 (β-secretase) cleavage of APP. To further confirm the protective effect of A673T against AD, an in vitro BACE1 cleavage assay was performed with a WT synthetic APP peptide and a peptide bearing the A673T substitution. The A673T APP peptide was processed ∼50% less efficiently than the WT substrate, suggesting that A673T carriers impaired BACE1 cleavage of APP (Jonsson et al. 2012). It has been shown that the A673T substitution decreases the catalytic turnover rate of APP by BACE1, thereby reducing Aβ aggregation (Maloney et al. 2014). Thus, this mutation prevents Aβ aggregation by inhibiting the generation of Aβ peptide from APP. The observation of this rare protective variant that decreases Aβ production and reduces the risk of AD provides compelling evidence in support of the amyloid hypothesis: mutations that increase Aβ levels increase the risk for AD, whereas those that decrease Aβ levels reduce the risk for the disease (Fig. 3) (Hardy and Selkoe 2002).
Figure 3.
Proposed sequence of pathogenic events leading to Alzheimer’s disease.
CONCLUDING REMARKS
The discovery of mutations in APP that increase or decrease Aβ production and the risk for AD provides strong support for the amyloid cascade hypothesis, which posits that accumulation of Aβ is the primary effector of AD pathogenesis (Fig. 3) (Hardy and Selkoe 2002). The amyloid hypothesis proposes that AD is caused by altered APP expression or APP-mutation-induced Aβ aggregation, following an imbalance between Aβ production and Aβ clearance.
Genetics and genomic studies of APP have identified 52 pathogenic mutations in APP that can lead to Aβ deposition in the brain parenchyma and in cerebral blood vessels. Studies of these families have conclusively shown that overexpression of the normal APP sequence (trisomy 21 or APP duplication) or mutations that lead to elevated total Aβ, elevated Aβ42, or increased Aβ aggregation lead to dementia and AD neuropathology. In the presence of elevated total Aβ, there is also widespread CAA and hemorrhagic stroke. In contrast, mutations that decrease Aβ levels substantially reduce the risk of developing AD.
Introduction of missense mutations in human APP into transgenic mice has enabled the recapitulation of at least some aspects of AD. These mice show an age-dependent deposition of Aβ in the brain. As is observed in human patients, mice carrying the Swedish mutation (Tg2576) develop diffuse and dense-cored plaques in the brain as well as severe CAA (Hsiao et al. 1996), whereas mice expressing mutations at the C terminus of Aβ, such as the V717F mutation, develop plaques but not CAA (Games et al. 1995). These animals do not exhibit neurofibrillary tangles or widespread neuronal loss, but they do exhibit some synapse loss. Furthermore, mice unable to generate Aβ, such as the BACE−/− mouse, show no neuronal loss and improved cognitive function (Ohno et al. 2004). Together, the human and animal studies strongly support the notion that a therapeutic strategy aimed at reducing Aβ levels by either inhibiting production or increasing clearance could be useful, particularly if applied early enough during the course of disease.
ACKNOWLEDGMENTS
A.G. and J.TCW are supported by grants from the National Institute on Aging, the JPB Foundation, and the Ronald M. Loeb Center for Alzheimer’s Disease.
Footnotes
Editor: Stanley B. Prusiner
Additional Perspectives on Prion Diseases available at www.perspectivesinmedicine.org
REFERENCES
*Reference is also in this collection.
- Bertram L, Tanzi RE. 2005. The genetic epidemiology of neurodegenerative disease. J Clin Invest 115: 1449–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugiani O, Giaccone G, Rossi G, Mangieri M, Capobianco R, Morbin M, Mazzoleni G, Cupidi C, Marcon G, Giovagnoli A, et al. 2010. Hereditary cerebral hemorrhage with amyloidosis associated with the E693K mutation of APP. Arch Neurol 67: 987–995. [DOI] [PubMed] [Google Scholar]
- Burger PC, Vogel FS. 1973. The development of the pathologic changes of Alzheimer's disease and senile dementia in patients with Down's syndrome. Am J Pathol 73: 457–476. [PMC free article] [PubMed] [Google Scholar]
- Busciglio J, Gabuzda DH, Matsudaira P, Yankner BA. 1993. Generation of β-amyloid in the secretory pathway in neuronal and nonneuronal cells. Proc Natl Acad Sci 90: 2092–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellani RJ, Smith MA, Perry G, Friedland RP. 2004. Cerebral amyloid angiopathy: Major contributor or decorative response to Alzheimer’s disease pathogenesis. Neurobiol Aging 25: 599–602; discussion 603–594. [DOI] [PubMed] [Google Scholar]
- Citron M, Vigo-Pelfrey C, Teplow DB, Miller C, Schenk D, Johnston J, Winblad B, Venizelos N, Lannfelt L, Selkoe DJ. 1994. Excessive production of amyloid β-protein by peripheral cells of symptomatic and presymptomatic patients carrying the Swedish familial Alzheimer disease mutation. Proc Natl Acad Sci 91: 11993–11997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Fede G, Catania M, Morbin M, Giaccone G, Moro ML, Ghidoni R, Colombo L, Messa M, Cagnotto A, Romeo M, et al. 2012. Good gene, bad gene: New APP variant may be both. Prog Neurobiol 99: 281–292. [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Olichney JM, Thal LJ, Mirra SS, Morris JC, Beekly D, Heyman A. 1996. Cerebral amyloid angiopathy in the brains of patients with Alzheimer’s disease: The CERAD experience, Part XV. Neurology 46: 1592–1596. [DOI] [PubMed] [Google Scholar]
- Games D, Adams D, Alessandrini R, Barbour R, Berthelette P, Blackwell C, Carr T, Clemens J, Donaldson T, Gillespie F, et al. 1995. Alzheimer-type neuropathology in transgenic mice overexpressing V717F β-amyloid precursor protein. Nature 373: 523–527. [DOI] [PubMed] [Google Scholar]
- Giaccone G, Morbin M, Moda F, Botta M, Mazzoleni G, Uggetti A, Catania M, Moro ML, Redaelli V, Spagnoli A, et al. 2010. Neuropathology of the recessive A673V APP mutation: Alzheimer disease with distinctive features. Acta Neuropathol 120: 803–812. [DOI] [PubMed] [Google Scholar]
- Goate A, Chartier-Harlin MC, Mullan M, Brown J, Crawford F, Fidani L, Giuffra L, Haynes A, Irving N, James L, et al. 1991. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature 349: 704–706. [DOI] [PubMed] [Google Scholar]
- Goldgaber D, Lerman MI, McBride OW, Saffiotti U, Gajdusek DC. 1987. Characterization and chromosomal localization of a cDNA encoding brain amyloid of Alzheimer’s disease. Science 235: 877–880. [DOI] [PubMed] [Google Scholar]
- Guyant-Marechal I, Berger E, Laquerriere A, Rovelet-Lecrux A, Viennet G, Frebourg T, Rumbach L, Campion D, Hannequin D. 2008. Intrafamilial diversity of phenotype associated with app duplication. Neurology 71: 1925–1926. [DOI] [PubMed] [Google Scholar]
- Haass C. 2004. Take five—BACE and the γ-secretase quartet conduct Alzheimer’s amyloid β-peptide generation. EMBO J 23: 483–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C, Hung AY, Selkoe DJ, Teplow DB. 1994. Mutations associated with a locus for familial Alzheimer’s disease result in alternative processing of amyloid β-protein precursor. J Biol Chem 269: 17741–17748. [PubMed] [Google Scholar]
- Hardy J. 1997. Amyloid, the presenilins and Alzheimer’s disease. Trends Neurosci 20: 154–159. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. 2002. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 297: 353–356. [DOI] [PubMed] [Google Scholar]
- Hattori M, Fujiyama A, Taylor TD, Watanabe H, Yada T, Park HS, Toyoda A, Ishii K, Totoki Y, Choi DK, et al. 2000. The DNA sequence of human chromosome 21. Nature 405: 311–319. [DOI] [PubMed] [Google Scholar]
- Hendriks L, van Duijn CM, Cras P, Cruts M, Van Hul W, van Harskamp F, Warren A, McInnis MG, Antonarakis SE, Martin JJ, et al. 1992. Presenile dementia and cerebral haemorrhage linked to a mutation at codon 692 of the β-amyloid precursor protein gene. Nat Genet 1: 218–221. [DOI] [PubMed] [Google Scholar]
- Henrichsen CN, Chaignat E, Reymond A. 2009. Copy number variants, diseases and gene expression. Hum Mol Genet 18: R1–R8. [DOI] [PubMed] [Google Scholar]
- Hof PR, Bouras C, Perl DP, Sparks DL, Mehta N, Morrison JH. 1995. Age-related distribution of neuropathologic changes in the cerebral cortex of patients with Down’s syndrome. Quantitative regional analysis and comparison with Alzheimer’s disease. Arch Neurol 52: 379–391. [DOI] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. 1996. Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science 274: 99–102. [DOI] [PubMed] [Google Scholar]
- *.Johnson DS, Li Y-M, Pettersson M, St George-Hyslop PH. 2016. Structural and chemical biology of presenilin complexes. Cold Spring Harb Perspect Med 10.1101/cshperspect.a024067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson T, Atwal JK, Steinberg S, Snaedal J, Jonsson PV, Bjornsson S, Stefansson H, Sulem P, Gudbjartsson D, Maloney J, et al. 2012. A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline. Nature 488: 96–99. [DOI] [PubMed] [Google Scholar]
- Kaminsky YG, Marlatt MW, Smith MA, Kosenko EA. 2010. Subcellular and metabolic examination of amyloid-β peptides in Alzheimer disease pathogenesis: Evidence for Aβ(25–35). Exp Neurol 221: 26–37. [DOI] [PubMed] [Google Scholar]
- Kang J, Lemaire HG, Unterbeck A, Salbaum JM, Masters CL, Grzeschik KH, Multhaup G, Beyreuther K, Muller-Hill B. 1987. The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor. Nature 325: 733–736. [DOI] [PubMed] [Google Scholar]
- Kasuga K, Shimohata T, Nishimura A, Shiga A, Mizuguchi T, Tokunaga J, Ohno T, Miyashita A, Kuwano R, Matsumoto N, et al. 2009. Identification of independent APP locus duplication in Japanese patients with early-onset Alzheimer disease. J Neurol Neurosurg Psychiatry 80: 1050–1052. [DOI] [PubMed] [Google Scholar]
- Kero M, Paetau A, Polvikoski T, Tanskanen M, Sulkava R, Jansson L, Myllykangas L, Tienari PJ. 2013. Amyloid precursor protein (APP) A673T mutation in the elderly Finnish population. Neurobiol Aging 34: e1511–e1513. [DOI] [PubMed] [Google Scholar]
- Korenberg JR, Kawashima H, Pulst SM, Allen L, Magenis E, Epstein CJ. 1990. Down syndrome: Toward a molecular definition of the phenotype. Am J Med Genet 7: 91–97. [DOI] [PubMed] [Google Scholar]
- Kumar-Singh S, Cras P, Wang R, Kros JM, van Swieten J, Lubke U, Ceuterick C, Serneels S, Vennekens K, Timmermans JP, et al. 2002. Dense-core senile plaques in the Flemish variant of Alzheimer’s disease are vasocentric. Am J Pathol 161: 507–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy E, Carman MD, Fernandez-Madrid IJ, Power MD, Lieberburg I, van Duinen SG, Bots GT, Luyendijk W, Frangione B. 1990. Mutation of the Alzheimer’s disease amyloid gene in hereditary cerebral hemorrhage, Dutch type. Science 248: 1124–1126. [DOI] [PubMed] [Google Scholar]
- Levy E, Prelli F, Frangione B. 2006. Studies on the first described Alzheimer’s disease amyloid β mutant, the Dutch variant. J Alzheimers Dis 9: 329–339. [DOI] [PubMed] [Google Scholar]
- Ling Y, Morgan K, Kalsheker N. 2003. Amyloid precursor protein (APP) and the biology of proteolytic processing: Relevance to Alzheimer’s disease. Int J Biochem Cell Biol 35: 1505–1535. [DOI] [PubMed] [Google Scholar]
- Liu YW, He YH, Zhang YX, Cai WW, Yang LQ, Xu LY, Kong QP. 2014. Absence of A673T variant in APP gene indicates an alternative protective mechanism contributing to longevity in Chinese individuals. Neurobiol Aging 35: e911–e932. [DOI] [PubMed] [Google Scholar]
- Maloney JA, Bainbridge T, Gustafson A, Zhang S, Kyauk R, Steiner P, van der Brug M, Liu Y, Ernst JA, Watts RJ, et al. 2014. Molecular mechanisms of Alzheimer disease protection by the A673T allele of amyloid precursor protein. J Biol Chem 289: 30990–31000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann DM, Esiri MM. 1989. The pattern of acquisition of plaques and tangles in the brains of patients under 50 years of age with Down’s syndrome. J Neurol Sci 89: 169–179. [DOI] [PubMed] [Google Scholar]
- Mullan M, Crawford F, Axelman K, Houlden H, Lilius L, Winblad B, Lannfelt L. 1992. A pathogenic mutation for probable Alzheimer’s disease in the APP gene at the N-terminus of β-amyloid. Nat Genet 1: 345–347. [DOI] [PubMed] [Google Scholar]
- Nilsberth C, Westlind-Danielsson A, Eckman CB, Condron MM, Axelman K, Forsell C, Stenh C, Luthman J, Teplow DB, Younkin SG, et al. 2001. The “Arctic” APP mutation (E693G) causes Alzheimer’s disease by enhanced Aβ protofibril formation. Nat Neurosci 4: 887–893. [DOI] [PubMed] [Google Scholar]
- Obici L, Demarchi A, de Rosa G, Bellotti V, Marciano S, Donadei S, Arbustini E, Palladini G, Diegoli M, Genovese E, et al. 2005. A novel AβPP mutation exclusively associated with cerebral amyloid angiopathy. Ann Neurol 58: 639–644. [DOI] [PubMed] [Google Scholar]
- Ohno M, Sametsky EA, Younkin LH, Oakley H, Younkin SG, Citron M, Vassar R, Disterhoft JF. 2004. BACE1 deficiency rescues memory deficits and cholinergic dysfunction in a mouse model of Alzheimer’s disease. Neuron 41: 27–33. [DOI] [PubMed] [Google Scholar]
- Oyama F, Cairns NJ, Shimada H, Oyama R, Titani K, Ihara Y. 1994. Down's syndrome: up-regulation of β-amyloid protein precursor and tau mRNAs and their defective coordination. J Neurochem 62: 1062–1066. [DOI] [PubMed] [Google Scholar]
- Patterson D. 2009. Molecular genetic analysis of Down syndrome. Hum Genet 126: 195–214. [DOI] [PubMed] [Google Scholar]
- Pfeifer LA, White LR, Ross GW, Petrovitch H, Launer LJ. 2002. Cerebral amyloid angiopathy and cognitive function: The HAAS autopsy study. Neurology 58: 1629–1634. [DOI] [PubMed] [Google Scholar]
- Prasher VP, Farrer MJ, Kessling AM, Fisher EM, West RJ, Barber PC, Butler AC. 1998. Molecular mapping of Alzheimer-type dementia in Down’s syndrome. Ann Neurol 43: 380–383. [DOI] [PubMed] [Google Scholar]
- *.Prusiner SB. 2016. Aβ prions and pathobiology of Alzheimer’s disease. Cold Spring Harb Perspect Med 10.1101/cshperspect.a030676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmani Z, Blouin JL, Creau-Goldberg N, Watkins PC, Mattei JF, Poissonnier M, Prieur M, Chettouh Z, Nicole A, Aurias A, et al. 1989. Critical role of the D21S55 region on chromosome 21 in the pathogenesis of Down syndrome. Proc Natl Acad Sci 86: 5958–5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocchi A, Pellegrini S, Siciliano G, Murri L. 2003. Causative and susceptibility genes for Alzheimer's disease: A review. Brain Res Bull 61: 1–24. [DOI] [PubMed] [Google Scholar]
- Rossi G, Giaccone G, Maletta R, Morbin M, Capobianco R, Mangieri M, Giovagnoli AR, Bizzi A, Tomaino C, Perri M, et al. 2004. A family with Alzheimer disease and strokes associated with A713T mutation of the APP gene. Neurology 63: 910–912. [DOI] [PubMed] [Google Scholar]
- Rovelet-Lecrux A, Hannequin D, Raux G, Le Meur N, Laquerriere A, Vital A, Dumanchin C, Feuillette S, Brice A, Vercelletto M, et al. 2006. APP locus duplication causes autosomal dominant early-onset Alzheimer disease with cerebral amyloid angiopathy. Nat Genet 38: 24–26. [DOI] [PubMed] [Google Scholar]
- Rumble B, Retallack R, Hilbich C, Simms G, Multhaup G, Martins R, Hockey A, Montgomery P, Beyreuther K, Masters CL. 1989. Amyloid A4 protein and its precursor in Down’s syndrome and Alzheimer’s disease. N Engl J Med 320: 1446–1452. [DOI] [PubMed] [Google Scholar]
- Sadowski M, Wisniewski HM, Tarnawski M, Kozlowski PB, Lach B, Wegiel J. 1999. Entorhinal cortex of aged subjects with Down’s syndrome shows severe neuronal loss caused by neurofibrillary pathology. Acta Neuropathol 97: 156–164. [DOI] [PubMed] [Google Scholar]
- Salehi A, Delcroix JD, Belichenko PV, Zhan K, Wu C, Valletta JS, Takimoto-Kimura R, Kleschevnikov AM, Sambamurti K, Chung PP, et al. 2006. Increased App expression in a mouse model of Down’s syndrome disrupts NGF transport and causes cholinergic neuron degeneration. Neuron 51: 29–42. [DOI] [PubMed] [Google Scholar]
- Sandbrink R, Masters CL, Beyreuther K. 1996. APP gene family. Alternative splicing generates functionally related isoforms. Ann NY Acad Sci 777: 281–287. [DOI] [PubMed] [Google Scholar]
- Sleegers K, Brouwers N, Gijselinck I, Theuns J, Goossens D, Wauters J, Del-Favero J, Cruts M, van Duijn CM, Van Broeckhoven C. 2006. APP duplication is sufficient to cause early onset Alzheimer’s dementia with cerebral amyloid angiopathy. Brain 129: 2977–2983. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Cheung TT, Cai XD, Odaka A, Otvos L Jr, Eckman C, Golde TE, Younkin SG. 1994. An increased percentage of long amyloid β protein secreted by familial amyloid β protein precursor (β APP717) mutants. Science 264: 1336–1340. [DOI] [PubMed] [Google Scholar]
- Tamaoka A, Odaka A, Ishibashi Y, Usami M, Sahara N, Suzuki N, Nukina N, Mizusawa H, Shoji S, Kanazawa I, et al. 1994. APP717 missense mutation affects the ratio of amyloid β protein species (Aβ1-42/43 and Aβ1-40) in familial Alzheimer’s disease brain. J Biol Chem 269: 32721–32724. [PubMed] [Google Scholar]
- Tanzi RE, Gusella JF, Watkins PC, Bruns GA, St George-Hyslop P, Van Keuren ML, Patterson D, Pagan S, Kurnit DM, Neve RL. 1987. Amyloid β protein gene: cDNA, mRNA distribution, and genetic linkage near the Alzheimer locus. Science 235: 880–884. [DOI] [PubMed] [Google Scholar]
- Teller JK, Russo C, DeBusk LM, Angelini G, Zaccheo D, Dagna-Bricarelli F, Scartezzini P, Bertolini S, Mann DM, Tabaton M, et al. 1996. Presence of soluble amyloid β-peptide precedes amyloid plaque formation in Down’s syndrome. Nat Med 2: 93–95. [DOI] [PubMed] [Google Scholar]
- Thonberg H, Fallstrom M, Bjorkstrom J, Schoumans J, Nennesmo I, Graff C. 2011. Mutation screening of patients with Alzheimer disease identifies APP locus duplication in a Swedish patient. BMC Res Notes 4: 476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Tycko R. 2016. Molecular structure of aggregated amyloid-β: Insights from solid-state nuclear magnetic resonance. Cold Spring Harb Perspect Med 8: a024083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duinen SG, Castano EM, Prelli F, Bots GT, Luyendijk W, Frangione B. 1987. Hereditary cerebral hemorrhage with amyloidosis in patients of Dutch origin is related to Alzheimer disease. Proc Natl Acad Sci 84: 5991–5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltfang J, Esselmann H, Bibl M, Smirnov A, Otto M, Paul S, Schmidt B, Klafki HW, Maler M, Dyrks T, et al. 2002. Highly conserved and disease-specific patterns of carboxyterminally truncated Aβ peptides 1–37/38/39 in addition to 1–40/42 in Alzheimer’s disease and in patients with chronic neuroinflammation. J Neurochem 81: 481–496. [DOI] [PubMed] [Google Scholar]
- Wisniewski KE, Wisniewski HM, Wen GY. 1985. Occurrence of neuropathological changes and dementia of Alzheimer’s disease in Down’s syndrome. Ann Neurol 17: 278–282. [DOI] [PubMed] [Google Scholar]
- Wisniewski T, Ghiso J, Frangione B. 1991. Peptides homologous to the amyloid protein of Alzheimer’s disease containing a glutamine for glutamic acid substitution have accelerated amyloid fibril formation. Biochem Biophys Res Commun 180: 1528. [DOI] [PubMed] [Google Scholar]
- Wolfe MS, Guenette SY. 2007. APP at a glance. J Cell Sci 120: 3157–3161. [DOI] [PubMed] [Google Scholar]
- Yoshikai S, Sasaki H, Doh-ura K, Furuya H, Sakaki Y. 1990. Genomic organization of the human amyloid β-protein precursor gene. Gene 87: 257–263. [DOI] [PubMed] [Google Scholar]
- Younkin SG. 1994. The amyloid β protein precursor mutations linked to familial Alzheimer’s disease alter processing in a way that fosters amyloid deposition. Tohoku J Exp Med 174: 217–223. [DOI] [PubMed] [Google Scholar]
- Zarrei M, MacDonald JR, Merico D, Scherer SW. 2015. A copy number variation map of the human genome. Nat Rev Genet 16: 172–183. [DOI] [PubMed] [Google Scholar]
- Zigman WB. 2013. Atypical aging in Down syndrome. Dev Disabil Res Rev 18: 51–67. [DOI] [PubMed] [Google Scholar]